Abstract
Tumor-infiltrating immune cells can promote chemoresistance and metastatic spread in aggressive tumors. Consequently, the type and quality of immune responses present in the neoplastic stroma are highly predictive of patient outcome in several cancer types. In addition to host immune responses, intrinsic tumor cell activities that mimic stem cell properties have been linked to chemoresistance, metastatic dissemination and the induction of immune suppression. Cancer stem cells are far from a static cell population; rather, their presence appears to be controlled by highly dynamic processes that are dependent on cues from the tumor stroma. However, the impact immune responses have on tumor stem cell differentiation or expansion is not well understood. In this study, we demonstrate that targeting tumor-infiltrating macrophages and inflammatory monocytes by inhibiting either the myeloid cell receptors CSF1R or CCR2 decreases the number of tumor-initiating cells in pancreatic tumors. Targeting CCR2 or CSF1R improves chemotherapeutic efficacy, inhibits metastasis and increases anti-tumor T-cell responses. Tumor-educated macrophages also directly enhanced the tumor-initiating capacity of pancreatic tumor cells by activating the transcription factor STAT3, thereby facilitating macrophage-mediated suppression of CD8+ T lymphocytes. Together, our findings show how targeting tumor-infiltrating macrophages can effectively overcome therapeutic resistance mediated by tumor-initiating cells.
Keywords: macrophage, chemoresistance, stem cell, pancreatic cancer, innate immunity, cytokines
Introduction
Tumor-infiltrating immune cells are a hallmark of most solid tumors, and the presence of varied immune populations significantly affects clinical outcomes for cancer patients (1, 2). Historically, tumor-infiltrating immune cells have been viewed as restraining tumor progression (3), but in recent years, it has become more widely appreciated that chronic immune responses play critical roles in promoting tumor progression, metastasis, and resistance to cytotoxic therapies (1). Therefore, understanding the molecular mechanisms by which malignant cells derail antitumor immune responses to favor disease progression is critical to identify potential therapeutic targets. Recently, we reported that selective depletion of tumor-infiltrating macrophages by neutralizing colony stimulating factor-1 (CSF1) or inhibiting CSF1 receptor (CSF1R) activity improves the efficacy of chemotherapy in mammary tumors, in part by instigating antitumor responses by CD8+ T cells (4). Similarly, deficiency in the CSF1 gene in op/op mice leads to decreased mammary tumor metastasis and slows pancreatic neuroendocrine tumor development (5, 6). Although the potent capacity of macrophages to induce tumor progression has been well established, the mechanisms by which macrophage affect chemoresistance are not well defined.
In addition to immune regulation of cancer progression and chemoresistance, tumor cells that acquire stem-like or tumor-initiating properties (often called “cancer stem cells”) exhibit enhanced resistance to cytotoxic therapy and increased propensity for metastatic dissemination (7, 8). Several lines of evidence suggest that the tumor-initiating capacity of malignant cells is rooted in inflammatory signals (9). However, the mechanisms by which different populations of leukocytes might support the expansion of tumor-initiating cells (TICs) are unknown. One possibility is that reciprocal crosstalk between tumor-infiltrating leukocytes and malignant cells regulates the development of cells with stem-like properties, which in turn facilitates resistance to therapeutic interventions. A recent study showed that macrophages can induce tumor stem-like properties in vitro in murine lung and colon cancer cell lines (10). However, it is unclear whether this interaction can be exploited pharmacologically, and if so, whether it also affects tumor-derived immunosuppression.
In this study, we investigated the mechanisms by which macrophages and TICs collaborate to regulate pancreatic ductal adenocarcinoma (PDAC) progression, immunosuppression, and responses to chemotherapy. We demonstrate that targeting tumor-infiltrating macrophages (TAMs) by inhibiting either CSF1R or chemokine (C-C motif) receptor 2 (CCR2) decreases the numbers of pancreatic TICs and improves chemotherapeutic efficacy in vivo. We also found that tumor-infiltrating macrophages directly induce TIC properties in pancreatic cancer cells by activating signal transducer and activator of transcription 3 (STAT3). In turn, TICs induce immunosuppressive behavior in TAMs and thus block antitumor CD8+ T lymphocyte responses during chemotherapeutic treatment.
Methods
Pancreatic cancer tissue microarray (TMA) cohort and analysis
TMA studies were conducted on a patient cohort constructed from 60 cases of invasive PDAC diagnosed at the Department of Pathology at Washington University. Patients had not received neoadjuvant therapy and underwent pancreaticoduodenectomy, typically followed by adjuvant chemotherapy. To assemble tissue microarrays, clearly defined areas of tumor tissue were demarked and two biopsies (1.0-mm diameter) taken from each donor block. We used 4.0-mm paraffin sections for immunohistochemical analyses. The Washington University School of Medicine ethical committee approved this study. Fully automated image acquisition was employed using Aperio ScanScope XT Slide Scanner (Aperio Technologies) system to capture whole-slide digital images with a ×20 objective. A tumor-specific nuclear algorithm (IHC-MARK) developed in-house was modified to quantify CD8 and CD68 expression as previously published (4, 11).
CCR2 kinase, CSF1R kinase, and STAT3 inhibitors
CCR2 inhibitor PF-04136309 was provided by Pfizer and administered to mice at a concentration of 100 mg/kg in twice-daily subcutaneous injections. PF-04136309 details have been previously published (12). Inhibitors of CSF1R1 and CSF1R2 were provided by Plexxikon Inc. CSF1Ri1 is PLX6134 that contains the GW2580 compound, which was described in detail elsewhere (13). CSF1Ri2 is PLX3397, a selective bispecific inhibitor for c-Fms and the c-Kit receptor tyrosine kinases, with biochemical IC50 values of 0.02 and 0.01 μM, respectively. PLX3397 was used as a confirmatory compound for PLX6134/GW2580 with better specificity for CSF1R, and details were presented elsewhere (4, 14, 15). Both CSF1R inhibitors were administered to mice in a formulated diet at a concentration of 800 mg/kg chow. STATIC was obtained from Calbiochem/EMD, used at doses less than the reported IC50 (<10 μM) in vitro, and handled according to manufacturer instructions.
Cell lines and constructs
PAN02 cells were obtained from Dr. Linehan, KCM cells from Dr. Mukherjee, and Kras-INK from Dr. Hanahan’s laboratory and have been published else where (16–18). Briefly, KCM and Kras-INK were derived from pancreatic adenocarcinomas from p48-CRE/LSL-KrasG12D/Muc1.Tg (17, 18)) and p48-CRE/LSL-KrasG12D/INK4aflox (18, (19) mice respectively. All cell lines were Mycoplasma tested and labeled with a polycistronic click beetle red luciferase-mCherry reporter by lentiviral infection, and positive cells were selected by FACS sorting. These constructs were supplied by Dr. Piwnica-Worms and the BRIGHT Institute.
Orthotopic model and preclinical animal cohorts
Syngeneic orthotopic PDAC tumors were established by surgical implantation as previously described (20). Briefly, we injected 50,000–200,000 cells with 50 μl Matrigel (BD-Biosciences) and cohorts of mice were randomized to treatment groups by bioluminescence imaging of click beetle red luciferase at day 21 (21) or gross palpation of the pancreas. Gemcitabine (GEM; Hospira) was obtained from the Washington University School of Medicine pharmacy and diluted in phosphate-buffered-saline. Mice were treated with 50 mg/kg GEM by intravenous injection into the right retro-orbital sinus every 4–5 days. Preclinical studies were conducted with 10–15 age-matched 10-week-old female mice/group. In survival studies, a death event was classified as a loss of 15% of body weight or poor body conditioning score. Disease and tumor burden were measured by the gross wet weight of the pancreas. Metastatic and disseminated tumors were scored by gross evaluation, which was validated by either bioluminescence or tissue pathology.
Additional methodological detail is in Supplemental Data.
Results
ALDH+ PDAC cells have high tumor-initiating capacity
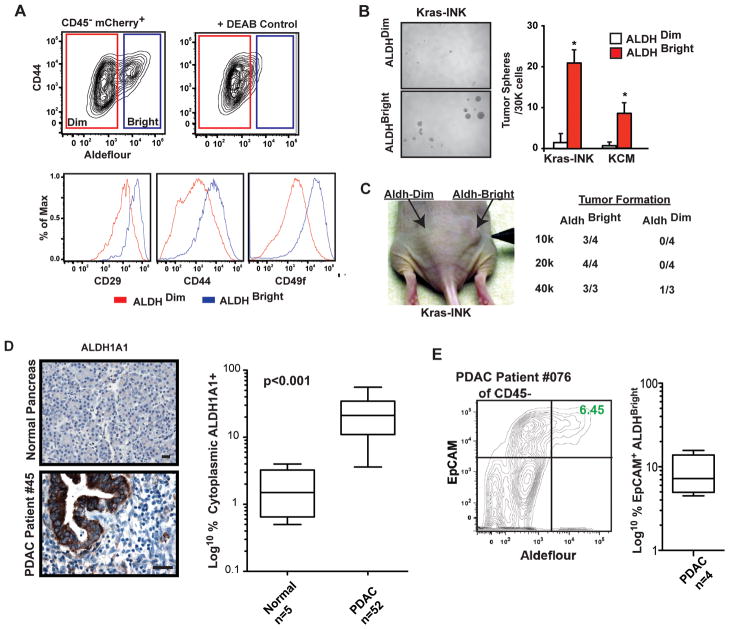
To investigate how macrophages impact the presence of TICs, we utilized three distinct murine pancreatic tumor cell lines, denoted as Kras-INK, KCM, and PAN02, derived from PDACs arising from genetic models (p48-CRE/LSL-Kras/INK4Aflox/wt or Pdx-CRE/LSL-Kras/Tg-Muc1) or tumors arising from 3-methylcholanthrene carcinogenesis (PAN02) (16, 17, 22). To identify potential TIC cellular subsets, these cell lines were labeled with mCherry and CBR luciferase and analyzed for EpCAM, CD24, CD44, CD29, CD49f, CD133, and cMet expression and Aldefluor activity (a measure of aldehyde dehydrogenase activity) using mCherry to identify implanted tumor cells in vivo. Our analysis revealed a distinct population of tumor cells with high Aldefluor activity (ALDHBright, Figure 1A). No distinct populations of CD133+ or cMet+ cells were observed. Analysis of cell sorted from orthotopic Kras-INK and KCM PDAC tumors illustrated that ALDHBright cells express higher levels of CD29, CD44, and CD49f, display increased tumor spheroid formation in vitro, and have increased tumorigenic potential in nude mice (Figure 1A–C, data not shown). Analysis of fresh human PDAC tissue also revealed an identifiable population of ALDHBright tumor cells with a frequency ranging from 2–15% of the total CD45− EpCAM+ cells (Figure 1E). Immunohistochemical (IHC) analysis of ALDH1A1 revealed a significant increase in positive cells in PDAC tissue compared to the normal pancreas (Figure 1D). These results are consistent with previous reports demonstrating the tumorigenic potential of ALDH1+ cells in human tumors (23, 24).
Figure 1. ALDH1+ PDAC cells have high tumor-initiating potential.
A) FACS analysis of ALDHBright cells from Kras-INK tumors. Plots of cells gated on CD45−mCherry+ and stained with Aldefluor ± a DEAB inhibitor. ALDHBright (blue) and ALDHDim (red) cells are depicted.
B) Analysis tumor spheroid formation from ALDHBright and ALDHDim Kras-INK and KCM cells. The mean number of tumor spheroids formed after 2 weeks is depicted.
C) Paired aliquots of FACS-sorted ALDHBright and ALDHDim Kras-INK cells were injected into subcutaneous in nude mice, and tumor formation was accessed.
D) Automated quantitation of cytoplasmic ALDH1A1+ cells in normal human pancreas and PDAC tissues (n=52 PDAC and 4 normal).
E) Flow cytometry analysis of human PDAC tissue gated on CD45−and stained for EpCAM and Aldefluor positivity.
All error bars are SEM, and * denotes p<0.05 by Mann-Whitney.
Depletion of TAMs decreased the presence of ALDHBright TICs
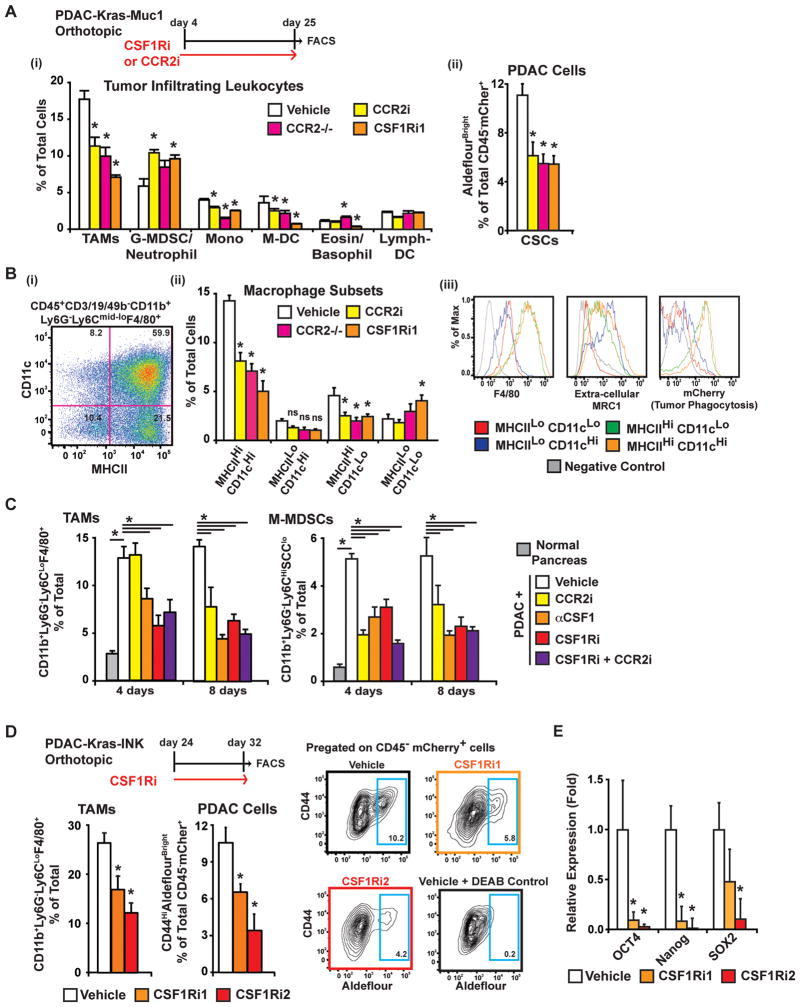
To determine whether targeting tumor-infiltrating macrophages alters the frequency of TICs, we treated mice bearing orthotopic KCM tumors with two CSF1R tyrosine kinase antagonists and a CCR2 antagonist and used CCR2 knockout mice (CCR2−/−). The CSF1R kinase inhibitors used were PLX6134, a preparation of the GW2580 compound (CSF1Ri1), and PLX3397 (CSF1Ri2). To test CCR2 antagonism, we used PF04136309 (CCR2i). Additional details and structures for these compounds are in the methods and published elsewhere (12–15, 25). Analysis of tumor tissue after 21 days of treatment revealed significant reductions in numbers of tumor-infiltrating CD11b+Ly6G−Ly6CLoF4/80HiMHCII+ macrophages, CD11b+Ly6G−Ly6CHiF4/80MidMHCII+ inflammatory monocytes, and CD11b+Ly6G−Ly6CloCD11cHiMHCIIHi dendritic cells (DCs; presumably myeloid-derived DCs) in response to blockade of CCR2 or CSF1R signaling. By contrast, we observed no alteration in the number of CD11bLoLy6G−Ly6C−CD11cHiMHCIIHi cells (presumably lymphoid-derived DCs), and the numbers of CD11b+Ly6GHiLy6C+ immature granulocytes/neutrophils were modestly increased (~30%; Figure 2A). Despite possible cellular diversity, we will use the terms granulocytic myeloid-derived suppressor cells (G-MDSCs) for CD11b+Ly6GHiLy6C+ cells and monocytic MDSCs (M-MDSCs) for CD11b+Ly6G−Ly6CHiF4/80MidMHCII+ cells.
Figure 2. Depletion of TAMs results in reduced ALDHBright TICs.
A) Analysis of leukocyte and ALDHBright TIC frequency in KCM tumors from mice treated for 21 days with vehicle, CSF1Ri1, CCR2i or in CCR2−/− hosts. (i) The presence of CD11b+Ly6G− Ly6CLoF4/80HiMHCII+ macrophage, CD11b+Ly6GHiLy6C+ (G-MDSC/neutrophil), CD11b+Ly6G− Ly6CHiF4/80+MHCII+SSCLo monocyte (mono), CD11b+Ly6G− Ly6CLoCD11cHiMHCIIHi (M-DC), CD11b+Ly6G+Ly6C− MHCII+ (basophil), and CD11bLoLy6G−Ly6C− CD11cHiMHCIIHi (Lymph-DC) subsets is depicted as the mean % of total live cells. (ii) ALDHBright TICs are depicted as the mean % of total live CD45−mCherry+ cells.
B) Analysis of macrophage subsets following CCR2 or CSF1R inhibition. (i) CD11b+CD3/19/49b− Ly6G−Ly6CLoF4/80+ macrophages were subdivided by MHCII and CD11c expression, and (ii) the mean frequency of each subset is displayed for all treatment groups. (iii) Relative expression of F4/80, CD206, and mCherry (indicator of phagocytosis) is depicted.
C) Flow cytometry analysis of TAMs and M-MDSCs infiltrating PAN02 tumors in mice treated for 4 or 8 days with vehicle, anti-CSF1, CCR2i, and/or CSF1Ri2 is depicted.
D) The mean frequency of macrophages and CD45−mCherry+ALDHBright TICs in Kras-INK tumors following 8 days of CSF1Ri treatment is depicted. Representative flow cytometry plots of mCherry+ ALDHBright tumor cells (Blue gate) are shown.
E) Quantitative-RT-PCR analysis in orthotopic Kras-INK tumor tissue following treatment with CSF1Ri for 14 days. Graph depicts the mean fold change compared vehicle.
Flow cytometry and quantitative-RT-PCR data depict the mean values from 5–10 mice ± SEM. * denotes statistically significant differences at p<0.05 (Mann-Whitney U-test).
Analysis of the impact of CCR2 or CSF1R inhibition on TAM subsets found that these inhibitors significantly deplete macrophages expressing high levels of MHCII, but not MHCIILo−or Tie2+ TAMs (Figure 2B, S1A). Analysis of the MHCIIHi macrophages demonstrated that these cells express the highest levels of F4/80, display high tumor phagocytosis as measured by mCherry fluorescence, and express modest levels of extracellular MRC1 (features consistent with mature macrophages).
To determine whether CSF1R and CCR2 blockade have differential effects on tumor-infiltrating myeloid cells, we analyzed the impact of CSF1R and CCR2 inhibitors as single agents or in combination (Figure 2C). Mice bearing established (~1 cm) PDAC tumors were treated for 4 or 8 days with CCR2i, anti-CSF1 IgG (5A1), CSF1Ri2, or CCR2i plus CSF1Ri2. Analysis of tumor-infiltrating M-MDSCs and TAMs revealed no additive effects of combined inhibition. Individually, anti-CSF1, CSF1Ri, and CCR2i all effectively depleted monocytes within 4 days. However, blockade of CSF1/CSF1R depleted mature TAMs in the first 4 days, whereas CCR2 inhibition did not effectively deplete TAMs until after 8 days of treatment. These results suggest that CSF1R and CCR2 have redundant rather than divergent activities on depleting TAMs and M-MDSCs.
To assess the effects of these inhibitors on TICs, we performed a parallel analysis of the KCM tumor cells, identified by CD45−mCherry+, and found a 40–55% reduction in CD44HiALDHBright TICs following CCR2 or CSF1R blockade (Figure 2Aii). To determine whether macrophage depletion can rapidly alter the presence of ALDHBright TICs, we treated mice bearing ~1-cm Kras-INK tumors with CSF1R inhibitors for 8 days and found a 40–70% decrease in CD44HiALDHBright TICs (Figure 2C). We observed similar results in orthotopic PAN02 tumors (Figure S1B). Correlating with these observed decreases in ALDHBright cells, we found that OCT4, Nanog, and SOX2 mRNA expression was decreased following treatment with CSF1R or CCR2 inhibitors (Figures 2E, S1C). Notably, IHC and mRNA analysis of CCR2 or CSF1R expression in these PDAC tumors revealed they do not express significant levels of CCR2 or CSF1R in vivo and in vitro (Figure S1D–E). Taken together, these results suggest that targeting TAMs can rapidly reduce the numbers of ALDHBright TICs.
TAMs can directly enhance the tumor-initiating properties of PDAC cells
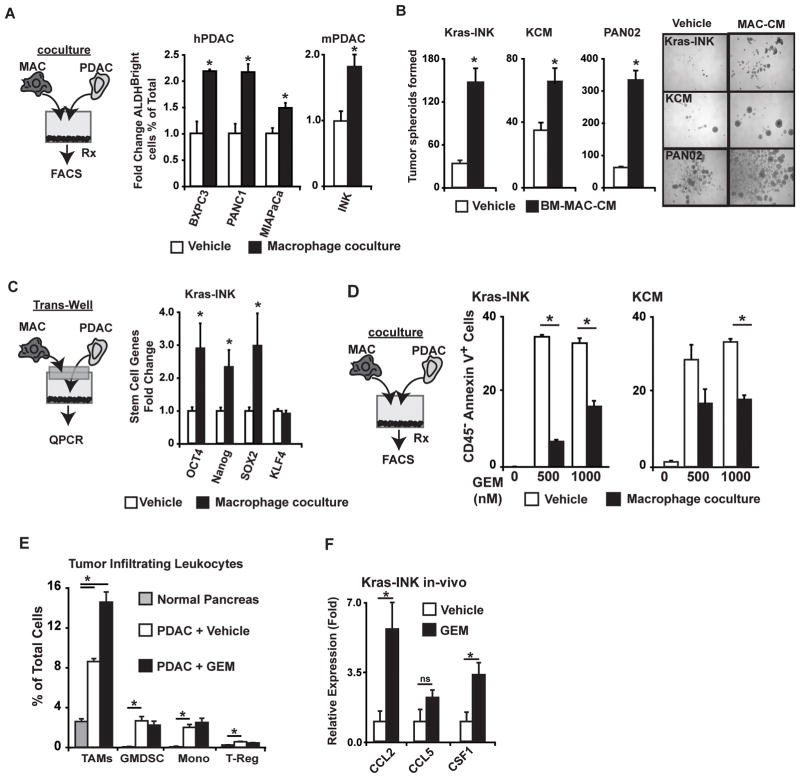
To determine whether macrophages can directly enhance the tumor-initiating properties of pancreatic cancer cells, we cocultured macrophages with PDAC cells. Coculture with macrophages increased the frequency of ALDHBright cells in murine and human PDAC cell lines (Figure 3A). To determine whether soluble factors derived from tumor-educated macrophages enhanced TIC properties, we first cultured bone-marrow-derived macrophages (BM-MACs) in PDAC-conditioned medium (CM) and then used the resultant “tumor-educated” BM-MACs to create CM for tumor spheroid assays. CM from tumor-activated BM-MACs enhanced the formation of tumor spheres in PAN02, Kras-INK, and KCM cells (Figure 3B). Similar results were also seen in Kras-INK cells cultured in Transwells with BM-MACs (Figure S2A). Consistent with enhanced tumor-initiating properties, we observed that BM-MAC coculture increased CD29 and CD49f protein and OCT4, Nanog, and SOX2 mRNA expression in PDAC cells (Figures 3C, S2B).
Figure 3. Macrophages promote TIC properties in vitro.
A) Analysis of ALDHBright cells frequency in human and mouse PDAC cells cocultured with human blood monocyte-derived macrophages or murine BM-MACs for 36 h. Mean fold changes in CD45−ALDHBright cells is depicted.
B) Quantitation of PDAC tumor spheroids following 14 days of culture in vehicle or BM-MAC conditioned medium.
C) Quantitative-RT-PCR results from Kras-INK tumor cells cocultured for 24 h with BM-MACs in a Transwell chamber. Normalized mean fold changes is depicted.
D) Kras-INK or KCM cells in coculture with BM-MACs were treated with GEM for 36hrs. The mean % of CD45−mCherry+Annnexin V+ tumor cells is depicted (n=3).
E) Analysis of immune cell infiltration following GEM treatment of mice bearing Kras-INK tumors. The mean frequency of macrophages, G-MDSC/neutrophil, monocytes (mono), and CD3+CD4+FOXP3+ TRegs is depicted (n=5 mice/group).
F) Analysis of mRNA expression from Kras-INK tumor tissue from mice treated with either vehicle or GEM. Normalized mean fold change is depicted (n=5 mice/group).
All graphs displayed as means ± SEM, and * denotes p<0.05 (Mann-Whitney U-test).
Another feature commonly associated with TICs is increased resistance to chemotherapy. Fitting this, ALDHBright cells isolated from Kras-INK tumors displayed decreased response to Gemcitabine (GEM; Figure S2C). To elucidate whether macrophages can enhance the resistance to chemotherapy, we treated PDAC cells with GEM and found reduced numbers of Annexin V+ cells when BM-MACs were present in coculture (Figures 3D, S2D). Similar results were observed using a Transwell system or BM-MAC-CM (Figure S2E–F). Together, these results suggest that tumor-educated macrophages produce soluble factors that can regulate both tumor-initiating capacity and chemoresistance in PDAC cells.
GEM treatment increases macrophage infiltration into PDAC tumors
Common chemotherapeutics have been reported to induce the recruitment of myeloid cells to regressing tumors (4, 26, 27). To assess whether GEM treatment alters myeloid cell recruitment in PDAC tumors, we analyzed pancreatic tissue from normal mice or mice bearing Kras-INK tumors ± GEM treatment. The numbers of TAMs, G-MDSCs, inflammatory monocytes, and CD4+FOXP3+ regulatory T cells (TRegs) were increased by the presence of PDAC tumors, but only TAMs increased in number following GEM treatment (Figure 3E). Corresponding with increased TAM infiltration, CSF1 and CCL2 but not CCL5 were upregulated by GEM treatment (Figure 3F). Taken together, these results suggest that blockade of CCR2 and/or CSF1R would improve the response to GEM in PDAC tumors.
Targeting TAMs enhances the response to chemotherapy and reduces metastasis
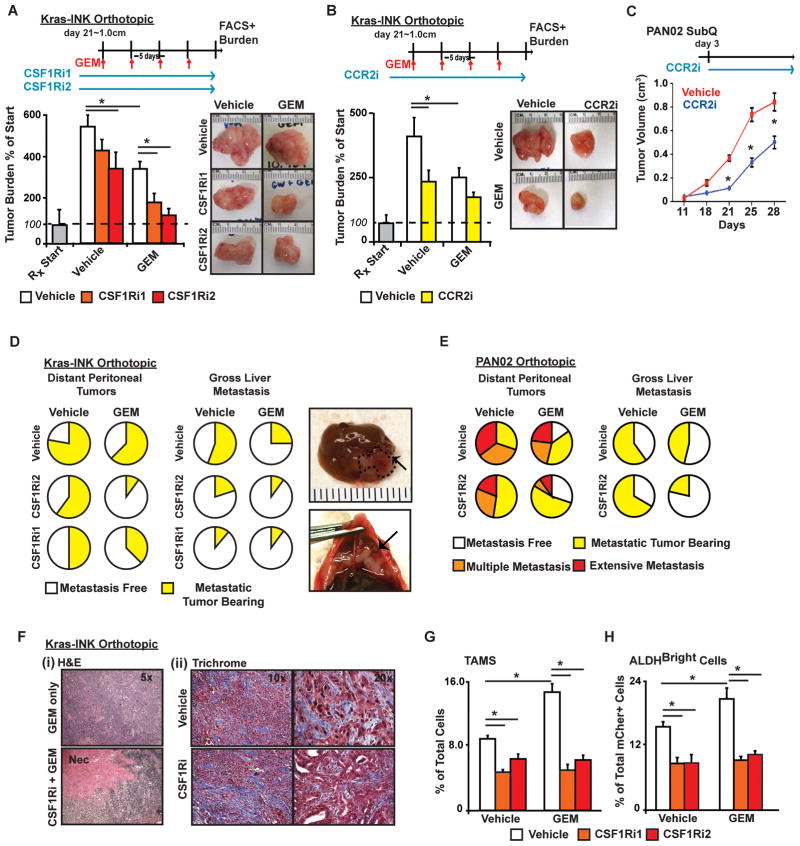
CSF1R and CCR2 inhibitors as single agents modestly slow PDAC tumor growth, similar to GEM therapy (Figures 4A–C, S3A–C). Similar results were also observed in CCR2−/− mice (Figure S3B), suggesting that these effects are due to alterations in the tumor stroma. To determine whether inhibiting CSF1R or CCR2 could improve responses to chemotherapy, we treated mice bearing established orthotopic Kras-INK or Pan02 tumors with GEM alone or in combination with CSF1R or CCR2 inhibitors. We found that CSF1Ri plus GEM dramatically slowed tumor progression in both Pan02 and Kras-INK orthotopic tumors (Figures 4A, S3A,C). For example, in Kras-INK tumors, GEM reduced tumor growth (compared to parallel mice sacrificed at the start of treatment, “Rx Start”) by 32% compared to vehicle-treated tumors, whereas CSF1Ri plus GEM reduced tumor growth by 81%. Similar but somewhat less dramatic results were obtained with GEM in combination with CCR2i (Figure 4B). Similar results were observed with paclitaxel plus CSF1Ri2 (Figure S3C). Detailed analysis of primary tumor pathology revealed a high level of necrosis in tumors from mice treated with GEM plus CSF1Ri, but not either agent alone. No alterations in stromal desmoplasia were observed (Figure 4F).
Figure 4. Inhibition of CSF1R or CCR2 overcomes chemoresistance.
A–B) Mice bearing established Kras-INK tumors were treated with vehicle or CSF1Ri1, CSF1Ri2, or CCR2i (B) ± GEM. The tumor burden was accessed by wet tumor weight, and it is displayed as the mean % increase compared to five mice sacrificed at the start of treatment (“RXStart”).
C) The growth of subcutaneous PAN02 tumors in mice treated with ± CCR2i is depicted (n=5/group).
D–E) The frequency and/or severity of disseminated tumors in the perennial cavity or liver is depicted as the proportion of mice with gross tumors at necropsy (n=10–15 mice/group).
F) (i) Images of hematoxylin and eosin staining depicts necrotic tissue (Nec) following CSF1Ri and GEM treatment, and (ii) TriChrome staining depicts collagen deposition (blue).
G–H) Flow cytometry analysis of TAM and ALDHBright TIC frequency in Kras-INK tumors is depict the mean % of the total number of live cells or CD45−mCherry+ cells (n=5–8 mice/group). * denotes p<0.05 (Mann-Whitney U-test or unpaired t-test) in all panels.
Similar to human PDAC, orthotopic Kras-INK and Pan02 tumors develop hepatic and peritoneal metastatic disease. In mice bearing Kras-INK tumors, GEM or CSF1R inhibition alone reduced peritoneal metastases, and combined treatment regimens had an additive effect (Figure 4D). We observed similar results in the Pan02 model (Figure 4E). GEM or CSF1R inhibition alone also decreased the frequency of hepatic metastases; however, an additive reduction in the liver metastatic frequency was only observed in the Pan02 model (Figure 4D–E). Consistent with these results, CSF1Ri plus GEM also increased the survival of Pan02 tumor-bearing mice (Figure S3D).
Paralleling the observed efficacy of CSF1Ri plus GEM, although GEM treatment significantly increased the frequency of both TAMs and ALDHBright TICs, this increase was abrogated by CSF1R inhibition (Figure 4G–H). Similar but less dramatic effects were observed with CCR2 inhibition (Figure S3E). Taken together, these results suggest that blocking macrophage infiltration into tumors during therapy reduces the number of TICs and improves the efficacy of chemotherapy.
Depletion of TAMs results in increased cytotoxic T lymphocyte response during chemotherapy
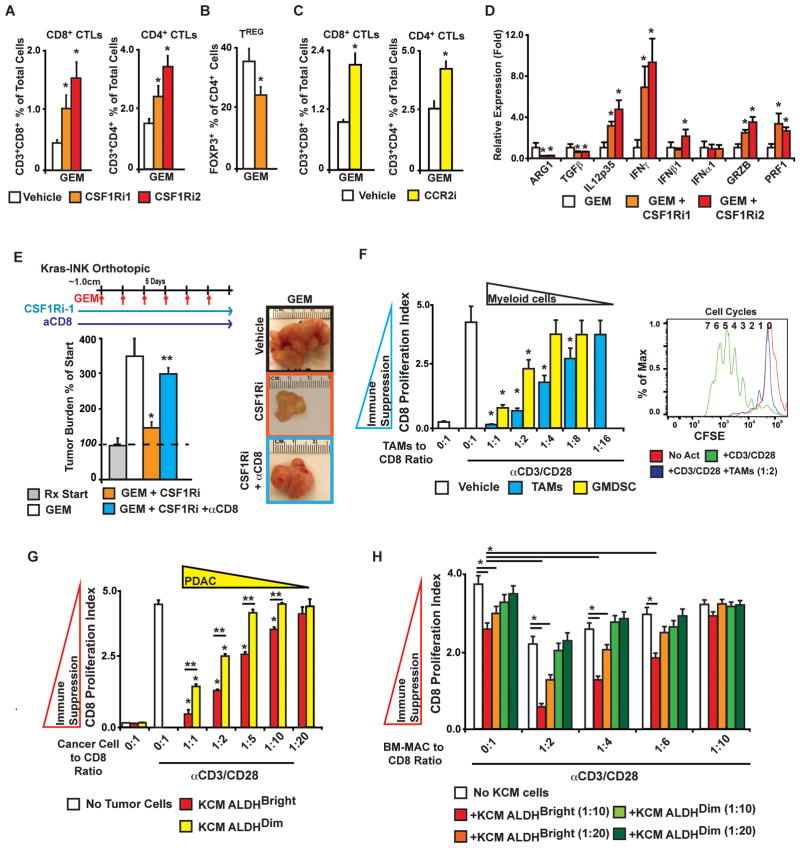
Previous studies demonstrated that TAMs have significant immunosuppressive capacity (4, 28, 29). To determine whether macrophage depletion would restore antitumor T-cell activity in PDAC tumors, we analyzed tumor-infiltrating T lymphocytes in mice treated with CCR2i or CSF1Ri ±GEM. Analysis of tumor-infiltrating lymphocytes revealed significantly increased CD4+ and CD8+ T-cell and reduced FOXP3+ TReg infiltration when CSF1Ri or CCR2i was given in combination with GEM (Figure 5A–C). Thus, despite eliciting decreased tumor growth, single-agent GEM, CCR2i, or CFS1Ri did not alter T-cell infiltration, suggesting that both tumor cell destruction by chemotherapy and macrophage depletion are necessary to sustain cytotoxic T lymphocyte (CTL) infiltration. Consistent with elevated CTL responses, we also observed increased interferon-γ, interferon-β, granzyme B, perforin, and interleukin (IL)-12 p35 and decreased transforming growth factor-β and arginase-1 mRNA expression in tumors (Figure 5D). To determine the role of CD8+ T lymphocytes in the efficacy of combined therapy, we used CD8-depleting antibodies (clone 2.43) in the context of CSF1Ri plus GEM treatment. While treatment with CSF1Ri1 plus GEM significantly blunted tumor growth compared to the effects of GEM alone (−80%), this therapeutic efficacy was largely dependent on CD8+ T lymphocytes (Figure 5E).
Figure 5. TAM and TIC crosstalk to suppress CTLs.
A–C) Analysis of CD3+CD4+ and CD3+CD8+ T-cell (A, C) and FOXP3+ CD4+ Treg (B) infiltration into Kras-INK tumors from mice is depicted as the mean % of total live cells (n=5–6 mice/group).
D) Cytokine mRNA expression assessed in Kras-INK tumors from mice treated with GEM ± CSF1Ri is depicted as mean normalized fold change compared to the GEM alone treatment group (5 animals/group).
E) Mice bearing orthotopic Kras-INK tumors were treated with GEM ± CSF1Ri2 ± anti-CD8IgG. Tumor burden is depicted as the mean % increase compared to the start of treatment (“RXStart”). n=10–14 mice/group.
F) CD8+ CTL suppression by tumor-infiltrating leukocytes. TAMs or G-MDSCs isolated by flow sorting were assayed for their ability to repress splenic CD8+ proliferation following anti-CD3/CD28 stimulation. The mean number of proliferation cycles is measured by CFSE dilution after 70 h of activation. A representative plot of CFSE signal intensity in CD8+ cells is depicted for un-activated, activated, and TAM cocultures (right panel).
G) CD8+ CTL suppression by TICs. Isolated ALDHBright and ALDHDim KCM cells assayed for their ability to repress CD8+ proliferation following anti-CD3/CD28 stimulation.
H) Suppression of CD8+ proliferation was assayed in cocultures with BM-MACs and/or isolated ALDHBright and ALDHDim KCM cells. The ratio of all cells to CD8+ CTLs is depicted.
* or ** denotes p<0.05 by Mann-Whitney in all panels.
TAMs and TICs crosstalk to repress CD8+ T lymphocytes
Immunosuppression in the tumor microenvironment can be mediated by TAMs, M-MDSCs, G-MDSCs, TRegs, and immature DCs. Although TAMs are the most prevalent of these cells, two recent reports demonstrated that G-MDSCs mobilized by tumor-derived granulocyte-macrophage colony-stimulating factor (GM-CSF) can also suppress CTL activation in PDAC tumors (30, 31). To assess the immunosuppressive capacity of these leukocytes, we isolated tumor-infiltrating TAMs and G-MDSCs from Kras-INK tumors and compared their ability to suppress CD8+ T lymphocyte proliferation (by CFSE dilution) following polyclonal anti-CD3/CD28 activation. Although both TAMs and G-MDSCs are capable of suppressing CD8+ T-cell proliferation, TAMs were slightly more suppressive at lower dilutions (Figure 5F).
Enhanced T cell-suppressive activity in TICs has been reported in glioblastoma (32). To test if PDAC TICs are highly immunosuppressive, we analyzed the suppressive capacity of ALDHBright and ALDHDim KCM cells and found that although both subsets inhibit CD8+ T-cell proliferation at high concentrations, ALDHBright cells exhibited modestly greater suppressive activity (Figure 5G).
Activation of the immunosuppressive properties in innate immune cells by tumor cells is a common feature of aggressive tumors. To determine whether PDAC TICs increase the immunosuppressive capacity of macrophages, we cocultured small numbers of ALDHBright and ALDHDim KCM cells with BM-MACs (Figure 5H). Whereas tumor-naive BM-MACs exhibited decreased CD8-suppressive capacity compared to that of TAMs, CD8 suppression by BM-MACs was significantly elevated by the presence of ALDHBright but not ALDHDim KCM cells. Taken together, these data suggest that inhibiting CSF1R or CCR2 in the context of chemotherapy allows productive CD8+ T-cell responses via the combined action of the 1) reduced presence of immunosuppressive ALDHBright TICs, 2) reduced presence of immunosuppressive TAMs (as well as M-MDSC), and 3) disruption of TIC-induced macrophage immunosuppression.
STAT3 activation is necessary for macrophage-dependent increases in TIC numbers and chemoresistance
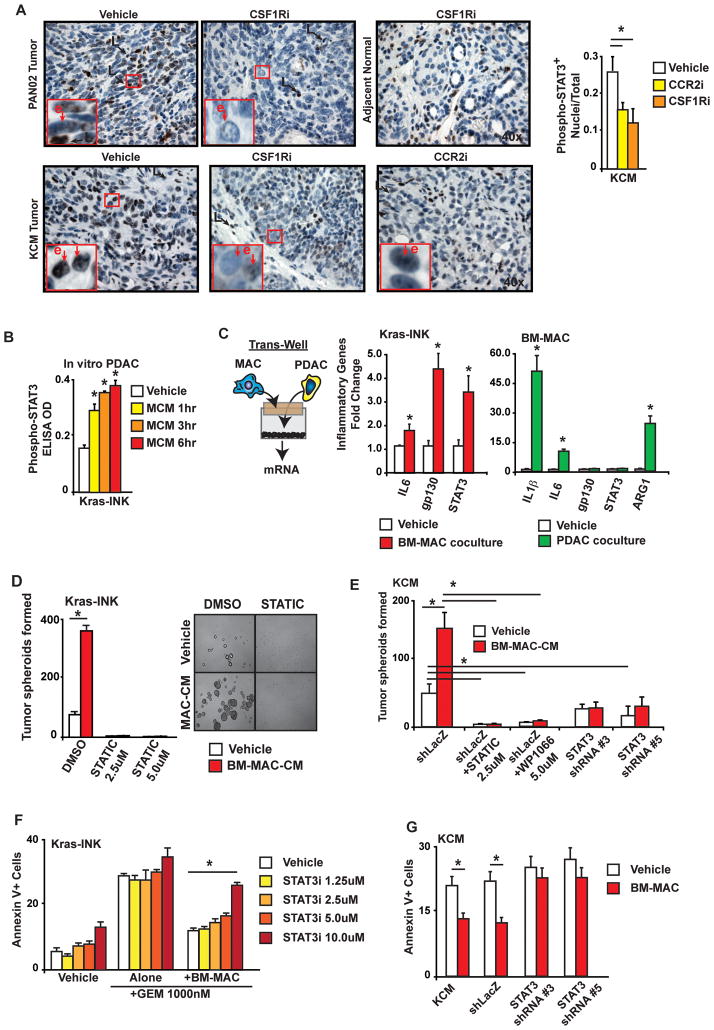
STAT3 is a key mediator of proinflammatory cytokines and immune suppression in both leukocytes and neoplastic cells, and has also been linked to TIC survival and chemoresistance in several cancer types (33–36). IHC analysis of tumor tissue from mice treated with CSF1R or CCR2 inhibitors revealed significantly reduced levels of phospho-STAT3 (pSTAT3, Ser205) in malignant cells, but not adjacent normal or leukocytes (Figure 6A). Correlating with these results macrophage CM increased pSTAT3 levels in Kras-INK cells in vitro (Figure 6B). Corresponding with the activation of STAT3-mediated transcription, we observed increased IL6,GP130, and STAT3 mRNA expression in PDAC cells and increased IL1β, IL6, and ARG1 mRNA expression in macrophages when cocultured in a Transwell (Figure 6C). To determine if STAT3 was necessary for TAM-mediated regulation of PDAC TICs, we used small-molecule inhibitors of STAT3 signaling (STATIC(37) and WP1066) and STAT3 shRNA constructs. Treatment with STATIC or WP1066 abrogated the formation of tumor spheroids in the presence or absence of BM-MAC-CM (Figure 6D–E). Additionally, partial suppression of STAT3 expression (~50–60%) using shRNA reduced the induction of tumor spheroid formation by BM-MAC-CM (Figure 6E). Inhibition of STAT3 by either STATIC or shRNA was sufficient to overcome the chemoprotective effects of macrophage coculture (Figure 6F–G). These data suggest that TAMs induce TIC properties and chemoresistance through the activation of STAT3.
Figure 6. Macrophage-induced chemoresistance and stem-like properties require STAT3 signaling.
A) Quantitation and ×40 images of pSTAT3+ tumor cells are depicted. The mean frequency of positive cells in PAN02 and KCM tumors treated with either vehicle or CSF1R or CCR2 inhibitors is shown (n>5/group).
B) ELISA analysis of STAT3 phosphorylation in Kras-INK cells following treatment with macrophage conditioned medium (MCM).
C) Normalized mean fold changes in gene expression are depicted from Kras-INK tumor cells and BM-MACs alone or in coculture for 24hrs using a Transwell chamber.
D) MCM was added to adherence-free Kras-INK tumor spheroid cultures ± the STAT3 inhibitor STATIC, and the number of tumor spheroids after 14 days is depicted.
E) MCM was added to adherence-free KCM-shLacZ, KCM-shSTAT3#3, or KCM-shSTAT3#5 tumor spheroid cultures ± the STAT3 inhibitors and the number of tumor spheroids after 14 days is depicted.
F) Analysis of Annexin V+ Kras-INK cells in direct coculture with BM-MACs ± STATIC and treated with GEM for 36 h is depicted as % of CD45−mCherry+ tumor cells (n=3/group).
G) Analysis of Annexin V+ KCM, KCM-shLacZ, KCM-shSTAT3#3, or KCM-shSTAT3#5 cells cocultured with BM-MACs ± GEM is depicted (n=3/group).
STAT3 signaling is necessary for TIC-induced immunosuppression
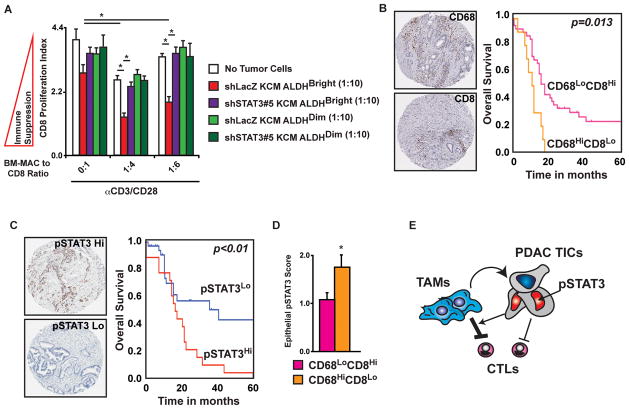
We next sought to understand how STAT3 activation in TICs regulated the immunosuppressive capacity of TAMs. To accomplish this, we analyzed the effects of shRNA against STAT3 on PDAC-induced immunosuppression. Corresponding to our previous results, ALDHBright KCM cells, but not KCM ALDHDim cells, stably expressing shRNA against LacZ robustly induced BM-MAC-mediated suppression of CD8+ T-cell proliferation. By contrast, ALDHBright KCM cells expressing STAT3 shRNA were unable to enhance BM-MAC-mediated CD8+ T-cell suppression (Figure 7A).
Figure 7. Immune status and STAT3 activation predict patient survival.
A) STAT3+ TICs augment macrophage-mediated immunosuppression. Suppression of CD8 proliferation was assayed in cocultures with BM-MACs and/or isolated ALDHBright and ALDHDim KCM-shLacZ or KCM-shSTAT3#5 cells. The ratio of all cells to CD8+ CTLs and the mean number of proliferation cycles is depicted.
B) The CD8 to CD68 ratio predicts PDAC patient survival. Automated analysis of CD68+ and CD8+ IHC reveals the relationship between leukocyte density and overall survival. The Kaplan-Meier estimate of overall survival comparing CD68Hi/CD8Low to all other groups, denoted as CD68Low/CD8High, is shown.
C) STAT3 phosphorylation predicts PDAC patient survival. The Kaplan-Meier estimate of overall survival comparing patients divided into pSTAT3Hi and pSTAT3Lo subgroups by the mean intensity score is shown.
D) Mean epithelial pSTAT3 score is depicted for tumors classified as either CD68Hi/CD8Lo or CD68Hi/CD8Lo.
E) Schematic depiction of the coordinated suppression of CD8+ CTLs by tumor-infiltrating macrophages and TICs.
* denotes p<0.05 by Mann-Whitney, and survival p-values are log-rank (Mantel-Cox).
To understand the clinical implications of these interactions, we analyzed a TMA containing specimens from 59 PDAC patients. Tumors were scored for the presence of CD68+ and CD8+ leukocytes and stratified into two groups (CD68Hi/CD8Lo and CD68Lo/CD8Hi). We observed that in patients in whom CD68+ macrophages were the dominant tumor-infiltrating leukocyte (CD68Hi/CD8Lo), overall survival was significantly reduced compared to all other groups (denoted as CD68Lo/CD8Hi; Figure 7B). Similarly, stratification of patients by epithelial pSTAT3 revealed that high pSTAT3 indicated reduced survival (Figure 7C). Combined analysis found that CD68+ leukocytes, but not CD8+ T cells, correlated with epithelial pSTAT3 intensity (Spearman r=0.32, p=0.024) and tumors classified as CD68Hi/CD8Lo had increased epithelial pSTAT3 (Figure 7D). Taken together, these results suggest that epithelial STAT3 signaling is high in tumors where macrophages likely play an immunosuppressive role (e.g., CD68Hi/CD8Lo tumors) and targeting TAMS in these patients could result in improved survival.
Discussion
Our results demonstrate that inhibiting CSF1R or CCR2 signaling can increase chemotherapeutic efficacy and block metastasis by the combined action of reducing TIC numbers, and overcome macrophage-induced CD8+ CTL suppression. We illustrate that macrophages can directly induce TIC properties in PDAC cells by enhancing STAT3 activation and that STAT3+ TICs enhance TAM-mediated immunosuppression. Thus, crosstalk between TAMs and TICs through STAT3 regulates the chemotherapeutic response by repressing antitumor CTL activity (Figure 7E).
CCR2 and CSF1R as regulators of myeloid responses
Tumor-infiltrating innate immune cells are composed of diverse cellular populations including G-MDSCs, M-MDSCs, tie-2+ angiogenic monocytes and myeloid-derived DCs. We found that blockade of CSF1R or CCR2 result in a very similar spectrum of alterations in tumor-infiltrating myeloid cells both reducing mature CD11b+Ly6G−L6CMid-LoMHCIIHiF480+ macrophages and CD11b+Ly6G−L6CHiMHCII+F480+ monocytes. Although the effects of CCR2 and CSF1R blockade on tumor-infiltrating leukocytes are similar, their mechanisms of action are likely divergent. Studies have suggested that CCR2 mediates the trafficking of circulating Ly6CHiCCR2+CX3CR1Lo inflammatory monocytes to target tissues (38, 39), whereas CSF1R is more likely involved in maturation and/or survival of these cells at inflamed sites. Both receptors can affect bone marrow mobilization. In this and previous studies, significantly reduced macrophage infiltration was observed as early as 2–4 days after CSF1R inhibition (4). By contrast, CCR2 inhibition reduced monocyte numbers in 4 days but only depleted TAMs after 8 days of treatment. Neither CSF1R nor CCR2 blockade resulted in dramatic alterations in circulating monocyte numbers over these periods. Thus, these results suggest that CCR2 inhibition may affect recruitment of inflammatory monocytes from the circulation, whereas CSF1R inhibition may affect survival of monocytes/macrophages at the tumor site.
Tumor-infiltrating immature granulocytes delineated as CD11b+Ly6GHi are often composed of highly heterogeneous populations, which can include tumor-activated neutrophils and G-MDSCs (40). GM-CSF–dependent mobilization and activation of these cells have been demonstrated to mediate suppression of CTL responses in PDAC tumors (30, 31). However, in this study, targeting either CCR2 or CSF1R did not reduce the presence of CD11b+Ly6GHi cells (Figure 2). These findings suggest that in the context of chemotherapy, CD11b+Ly6GHi cells cannot overcome the loss of TAMs and M-MDSC. While not observed in this model, GEM treatment has been shown to reduce the numbers of G-MDSCs in mammary tumors (41) and may affect the immunosuppressive capacity of G-MDSCs in PDAC. Alternatively, the depletion of TAMs may alter the cellular activity CD11b+Ly6GHi cells, pushing them toward more mature, less immunosuppressive phenotypes. These differences in immune responses following CCR2 or CSF1/CSF1R inhibition are likely important considerations for their clinical application.
Inflammation and TICs
STAT3 activity has been demonstrated to be required for the expansion and maintenance of cancer stem cells in several cancers (33, 34). In pancreatic cancer, epithelial deletion of STAT3 results in significantly reduced Kras-induced tumor formation in part through alterations in MMP7 expression and decreased immune infiltration (35). These data are consistent with the idea that reciprocal crosstalk between leukocytes and cancer cells sustain tumor progression. Work by Jinushi et al demonstrated that macrophage-derived MGF-E8 and IL6 enhance stem-like properties in lung and colon cancer cells by activating STAT3 (10). Similarly, we revealed that depletion of TAMs by CSF1R or CCR2 inhibition leads to reduced STAT3 phosphorylation (Figure 6) and decreased numbers of ALDHBright TICs. However, regulation of STAT3 activation may be one of several pathways regulating this process. TAMs are potent producers of WNTs and Sonic hedgehog ligands as well as growth factors such as epidermal growth factor, basic fibroblast growth factor, platelet-derived growth factor, and hepatocyte growth factor, many of which have been implicated in stimulating cancer stem-like properties (42, 43). Additionally, factors such as vascular endothelial growth factor, matrix metallopeptidase 9, and Bv8 emanating from tumor-infiltrating myeloid cells can significantly alter the quality of tumor vasculature, and endothelial cell/tumor cell interactions may be a critical component of the cancer stem cell niche (44–46). Thus, multiple pathways are likely regulated by tumor inflammation to influence the prevalence of TICs.
Macrophages and chemosensitivity
Several recent studies revealed that macrophages can directly regulate tumor cell chemoresistance. Previous work by Joyce and colleagues has illustrated that macrophage-derived cathepsins regulate pancreatic neuroendocrine tumor progression and mammary tumor response to paclitaxel (27, 47). Intriguingly, cathepsin activity has also been revealed as necessary for the secretion and processing of proinflammatory cytokines. However, the manner in which these activities affect mammary TICs or STAT3 activation is not known. Additionally, targeting CCR2 in the tumor microenvironment has been demonstrated to improve the delivery of chemotherapeutic regimens by regulating the tumor vasculature (48). These observations further stress the importance of tumor-infiltrating macrophages and the complexity of their roles in the tumor microenvironment.
Clinical prospective
Therapeutic resistance and metastatic spread define the lethality of aggressive cancers. Thus, understanding and targeting the mechanisms responsible is critical to improving therapeutic outcomes. Several studies have indicated that targeting key pathways regulating TIC survival and/or differentiation can overcome therapeutic resistance. However, the durability of such therapies remains unproven, as such targeting the tumor stromal responses that support tumor “stemness” is an attractive alternative.
Supplementary Material
Acknowledgments
DCL acknowledges funding from the WU/Pfizer Biomedical Research Grant PW0457. DGD acknowledges generous support from the Lustgarten Foundation, V Foundation, Edward Mallinckrodt Jr. Award, the Cancer Research Foundation and Siteman Cancer Center Career Development Award. DCL and DGD acknowledge the Siteman Frontier Funds Team Science Award, Drs. Mukherjee and Hanahan for providing cell lines, and the efforts of the Bright Imaging Center. The authors also thank Drs. Hawkins, Stewart, and Webber for discussions and input. DPW acknowledges funding from NIH P50 CA 94056. JBM and DES acknowledge funding from NCI grant T32 CA 009621.
Abbreviations
- CCR2
chemokine (C-C motif) receptor 2
- CSF1R
colony-stimulating factor 1 receptor
- STAT3
signal transducer and activator of transcription 3
- CTL
cytotoxic T lymphocyte
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- MDSC
myeloid derived suppressor cell
- PDAC
pancreatic ductal adenocarcinoma
- TAM
tumor-associated macrophage
- TIC
tumor-initiating cell
- GEM
gemcitabine
- G-MDSC
granulocytic myeloid-derived suppressor cell
- M-MDSC
monocytic myeloid-derived suppressor cell
- TReg
regulatory T cell
- IL
interleukin
Footnotes
Conflict of Interest: Author David Linehan received research support from Pfizer Oncology and Novartis. Author Donal Brennan has ownership interest in his patent in for a CD8:CD68 immune signature.
References
- 1.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–7. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 4.DeNardo DG, Brennan D, Rexhapaj E, Ruffel B, Shiao S, Gallagher WM, et al. Leukocyte complexity in breast cancer predicts overall survival and functionally regulates response to chemotherapy. Cancer Discovery. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pyonteck SM, Gadea BB, Wang HW, Gocheva V, Hunter KE, Tang LH, et al. Deficiency of the macrophage growth factor CSF-1 disrupts pancreatic neuroendocrine tumor development. Oncogene. 2011 doi: 10.1038/onc.2011.337. [DOI] [PubMed] [Google Scholar]
- 6.Lin EY, Gouon-Evans V, Nguyen AV, Pollard JW. The macrophage growth factor CSF-1 in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia. 2002;7:147–62. doi: 10.1023/a:1020399802795. [DOI] [PubMed] [Google Scholar]
- 7.Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–9. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 8.Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nature reviews Cancer. 2008;8:545–54. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- 9.Korkaya H, Liu S, Wicha MS. Regulation of cancer stem cells by cytokine networks: attacking cancer’s inflammatory roots. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:6125–9. doi: 10.1158/1078-0432.CCR-10-2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, et al. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12425–30. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rexhepaj E, Brennan DJ, Holloway P, Kay EW, McCann AH, Landberg G, et al. Novel image analysis approach for quantifying expression of nuclear proteins assessed by immunohistochemistry: application to measurement of oestrogen and progesterone receptor levels in breast cancer. Breast Cancer Res. 2008;10:R89. doi: 10.1186/bcr2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue CBWA, Han Q, Zhang X, Cao G, Feng H, Huang T, Zheng C, Xia M, Zhang K, Kong L, Glenn J, Rajan A, Meloni D, Robinson DJ, Shao L, Stoace L, Mei L, Hughes RO, Devraj R, Morton PA, Rogier DJ, Covington M, Scherle P, Diamond S, Emm T, Yeleswaram S, Contel N, Vaddi K, Newton R, Hollis G, Metcalf B. Discovery of INCB8761/PF-4136309, a Potent, Selective, and Orally Bioavailable CCR2 Antagonist. ACS Med Chem Lett. 2011;2:913–18. doi: 10.1021/ml200199c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conway JG, McDonald B, Parham J, Keith B, Rusnak DW, Shaw E, et al. Inhibition of colony-stimulating-factor-1 signaling in vivo with the orally bioavailable cFMS kinase inhibitor GW2580. Proc Natl Acad Sci U S A. 2005;102:16078–83. doi: 10.1073/pnas.0502000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–6. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plexxikon, Inc., assignee Molecular Scaffolds for Kinase Ligand Development. United States: 2005. [Google Scholar]
- 16.Corbett TH, Roberts BJ, Leopold WR, Peckham JC, Wilkoff LJ, Griswold DP, Jr, et al. Induction and chemotherapeutic response of two transplantable ductal adenocarcinomas of the pancreas in C57BL/6 mice. Cancer research. 1984;44:717–26. [PubMed] [Google Scholar]
- 17.Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nature medicine. 2011;17:500–3. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy LD, Sahraei M, Subramani DB, Besmer D, Nath S, Tinder TL, et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene. 2011;30:1449–59. doi: 10.1038/onc.2010.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes & development. 2003;17:3112–26. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim MP, Evans DB, Wang H, Abbruzzese JL, Fleming JB, Gallick GE. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nature protocols. 2009;4:1670–80. doi: 10.1038/nprot.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross S, Piwnica-Worms D. Real-time imaging of ligand-induced IKK activation in intact cells and in living mice. Nature methods. 2005;2:607–14. doi: 10.1038/nmeth779. [DOI] [PubMed] [Google Scholar]
- 22.Besmer DM, Curry JM, Roy LD, Tinder TL, Sahraei M, Schettini J, et al. Pancreatic ductal adenocarcinoma mice lacking mucin 1 have a profound defect in tumor growth and metastasis. Cancer research. 2011;71:4432–42. doi: 10.1158/0008-5472.CAN-10-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charafe-Jauffret E, Ginestier C, Iovino F, Tarpin C, Diebel M, Esterni B, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Resetkova E, Reis-Filho JS, Jain RK, Mehta R, Thorat MA, Nakshatri H, et al. Prognostic impact of ALDH1 in breast cancer: a story of stem cells and tumor microenvironment. Breast Cancer Res Treat. 2010;123:97–108. doi: 10.1007/s10549-009-0619-3. [DOI] [PubMed] [Google Scholar]
- 25.Schubert C, Schalk-Hihi C, Struble GT, Ma HC, Petrounia IP, Brandt B, et al. Crystal structure of the tyrosine kinase domain of colony-stimulating factor-1 receptor (cFMS) in complex with two inhibitors. The Journal of biological chemistry. 2007;282:4094–101. doi: 10.1074/jbc.M608183200. [DOI] [PubMed] [Google Scholar]
- 26.Nakasone ES, Askautrud HA, Kees T, Park JH, Plaks V, Ewald AJ, et al. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer cell. 2012;21:488–503. doi: 10.1016/j.ccr.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shree T, Olson OC, Elie BT, Kester JC, Garfall AL, Simpson K, et al. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes & development. 2011;25:2465–79. doi: 10.1101/gad.180331.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Engelhardt JJ, Boldajipour B, Beemiller P, Pandurangi P, Sorensen C, Werb Z, et al. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer cell. 2012;21:402–17. doi: 10.1016/j.ccr.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bayne LJ, Beatty GL, Jhala N, Clark CE, Rhim AD, Stanger BZ, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer cell. 2012;21:822–35. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-Induced GM-CSF Production Promotes the Development of Pancreatic Neoplasia. Cancer cell. 2012;21:836–47. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei J, Barr J, Kong LY, Wang Y, Wu A, Sharma AK, et al. Glioma-associated cancer-initiating cells induce immunosuppression. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:461–73. doi: 10.1158/1078-0432.CCR-09-1983. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, et al. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16158–63. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherry MM, Reeves A, Wu JK, Cochran BH. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells. 2009;27:2383–92. doi: 10.1002/stem.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukuda A, Wang SC, Morris JPt, Folias AE, Liou A, Kim GE, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer cell. 2011;19:441–55. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corcoran RB, Contino G, Deshpande V, Tzatsos A, Conrad C, Benes CH, et al. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer research. 2011;71:5020–9. doi: 10.1158/0008-5472.CAN-11-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13:1235–42. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nature biotechnology. 2011;29:1005–10. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nature reviews Immunology. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–83. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 42.Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nature cell biology. 2010;12:468–76. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 43.Zhu X, Zhou X, Lewis MT, Xia L, Wong S. Cancer stem cell, niche and EGFR decide tumor development and treatment response: A bio-computational simulation study. J Theor Biol. 2011;269:138–49. doi: 10.1016/j.jtbi.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–31. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 45.Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, et al. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer research. 2010;70:9969–78. doi: 10.1158/0008-5472.CAN-10-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403. doi: 10.1038/nature10525. [DOI] [PubMed] [Google Scholar]
- 47.Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–55. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, et al. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–8. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.