Abstract
T cell Ig and mucin domain (Tim)-3 is well known to interact with its natural ligand, Galectin-9 (Gal-9), to regulate T cell function. However, little is known about the function of Tim-3/Gal-9 signaling in the pathogenesis of nonalcoholic fatty liver disease (NAFLD) mediated by hepatic NKT cells that also express Tim-3. In the current study, we define the role and the mechanism of Tim-3/Gal-9 signaling in hepatic NKT cell regulation in a mouse model of diet-induced NAFLD. Adult male wild-type or CD1d knockout C57BL/6 mice were fed a high-fat diet to induce steatosis. Some of the mice also received one or a combination of Gal-9, anti–IL-15R/IL-15 mAb, rIL-15, α-galactosylceramide, and multilamellar liposomes containing Cl2MDP. The expression of Tim-3 and various markers reflecting cell proliferation, activation, cytokine production, and apoptosis was analyzed. Liver histology, steatosis grade, and hepatic triglyceride content were also evaluated. In the liver, Tim-3+ NKT cells are in an activated state, and Gal-9 directly induces Tim-3+ NKT cell apoptosis and contributes to the depletion of NKT cells in diet-induced steatosis. However, Gal-9 also interacts with Tim-3–expressing Kupffer cells to induce secretion of IL-15, thus promoting NKT cell proliferation. Exogenous administration of Gal-9 significantly ameliorates diet-induced steatosis by modulating hepatic NKT cell function. In summary, the Tim-3/Gal-9–signaling pathway plays a critical role in the homeostasis of hepatic NKT cells through activation-induced apoptosis and secondary proliferation and, thus, contributes to the pathogenesis of NAFLD.
Introduction
As a member of the T cell Ig and mucin domain (Tim) family, Tim-3 is specifically expressed on terminally differentiated CD4+ Th1 cells but not Th2 cells (1, 2). Numerous studies demonstrated the expression of Tim-3 on cells of the adaptive immune system, as well as on cells of the innate immune system, including dendritic cells (DCs) (3), macrophages (3), and mast cells (4). The natural ligand for Tim-3 is Galectin-9 (Gal-9), a β-galactoside–binding lectin (5). Cumulative findings indicate that Tim-3/Gal-9 interaction plays crucial roles in immune regulation. Gal-9 binds to Tim-3 to induce Th1 cell apoptosis to dampen Th1 immunity and induce peripheral tolerance (5). Tim-3/Gal-9 mediate proinflammatory cytokine secretion in dendritic cells and macrophages and promote inflammation (3). Recently, studies showed that, in chronic hepatitis C virus infection, the expression of Tim-3 is increased on both CD4+ and CD8+ T cells and that those cells fail to produce cytokines or to proliferate in response to Ag. Treatment with Tim-3 mAb strongly enhanced T cell proliferation and IFN-γ production (6). Also, blockade of Tim-3 enhances the proliferation and the cytotoxicity of hepatitis C virus–specific CTLs (7). Therefore, Tim-3+ T cells are labeled as “exhausted” T cells. However, among T lymphocytes there is a subset of cells with distinct immunological phenotypes that is characterized by the expression of an invariant TCR (Vα14-Jα18 in mouse and Vα24-Jα18 in human) in addition to NK cell markers. These cells are referred to as NKT cells (8, 9). Numerous studies showed that NKT cells exhibit features of both innate and adaptive immune cells and act as a bridging system between innate and adaptive immunity (8, 9). Despite evidence of Tim-3 expression on NKT cells (10), the function of Tim-3/Gal-9 signaling in NKT cells has not been fully investigated.
NKT cells are particularly enriched within the liver and regulate immune response through rapid secretion of large amounts of both Th1 and Th2 cytokines following stimulation (11). We previously reported that NKT cells play an important role in the pathogenesis of nonalcoholic fatty liver disease (NAFLD), the most common chronic liver disorder in the world (12). The depletion of hepatic NKT cells by local and environmental factors leads to chronic inflammatory conditions that contribute to insulin resistance and steatosis (12). Upregulation of hepatic NKT cells improves high-fat (HF) diet–induced steatosis and insulin resistance (13). However, little is known about the function of Tim-3/Gal-9 signaling in the pathogenesis of NAFLD mediated by hepatic NKT cells.
In the current study, we examine the role of Tim-3/Gal-9 signaling in hepatic NKT cell regulation. We further evaluate the function of Tim-3/Gal-9 signaling in the pathogenesis of NAFLD. Understanding the function of the Tim-3/Gal-9 pathway in the homeostasis of hepatic NKT cells that regulate the local immune microenvironment in liver may result in a valid therapeutic target for NAFLD.
Materials and Methods
Animal experiments
Adult male wild-type C57BL/6 mice, 6–8 wk old, were purchased from The Jackson Laboratory (Bar Harbor, ME). CD1d knockout (CD1dko) mice originated in Dr. Albert Bendelac’s laboratory (University of Chicago, Chicago, IL) and were back-crossed to the C57BL/6 background for >10 generations. Mice were fed commercial diets containing either a high amount of fat (50% of the total kilocalories; F3282; BioServ, Frenchtown, NJ) or a normal amount of fat (11% of the total kilocalories) for 12–24 wk. Some of the mice also received one or a combination of the following: sGal-9 (stable form of recombinant Gal-9, a gift of Dr. Toshiro Niki, GalPharma, Kagawa, Japan; 0.6–1.2 μg/g body weight, i.p.); anti-mouse IL-15R/IL-15 complex mAb (1 μg/g body weight; eBioscience, San Diego, CA) or isotype control Ab i.p.; rIL-15 protein (25 ng/g body weight i.p. once every 3 d for 9 d; eBioscience); α-galactosylceramide (α-GalCer; 2 μg i.p. once daily for 3 d; Axxora, San Diego, CA); or multilamellar liposomes containing Cl2MDP (Sigma-Aldrich, St. Louis, MO) or PBS (200 μl 1 mg/ml, prepared as previously described) (14, 15). All mice were maintained in a pathogen-free, temperature- and light-controlled facility at The Johns Hopkins University. All animal experiments fulfilled the National Institutes of Health and The Johns Hopkins University criteria for the humane treatment of laboratory animals and were approved by The Johns Hopkins University Animal Care and Use Committee.
Isolation and purification of hepatic mononuclear cells, NKT cells, Kupffer cells, and DCs
Hepatic mononuclear cells (HMNCs) were isolated as previously described (12, 13). After isolation, HMNCs were labeled with surface markers CD3 and NK1.1 (BD Bioscience, San Diego, CA), and NKT cells (CD3+NK1.1+ cells) were isolated with a FACSVantage SE high-speed sorter (Becton Dickinson). Kupffer cells were isolated as described, with slight modification (16). The liver was perfused with type IV collagenase (Sigma-Aldrich) solution, and several centrifugation steps were performed to eliminate the majority of parenchymal cells. Nonparenchymal cells were then separated by isopycnic sedimentation in Percoll; finally, purified Kupffer cells were obtained based on the selective substrate–attachment properties on plastic Flasche. The viability and purity of Kupffer cells was determined by trypan blue exclusion and ED2 staining, respectively. DCs were isolated from spleens as previously described (17). After homogenization and centrifugation, splenocytes were labeled with anti-CD11c Ab (Invitrogen, Carlsbad, CA). DCs (CD11c+) were isolated using the MACS system (Miltenyi Biotec, Auburn, CA)
Flow cytometry analysis
For cell surface marker labeling, the single-cell suspensions were stained for different anti-mouse Abs, including CD3, CD4, CD8, NK1.1, CCR7, CD62L, CD25, CD69, CD44, Annexin V (all from BD Biosciences) or Tim-3 or CD11b (both from eBioscience). α-GalCer–CD1d tetramer–allophycocyanin was obtained from the National Institutes of Health tetramer facility. Intracellular cytokine staining was performed as previously described (12, 13) with anti–IFN-γ, IL-4 Abs (BD Bioscience). Data were collected with a FACSCalibur (Becton Dickinson) and analyzed with FlowJo software (TreeStar).
Cell culture
For cell proliferation assay, freshly isolated HMNCs were resuspended in culture media (RPMI 1640) and seeded on 24-well microtiter plates at a density of 1 × 106 cells/ml/well. After 5 h of incubation in the presence of anti-CD3 Ab (0.5 μg/ml; eBioscience) and anti-CD28 (0.5 μg/ml; eBioscience), 10 μl BrdU-labeling solution (1 mM; BD Biosciences) was added directly to each milliliter of cell culture medium and incubated for 1 h. After removing the culture medium, the BrdU-pulsed cells were stained with various cell surface markers (NK1.1, CD3, CD4, Tim-3, and CD3), as described above, and analyzed using a BrdU flow kit (BD Biosciences), according to the manufacturer’s protocol. For Kupffer cell culture, purified Kupffer cells from two mice were pooled and placed in a 24-well cell culture plate (3 × 105 cells/well) with culture media (RPMI 1640). Kupffer cells were incubated with sGal-9 (0.5 μM), α-lactose (competitive inhibitor of sGal-9; 40 mM), anti–Tim-3 mAb (20 μg/ml; eBioscience), and LPS (positive control; 5 μg/ml), either alone or in combination, for 24 h.
Real-time RT-PCR
Total liver RNA was isolated as previously described (12, 13). cDNA was synthesized from 4 μg total RNA, using High-Capacity cDNA Reverse Transcription Kits (Applied Biosystems, Foster City, CA). The primer sets for Gal-9, Tim-3, IL-4, IFN-γ, and IL-15 and housekeeping gene GAPDH were obtained commercially (QIAGEN, Valencia, CA and Sigma-Aldrich). Quantified PCR amplifications were performed using SYBR GREEN PCR Master Mix with the Prism 7900HT detection system (Applied Biosystems). Negative controls were performed without cDNA in the reaction mixture. Gene-expression levels of sample were normalized to GAPDH using ΔCt calculations.
ELISA
Total liver protein was extracted from 20 mg liver tissue using 2 ml lysis buffer (Cell Signaling) with protease inhibitors (Sigma-Aldrich). The concentrations of IL-15 in tissue extract or culture media were measured with a mouse IL-15/IL-15R complex ELISA kit, according to the manufacturer’s instruction (eBioscience). Total protein concentrations were determined by a BCA Protein Assay Kit (Pierce).
Liver histology, hepatic triglyceride content, and steatosis score
The liver tissue was fixed in 10% formaldehyde and embedded in paraffin. Thin 10-μm slices of liver tissue were stained with H&E. Total lipids were extracted from liver tissue using the method that we described previously (13). Triglyceride content was measured with a kit, according to the manufacturer’s instructions (Sigma-Aldrich). Ten 200× fields were assessed in each section, using a light microscope, and scored for the severity of steatosis according to the following criteria: grade 0 = no fat, grade 1 = fatty hepatocytes occupying <33% of the hepatic parenchyma, grade 2 = fatty hepatocytes occupying 34–66% of the hepatic parenchyma, and grade 3 = fatty hepatocytes occupying >66% of the hepatic parenchyma.
Statistical analysis
All values are expressed as mean ± SD. Treatment-related differences were evaluated by ANOVA. The paired individual means were compared using the Student t test. Statistical significance was set at a p value < 0.05.
Results
Characterization of Tim-3+ hepatic NKT cells
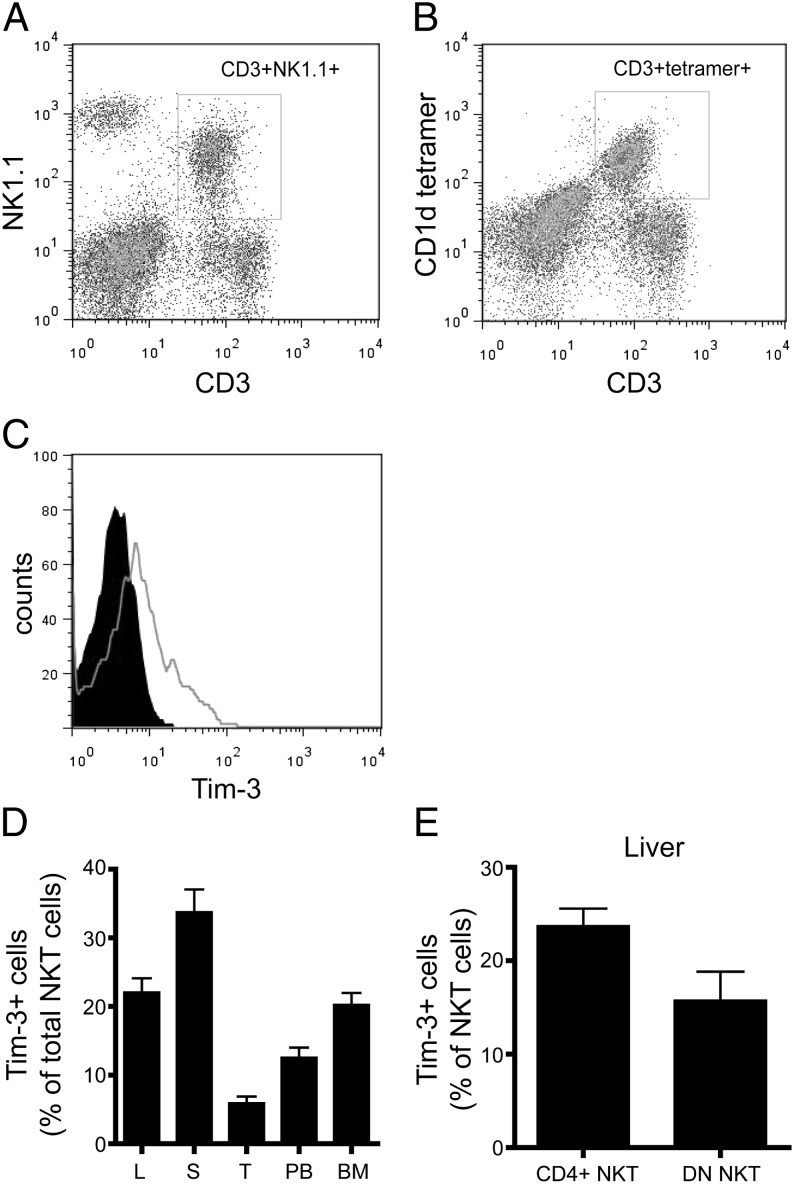
To understand Tim-3/Gal-9 regulation on NKT cells, we first examined Tim-3 expression on NKT cells. We used either CD3+NK1.1+ (Fig. 1A) or CD1d tetramer (Fig. 1B) to identify NKT cells and found that the majority of NKT cells overlapped between these two populations. Our studies showed that 23 ± 4% of NKT cells express Tim-3 in the liver compared with 35 ± 8%, 6 ± 2%, 13 ± 3%, and 20 ± 4% of NKT cells in the spleen, thymus, peripheral blood, and bone marrow, respectively (Fig. 1C, 1D). Hepatic NKT cells can be further divided into CD4+ or CD4−CD8− double-negative (DN) subsets. Approximately 23 ± 4% of CD4+ NKT cells and 15 ± 7% of DN NKT cells expressed Tim-3 (Fig. 1E).
FIGURE 1.
Tim-3 expression on murine NKT cells. Total mononuclear cells (TMNCs) were obtained from the liver (L), spleen (S), thymus (T), peripheral blood (PB), and bone marrow (BM) of wild-type C57BL/6 mice fed ND and labeled with anti-mouse Abs to NK1.1, CD3, CD4, CD8, and Tim-3. (A) Representative dot plot of CD3 and NK1.1 staining (gated on hepatic TMNCs). (B) Representative dot plot of CD3 and CD1d tetramer staining (gated on hepatic TMNCs). (C) Representative graphs of Tim-3 labeling on hepatic NKT cells (gated on NK1.1+CD3+ cells). Anti–Tim-3: open graph; isotype control: filled graph). (D) Percentage of Tim-3+ cells (gated on NK1.1+CD3+ cells) from various tissues. (E) Percentage of Tim-3+ cells among CD4+ NKT cells (NK1.1+CD3+CD4+) and DN NKT cells (NK1.1+CD3+CD4−CD8−) in the liver. Data are mean ± SD of five to seven independent experiments.
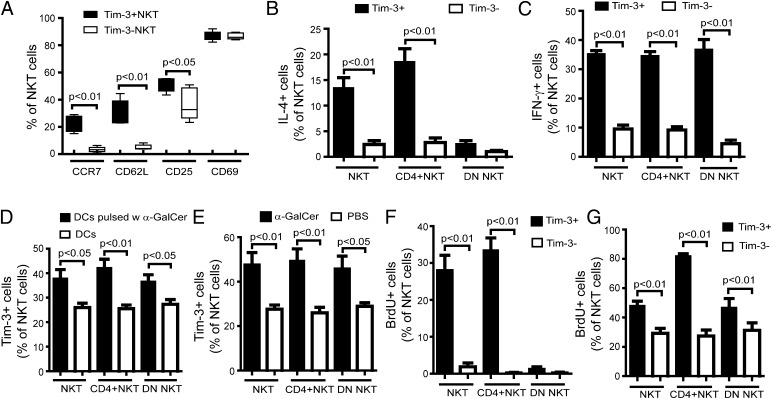
To explore whether there is a correlation between Tim-3 expression and the functional status of NKT cells, we assessed their phenotypic profiles, including activation, cytokine production, and proliferation. First, we evaluated several surface markers associated with central memory (CCR7, CD62L) and activation (CD25, CD69) among hepatic Tim-3+ or Tim-3− NKT cells. As shown in Fig. 2A, hepatic Tim-3+ NKT cells had much higher expression of CCR7 and CD62L than did Tim-3− NKT cells. In contrast, the expression of CD69 was similar and the expression of CD25 was slightly higher among hepatic Tim-3+ NKT cells compared with Tim-3− NKT cells (Fig. 2A).
FIGURE 2.
Characteristics of hepatic Tim-3+ NKT cells. HMNCs were isolated and labeled with various surface markers and intracellular cytokine staining. (A) Expression of CCR7, CD62L, CD25, and CD69 on Tim-3+ or Tim-3− NKT cells. Intracellular staining of IFN-γ (B) and IL-4 (C) among Tim-3+ or Tim-3− NKT, CD4+ NKT, and DN NKT cells. (D) Hepatic NKT cells were isolated and cocultured with splenic DCs pulsed or not with α-GalCer. Tim-3 expression was evaluated on NKT, CD4+ NKT, and DN NKT cells. (E) Wild-type C57BL/6 mice were injected i.p. with 2 μg of α-GalCer. HMNCs were isolated 3 d later, and Tim-3 expression on NKT, CD4+ NKT, and DN NKT cells was evaluated. (F) Hepatic NKT cells were isolated and stimulated with PHA (10 μg/ml) for 5 h. BrdU (1 mM) was then added for another hour. The proliferation of Tim-3+ or Tim-3− NKT, CD4+ NKT, and DN NKT cells was evaluated. (G) Wild-type C57BL/6 mice were injected i.p. with 2 μg of α-GalCer for 2 d, followed by i.p. BrdU during the last 24 h. HMNCs were isolated 3 d later, and the proliferation of Tim-3+ or Tim-3− NKT, CD4+ NKT, and DN NKT cells was evaluated. All results are mean ± SD of five independent experiments.
NKT cells have the unique ability to produce both Th1 and Th2 cytokines, including IFN-γ and IL-4. We evaluated intracellular cytokine staining of hepatic Tim-3+ and Tim-3− NKT cells to determine the correlation between Tim-3 expression and cytokine production in NKT cells. Many more Tim-3+ NKT cells produced IL-4 compared with Tim-3− NKT cells. They were mainly from the CD4+ NKT cell subgroup, whereas DN NKT cells produced similar levels of IL-4 in the Tim-3+ and Tim-3− cells (Fig. 2B). In contrast, all groups of Tim-3+ NKT cells, including CD4+ and DN cells, produced much more IFN-γ than did groups of Tim-3− NKT cells (Fig. 2C).
α-GalCer is an exogenous Ag that causes specific activation of NKT cells. Next, we examined whether Tim-3 expression was associated with the activation of NKT cells. Freshly isolated primary hepatic NKT cells were cultured with splenic DCs that were pulsed or not with α-GalCer to evaluate the Tim-3 expression on NKT cells after their activation. Activation of NKT cells by α-GalCer led to a significant upregulation of Tim-3 expression (Fig. 2D). Similar results were also observed in vivo, with significantly increased expression of Tim-3 among hepatic NKT cells after α-GalCer injection (Fig. 2E). The proliferative ability of Tim-3+ NKT cells was also investigated. In vitro stimulation of NKT cells caused cell proliferation and incorporation of BrdU among Tim-3+ NKT cells but not in Tim-3− NKT cells. Almost all proliferative Tim-3+ NKT cells were CD4+ NKT cells (Fig. 2F). Similar results were also shown in vivo by stimulation of NKT cells with injection of α-GalCer. The majority of proliferative hepatic NKT cells were a Tim-3+ subset (Fig. 2G). The splenic Tim-3+ NKT cells displayed phenotypic and functional characteristics similar to hepatic Tim-3+ NKT cells (data not shown). Therefore, we showed that Tim-3+ was preferentially expressed on proliferative NKT cells that actively produce cytokines.
Tim-3/Gal-9 signaling contributes to NKT cell apoptosis in HF diet–induced fatty liver
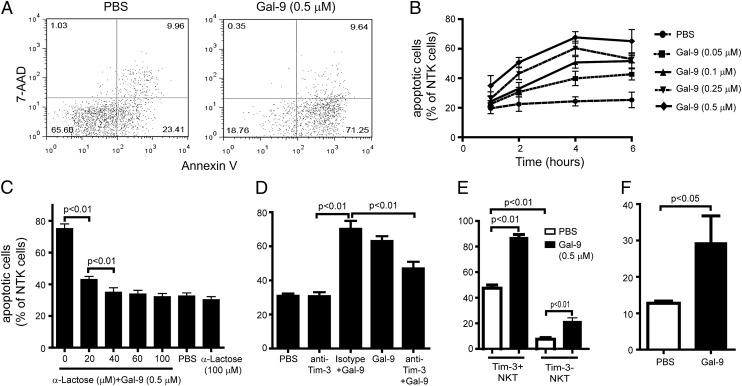
Growing evidence has indicated that Tim-3/Gal-9 signaling regulates lymphocyte function by specifically inducing apoptosis (5, 18, 19). Therefore, we examined the effect of Tim-3/Gal-9 signaling on hepatic NKT cells. Isolated hepatic NKT cells were incubated with rGal-9, and NKT cell apoptosis was determined by Annexin V/7-aminoactinomycin D (7-AAD) staining. Gal-9–induced apoptosis of hepatic NKT cells was dose and time dependent (Fig. 3A, 3B) and could be blocked by α-lactose (competitive inhibitor of Gal-9) or anti–Tim-3 Ab (Fig. 3C, 3D). The effect of Gal-9 on NKT cell apoptosis was much more significant among Tim-3+ NKT cells than Tim-3− NKT cells, suggesting the effect is via Tim-3/Gal-9 signaling (Fig. 3E). Interestingly, Gal-9 also induced apoptosis in Tim-3− NKT cells (Fig. 3E). Similar results were observed in Tim-3−CD4+ T cells (5), indicating that other pathways may be involved in Gal-9–induced lymphocyte apoptosis. We further confirmed Gal-9–induced hepatic NKT cell apoptosis in vivo by administering Gal-9 via i.p. injection (Fig. 3F).
FIGURE 3.
Gal-9 induced Tim-3+ NKT cell apoptosis. Hepatic NKT cells were isolated and treated with different concentrations of Gal-9. NKT cell apoptosis assays were performed with Annexin V staining and concurrent incubation with 7-AAD. Apoptotic cells are Annexin V+/7-AAD−. (A) Representative dot plot of apoptosis assay of NKT cells. (B–F) Percentage of apoptotic cells among NKT cells. (B) Time and dose curve of Gal-9–induced NKT cell apoptosis. (C) Hepatic NKT cells were treated with Gal-9 or PBS in the presence or absence of anti-lactose, a Gal-9 antagonist. NKT cell apoptosis was evaluated. (D) Hepatic NKT cells were treated with Gal-9 or PBS in the presence of anti–Tim-3 Ab or isotype control. NKT cell apoptosis was evaluated. (E) Hepatic NKT cells were treated with Gal-9 or PBS. Apoptosis among Tim-3+ or Tim-3− NKT cells was evaluated. (F) Wild-type C57BL/6 mice were treated with Gal-9 (1.2 μg/g body weight, i.p.) or PBS once every 2 d for a total of 6 d. Hepatic NKT cells were isolated, and the percentage of apoptosis was evaluated. Data are mean ± SD of five independent experiments.
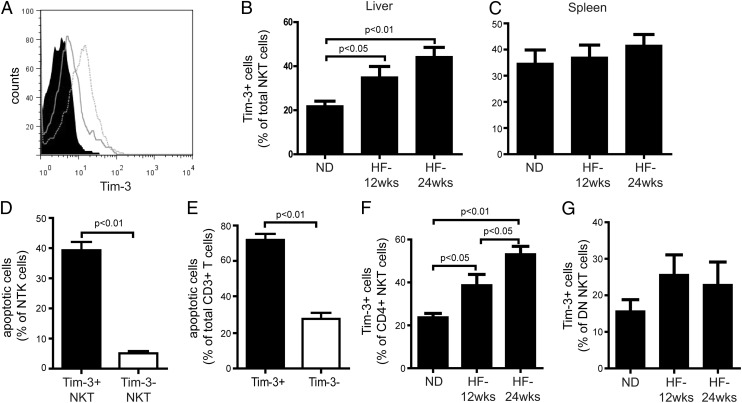
Our previous studies showed that HF diets increase hepatic NKT cell apoptosis, leading to hepatic NKT cell depletion and local and systemic inflammation, thus contributing to insulin resistance and hepatic steatosis (12). Because Tim-3/Gal-9 signaling contributed to NKT cell apoptosis and there was a very close correlation between Tim-3 expression and the functional status of hepatic NKT cells (Figs. 2, 3), we compared Tim-3 expression on hepatic NKT cells after mice were fed a normal diet (ND) or HF diet for up to 24 wk. Similar to our previous findings, mice fed HF diet had significantly reduced hepatic NKT cell number, as well as increased hepatic NKT cell apoptosis and hepatic steatosis (data not shown). Also, Tim-3 expression on hepatic NKT cells was dramatically enhanced in mice fed HF diet (Fig. 4A, 4B). Moreover, upregulation of Tim-3 expression was closely correlated with the progression of steatosis with prolonged HF diet feeding (Fig. 4B) and previously published data (12, 13). The upregulation of Tim-3 expression on NKT cells was limited to the liver since splenic NKT cells had steady Tim-3 expression after HF diet feeding (Fig. 4C). There was also no difference in Tim-3 expression among CD4+ Th cells (CD3+CD4+NK1.1−) in the liver and the spleen after HF diet feeding (data not shown). Furthermore, there was no difference in the protein and mRNA expression of Gal-9 in the liver between mice fed HF diet or ND (data not shown).
FIGURE 4.
Upregulation of Tim-3 expression among hepatic NKT cells of mice fed HF diet. Mononuclear cells were isolated from the liver or the spleen of C57BL/6 mice fed ND or HF diet for up to 24 wk and stained with Abs to NK1.1, CD3, CD4, CD8, and Tim-3. (A) Representative graphs of Tim-3 labeling on hepatic NKT cells from mice fed ND (dashed line), mice fed HF diet for 24 wk (open graph), or isotype control (filled graph) (gated on NK1.1+CD3+ cells). Percentage of Tim-3+ cells among hepatic (B) or splenic (C) NKT cells (gated on CD3+NK1.1+ cells). (D) Percentage of apoptotic cells (Annexin V+/7-AAD−) among Tim-3+ NKT cells (gated on Tim-3+CD3+NK1.1+ cells) or Tim-3− NKT cells (gated on Tim-3−CD3+NK1.1+ cells). (E) Percentage of apoptotic NKT cells (gated on CD3+NK1.1+Annexin V+7-AAD− cells) among Tim-3+ T cells (gated on Tim-3+CD3+ cells) or Tim-3− T cells (gated on Tim-3−CD3+ cells). Percentage of Tim-3+ cells among hepatic CD4+ NKT cells (gated on CD3+CD4+NK1.1+ cells) (F) or DN NKT cells (gated on CD3+CD4−CD8−NK1.1+ cells) (G). Results are mean ± SD of five independent experiments.
In mice fed HF diet, hepatic Tim-3+ NKT cells were more prone to apoptosis than were Tim-3− NKT cells (Fig. 4D), and the majority of the apoptotic hepatic NKT cells were associated with Tim-3+ cells (Fig. 4E). In addition, Tim-3+ NKT cells had a higher baseline apoptosis rate than did Tim-3− NKT cells, even without Gal-9 induction (Fig. 3E), possibly contributing to a higher proportion of Tim-3+ NKT cells in active states (Fig. 2). The increased hepatic Tim-3+ NKT cells in mice fed HF diet were predominantly attributable to Tim-3+CD4+ NKT cells (Fig. 4F), because Tim-3+ DN NKT cells remained at the same level (Fig. 4G). These results were consistent with our previous findings that HF diet selectively reduced hepatic CD4+ NKT cells (12, 13) (see data below). Thus, HF diet–upregulated Tim-3 expression in hepatic NKT cells might contribute to increased hepatic NKT cell apoptosis and depletion.
Gal-9 regulates hepatic NKT cells through IL-15 secreted by Kupffer cells
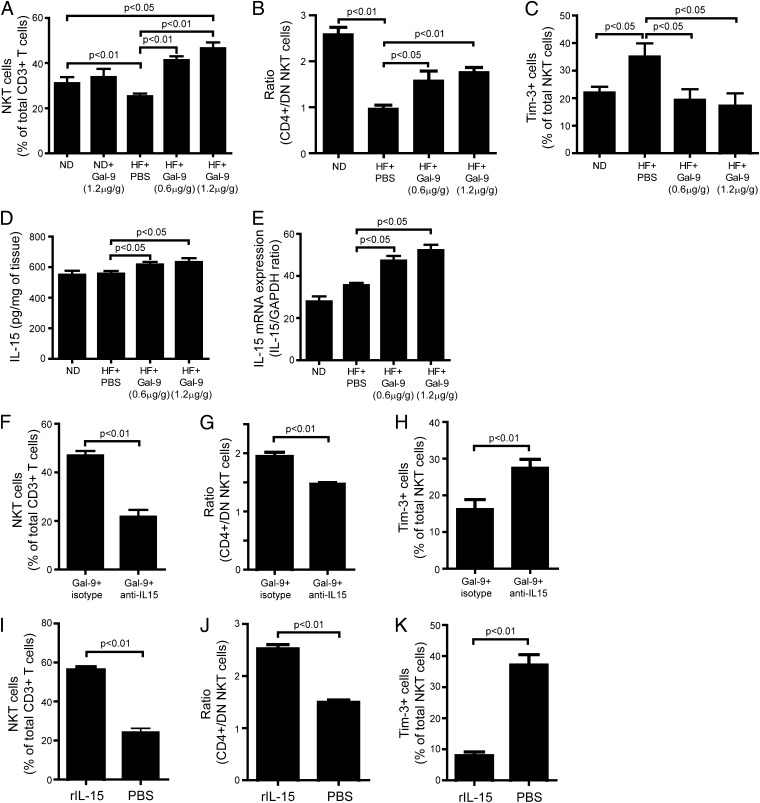
Because hepatic NKT cells play an important role in regulating inflammation, insulin resistance, and the pathogenesis of NAFLD (12, 13), we examined the effect of activation of the Tim-3/Gal-9 signal on the regulation of hepatic NKT cells in vivo. Mice fed ND or an HF diet were given Gal-9 i.p., which had little effect on the hepatic NKT cell content in animals fed ND (data not shown). Surprisingly, Gal-9 significantly increased hepatic NKT cells in mice fed HF diet (Fig. 5A). In addition, Gal-9 treatment altered the ratio of CD4+/DN NKT cells by selectively enhancing the CD4+ NKT cell population in the liver of mice fed HF diet (Fig. 5B). This was unexpected, because activation of Tim-3 signaling induced NKT cell apoptosis (Fig. 3), and HF diet increased Tim-3+ expression in hepatic NKT cells (Fig. 4), which should lead to a further decline in hepatic NKT cells after Gal-9 treatment. However, it appeared that there was a significant expansion of Tim-3− NKT cells in the liver of mice fed HF diet after Gal-9 treatment (Fig. 5C). Gal-9 not only increased hepatic NKT cells, it also induced significant proliferation of hepatic NK cells and CD8+ T cells (data not shown), while CD4+ Th cells remained constant (data not shown). This provided the important clue that IL-15 may be a crucial factor mediating the effect of Gal-9 in animals fed HF diet, because abundant evidence demonstrated that the proliferation and maintenance of NKT, NK, and CD8+ T cells are highly dependent on IL-15 (20). Our previous study showed that IL-15 directly contributes to reduced NKT cells in mice with fatty liver (21). Gal-9 treatment significantly increased both mRNA and protein expression of IL-15 in the liver of mice fed and HF diet (Fig. 5D, 5E). Other critical factors associated with NKT cell development, such as IL-7 and IL-21, remained the same (data not shown). Blocking IL-15 with anti–IL-15 Ab abolished Gal-9–induced hepatic NKT cell proliferation (Fig. 5F), CD4+/DN NKT cell ratio reversion (Fig. 5G), and Tim-3− NKT expansion (Fig. 5H) in mice fed HF diet. Treatment of these mice with rIL-15 protein resulted in NKT cell proliferation (Fig. 5I); it also reversed the ratio of CD4+/DN NKT cells (Fig. 5J) and increased Tim-3− NKT cells (Fig. 5K), further indicating that the effect of Gal-9 was mediated by IL-15.
FIGURE 5.
Gal-9 increased hepatic IL-15 expression and NKT cell proliferation in animals fed HF diet. C57BL/6 mice were fed ND or HF diet for 16 wk. Some of the mice fed HF diet also received Gal-9 (0.6 or 1.2 μg/g body weight, i.p.) every three days for the last 4 wk of feeding, with or without anti–IL-15 Ab (1 μg/g body weight, i.p.). Other mice fed HF diet received rIL-15 protein (25 ng/g body weight) once every 3 d for 9 d before sacrifice. HMNCs were isolated and stained with Abs to NK1.1, CD3, CD4, CD8, and Tim-3. Gal-9 treatment caused a significant increase in NKT cells (A), reversal of CD4+/DN NKT cell ratio (B), and expansion of Tim-3− NKT cells (C) in the liver of mice fed HF diet. Gal-9 treatment significantly increased both protein (D) and mRNA (E) expression of IL-15 in the liver of mice fed HF diet. Anti–IL-15 Ab treatment abolished Gal-9–induced NKT cell proliferation (F), reversal of CD4+/DN NKT cell ratio (G), and Tim-3− NKT cell expansion (H) in the liver of mice fed HF diet. rIL-15 protein treatment had similar effects on Gal-9 that caused NKT cell proliferation (I), reversal of CD4+/DN NKT cell ratio (J), and expansion of Tim-3− NKT cells (K) in the liver of mice fed HF diet. Results are mean ± SD of five independent experiments.
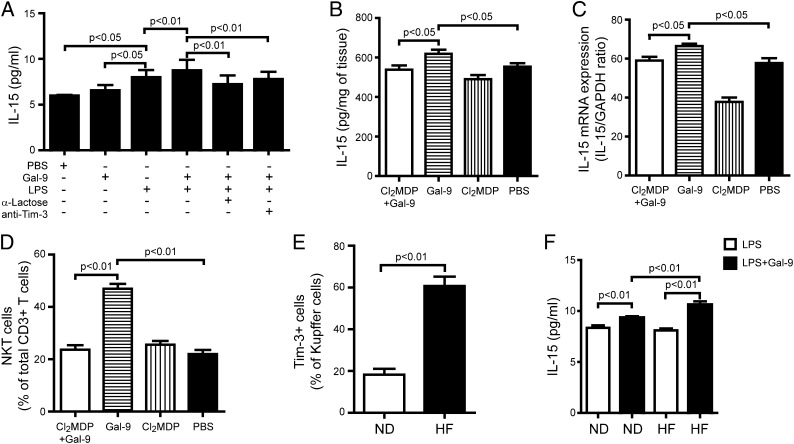
Previous studies, including ours, indicated that Kupffer cells are a major source of IL-15 in the liver (21, 22). In the current study, we found that although Gal-9 alone did not stimulate Kupffer cells to secret more IL-15, it significantly enhanced LPS-induced IL-15 production (Fig. 6A). Our results were in agreement with previous reports that Tim-3/Gal-9 may synergize with TLRs to influence a range of immune inflammatory reactions (3, 23). These effects could be blocked by the Gal-9 antagonist, α-lactose, or anti–Tim-3 Ab, demonstrating that the effect was mediated through Tim-3/Gal-9 signaling (Fig. 6A). To confirm Kupffer cells as the source of IL-15 induced by Gal-9, mice fed HF diet were injected i.v. with a suspension of clodronate-loaded liposomes to deplete Kupffer cells; this resulted in effective elimination of Gal-9–induced IL-15 production in the livers of HF-fed mice at both the mRNA and protein levels (Fig. 6B, 6C). Furthermore, depleting Kupffer cells also abolished Gal-9–induced NKT cell proliferation in the mice fed HF diet (Fig. 6D). In addition, Tim-3 expression was significantly elevated on Kupffer cells isolated from mice fed HF diet compared with those fed ND (Fig. 6E). Gal-9 in association with LPS caused significantly higher production of IL-15 from Kupffer cells isolated from mice fed HF diet versus ND (Fig. 6F). Taken together, these results suggest that Kupffer cells from mice fed HF diet exhibit high potency to Gal-9 stimulation due to an increased Tim-3 expression and respond with more IL-15 production, leading to hepatic NKT cell proliferation.
FIGURE 6.
Gal-9 induced Kupffer cell secretion of IL-15 in mice fed HF diet. C57BL/6 mice were fed ND or HF diet for 16 wk. Some mice also received Gal-9 (1.2 μg/g body weight, i.p.) every 3 d for the last 4 wk of feeding. In addition, some mice also received clodronate- or PBS-loaded liposomes (40 mg/g body weight, i.v.) every 3 d during the Gal-9 treatment. (A) Kupffer cells isolated from animal fed ND were cultured with Gal-9 (0.5 μM), α-lactose (40 mM), anti–Tim-3 mAb (20 μg/ml), or LPS (5 μg/ml), alone or in combination, for 24 h. IL-15 released to the media was measured by ELISA. Hepatic expression of IL-15 from animals fed HF diet treated with liposomes loaded with clodronate or PBS and stimulated or not with Gal-9 were determined by ELISA as pg/mg of liver protein (B) and quantitative PCR (C). (D) Percentage of hepatic NKT cells (gated on CD3+ T cells) from animals treated as above. (E) Kupffer cells were isolated from mice fed ND or HF diet. Percentage of Tim-3+ cells was determined by FACS. (F) Kupffer cells were isolated from mice fed ND or HF diet and cultured with LPS (5 μg/ml), with or without Gal-9 (0.5 μM), for 24 h. IL-15 released to the media was measured by ELISA. Results are mean ± SD of five independent experiments.
Gal-9 treatment improves steatosis in mice fed HF diet
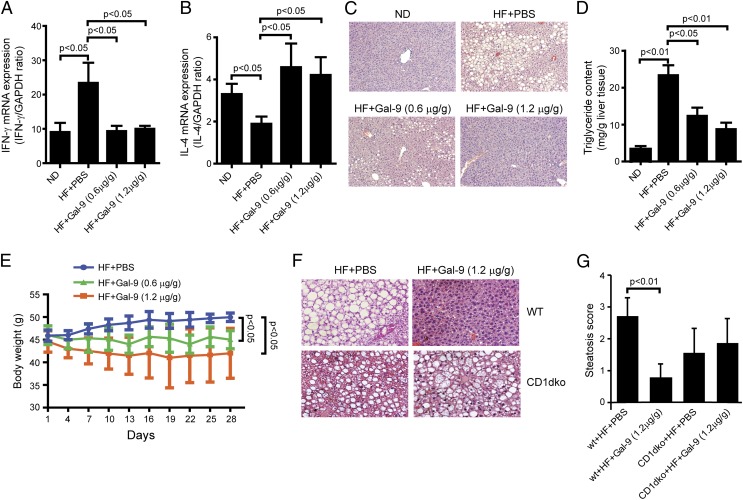
Our previous studies showed that depletion of hepatic NKT cells in mice fed HF diet contributes to the pathogenesis of NAFLD. This was likely due to increased inflammation and insulin resistance caused by NKT cell depletion in the liver. Adoptive transfer of NKT cells significantly improved insulin resistance and steatosis (12, 13). In this study, we evaluated whether Gal-9–induced hepatic NKT cell proliferation in mice fed HF also improved NAFLD. As shown previously, HF diet caused a proinflammatory cytokine profile, with increased IFN-γ expression and decreased IL-4 expression (12). Gal-9 treatment significantly reversed the profile by reducing IFN-γ and increasing IL-4 (Fig. 7A, 7B). Gal-9 treatment also significantly improved HF diet–induced hepatic steatosis on histology (Fig. 7C) and reduced hepatic triglyceride contents (Fig. 7D) in a dose-dependent manner. Furthermore, Gal-9 treatment also reduced animal weight and improved obesity in animals fed HF diet (Fig. 7E). To prove that the effect of Gal-9 on diet-induced hepatic steatosis is NKT cell dependent, we treated HF-fed CD1dko mice with Gal-9 in the same manner as wild-type mice fed HF diet. Gal-9’s effect on hepatic steatosis was greatly diminished among CD1dko mice that lack NKT cells (Fig. 7F, 7G).
FIGURE 7.
Gal-9 treatment ameliorated HF diet–induced obesity and hepatic steatosis. C57BL/6 wild-type or CD1dko mice were fed ND or HF diet for 16 wk. Some mice also received Gal-9 (0.6 or 1.2 μg/g body weight, i.p.) every 3 d for the last 4 wk of feeding. Hepatic expression of IFN-γ (A) and IL-4 (B) was determined by quantitative PCR. (C and F) Representative H&E staining of liver histology [original magnification ×100 (C) and ×200 (F)]. (D) Hepatic triglyceride contents. (E) Animal weight. (G) NAFLD histological score. Results are mean ± SD of five independent experiments (n = 5/group).
Discussion
Tim-3 is selectively expressed on a subset of T cells and is involved in the regulation of immunity and tolerance in vivo. Previous studies showed that Tim-3+ T cells are unresponsive to stimulation, as evidenced by their inability to proliferate or to produce cytokines (6, 24). Therefore, Tim-3+ T cells are identified as noneffector T cells or exhausted T cells. However, in our current study, we demonstrated that a large proportion of Tim-3+ T cells in the liver are NKT cells. These Tim-3+ NKT cells have the ability to proliferate and to produce more cytokines upon stimulation, suggesting that they are in a functional activated state. This distinguishes Tim-3+ NKT cells from other Tim-3+ T cells and may help to explain some paradoxical results on the function of Tim-3+ T cells. For example, Tim-3 is thought to be expressed only on Th1 CD4+ T cells (2); however, recent studies showed that some Tim-3+CD4+ T cells secret IL-4, which is a Th2 marker (25). We believe that those Tim-3+ IL-4–secreting cells are very likely to be CD4+ NKT cells.
The hallmark of NKT cell functional responses to specific stimulation is their ability to rapidly and simultaneously produce large amounts of Th1 (IFN-γ) and Th2 (IL-4) cytokines. Based on their distinct expression profiles of Th1 and Th2 cytokines, NKT cells can be further divided into CD4+ or CD4−CD8− DN subgroups. The CD4+ NKT cells produce both IFN-γ and IL-4, and the DN NKT cells produce predominantly IFN-γ (Fig. 2) (25, 26). The balance between these two NKT cell subgroups is important to initiate and regulate the Th1 and Th2 immune responses. The ratio of CD4+/DN NKT cells can be used as an index for evaluating the balance and the outcome of an immune response derived from NKT cell regulation (27, 28). Previously, we showed that the depletion of NKT cells in fatty liver is mainly due to a reduction in CD4+ NKT cells, which decreases the ratio of CD4+/DN NKT cells (12, 13). In the current study, we found that Tim-3+ NKT cells are predominantly CD4+ NKT cells (Fig. 1F). This may well explain why CD4+ NKT cells are more susceptible to Gal-9–induced apoptosis; exhibit a more spontaneous apoptosis tendency, resulting from enhanced Tim-3 expression induced by HF diets; and exhibit a decreased ratio of CD4+/DN NKT cells, leading to a Th1-biased immune response. This finding may further help us to understand NKT cell subtypes and their related function.
Many studies have provided evidence that the Tim-3/Gal-9 pathway may regulate chronic inflammatory diseases, such as autoimmune hepatitis (29), multiple sclerosis (30), and inflammatory bowel disease (31). Growing evidence also indicates local chronic inflammation as the key feature of NAFLD. (32, 33), which is closely related to obesity, has emerged as a major health problem in the U.S. (34). Understanding its disease mechanisms and finding appropriate therapies will have a huge impact on public health. We previously reported that hepatic NKT cell depletion plays an important role in the pathogenesis of NAFLD (12, 13). The mechanisms of hepatic NKT cell depletion can be multiple, including dietary factors (35) and gut flora (13). In the current study, we show that Tim-3/Gal-9 play an important role in maintaining the homeostasis of hepatic NKT cells. They not only cause activation-induced apoptosis through Tim-3 expression on NKT cells but also cause activation-induced proliferation through Kupffer cells in an IL-15–dependent manner (Figs. 5, 6). Our study shows that Gal-9 can synergize with LPS to activate Kupffer cells to secrete IL-15. Additionally, studies recently reported that Tim-3/Gal-9 may synergize with the TLR system to influence a range of immune inflammatory reactions (3, 23). To our knowledge, our study shows for the first time that exogenous Gal-9 can significantly improve diet-induced steatosis and obesity in an NKT cell–dependent manner (Fig. 7). Using adoptive transfer and NKT deletion, we previously showed the critical role for hepatic NKT cells in regulating diet-induced inflammation and steatosis (13). Taken together, they demonstrate that modulating NKT cells may have a significant therapeutic impact in fighting the ever-growing trend of obesity and fatty liver diseases.
The Tim-3/Gal-9 pathway is thought to play a complex role in regulating innate and adaptive immunity during various phases of the immune response. During the priming phase, Tim-3–expressing innate immune cells, such as DCs and macrophages, engage with Gal-9 and produce proinflammatory cytokines that drive Th1 cell responses. During the effector phase, Gal-9 specifically induces apoptosis of adaptive immune cells, such as Th1 cells, through binding to Tim-3, downregulating effector Th1 responses, and inducing tolerance (3, 5, 36). In the current study, we discovered a similar, but more complex, paradigm that regulates the homeostasis of hepatic NKT cells. Our study indicates that, upon activation by either endogenous (iGb3) (37) or exogenous (bacterial pathogen) Ags (38–40), NKT cells secrete many cytokines, including IFN-γ and IL-4, and upregulate the expression of Tim-3 (Fig. 2). IFN-γ induces the production of Gal-9 by Kupffer cells (41), leading to Tim-3+ NKT cell apoptosis, limiting the inflammatory response, and avoiding destructive immunity. In the meantime, Gal-9 also interacts with Tim-3 expressed on Kupffer cells to produce IL-15 that induces the proliferation of NKT cells. These eventually lead to the homeostasis of NKT cells and balance of the local immune microenvironment. Interestingly, our study also identified Tim-3–independent, Gal-9–induced NKT cell apoptosis (Fig. 3E). Several studies showed Tim-3–independent T cell regulation by Gal-9 (5, 42, 43), indicating that other pathways may be involved in Gal-9 signaling. Currently, we do not know which pathway is involved in Tim-3–independent Gal-9 signaling in NKT cells.
In summary, we showed in this study that the Tim-3/Gal-9 signaling pathway plays a critical role in the homeostasis of hepatic NKT cells. Through activation-induced apoptosis and secondary proliferation, the Tim-3/Gal-9 signaling pathway accurately maintains a balanced local immune microenvironment in the liver. Understanding its mechanism will provide an attractive approach to experimental therapies for obesity-related fatty liver diseases.
Acknowledgments
We thank Dr. Toshiro Niki for providing sGal-9 and anti-mouse Gal-9 Ab.
This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK075990 (to Z.L.), National Natural Science Foundation of China Grants 30700799 and 81172803 (to Z.-H.T.), Doctoral Fund of Ministry of Education of China Grant 20070487119 (to Z.-H.T.), and Natural Science Foundation of Hubei Province Grant 2007ADA201 (to Z.-H.T.).
- 7-AAD
- 7-aminoactinomycin D
- CD1dko
- CD1d knockout
- DC
- dendritic cell
- DN
- double negative
- α-GalCer
- α-galactosylceramide
- Gal-9
- Galectin-9
- HF
- high fat
- HMNC
- hepatic mononuclear cell
- NAFLD
- nonalcoholic fatty liver disease
- ND
- normal diet
- sGal-9
- stable form of recombinant Galectin-9
- Tim
- T cell Ig and mucin domain.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Monney L., Sabatos C. A., Gaglia J. L., Ryu A., Waldner H., Chernova T., Manning S., Greenfield E. A., Coyle A. J., Sobel R. A., et al. 2002. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415: 536–541 [DOI] [PubMed] [Google Scholar]
- 2.Sánchez-Fueyo A., Tian J., Picarella D., Domenig C., Zheng X. X., Sabatos C. A., Manlongat N., Bender O., Kamradt T., Kuchroo V. K., et al. 2003. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 4: 1093–1101 [DOI] [PubMed] [Google Scholar]
- 3.Anderson A. C., Anderson D. E., Bregoli L., Hastings W. D., Kassam N., Lei C., Chandwaskar R., Karman J., Su E. W., Hirashima M., et al. 2007. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science 318: 1141–1143 [DOI] [PubMed] [Google Scholar]
- 4.Nakae S., Iikura M., Suto H., Akiba H., Umetsu D. T., Dekruyff R. H., Saito H., Galli S. J. 2007. TIM-1 and TIM-3 enhancement of Th2 cytokine production by mast cells. Blood 110: 2565–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu C., Anderson A. C., Schubart A., Xiong H., Imitola J., Khoury S. J., Zheng X. X., Strom T. B., Kuchroo V. K. 2005. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 6: 1245–1252 [DOI] [PubMed] [Google Scholar]
- 6.Golden-Mason L., Palmer B. E., Kassam N., Townshend-Bulson L., Livingston S., McMahon B. J., Castelblanco N., Kuchroo V., Gretch D. R., Rosen H. R. 2009. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J. Virol. 83: 9122–9130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMahan R. H., Golden-Mason L., Nishimura M. I., McMahon B. J., Kemper M., Allen T. M., Gretch D. R., Rosen H. R. 2010. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J. Clin. Invest. 120: 4546–4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ronchi F., Falcone M. 2008. Immune regulation by invariant NKT cells in autoimmunity. Front. Biosci. 13: 4827–4837 [DOI] [PubMed] [Google Scholar]
- 9.Taniguchi M., Seino K., Nakayama T. 2003. The NKT cell system: bridging innate and acquired immunity. Nat. Immunol. 4: 1164–1165 [DOI] [PubMed] [Google Scholar]
- 10.Liu Y., Shu Q., Gao L., Hou N., Zhao D., Liu X., Zhang X., Xu L., Yue X., Zhu F., et al. 2010. Increased Tim-3 expression on peripheral lymphocytes from patients with rheumatoid arthritis negatively correlates with disease activity. Clin. Immunol. 137: 288–295 [DOI] [PubMed] [Google Scholar]
- 11.Godfrey D. I., Hammond K. J., Poulton L. D., Smyth M. J., Baxter A. G. 2000. NKT cells: facts, functions and fallacies. Immunol. Today 21: 573–583 [DOI] [PubMed] [Google Scholar]
- 12.Li Z., Soloski M. J., Diehl A. M. 2005. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology 42: 880–885 [DOI] [PubMed] [Google Scholar]
- 13.Ma X., Hua J., Li Z. 2008. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J. Hepatol. 49: 821–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Rooijen N., Sanders A. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174: 83–93 [DOI] [PubMed] [Google Scholar]
- 15.Van Rooijen N., Kors N., vd Ende M., Dijkstra C. D. 1990. Depletion and repopulation of macrophages in spleen and liver of rat after intravenous treatment with liposome-encapsulated dichloromethylene diphosphonate. Cell Tissue Res. 260: 215–222 [DOI] [PubMed] [Google Scholar]
- 16.Smedsrød B., Pertoft H. 1985. Preparation of pure hepatocytes and reticuloendothelial cells in high yield from a single rat liver by means of Percoll centrifugation and selective adherence. J. Leukoc. Biol. 38: 213–230 [DOI] [PubMed] [Google Scholar]
- 17.Ohteki T., Suzue K., Maki C., Ota T., Koyasu S. 2001. Critical role of IL-15-IL-15R for antigen-presenting cell functions in the innate immune response. Nat. Immunol. 2: 1138–1143 [DOI] [PubMed] [Google Scholar]
- 18.Lv K., Zhang Y., Zhang M., Zhong M., Suo Q. 2012. Galectin-9 ameliorates Con A-induced hepatitis by inducing CD+CD25low/int effector T-cell apoptosis and increasing regulatory T cell number. PLoS One 7: e48379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kashio Y., Nakamura K., Abedin M. J., Seki M., Nishi N., Yoshida N., Nakamura T., Hirashima M. 2003. Galectin-9 induces apoptosis through the calcium-calpain-caspase-1 pathway. J. Immunol. 170: 3631–3636 [DOI] [PubMed] [Google Scholar]
- 20.Ranson T., Vosshenrich C. A., Corcuff E., Richard O., Laloux V., Lehuen A., Di Santo J. P. 2003. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc. Natl. Acad. Sci. USA 100: 2663–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z., Lin H., Yang S., Diehl A. M. 2002. Murine leptin deficiency alters Kupffer cell production of cytokines that regulate the innate immune system. Gastroenterology 123: 1304–1310 [DOI] [PubMed] [Google Scholar]
- 22.Golden-Mason L., Kelly A. M., Doherty D. G., Traynor O., McEntee G., Kelly J., Hegarty J. E., O’Farrelly C. 2004. Hepatic interleuklin 15 (IL-15) expression: implications for local NK/NKT cell homeostasis and development. Clin. Exp. Immunol. 138: 94–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uchida, Y., B. Ke, M. C. Freitas, H. Yagita, H. Akiba, R. W. Busuttil, N. Najafian, and J. W. Kupiec-Weglinski. 2010. T cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in mice in TLR4-dependent manner. Gastroenterology 139: 2195–2206. [DOI] [PMC free article] [PubMed]
- 24.Hastings W. D., Anderson D. E., Kassam N., Koguchi K., Greenfield E. A., Kent S. C., Zheng X. X., Strom T. B., Hafler D. A., Kuchroo V. K. 2009. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur. J. Immunol. 39: 2492–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gumperz J. E., Miyake S., Yamamura T., Brenner M. B. 2002. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J. Exp. Med. 195: 625–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee P. T., Benlagha K., Teyton L., Bendelac A. 2002. Distinct functional lineages of human V(alpha)24 natural killer T cells. J. Exp. Med. 195: 637–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seino K., Taniguchi M. 2005. Functionally distinct NKT cell subsets and subtypes. J. Exp. Med. 202: 1623–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson S. B., Delovitch T. L. 2003. Janus-like role of regulatory iNKT cells in autoimmune disease and tumour immunity. Nat. Rev. Immunol. 3: 211–222 [DOI] [PubMed] [Google Scholar]
- 29.Liberal R., Grant C. R., Holder B. S., Ma Y., Mieli-Vergani G., Vergani D., Longhi M. S. 2012. The impaired immune regulation of autoimmune hepatitis is linked to a defective galectin-9/tim-3 pathway. Hepatology 56: 677–686 [DOI] [PubMed] [Google Scholar]
- 30.Koguchi K., Anderson D. E., Yang L., O’Connor K. C., Kuchroo V. K., Hafler D. A. 2006. Dysregulated T cell expression of TIM3 in multiple sclerosis. J. Exp. Med. 203: 1413–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X., Chen G., Li Y., Wang R., Wang L., Lin Z., Gao X., Feng J., Ma Y., Shen B., et al. 2010. Involvement of T cell Ig Mucin-3 (Tim-3) in the negative regulation of inflammatory bowel disease. Clin. Immunol. 134: 169–177 [DOI] [PubMed] [Google Scholar]
- 32.Hotamisligil G. S. 2006. Inflammation and metabolic disorders. Nature 444: 860–867 [DOI] [PubMed] [Google Scholar]
- 33.Brunt E. M. 2010. Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol. Hepatol. 7: 195–203 [DOI] [PubMed] [Google Scholar]
- 34.Mokdad A. H., Ford E. S., Bowman B. A., Dietz W. H., Vinicor F., Bales V. S., Marks J. S. 2003. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289: 76–79 [DOI] [PubMed] [Google Scholar]
- 35.Hua J., Ma X., Webb T., Potter J. J., Oelke M., Li Z. 2010. Dietary fatty acids modulate antigen presentation to hepatic NKT cells in nonalcoholic fatty liver disease. J. Lipid Res. 51: 1696–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Manzanet R., DeKruyff R., Kuchroo V. K., Umetsu D. T. 2009. The costimulatory role of TIM molecules. Immunol. Rev. 229: 259–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou D., Mattner J., Cantu C., III, Schrantz N., Yin N., Gao Y., Sagiv Y., Hudspeth K., Wu Y.-P., Yamashita T., et al. 2004. Lysosomal glycosphingolipid recognition by NKT cells. Science 306: 1786–1789 [DOI] [PubMed] [Google Scholar]
- 38.Mattner J., Debord K. L., Ismail N., Goff R. D., Cantu C., III, Zhou D., Saint-Mezard P., Wang V., Gao Y., Yin N., et al. 2005. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434: 525–529 [DOI] [PubMed] [Google Scholar]
- 39.Kinjo Y., Tupin E., Wu D., Fujio M., Garcia-Navarro R., Benhnia M. R., Zajonc D. M., Ben-Menachem G., Ainge G. D., Painter G. F., et al. 2006. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat. Immunol. 7: 978–986 [DOI] [PubMed] [Google Scholar]
- 40.Kinjo Y., Wu D., Kim G., Xing G. W., Poles M. A., Ho D. D., Tsuji M., Kawahara K., Wong C. H., Kronenberg M. 2005. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434: 520–525 [DOI] [PubMed] [Google Scholar]
- 41.Mengshol J. A., Golden-Mason L., Arikawa T., Smith M., Niki T., McWilliams R., Randall J. A., McMahan R., Zimmerman M. A., Rangachari M., et al. 2010. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS ONE 5: e9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su E. W., Bi S., Kane L. P. 2011. Galectin-9 regulates T helper cell function independently of Tim-3. Glycobiology 21: 1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaitaitis G. M., Wagner D. H., Jr. 2012. Galectin-9 controls CD40 signaling through a Tim-3 independent mechanism and redirects the cytokine profile of pathogenic T cells in autoimmunity. PLoS ONE 7: e38708. [DOI] [PMC free article] [PubMed] [Google Scholar]