Abstract
The germinal center (GC) is the dymanic microenvironment where Ag-activated B cells rapidly expand and differentiate, generating plasma cells (PC) that produce high affinity antibodies. B cells within the GC have great heterogeneity, containing B cells at different stages of activation and differentiation. However, there are few surface markers that allow subsets of GC-B cells to be distinguished. In the present study, we show that GC-B cells in human tonsils contain two distinct populations regarding CD9 expression; CD9− and CD9+ cells. CD9+ GC-B cells are functionally more differentiated towards PC based upon the following evidence; (1) CD9+ cells express higher levels of PC transcription factor, Blimp-1 while lower levels of B cell transcription factors, Bcl-6 and Pax-5, compared to CD9− cells, (2) CD9+ cells differentiate into plasmablasts faster than CD9− cells in the presence of cytokines that generate PC, and (3) CD9 expression was induced in CD9− GC-B cells under PC generating condition and gradually increased in the course of PC differentiation. Taken together, our data suggest that CD9 is a novel marker for a human GC-B cell subset that is committed to PC lineage.
Keywords: CD9, Germinal Center, B cells, Plasma cells, differentiation
Introduction
The tetraspanins are a large superfamily of four transmembrane proteins. Tetraspanins organize transmembrane proteins and signaling molecules into functional microdomains in the plasma membrane, regulating fundamental cellular processes such as migration, proliferation, and differentiation [1, 2]. CD9 belongs to the family of tetraspanin and has been implicated in various biological functions, including cell adhesion, motility, metastasis, growth, signal transduction, differentiation, and sperm-egg fusion [2–5]. CD9 is widely distributed on the surface of normal and malignant cells as well as on a variety of cell lines [3, 6]. Although this molecule was originally identified on the surface of human B lineage cell [7], its expression and function in human B cells have not been studied extensively. In the mouse system, CD9 was reported to be a unique marker for B1 and marginal zone B cells and plasma cells [8]. In addition, CD9 on B1 cells play a functional role in retention of B1 cells within the peritoneum-associated tissues via integrins [9]. However, CD9 expression and its function in mature human B cells and humoral immune response have not been explored yet.
Humoral immune response, which is governed by B cells, protects the body from invading pathogens by producing immunoglobulin against them. Shortly after exposure to antigens, some B cells become short-lived plasmablasts, generating an early burst of antibody that plays an initial protective role. Simultaneously, some selected B cells migrate to the germinal center (GC) to generate long-lived plasma cells and memory B cells for long-term protection. The germinal center serves as a niche for antibody diversification processes such as somatic hypermutation and class-switch recombination, leading to the generation of high affinity antibodies. Somatic hypermutation introduces point mutations into the IgV gene and class-switch recombination alters the effector function of the antibody by switching the constant region of IgM to IgG, IgE, or IgA isotype. Subsequently, newly mutated cells compete for the antigen bound to the surface of follicular dendritic cells (FDC) and for T-cell help by follicular helper T cells. Those that successfully bind the antigen receive survival and maturation signals and differentiate into memeory B cells or plasma cells (PC). B cells within the GC have great heterogeneity, containing B cells at different stages of differentiation as a consequence of the ongoing events.
In the present study, we show that GC-B cells in human tonsils contain two distinct populations regarding CD9 expression; CD9− and CD9+ cells. In vitro comparison experiments and in situ tonsil tissue staining revealed that CD9+ GC-B cells are in more advanced stages of PC differentiation, compared to CD9− GC-B cells.
Materials and Methods
Antibodies
FITC- or PE- conjugated anti-CD9 (clone MM2/57), FITC- or -PE- conjugated isotype-matched control (clone A1), and FITC-conjugated anti-CD20 were obtained from Southern biotech (Birmingham, AL); PE- conjugated anti-IgD, FITC-, PE-, PerCP-Cy5.5-, or APC- conjugated anti-CD38, PE- or PerCP-Cy5.5- conjugated anti-CD27, FITC- or PerCP-Cy5.5- conjugated anti-CD20, PE- conjugated or unconjugated anti-CD138, and FITC- conjugated anti-CD10 (BD PharMingen, San Diego, CA); rat anti-mouse IgG1 microbeads (Miltenyi Biotech, Sunnyvale, CA). Anti-CD9 (clone 10E5) was prepared as described previously [10].
Cytokines and reagents
Cytokines used were IL-2 (Hoffman-La Roche, Nutley, NJ), IL-4 (a generous gift from Schering-Plough, Union, NJ), and IL-10 (Biovision, Mountain View, CA). Soluble human CD40L was generously provided by Dr. R. Armitage (Amgen Corporation, Seattle, WA).
Preparation and culture of B cell subsets
Blood and tonsils were obtained through Blood Bank and Surgery Department within the Ochsner Clinic Foundation. Informed consent was not required in our institute as these materials are considered as waste generated during either blood donation or surgery. In addition, patient samples arrive de-identified in the laboratory. Peripheral blood B cells (PBB) was isolated from buffy coat using B cell isolation kit (Miltenyi Biotech). GC-B cells were isolated from tonsils as described previously with a minor modification [11]. Briefly, T cells were depleted from mononuclear cells isolated from tonsils by rosetting with SRBC; the resulting cells contained > 97% CD20+ B cells as analyzed by FACScan (Becton Dickinson, Sunnyvale, CA). GC-B cells were purified by magnetic cell separation (MACS; Miltenyi Biotec) as follows; Nonrosetting tonsillar B cells were incubated with anti-IgD (clone HJ9), anti-CD44 (clone NK1P1), and anti-CD138 (clone DL-101) mAbs for 15 min and then with rat anti-mouse IgG1- microbeads (Miltenyi Biotec) for 15 min on ice. The cells were loaded onto MACS columns and the negative fraction was collected, and the purity of GC-B cells was assessed by FACScan (> 96% IgD-CD38+ cells from all experiments; see Figure 1C). GC-B cells were further separated by CD9 expression. Anti-CD9 (clone 10E5) was added to the isolated GC-B cells and followed by anti-mouse IgG1- microbeads.
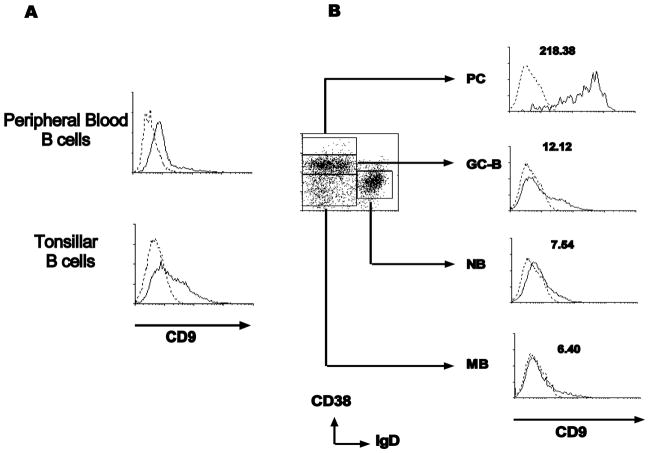
Figure 1. Expression of CD9 on human tonsillar B cell subsets.
(A) Peripheral blood B cells and tonsillar B cells were stained with anti-CD9 (solid line) or isotype control antibody (dotted line). (B) Tonsillar B cells were further stained with anti-CD38 and anti-IgD, gated by the expression of these two markers, and the expression of CD9 on each subset was examined (PC, plasma cells; GC-B, germinal center B cells; NB, naive B cells; MB, memory B cells).
CD9+ and CD9− GC-B cells (2 × 105 cells/well) were cultured in 24-well plates in the presence of irradiated HK cells (2 × 104 cells/well; 5000 rad), CD40L (100 ng/mL), and a different combination of cytokines (IL-2 (30 U/mL), IL-4 (50 U/mL), IL-10 (50 ng/mL)) for 4 days. The culture medium was IMDM (Irvine Scientific, Santa Ana, CA) supplemented with 10% FCS (LifFe Technologies, Grand Island, NY), 2 mM glutamine, 100 U/mL penicillin G, and 100 μg/mL streptomycin (Irvine Scientific). HK cells [12] were maintained in RPMI1640 with 10% FCS.
Flow cytometry and ELISA
Cells were stained with a panel of Abs directly conjugated with PE, FITC, PerCp, or APC as previously described [11]. Briefly, cells were incubated with the appropriate concentration of Ab for 15 min at 4°C. After washing with PBS containing 0.2% BSA and 0.1% sodium azide, cells were fixed with 1% paraformaldehyde and analyzed by flow cytometry. The amount of IgG in the culture supernatant was measured by ELISA as described previously [13].
Gene expression analysis by quantitative real-time PCR
Total cellular RNAs were isolated from CD9+ and CD9− GC-B cells using RNeasy protect kit (Qiagen, Valencia, CA). Total RNA was DNase I (Promega, Madison, WI) treated and reverse transcribed with Promega ImPromII reverse transcriptase kit. Real-time PCR reactions were performed in a 10 μl volume containing 25 ng of cDNA, QuantiTect SYBR Green PCR Master Mix (Qiagen), and 2.5 μM of each gene-specific primer. The DNA Engine Opticon system with PTC-200 DNA Engine cycler and CFD-3200 Opticon Detector (MJ Research Inc, Waltham, MA) were used at 60 °C for 1 min, and 72 °C for 1 min. After cycling, the specificity of amplification was validated by generating a melting curve by slow denaturation of the PCR products. The primer sequences used are as follow. Pax-5 (forward: 5′-GGAGGAGTGAATCAGCTTGG-3′, reverse: 5′-GGCTTGATGCTTCCTGTCTC-3′) [14], Bcl-6 (forward: 5′-CTGGCTTTTGTGACGGAAAT-3′, reverse: 5′-AACCTGAAAACCCACACTCG-3′) [15], Blimp-1 (forward: 5′-GTGTCAGAACGGGATGAACA-3′, reverse: 5′-GCTCGGTTGCTTTAGACTGC-3′) [15], and β2m (forward: 5′-TGA GTG CTG TCT CCA TGT TTG A-3′, reverse: 5′-TCT GCT CCC CAC CTC TAA GTT G-3′) [13]. Ct values were analyzed with the ΔΔCt method by using β2m expression for normalization in each experiment. Results were expressed as fold change with respect to that of CD9− GC-B cells and plotted in Graphpad Prism 4.0 (GraphPad, San Diego, CA).
Immunofluorescence
Cryosections of human tonsils (5μm thick) were air-dried overnight at room temperature and fixed in cold acetone for 10 min. Nonspecific binding of antibody was blocked by incubating the slides with 1% BSA-PBS for 30 min at room temperature in a humidified chamber. Slides were incubated with anti-CD9-PE in combination with anti-CD20-FITC, anti-CD10-FITC, anti-CD38-FITC, or anti-CD138-FITC. Isotype control-FITC and isotype control-PE were used side by side to exclude false positive. Slides were washed and mounted with anti-fade mounting medium (Invitrogen). Slides were then examined and images collected on a deconvolution microscope (Axiovert 200M; Carl Zeiss Microimaging). Images were processed using the slidebook software (Intelligent Imaging Innovations).
Statistical analysis
Statistical analysis and graphic presentation were carried out with GraphPad Prism 4.0 (GraphPad). Results are presented as means of triplicate assays plus SEM. The statistical significance of differences was determined by Student’s t- test; P < 0.05 was considered significant.
Results and Discussion
CD9 is differentially expressed among human tonsillar B cell subsets
Mature human B cells were isolated from human blood and tonsil and their CD9 expression was measured by FACS. Peripheral blood B cells expressed little CD9 while tonsillar B cells expressed CD9 in a heterogeneous manner (Figure 1A). CD9 expression in different subsets of tonsillar B cells was further examined by gating B cell subsets defined by CD38 and IgD expression (Figure 1B): IgD+CD38− naïve B cells (NB), IgD-CD38− memory B cells (MB), IgD-CD38+ GC-B cells, and IgD-CD38++ plasma cells (PC) [16]. As shown in Figure 1B, IgD-CD38++ PC showed the highest CD9 expression (MFI: 218.4) while naïve and memory B cells had negligible CD9 expression (MFI: 7.5 and 6.4, respectively). GC-B cells expressed modest levels of CD9 (MFI: 12.1) and contained two distinct populations, CD9+ and CD9−. The CD9 expression pattern among B cell subsets is consistent with different donors; however, the ratio of CD9+ to CD9− population in the GC-B cells significantly varied from donor to donor (the percentage of CD9+ GC-B cells ranged from 23 % to 70% from 7 donors, Table 1). Different from human GC-B cells, it has been reported that mouse GC-B cells did not express CD9 [8]. The disparity may be attributed to the difference between chronically inflamed GC (human tonsil) and the acutely induced GC (mouse spleen) because the proportion of CD9+ population in human GC-B cells varied among donors. This finding also suggests that CD9 expression on human GC-B cells may reflect the status of GC-B cell activation or differentiation.
Table 1.
Percentage of CD9+ GC-B cells from different donors
| Donors | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| #1 | #2 | #3 | #4 | #5 | #6 | #7 | |
| CD9+ GC-B cells (%) | |||||||
| 44.1 | 58.3 | 44.8 | 69.9 | 33.1 | 23.2 | 41.8 | |
CD9+ GC-B cells are more advanced cells than CD9- GC-B cells in the course of GC-B cell differentiation to PC
Based on our observations; (1) GC-B cells contained CD9+ and CD9− populations, (2) the germinal center is the site where plasma cells are generated, and (3) IgD-CD38++ plasma cells express high levels of CD9, we hypothesized that CD9+ GC-B cells were the cells in transit from GC-B cells to CD38++ PC. Since commitment of B cells to PC lineage cells is driven by specific transcription factors [17], we determined the expression of transcription factors crucial for PC differentiation in CD9+ and CD9− GC-B cell populations. First, we isolated GC-B cells as described in the Materials and Method, and CD9+ and CD9− GC-B cell populations were further separated using a MACS column (Figure 2A). Quantitative real-time PCR data showed CD9+ GC-B cells expressed higher levels of Blimp-1, a master transcription factor for PC differentiation [18], compared to CD9− GC-B cells (Figure 2B).
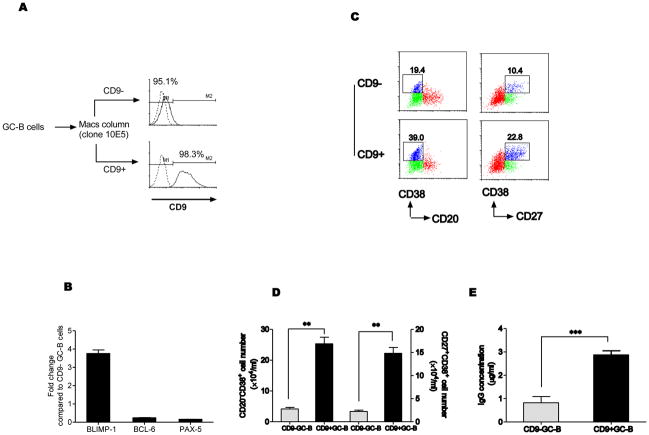
Figure 2. CD9+ GC-B cells are more advanced cells than CD9- GC-B cells in the course of GC-B cell differentiation to PC.
(A) CD9+ and CD9− GC-B cells were isolated with anti-CD9 antibody (10E5 clone) using a MACS column. Purity of the populations was determined by staining with FITC-conjugated anti-mouse IgG antibody. (B) Expression of transcription factors for PC differentiation was examined in isolated CD9+ and CD9− GC-B cells by a quantitative real-time PCR. The relative fold changes of each transcript in CD9+ GC-B cells are shown in comparison to the levels of the transcript in CD9− GC-B cells, which is assigned the value 1. One representative data from four reproducible experiments is shown. (C–E) CD9+ and CD9− GC-B cells were cultured for 4 days in the presence of FDC/HK cells with IL-2, IL-10, and CD40L. At the end of the culture, cell surface phenotype was determined by flow cytometry analysis after staining the cells with anti-CD38, anti-CD27, and anti-CD20. The percentage of plasmablasts (CD20-CD38+ or CD27+CD38+) is indicated (C). The numbers of CD20-CD38+ and CD27+CD38+ plasmablasts (D) and IgG concentration (E) were determined (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
At the same time, the expression of Pax-5 and Bcl-6, which are known to be switched off before PC differentiation was significantly lower in CD9+ GC-B cells (Figure 2B) [19, 20]. This data suggests that CD9+ population is a more differentiated population towards PC, compared to CD9− population, and corroborates a previous report that a subset of human GC-B cells express Blimp-1 [21].
To further confirm the differential expression in the transcription factors between CD9+ and CD9− populations functionally, we determined whether CD9+ GC-B cells generate PC faster than CD9− GC-B cells. CD9+ and CD9− GC-B cells were cultured with IL-2 and IL-10 in the presence of CD40L and an FDC line, HK cells [12] for 4 days to induce plasma cells [22] and at the end of the culture, cell surface phenotype and antibody production were examined. CD9+ GC-B cells generated a high percentage of CD20-CD38+ and CD27+CD38+ plasmablasts compared to CD9− GC-B cells (39.0% and 19.4% vs 22.8% and 10.4%, Figure 2B). Consistent with the phenotypic data, the numbers of CD20-CD38+ and CD27+CD38+ plasmablasts were significantly higher in the cultures of CD9+ GC-B cells compared to CD9− GC-B cells (Figure 2C). The amounts of the secreted IgG in the culture supernatants correlated with absolute numbers of plasmablasts generated (Figure 2D). This result is in agreement with a report using mouse B cells [8]. Although different target cells were used in the experiments, Won et al clearly demonstrated that CD9+ B1a cells could differentiate into CD138+ PC faster than CD9− B1a cells [8]. All together, the data suggest that CD9+ GC-B cells are more advanced cells than CD9− GC-B cells in the course of GC-B cell differentiation to PC.
CD9 is induced during GC-B cell differentiation to PC
Since CD9+ GC-B cells appear to be more differentiated towards PC, we examined whether CD9 is induced in the course of GC-B cell differentiation into PC. CD9− and CD9+ GC-B cells were cultured in the plasma cell generating culture condition for 4 days as described above or in the memory B cell generating culture condition by adding IL-2 plus IL-4 [22] in place of IL-2 plus IL-10, as a negative control and CD9 expression was quantified by FACS analysis. As shown in Figure 3A, both CD9− and CD9+ GC-B cells exhibited higher expression of CD9 when cultured with IL-2/IL-10 compared to IL-2/IL-4 (MFI 66.5 vs 25.4 for CD9−, MFI 183.3 vs 69.6 for CD9+). Furthermore, CD9 expression in CD20-CD38+ plasmablasts was higher than their precursors among the cells generated with IL-2/IL-10, suggesting that CD9 expression is upregulated during differentiation to PC (Figure 3B). Overall, CD9 expression is gradually increased in the course of GC-B cell differentiation to PC, confirming CD9 expression data obtained with ex vivo memory B cells and PC (Figure 1B).
Figure 3. CD9 is induced during GC-B cell differentiation to PC.
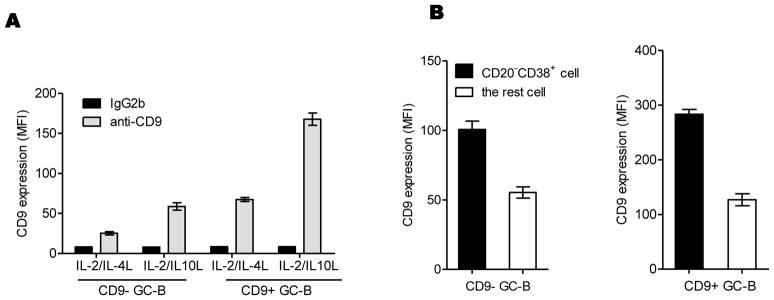
CD9− and CD9+ GC-B cells were cultured in the presence of FDC/HK cells with a different combination of cytokines (CD40L+ IL-2 + IL-4 or CD40L+ IL-2 + IL-10) for 4 days. (A) At the end of the culture, cells were stained with anti-CD9 or isotype control antibody. CD9 expression levels on the cells were quantified by FACS analysis and shown as MFIs. (B) Cells generated from the culture with CD40L+ IL-2 + IL-10 were further stained with anti-CD20 and anti-CD38, gated by the expression of these two markers, and the expression of CD9 on CD20-CD38+ plasmablasts and the rest was also quantified by FACS analysis and presented in MFIs. One representative data from three independent experiments with different donors is shown.
Localization of CD9+ GC-B cells in vivo
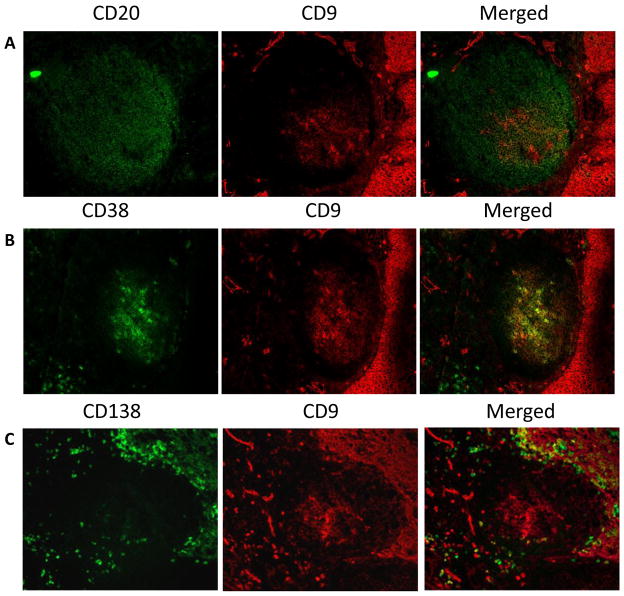
To localize CD9+ GC-B cells in vivo, human tonsillar tissue sections were stained with anti-CD9 antibody. Within the GC, cells in a certain area expressed CD9. To identify the CD9 expressing cells, tissue sections were costained with antibodies against GC-B cell and PC markers such as CD10, CD20, CD38, and CD138 (Figure 4). Anti-CD20 stained B cells in the GC while less than half of the CD20+ cells expressed CD9. Staining with anti-CD10 showed the same pattern as with anti-CD20 (data not shown). Majority of CD9+ cells in the GC were not mature PC because CD138+ cells are scarce within the GC and mostly reside outside the GC. However, most CD38 intermediate/bright cells in the GC express CD9, confirming that CD9 is a marker for PC lineage committed cells prior to the expression of CD38 and CD138. This immunohistochemical analysis of CD9+ GC-B cells in tonsillar tissue sections corroborate with our ex vivo and in vitro experimental data presented above, supporting our conclusion that CD9 is a marker for PC precursors.
Figure 4. Immunofluorescent staining for CD9 in the germinal centers of human tonsillar tissue sections.
The frozen tonsillar tissue sections were stained with anti-CD9 (red) in combination with anti-CD20 (A, green), anti-CD38 (B, green), and anti-CD138 (C, green).
Highlights.
Human tonsillar B cell subsets express CD9 differentially.
Germinal center (GC) B cells contain CD9+ and CD9− populations.
CD9+ GC-B cells are in more advanced stages of PC differentiation.
CD9 expression is induced in the course of GC-B cell differentiation to PC.
Acknowledgments
This work was supported by NIH grant R01CA121039 to YSC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wright MD, Moseley GW, van Spriel AB. Tetraspanin microdomains in immune cell signalling and malignant disease. Tissue Antigens. 2004;64:533–42. doi: 10.1111/j.1399-0039.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- 2.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–11. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 3.Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. Faseb J. 1997;11:428–42. [PubMed] [Google Scholar]
- 4.Boucheix C, Rubinstein E. Tetraspanins. Cell Mol Life Sci. 2001;58:1189–205. doi: 10.1007/PL00000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zoller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 2009;9:40–55. doi: 10.1038/nrc2543. [DOI] [PubMed] [Google Scholar]
- 6.Sincock PM, Mayrhofer G, Ashman LK. Localization of the transmembrane 4 superfamily (TM4SF) member PETA-3 (CD151) in normal human tissues: comparison with CD9, CD63, and alpha5beta1 integrin. J Histochem Cytochem. 1997;45:515–25. doi: 10.1177/002215549704500404. [DOI] [PubMed] [Google Scholar]
- 7.Kersey JH, LeBien TW, Abramson CS, Newman R, Sutherland R, Greaves M. P-24: a human leukemia-associated and lymphohemopoietic progenitor cell surface structure identified with monoclonal antibody. J Exp Med. 1981;153:726–31. doi: 10.1084/jem.153.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Won WJ, Kearney JF. CD9 is a unique marker for marginal zone B cells, B1 cells, and plasma cells in mice. J Immunol. 2002;168:5605–11. doi: 10.4049/jimmunol.168.11.5605. [DOI] [PubMed] [Google Scholar]
- 9.Ha SA, Tsuji M, Suzuki K, Meek B, Yasuda N, Kaisho T, Fagarasan S. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006;203:2541–50. doi: 10.1084/jem.20061041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko EM, Lee IY, Cheon IS, Kim J, Choi JS, Hwang JY, Cho JS, Lee DH, Kang D, Kim SH, Choe J. Monoclonal antibody to CD9 inhibits platelet-induced human endothelial cell proliferation. Mol Cells. 2006;22:70–7. [PubMed] [Google Scholar]
- 11.Choe J, Kim HS, Zhang X, Armitage RJ, Choi YS. Cellular and molecular factors that regulate the differentiation and apoptosis of germinal center B cells. Anti-Ig down-regulates Fas expression of CD40 ligand-stimulated germinal center B cells and inhibits Fas-mediated apoptosis. J Immunol. 1996;157:1006–16. [PubMed] [Google Scholar]
- 12.Kim H-S, Zhang X, Choi YS. Activation and proliferation of follicular dendritic cell-like cells by activated T lymphocytes. J Immunol. 1994;153:2951–2961. [PubMed] [Google Scholar]
- 13.Yoon SO, Zhang X, Berner P, Choi YS. IL-21 and IL-10 have redundant roles but differential capacities at different stages of Plasma Cell generation from human Germinal Center B cells. J Leukoc Biol. 2009;86:1311–8. doi: 10.1189/jlb.0409268. [DOI] [PubMed] [Google Scholar]
- 14.Diehl SA, Schmidlin H, Nagasawa M, van Haren SD, Kwakkenbos MJ, Yasuda E, Beaumont T, Scheeren FA, Spits H. STAT3-Mediated Up-Regulation of BLIMP1 Is Coordinated with BCL6 Down-Regulation to Control Human Plasma Cell Differentiation. J Immunol. 2008;180:4805–15. doi: 10.4049/jimmunol.180.7.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheeren FA, Naspetti M, Diehl S, Schotte R, Nagasawa M, Wijnands E, Gimeno R, Vyth-Dreese FA, Blom B, Spits H. STAT5 regulates the self-renewal capacity and differentiation of human memory B cells and controls Bcl-6 expression. Nat Immunol. 2005;6:303–13. doi: 10.1038/ni1172. [DOI] [PubMed] [Google Scholar]
- 16.Pascual V, Liu Y-J, Magalski A, de Bouteiller O, Banchereau J, Capra JD. Analysis of somatic mutation in five B cell subsets of human tonsil. J Exp Med. 1994;180:329–339. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calame KL, Lin KI, Tunyaplin C. Regulatory mechanisms that determine the development and function of plasma cells. Annu Rev Immunol. 2003;21:205–30. doi: 10.1146/annurev.immunol.21.120601.141138. [DOI] [PubMed] [Google Scholar]
- 18.Turner CA, Jr, Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 19.Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 20.Nera KP, Kohonen P, Narvi E, Peippo A, Mustonen L, Terho P, Koskela K, Buerstedde JM, Lassila O. Loss of Pax5 promotes plasma cell differentiation. Immunity. 2006;24:283–93. doi: 10.1016/j.immuni.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Angelin-Duclos C, Cattoretti G, Lin KI, Calame K. Commitment of B lymphocytes to a plasma cell fate is associated with Blimp-1 expression in vivo. J Immunol. 2000;165:5462–71. doi: 10.4049/jimmunol.165.10.5462. [DOI] [PubMed] [Google Scholar]
- 22.Choe J, Choi YS. Interleukin-10 interrupts memory B cell expansion in the germinal center by inducing differentiation into plasma. Eur J Immunol. 1998;28:508–515. doi: 10.1002/(SICI)1521-4141(199802)28:02<508::AID-IMMU508>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]