Abstract
As part of its Medical Technologies Evaluation Programme, the National Institute of Health and Clinical Excellence (NICE) invited the manufacturer, Covidien, to provide clinical and economic evidence for the evaluation of the Pipeline™ embolization device (PED) for the treatment of complex intracranial aneurysms. Cedar; a consortium between Cardiff and Vale University Health Board and Cardiff University, was commissioned to act as an External Assessment Centre (EAC) for NICE to independently critique the manufacturers’ submissions. This article gives an overview of the evidence provided, the findings of the EAC and the final guidance published by NICE.
The scope issued by NICE considered PED as the intervention in a patient population with complex unruptured intracranial aneurysms (IAs), specifically large/giant, wide-necked and fusiform aneurysms. The comparator treatments identified were stent-assisted coiling, parent vessel occlusion, neurosurgical techniques and conservative management. The manufacturer claimed that PED fulfils a currently unmet clinical need in the treatment of large or giant, wide-necked or fusiform IAs.
Thirteen studies were identified by the manufacturer as being relevant to the decision problem, with two of these included for data extraction. The EAC identified 16 studies as relevant, three of which had been published after the manufacturer’s search. Data extraction was carried out on these studies as, although many were low level research comprising of case reports and case series, they provided useful, pertinent safety and outcome data.
No relevant economic studies of the device were identified; therefore, a new economic model was designed by the manufacturer. The base-case scenario provided recognized the costs of PED to be higher than the costs for endovascular parent vessel occlusion, neurosurgical parent vessel occlusion, neurosurgical clipping and conservative management. However, PED was found to be cost saving compared with stent-assisted coiling, with a saving of £13,110 per patient.
Analysis of the clinical data suggested that treatment with PED has high rates of clinical success with high rates of aneurysm occlusion and acceptable adverse events for the patient population. Economic evidence suggested that the costs in the base-case for PED may have been underestimated, meaning that PED would only become cost saving in patients who would otherwise require treatment with 32 coils or more. NICE Medical Technologies Guidance MTG10, issued in May 2012, recommends the adoption of PED in selected patients within the UK National Health Service (NHS).
Key Points for Decision Makers
• The clinical evidence comparing the efficacy of the Pipeline™ embolization device (PED) with other interventions is very limited, but current data suggests high rates of successful device placement and occlusion.
• The ‘value’ of PED is case dependent. Evidence supports its use in patients with complex large or giant intracranial aneurysms that are not suitable for surgery and are being considered for stenting, when the number of PEDs does not exceed two and when 32 or more coils and one stent would be needed during stent-assisted coiling.
• A small number of patients have intracranial aneurysms that are unsuitable for conventional types of treatment and are at high risk of aneurysm rupture. For these patients, the PED offers the only possible means of treatment.
Introduction
The Medical Technologies Evaluation Programme (MTEP) has been established since 2009 when it was set up by the National Institute of Health and Clinical Excellence (NICE) to facilitate the adoption of efficient and value-for-money medical devices and diagnostics more rapidly and consistently in the UK National Health Service (NHS). Devices that meet the eligibility criteria and fall within the remit of the programme can be notified to NICE by a manufacturer. Following notification, MTEP selects devices that are likely to offer significant benefit to patients or the NHS at the same or reduced cost when compared with current practice for assessment under their programme. Once selected, the device manufacturer is asked to provide NICE with clinical and cost evidence submissions that meet a pre-defined scope. This evidence is independently critiqued, alongside the manufacturer’s submission, by an External Assessment Centre (EAC) and a report is produced. This report is presented to the NICE Medical Technologies Advisory Committee (MTAC), which is made up of 25 independent specialists. The committee uses the report in combination with other resources to produce guidance on the technology under evaluation. This article presents a summary of the EAC report for the Pipeline™ embolisation device for the treatment of complex intracranial aneurysms, and the development of the NICE guidance. It is one of a series of NICE Medical Technology Guidance summaries being published in Applied Health Economics and Health Policy [1, 2].
The Decision Problem
Disease Overview—Intracranial Aneurysms
Intracranial aneurysms (IAs), also known as cerebral or brain aneurysms, occur when a weakness develops in the wall of an artery supplying blood to the brain. This weakness allows the vessel to balloon or bulge and the resultant sac fills with blood to form an aneurysm. It has been estimated that approximately 2.8% of the population have a brain IA, with a higher prevalence in women at a ratio of 1.57 [3].
Types of aneurysms include saccular (or berry) and fusiform, with saccular aneurysms being spherical in shape with a distinctive neck while fusiform aneurysms have no distinct neck (Fig. 1). Small, unruptured aneurysms are usually asymptomatic; however, larger aneurysms can result in ‘mass effect’ due to compression of adjacent nerves and tissues causing features which can include headache, double vision, slurred speech and seizures. Untreated aneurysms are at significant risk of rupture leading to subarachnoid haemorrhage (SAH), which carries a high rate of morbidity and mortality. Thirty to forty percent of SAH patients die within 1 month and 10–20 % of survivors have long-term dependence due to brain damage [4]. Data suggest that larger aneurysms carry a higher risk of rupture than smaller ones. The International Study of Unruptured Intracranial Aneurysms (ISIUA) Investigators [5] found 5-year rupture rates of 2.6 % for aneurysms of 7–12 mm, 14.5 % for those 13–24 mm and 40 % rupture rates for aneurysms of 25 mm or greater.
Fig. 1.
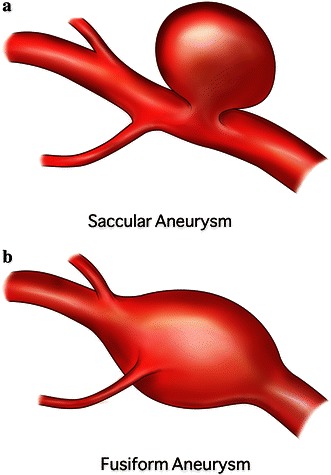
Representation of saccular (a) and fusiform (b) aneurysms
Current Treatment Options
Several neurosurgical treatment options are available for patients with IAs, including clipping, wrapping and bypass procedures. Surgical clipping was developed in the 1930s and was the standard therapy for IAs for many years; it continues to be used regularly due to its long-term efficacy. The technique involves a clip being placed across the neck of the aneurysm, excluding it from the circulation. In 1991, an endovascular technique using coils was introduced as an alternative therapy and its use has increased substantially, with coil embolization often used as a first-choice treatment due to the lower risk of morbidity and mortality [6]. Surgical clipping involves the insertion of a microcatheter into an artery and up into the targeted aneurysm. Platinum coils are fed into the aneurysm, filling the sac and preventing or reducing blood flow. Coiling is often used in conjunction with stents that are positioned in the main artery to prevent the coils migrating from the aneurysm.
Parent vessel occlusion (PVO) is an option for treatment of IAs where sufficient collateral circulation exists. In these cases the parent artery is occluded surgically or using an endovascular method (e.g., using balloons or coils), effectively preventing blood flow in the artery and thus the aneurysm. PVO is not suitable for all patients since it requires an adequate alternative circulation as without this, occluding the artery will result in a stroke. PVO is usually reserved for aneurysms that cannot be treated by other techniques [7].
In some patients, the shape, size or location of an aneurysm prohibits effective treatment using existing therapies, while for other patients previous attempts at treatment have failed. In these patients conservative management may be the only option available. This remains a high-risk option for many patients due to the poor long-term prognosis of large, complex aneurysms.
NICE has published several guidance documents to assist with the treatment of IAs; however, these do not specifically address the patient population defined within the scope of this review.
Pipeline™ Embolization Device
Manufactured by Covidien in the US, the Pipeline™ embolization device, or PED, is a stent-like structure made of braided cobalt chromium and platinum tungsten. It is delivered via a microcatheter and is designed to be placed across the neck of an IA, thereby disrupting the flow of blood within the aneurysm. This reduced flow increases the blood viscosity, eventually leading to clotting of the blood within the aneurysm itself. Furthermore, the PED forms a scaffold over which endothelial cells can grow, eventually incorporating the PED into the wall of the parent artery. This forms a biological seal and the aneurysm becomes excluded from the circulation completely. Over time the clotted aneurysm may reduce in size, and symptoms of mass effect may also diminish [8]. PED’s are available in various lengths between 10 mm and 35 mm and can expand between 2.5 mm and 5 mm in diameter. They can be deployed one within another or telescoped to increase the overall length or surface coverage over the area being treated. Due to their flexible nature they can be used in vessels with tortuous anatomy while their configuration allows continued circulation in perforators and side branches.
National Institute of Health and Clinical Excellence (NICE) Scope
The scope of the decision problem outlined by NICE identified the PED as the intervention to be studied. The patient population was defined as “Patients with complex intracranial aneurysms, specifically large/giant, wide necked and fusiform aneurysms”. Discussions during the preliminary stages of the assessment clarified that ruptured aneurysms should be excluded from the scope. Several comparators were identified for consideration: stent-assisted coiling (SAC); parent vessel occlusion; neurosurgical techniques and conservative management. Ten outcome measures were specified for consideration: successful device deployment; successful occlusion of the aneurysm; size of collective aneurysm-thrombus mass; resolution of symptoms; resource use outcomes; stroke; delayed parent vessel occlusion; subarachnoid haemorrhage/other major bleeding events requiring active treatment; neurovascular death; and device-related adverse events. The scope also requested three separate cost analyses:
Analysis 1 Population: patients with complex IAs for whom SAC is considered feasible (de novo or repeat treatment). Intervention: PED. Comparator: percutaneous interventional techniques including SAC and parent vessel occlusion.
Analysis 2 Population: Patients with complex IAs for whom SAC is not considered feasible (de novo or repeat treatment). Intervention: PED. Comparator: neurosurgical techniques (including bypass).
Analysis 3 Population: Patients with complex IAs for whom SAC and neurosurgical techniques are not considered feasible (de novo or repeat treatment). Intervention: PED. Comparator: conservative treatment.
External Assessment Centre (EAC) Review
Cedar, a consortium between Cardiff and Vale University Health Board and Cardiff University, was commissioned by NICE to act as the EAC to independently evaluate the PED. The role of the EAC is to review and critique the manufacturers’ submissions and produce a structured report on their findings. Nominated expert advisers are also available to the EAC to provide clinical advice if required during the process.
As per NICE requirements, Covidien made a submission based on the scope of the decision problem. The first part is a clinical submission comprising an overview of the disease and current treatment provision with a review of the available clinical literature relating to PED and its comparator technologies. This is followed by an economic submission comprising a search strategy and relevant economic evidence and a de novo economic model accompanied by a detailed description.
Clinical Effectiveness Evidence
The manufacturer used Hospital Episode Statistic (HES) data from 2009–2010, which identified 2,191 patients in England and Wales with a primary diagnosis of unruptured IA. Using data from the ISUIA study [5] to estimate the prevalence of large and giant aneurysms, Covidien calculated the number of patients with unruptured aneurysms eligible for treatment with PED in England and Wales to be between 460 and 580 annually.
Covidien identified 13 studies as being appropriate to the decision problem, with two multicentre, prospective, single-arm feasibility studies forming the main evidence base. PITA (the Pipeline™ Embolization Device for the Intracranial Treatment of Aneurysms trial) [9] was a feasibility study that monitored 31 patients (and 31 aneurysms) over 180 days. The study endpoints were successful device placement and the incidence of death or ipsilateral stroke at 30 days in a patient population with unruptured aneurysms that were wide necked (≥4 mm), had unfavourable dome/neck ratios (<2) or had failed previous therapy. In thirty cases, device placement was successful while in the remaining patient, diminished blood flow in the parent artery required corrective angioplasty that led to rupture of the artery and subsequent artery ligature. Two patients experienced periprocedural strokes. No other patients showed signs of neurological deterioration and there were no deaths. Aneurysm occlusion rates were high with a rate of 93.3 % observed in the thirty patients who received angiographic follow up at 180 days.
The Pipeline™ for Uncoilable or Failed Aneurysms Study or PUFS [8] is an ongoing unpublished study following 108 patients with 110 aneurysms, which is due to complete in June 2014. These aneurysms were both wide necked (≥4 mm) and either large (10–25 mm) or giant (>25 mm), with the primary effectiveness endpoint being complete occlusion of the target aneurysm at 180 days in the absence of major stenosis. Device placement was successful in 99 % of patients with rates of complete occlusion (without major stenosis when using PED alone) of 73.6 %. Ipsilateral stroke or death by 180 days after the procedure was the stated primary safety end point and occurred in six patients (5.6 %). The data from this trial are available from the Executive Device Summary for PED, published online by the US Food and Drug Administration (FDA) [8].
The remaining eleven identified studies [10–20] were excluded from qualitative synthesis by the manufacturer as they were not felt to be sufficiently robust to be included due to their design. Several of these are case reports or case series involving a relatively small number of patients. A degree of patient duplication was also given as a reason for study exclusion.
Critique of Clinical Effectiveness Evidence
EAC discussions with the clinical experts suggested the number of patients estimated by Covidien to be suitable for treatment with PED was excessively high. As NICE guidance is targeted at the NHS in England, data on Welsh patients were excluded, and repeat admissions on HES were identified for removal. This left an estimated 333–420 patients in England eligible annually.
The manufacturer provided a clear overview of the condition, and the advantages and disadvantages of current treatment options were well described and their comparison to PED clearly illustrated. A well structured literature search was carried out and most of the relevant studies identified; however, the manufacturer excluded most of these from the data extraction process. The two studies that formed the main evidence base were highly relevant to the decision problem and were discussed in detail throughout the manufacturer’s submission. Of the eleven additional studies that were identified by Covidien, one of these [16] presented data on ruptured aneurysms that were identified as being outside the scope of the decision problem and it was therefore excluded from data extraction by the EAC. The manufacturer excluded the remaining ten studies from the data extraction process due to concerns regarding study quality, but the EAC felt that despite their limitations, these studies provided valuable data pertinent to the scope of the submission.
Using an adapted literature search, the EAC identified an additional case report not found by the manufacturer [21]. Three further studies [22–24] were identified that were published after the date of the manufacturer’s literature search. This led to the EAC including a total of 16 studies comprising one unpublished trial, one journal letter, four conference abstracts and ten published manuscripts. The EAC considered that although there was a degree of patient duplication, it was important to carry out data extraction on all relevant studies to ensure pertinent evidence was not omitted. A total of approximately 379 patients were described in the included literature; one study [18] described the treatment of 42 aneurysms but did not specify the number of patients. As previously noted, the total number of patients treated is fewer than the sum from the literature included due to duplication across studies.
As well as concerns regarding the design of some of the studies reported in the literature, there were other limitations in the clinical evidence available for PED. These included an absence of control groups in any of the studies; lack of clarity in reporting inclusion and exclusion criteria; the duplication of patients between studies; and lack of detail in the conference abstracts. As PED is a relatively new device there is a shortage of long-term follow-up data. However, the nature of the disease, lack of clinical equipoise and small patient numbers makes comparative studies inappropriate in the population being studied.
The EAC considered adverse event data from the manufacturer’s own sources and from the MAUDE (Manufacturer and User Facility Device Experience) database was pertinent to the decision problem and should be included. Data from MAUDE was accessed, and Covidien provided adverse event data on request, with this information being incorporated into the EAC report.
Despite being primarily low-level studies, the clinical evidence provided a large amount of data that was highly relevant to the scope of the decision problem, with data available on nine of the ten specified outcome measures. The studies showed a high rate of clinical success with few difficulties in device deployment and only three cases of delayed parent vessel occlusion reported. Aneurysm occlusion rates were well documented, being reported in twelve studies with seven studies reporting 100 % occlusion [10, 11, 14, 17, 19, 21, 24]: the lowest rate reported was 69 % [23]. No data was available on resource use outcomes.
Cost Evidence
The manufacturer provided details of the search strategy used to identify economic studies relevant to the scope: no relevant papers were identified. One unpublished document was provided by Covidien, comprising a simple cost calculation produced by the previous manufacturer; however, this was felt to be inadequate for the economic evaluation. The manufacturer produced a de novo model consisting of a short-term ‘decision tree’ with an additional long-term Markov element reflecting a time horizon of 10 years with a 6-month cycle length. The short-term data separated surviving patients into three occlusion categories: complete occlusion, residual neck and residual aneurysm. There were three health states used in the long-term model, these were i) no-complications; ii) re-treatment; iii) rupture resulting in survival or death: patients can also die from all causes at any time in the model. The model used an NHS and personal social service perspective with discounting for costs and QALYs (quality-adjusted life-years) applied at a rate of 3.5 % as per NICE guidance. However, as MTEP does not look at the cost effectiveness of a device, the QALY data were not considered by the committee in their decision.
The steps of the model were:
Treatment (followed by survival/death)
Initial outcome for surviving patients (split into three groups: complete occlusion; residual neck; residual aneurysm)
Prediction of ongoing outcomes (defined as no complication; retreatment; rupture)
Results (this includes costs and incremental costs)
Additional options included adverse events defined as SAH and stroke.
Lack of data prevented sensitivity analysis on the structural assumptions within the model, but an extensive one-way sensitivity analysis was carried out to measure the impact of numerous inputs within the model. This identified the most critical areas and highlighted that the model was particularly responsive to the cost of consumables used both for PED and its comparators. The base-case scenario illustrated total procedure costs over the 10-year time horizon for PED as £24,341. This is compared with £16,893 for endovascular PVO, £11,654 for neurosurgical PVO, £11,658 for neurosurgical clipping and £10,352 for conservative management. Only SAC was shown to be more costly than PED at £37,451, showing PED at a cost saving of £13,110 per treatment, making PED dominant. This is based on list prices of £10,171.00 for PED and £526.04 for coils. Covidien estimated the number of PEDs required to be 1.46 based on their own data on file, while the inputs used for the number of coils was based on an opinion in an editorial [25] that estimated a requirement of 40 coils for SAC.
Critique of Cost Evidence
The EAC felt the search strategy used to identify relevant economic studies was appropriate in regard to both search terms and databases utilized and did not repeat the search. The search and selection criteria used by the manufacturer to identify data sources for cost and clinical outcomes used in the cost model were not specified, therefore the EAC were unable to quality check the selection used or to confirm that these were the most appropriate sources. This is also true of the sources used to justify the assumptions made throughout the model, and while those identified by the manufacturer were clearly described and referenced, it is unclear how they were chosen. The structure of the model itself was well executed, with data sources clearly labelled. However, in some instances secondary references were used, and some inputs relied on extrapolated data, which in several cases were combined from a number of studies including some not directly relevant to the decision problem. This led to uncertainties at each stage in the model.
Adverse events were inadequately explored in the submission, and the model only included subarachnoid haemorrhage and stroke, with some types of stroke being excluded for the comparator treatments. For the comparator, many of the complications listed as adverse events also resulted in death within 31 days. The structure of the model meant that some of this data was double counted when the option for adverse events was selected as they also contributed to the perioperative mortality figures used in the model at an earlier stage.
As identified by the manufacturer following sensitivity analysis, the cost of consumables was a key driver, with the model being particularly responsive to the cost and number of PEDs and coils used. Covidien quoted list prices for PED of £10,171 and coils at £526.04, and used data on file to estimate the number of PEDs required at 1.46. The number of coils required was estimated at 40, making PED dominant compared with SAC. The EAC determined that the numbers estimated for coils and PED may not be appropriate and following consultation with the expert advisers estimated that the average number of coils required may be lower at approximately 25 while data from the studies identified in the clinical review suggested the average number of PEDs used would be higher than in the model at an estimated 2.4 devices. This would significantly increase the cost of PED in comparison with SAC, meaning that PED became the more costly option. Lack of data means that these inputs remain uncertain; however, doubt particularly surrounds the appropriate number of coils. The uncertainty regarding the number of PEDs and coils required to treat aneurysms in the target population is a critical parameter that potentially has a significant impact on the outcome of the model. The EAC determined that, for 2.4 PEDs, the model shows PED to be cost saving when the number of coils required is equal to or greater than 36.
The scope issued by NICE requested three cost analyses to be performed; the model provided deviated in respect to cost analysis in several areas:
Analysis 1 This was generally implemented as requested, however the scope indicated that SAC is a feasible retreatment in the population analysed, and while the model does apply this correctly, the manufacturer’s report stated that retreatment would be via neurosurgical clipping.
Analysis 2 Although included in the scope, it is unclear if the manufacturer included bypass along with other neurosurgical techniques in the model. Retreatment after neurosurgical clipping is costed as SAC in the model although the scope indicates that the population should comprise patients for whom SAC is not feasible.
Analysis 3 In the scope, the population comprised patients treated with conservative management for whom SAC and neurosurgical techniques were not feasible. However, the model incorporated retreatment costs using SAC in this group; removing this element reduces the cost of conservative management further.
Conclusion of the EAC
As noted by the manufacturer, there are considerations regarding the use of low-level evidence such as case reports and case series due to the risk of selection and reporting bias. However, in the absence of more robust studies such as randomized controlled trials as is often the case for novel treatments, particularly for those such as PED for whom comparator studies are not appropriate, they can provide valuable information on initial treatment efficacy and adverse events. Despite the concerns regarding study quality and patient duplication, the results of the studies identified are encouraging with the outcome measures addressed within the scope of the decision problem showing promising results. Rates of successful device placement and aneurysm occlusion are high and adverse events such as stroke, neurovascular death and delayed parent vessel occlusion were relatively low for this patient population. PED offers the benefit of long-term vessel patency and resolution of symptoms in some patients, which would not be afforded via many treatment alternatives.
The economic analysis for PED relies on the economic model, the accuracy of which is dependent on the inputs, and many of these are surrounded by a degree of uncertainty. The key drivers in analysis 1 of the scope are the number of PEDs and coils required, and the data provided to support these estimates is weak. Under the scope of the decision problem as identified by the manufacturer, the only treatment option for the patient population identified against which PED may be potentially cost saving is SAC. Here the most economic option will vary on a case-by-case basis requiring clinical assessment to estimate the potential number of coils or PEDs required in an individual patient; however, the EAC feels that the number of coils required would be larger than estimated by the manufacturer before PED becomes dominant. There remains a group of patients who have failed previous treatments or cannot be treated via conventional methods: for these patients no other treatment options exist.
NICE Guidance
In line with the MTEP process, the Committee met to develop draft recommendations following which a medical technology consultation document was produced. NICE accepted comments on these draft recommendations as well as notification of inaccuracies and additional information. Following a consultation period, comments were collated and presented to the committee for discussion.
Draft Recommendations
The MTAC Committee met in October 2011 and, following review of the manufacturer’s submissions and the EAC report [26] together with evidence from expert advisers, the following provisional recommendations were made:
“The case for adopting the Pipeline embolisation device in the NHS is supported by the current evidence when it is used in patients with giant or complex intracranial aneurysms that are unsuitable for surgery, which are being considered for stenting and where large numbers of coils are needed during stent-assisted coiling.
The Pipeline embolisation device is estimated to be cost saving when compared with stent-assisted coiling, in patients with giant or complex intracranial aneurysms when the number of Pipeline embolisation devices inserted does not exceed two and when treatment would otherwise require the use of 29 or more coils combined with one stent for stent-assisted coiling. If two Pipeline embolisation devices are used the total procedure cost is estimated as £30,354 compared with £30,775 for the use of 29 coils for stent-assisted coiling (a saving of £421 using the Pipeline embolisation device).
Clinicians should submit details of all patients being treated with the Pipeline embolisation device to the UK Neurointerventional Radiology Group audit database, to increase the evidence base and guide future use of this technology.”
The Committee noted that for some patients with aneurysms unsuitable for treatment with existing therapy options PED may be the only treatment available. However these patients are beyond the scope of this recommendation.
Consultation Response
There were numerous comments sent to NICE during the PED public consultation period. These raised issues including the differences in practice between the UK and the US, where many of the published studies were carried out. Some of these suggested changes were felt to be outside of the scope of the guidance and remit of the programme; however, other comments were considered by the Committee for inclusion while some comments led to automatic changes being made to ensure that data reported were clear and accurate.
During the consultation period, the expert advisers suggested that five inputs in the economic model could be addressed more suitably. One of these changes was felt to be inappropriate as the manufacturer’s original inputs were correctly referenced and valid. However, it was determined that four of the suggested changes would better reflect UK practice. The EAC subsequently produced an additional report to explain these changes and their impacts on the cost model; this identified the following changes.
The model assumed that while treatment with PED requires one Marksman microcatheter at a list price of £1030.00, two would be needed for treatment via SAC at a total cost of £2060.00. The expert advisers confirmed that while two microcatheters are required for SAC, standard practice in the UK involves the use of cheaper alternatives to the Marksman, with the EAC calculating an average cost for microcatheters of £460.50 each, reducing the total cost of these to £921.00.
Balloon use was estimated to occur in 50 % of SAC procedures but no PED procedures in the model. While it was not possible to determine an absolute rate of use, it was felt by the expert advisers that their use is relatively uncommon for both methods of treatment and was therefore removed from the cost model.
An additional cost for endovascular equipment was included in the model but only for retreatment for SAC and not de novo treatment or for any other comparator or PED. Removal of this reduces the cost in favour of SAC.
Drug resource use was calculated using data from non-comparable studies due to the lack of comparator studies. The study selection criteria were not specified, but the data used was not inappropriate. However, two minor calculation errors were identified in the number of days of drug therapy making very small reductions in the cost of both PED and SAC.
Final Guidance
The final Medical Technology Guidance document for PED for the treatment of complex IAs was published by NICE on 30 May 2012 as MTG10 [27]. Due to the impact of the changes made following suggestions from the Expert Advisers, one of the recommendations was updated as detailed below to more accurately reflect the number of coils required before PED became cost saving:
“The Pipeline embolisation device is estimated to be cost saving when compared with stent-assisted coiling, in patients with complex giant or large intracranial aneurysms when the number of Pipeline embolisation devices inserted does not exceed two, and when treatment would otherwise require the use of 32 or more coils combined with one stent for stent-assisted coiling. If two Pipeline embolisation devices are used the total procedure cost is estimated as £30,346 compared with £30,838 for the use of 32 coils for stent assisted coiling (a saving of £492 using the Pipeline embolisation device).”
Challenges
There were several challenges and learning points identified throughout the evaluation process. These mainly arose from two primary issues and are potentially factors which may impact other emerging medical technologies.
Patient numbers The patient population suitable for treatment with PED is very small and difficult to quantify accurately, particularly regarding the sub-set of patients for whom no other treatment options exist. The small patient population means that it is not feasible to carry out randomized controlled trials or comparator studies, particularly as many of the patients suitable for treatment will have failed previous treatment or will not be suitable for alternative procedures. This means that the evidence for this type of device will rely on less robust data such as case series and case reports. Complexities in identifying the number of eligible patients also makes it difficult to calculate the overall cost impact of adopting the technology.
Sources of data Most of the study data for patients treated with PED were based on patients treated in the US; no study data were available from the UK. Differences in clinical practice can result in misleading data being used to evaluate factors such as cost and efficacy. For example, patient selection, surgical techniques and treatment options may differ between countries, thus affecting the number of patients treated and potentially distorting extrapolated outcome data. The absence of fully researched data on the comparator technologies may also lead to inaccuracies in the assessment and comparison of the available evidence.
Acknowledgements
The authors thank Joelle Williams and Judith White (Cedar), Alison Weightman (Support Unit for Research Evidence, Cardiff University, Wales) and Professor Ceri J. Phillips (Swansea Centre for Health Economics, Swansea University, Wales) for their contribution to the original External Assessment Centre (EAC) report for the National Institute of Health and Clinical Excellence (NICE), and Jan Sharp (Media Resources Centre, Cardiff and Vale UHB, Wales) for providing illustrations.
Disclosures
Cedar is funded by NICE to act as an EAC for the Medical Technologies Evaluation Programme. This summary of the Medical Technology Guidance was produced following the publication of the final guidance report. This summary has been reviewed by NICE but has not been externally peer reviewed by Applied Health Economics and Health Policy. The authors are NHS employees; the NHS has a financial interest in the guidance issued by NICE as a result of this work.
Author contributions
The cost-effectiveness section of the paper was written by GCR and MD. The clinical effectiveness and other sections of the paper were written by KW with support from GCR and MD. The guarantor for overall content is Grace Carolan-Rees.
References
- 1.White J, Carolan-Rees G. PleurX peritoneal catheter drainage system for vacuum-assisted drainage of treatment-resistant, recurrent malignant ascites: a NICE medical technology guidance. Appl Health Econ Health Policy. 2012;10(5):299–308. doi: 10.1007/BF03261864. [DOI] [PubMed] [Google Scholar]
- 2.Campbell B, Campbell M. NICE medical technologies guidance: a novel and rigorous methodology to address a new health technology assessment challenge. Appl Health Econ Health Policy. 2012;10(5):295–297. doi: 10.1007/BF03261863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vlak MH, Algra A, Brandenburg R, et al. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10(7):626–636. doi: 10.1016/S1474-4422(11)70109-0. [DOI] [PubMed] [Google Scholar]
- 4.Burns JD, Brown RD., Jr Treatment of unruptured intracranial aneurysms: surgery, coiling, or nothing? Curr Neurol Neurosci Rep. 2009;9(1):6–12. doi: 10.1007/s11910-009-0002-0. [DOI] [PubMed] [Google Scholar]
- 5.Unruptured intracranial aneurysms: risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Eng J Med. 1998;339(24):1725–33. [DOI] [PubMed]
- 6.Molyneux AJ, Kerr RSC, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez NR, Duckwiler G, Jahan R, et al. Challenges in the endovascular treatment of giant intracranial aneurysms. Neurosurgery. 2006;59(5 Suppl. 3):S113–24. [DOI] [PubMed]
- 8.FDA. Chestnut Medical Technologies. Pipeline embolization device executive summary P100018. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/NeurologicalDevicesPanel/UCM247160.pdf. Accessed 2011 Sep 6.
- 9.Nelson PK, Lylyk P, Szikora I, et al. The pipeline embolization device for the intracranial treatment of aneurysms trial. Am J Neuroradiol. 2011;32(1):34–40. doi: 10.3174/ajnr.A2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiorella D, Woo HH, Albuquerque FC, et al. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008;62(5):1115–1120. doi: 10.1227/01.neu.0000325873.44881.6e. [DOI] [PubMed] [Google Scholar]
- 11.Fiorella D, Kelly ME, Albuquerque FC, et al. Curative reconstruction of a giant midbasilar trunk aneurysm with the pipeline embolization device. Neurosurgery. 2009;64(2):212–217. doi: 10.1227/01.NEU.0000337576.98984.E4. [DOI] [PubMed] [Google Scholar]
- 12.Fiorella D, Hsu D, Woo HH, et al. Very late thrombosis of a pipeline embolization device construct: case report. Neurosurgery. 2010;67(3):E313–E314. doi: 10.1227/01.NEU.0000383875.08681.23. [DOI] [PubMed] [Google Scholar]
- 13.Hartmann M, Rohde S, Braun C, et al. Endovascular treatment of cerebral aneurysms with the pipeline embolization device. Proceedings of the Jahrestagung der Deutschen Gesellschaft fur Neuroradiologie; 2010 Sep 22–25; Mannheim, Germany. Clin Neuroradiol. 2010; 20(3):190–1.
- 14.Klisch J, Turk A, Turner R, et al. Very late thrombosis of flow-diverting constructs after the treatment of large fusiform posterior circulation aneurysms. Am J Neuroradiol. 2011;32(4):627–632. doi: 10.3174/ajnr.A2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64(4):632–642. doi: 10.1227/01.NEU.0000339109.98070.65. [DOI] [PubMed] [Google Scholar]
- 16.Matouk C, O’Kelly C, Ellis M, et al. Pipeline embolization device reconstruction of ruptured intracranial aneurysms: report of two cases. In: Proceedings of the 45th Annual Congress of the Canadian Neurological Sciences Federation; 2010 Jun 8–11; Quebec, Canada. Can J Neurol Sci. 2010;37(3 Suppl. 1):S88–9.
- 17.Phillips T, Mitchell P, Dowling R, et al. Endovascular treatment of intracranial aneurysms with new generation flow diverting stents: early experience in an Australian neurointerventional centre. In: Proceedings of the 61st Annual Scientific Meeting of the Royal Australian and New Zealand College of Radiologists; 2010 Oct 14–17; Perth, Australia. J Med Imag Radiat Oncol; 2010 Oct; 54(Suppl. 1):A122.
- 18.Szikora I, Berentei Z, Kulcsar Z, et al. Effect of flow modification on aneurysm induced mass effect. In: Proceedings of the 19th Symposium Neuroradiologicum: The World Congress of Diagnostic and Therapeutic Neuroradiology; 2010 Oct 4–9; Bologna, Italy. Neuroradiol J 2010;23(1):324.
- 19.Szikora I, Berentei Z, Kulcsar Z, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: the Budapest experience with the pipeline embolization device. Am J Neuroradiol. 2010;31(6):1139–1147. doi: 10.3174/ajnr.A2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rooij WJ, Sluzewski M. Perforator infarction after placement of a pipeline flow-diverting stent for an unruptured A1 aneurysm. Am J Neuroradiol. 2010;31(4):E43–E44. doi: 10.3174/ajnr.A2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiorella D, Albuquerque F, Gonzalez F, et al. Reconstruction of the right anterior circulation with the Pipeline embolization device to achieve treatment of a progressively symptomatic, large carotid aneurysm. J NeuroIntervent Surg. 2009;2(1):31–37. doi: 10.1136/jnis.2009.000554. [DOI] [PubMed] [Google Scholar]
- 22.Hampton T, Walsh D, Tolias C, et al. Mural destabilization after aneurysm treatment with a flow-diverting device: a report of two cases. J NeuroIntervent Surg. 2011;3(2):167–171. doi: 10.1136/jnis.2010.002873. [DOI] [PubMed] [Google Scholar]
- 23.O’Kelly C, Spears J, Chow M, et al. Canadian experience with the pipeline embolization device for repair of unruptured intracranial aneurysms. In: Proceedings of the 46th Annual Congress of the Canadian Neurological Sciences Federation; 2011 Jun 15–17; Vancouver, Canada. Can J Neurol Sci. 2011;38(3):S31.
- 24.Sararols L, Castillo L, Graell X, et al. Right giant internal carotid artery bifurcation aneurism: presentation with homonymous left hemianopsia and successful treatment with intraneurismatic bypass. In: Proceedings of the 10th European Neuro-Ophthalmology Society; 2011 Jun 18–21; Barcelona Spain. Conference Publication; 35:S65.
- 25.Wehman JC, Hanel RA, Levy EI, et al. Giant cerebral aneurysms: endovascular challenges. Neurosurgery. 2006;59(5):S125–S138. doi: 10.1227/01.NEU.0000237330.11482.90. [DOI] [PubMed] [Google Scholar]
- 26.Withers KL, Carolan-Rees G, Dale M, et al. External Assessment Centre report: Pipeline Embolisation Device for the treatment of complex intracranial aneurysms. http://www.nice.org.uk/nicemedia/live/13016/57154/57154.pdf. Accessed 2012 Sep 5. [DOI] [PMC free article] [PubMed]
- 27.National Institute for Health and Clinical Excellence. Pipeline embolisation device for the treatment of complex intracranial aneurysms: NICE medical technology guidance 10. http://www.nice.org.uk/nicemedia/live/13685/59340/59340.pdf. Accessed 2012 Sep 5. [DOI] [PMC free article] [PubMed]


