Abstract.
The developing fetal brain is vulnerable to a variety of environmental agents including maternal ethanol consumption. Preclinical studies on the development and amelioration of fetal teratology would be significantly facilitated by the application of high resolution imaging technologies like optical coherence tomography (OCT) and high-frequency ultrasound (US). This study investigates the ability of these imaging technologies to measure the effects of maternal ethanol exposure on brain development, ex vivo, in fetal mice. Pregnant mice at gestational day 12.5 were administered ethanol ( b.wt.) or water by intragastric gavage, twice daily for three consecutive days. On gestational day 14.5, fetuses were collected and imaged. Three-dimensional images of the mice fetus brains were obtained by OCT and high-resolution US, and the volumes of the left and right ventricles of the brain were measured. Ethanol-exposed fetuses exhibited a statistically significant, 2-fold increase in average left and right ventricular volumes compared with the ventricular volume of control fetuses, with OCT-derived measures of 0.38 and , respectively, whereas the boundaries of the fetal mouse lateral ventricles were not clearly definable with US imaging. Our results indicate that OCT is a useful technology for assessing ventriculomegaly accompanying alcohol-induced developmental delay. This study clearly demonstrated advantages of using OCT for quantitative assessment of embryonic development compared with US imaging.
Keywords: fetal ventricular volume, alcohol exposure, mouse fetus, optical coherence tomography, ventriculomegaly
1. Introduction
Maternal consumption of alcohol during pregnancy is the leading nongenetic cause of mental retardation in children in the United States, and can lead to a cluster of permanent physical and mental defects in fetuses that are collectively termed the fetal alcohol spectrum disorder (FASD). It is estimated that 2% to 5% of children in the United States suffer from alcohol related birth defects.1 Previous research has shown that ethanol can directly affect various aspects of brain development in the fetus.2–4 Similarities in development between humans and mice have led to wide usage of fetal mice as models for human developmental diseases. Alcohol-exposed pregnant mice have previously been used to study the emergence of FASD during fetal development.5,6 Traditionally, histological methods have been used to study the structures in the mouse embryonic brain, which are tedious and time consuming procedures and cannot be performed in vivo. With the advent of modern imaging systems such as magnetic resonance imaging, computed tomography and ultrasound (US) imaging, three-dimensional (3-D) imaging, live imaging and longitudinal studies have become possible. However, lack of imaging resolution and contrast limits the ability of these modalities to perform careful quantitative analysis. Optical techniques such as confocal microscopy offer very high resolution and contrast; however, limited depth of penetration and requirement of tissue labeling are the major disadvantages for whole body/organ imaging.
Optical coherence tomography (OCT) is a noninvasive imaging modality that is capable of 3-D and real-time imaging, offers high optical contrast and does not require any external contrast agents.7 High imaging resolution (in the μm range) and depth penetration of a few millimeters in tissue makes OCT an attractive tool to study the development of fetal mice. Our group has previously utilized swept source OCT (SSOCT) to perform ex vivo hemodynamic studies on mice embryos.8–10 More recently, we demonstrated the capacity of SSOCT to be adapted to longitudinal studies in mice embryos to monitor the development of morphological features such as the brain, limbs, and ocular structures, in utero.11,12 In this paper, we report pilot results from a study where we used SSOCT and high-resolution ultrasound imaging to compare the volumes of lateral ventricles between control fetal mice and fetal mice exposed to ethanol in utero, during the second trimester-equivalent period of pregnancy, a critical period for neurogenesis in the developing brain.13 These data show that the volume of lateral ventricles of ethanol-exposed fetuses is significantly larger than in the fetuses in control group, which indicates that SSOCT may be used in animal models, to successfully detect evidence for ventriculomegaly and perturbations in neural development following maternal exposure to a common drug of abuse.
2. Materials and Methods
2.1. Animal Model
All procedures were performed in accordance with approved Institutional Animal Care and Use Committee protocols. Timed-Pregnant mice (C57Bl6, Harlan Laboratories, Houston, Texas) were housed in an AAALAC-accredited animal facility at Texas A&M Health Science Center. On gestational day 12.5, pregnant mice were divided into a control and an ethanol-exposed group. Ethanol-exposed pregnant female mice received a binge-like bolus of ethanol ( b.wt) by intragastric gavage, twice daily, between GD12.5 and GD14.5, to model ethanol exposure during the second-trimester equivalent period of neurogenesis, whereas control animal received water by gavage at the same time intervals. Previous research showed that intragastric gavage with this dose of ethanol resulted in a mean peak maternal blood ethanol concentrations of ,6 representing a level of binge-like intoxication readily achieved in nonalcoholic human populations. Two hours following the final exposure period, pregnant dams were over-anesthetized with Ketamine and Xylazine and euthanized by cervical dislocation. Ethanol-exposed and control fetuses were dissected out of the uterine horns and fixed in 4% paraformaldehyde overnight and transferred to phosphate buffered saline for imaging. Five fetuses from two mothers were used each for ethanol-exposed and control experiments. Following the imaging procedures, fetuses were embedded in paraffin and serial sections obtained. Every 10th section was stained with Hematoxylin and Eosin, and the brain visualized by light microscopy.
2.2. Brain Imaging
Paraformaldehyde fixed fetuses were initially imaged using a high resolution Vevo 2100 US imaging system, coupled to a MS550D Microscan™ transducer on a motorized stage, with a center frequency of 40 MHz (Visualsonics, Canada). Image slices were acquired in B-mode, through the fetal head with a 0.032 mm step size. The same fetuses were then subjected to SSOCT imaging, for volumetric analysis and quantification of fetal mouse brains. The SSOCT system comprises a broadband laser source (Thorlabs, SL1325-P16), which has a central wavelength of 1325 nm, bandwidth of 100 nm, output power of 12 mW and A-line rate of 16 kHz. The axial and transverse resolutions of the system are 12 and 15 μm, respectively (in air). Each 3-D volume contained A-lines.
2.3. Data and Statistical Analyses
The measurement of the volume of left and the right brain ventricles was performed using a Matlab-based graphical user interface code. The region corresponding to the ventricles were manually segmented across individual two-dimensional (2-D) cross-sections and the number of pixels was measured and summed up across all the 2-D cross-sections containing the ventricles. The total volume was then calculated by multiplying the total number of pixels with the volume of each voxel.
Data were expressed as the deviation (SD). A two-sample Kolmogorov–Smirnov test was then performed to determine the statistical significance of the difference between ethanol-treated and control groups. Finally, Pearson’s correlation coefficients () were computed to determine the extent to which variance in SSOCT measurements were predictive of variance in ultrasound measurements.
3. Results and Discussion
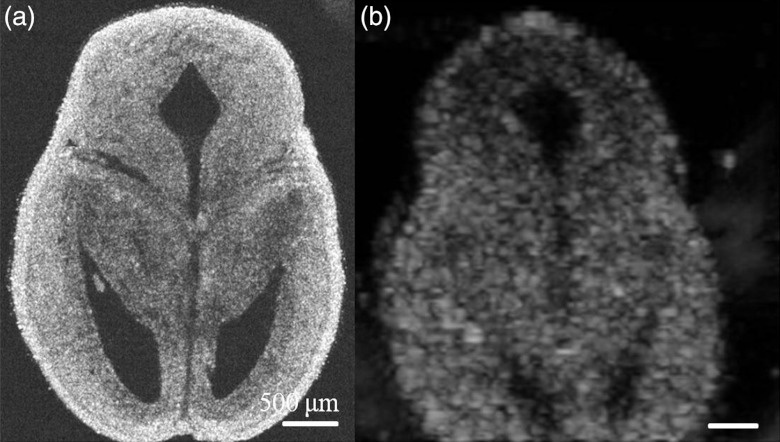
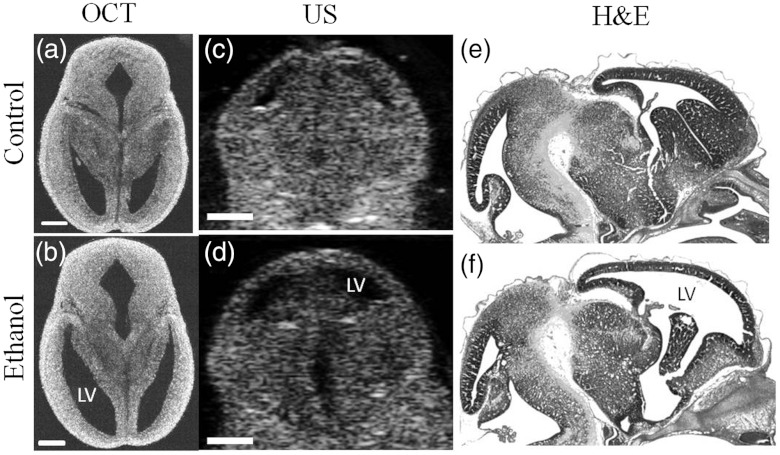
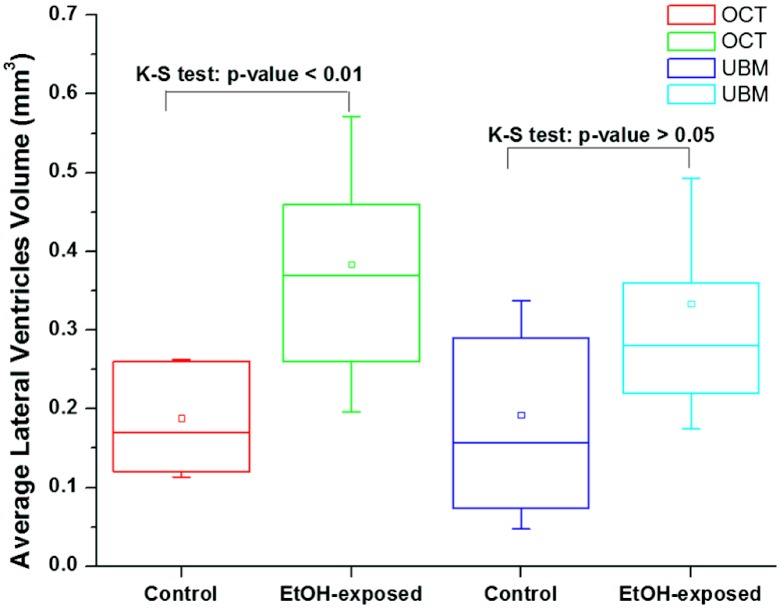
Ventricular defects of the brain are common symptoms of defective neurodevelopment,14 caused by a variety of agents including fetal alcohol exposure.15 High-resolution imaging and early accurate assessment of ventricular volume may facilitate the conduct of preclinical interventional studies and the testing of novel therapeutic approaches for ameliorating in utero damage. The challenge in measuring the volume of ventricles with high accuracy involves the segmentation of ventricular regions in consecutive 2-D images with high precision. The OCT offers high resolution and contrast to achieve it. To demonstrate this, 2-D transverse cross-sectional images obtained from SSOCT and ultrasound imaging, are presented in Fig. 1. It can be easily observed that OCT offers superior resolution and contrast compared to the US imaging. Typical 2-D transverse cross-sectional images for control and ethanol-exposed fetuses, obtained using SSOCT, are presented in Fig. 2(a) and 2(b), respectively. It can be also clearly seen that the ventricular regions are discernible and are dilated in the ethanol-exposed fetus compared to the control. The total number of pixels encompassing the entire 3-D lateral ventricular region was measured from individual 2-D images and multiplied by the voxel volume to obtain the total volume. The volumes of the right and left ventricles were then averaged for individual fetuses. The statistical measurement from five samples, each from control and ethanol-exposed fetuses is presented in Fig. 3. The averaged volume of lateral ventricles was measured to be for control and for ethanol-exposed fetuses. Interestingly, the standard deviation for the observed ventricular volume was also larger for the group of ethanol-exposed fetuses compared to the control group. This increased variance in ventricular volume reflects variation in susceptibility of individual fetuses to the teratogenic effects of ethanol, an observation that has been made in human populations as well.16 To verify the measurements made with OCT, high-resolution US was also was also used to obtain 3-D images of the same set of fetuses and the ventricular volume was measured using the same method. Typical 2-D transverse cross-sectional images for control and ethanol-exposed fetuses, obtained using US, are presented in Fig. 2(c) and 2(d), respectively. The averaged lateral ventricular volumes of control and ethanol-exposed fetuses were measured to be and , respectively (Fig. 3). The measurements from OCT are within the same range of the measurements obtained with US imaging. Moreover, there was an overall statistically significant correlation between ventricular volume estimates obtained by OCT and US imaging (Pearson’s , ). However, ventricular margins were not as clearly defined in the US-derived compared to OCT-derived images because of the lower contrast and resolution () of these images, and consequently, resulted in a 2-fold increase in the variance estimate of the ventricular volume in control fetuses compared to SSOCT imaging. The results from ex vivo US imaging alone suggest that there is no statistical difference between the lateral ventricle volumes from control and ethanol-exposed fetuses (two sample Kolmogorov–Smirnov test, ). Even though OCT has superior resolution and contrast, unlike US imaging, it has limited imaging depth due to light attenuation in tissues. Nevertheless, our group has recently demonstrated possibility of in utero imaging of mice embryonic organs, including brain.11
Fig. 1.
Comparison between images obtained using SSOCT and ultrasound. 2-D cross-sectional images obtained using (a) OCT (b) ultrasound imaging. Scale bar, 500 μm.
Fig. 2.
Effect of ethanol on mouse fetal brain development at GD14.5. Left panel represents OCT images (a) and (b) in horizontal section, middle panel shows US images (c) and (d) in the coronal plane, and right panel illustrates H&E images (e) and (f) in sagittal section of fetal brains from both control and ethanol treated pregnant dams. Ethanol induced increase in ventricular dilation can be observed with all the imaging techniques used in the study, and in all three planes of orientation. LV-lateral ventricles, US-ultrasound, OCT-optical coherence tomography, H&E- hematoxylin-eosin staining. Scale bar, 500 μm.
Fig. 3.
Measurements of lateral ventricles of control and ethanol-exposed fetuses at GD14.5 obtained from SSOCT and US images; the whiskers represent standard deviation, and small hollow squares represent the mean of the data distribution.
4. Conclusions
Maternal alcohol consumption during pregnancy is a well-established cause of fetal growth retardation, and the dilation of lateral ventricles is an important index of decreased brain growth. Our data suggest that binge-like episodes of ethanol exposure during the second trimester-equivalent period of neurogenesis, at levels below those attained in alcoholic populations, nevertheless result in a two-fold, statistically significant dilation of the lateral ventricles of the fetal brain. Moreover, OCT is more accurate than ex vivo US imaging, and is a useful and sensitive imaging modality for preclinical, whole animal studies, to assess the effects of drugs of abuse and perhaps other environmental teratogens on fetal growth and development. It will be important in future studies to identify the range of quantitative OCT-derived measurements that can serve as useful biomarkers for fetal teratogenesis.
Acknowledgments
The authors would like to thank Ms. Karishma Prasad (University of Houston) and Ms. Fatimah Hameed (University of Houston) for technical assistance. This work was supported, in part, by grants from the National Institutes of Health (R01HL095586 and R01AA013440) and Federal Target Program Scientific and Scientific-Pedagogical Personnel of Innovative Russia for 2009 to 2013 Grant 14.B37.21.1238.
References
- 1.May P. A., et al. , “Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies,” Dev. Disabil. Res. Rev. 15(3), 176–192 (2009). 10.1002/ddrr.68 [DOI] [PubMed] [Google Scholar]
- 2.Camarillo C., Miranda R. C., “Ethanol exposure during neurogenesis induces persistent effects on neural maturation: evidence from an ex vivo model of fetal cerebral cortical neuroepithelial progenitor maturation,” Gene Express. 14(3), 159–171 (2008). [PMC free article] [PubMed] [Google Scholar]
- 3.Goodlett C. R., Horn K. H., “Mechanisms of alcohol-induced damage to the developing nervous system,” Alcohol. Res. Health 25(3), 175–184 (2001). [PMC free article] [PubMed] [Google Scholar]
- 4.Carta M., Mameli M., Valenzuela C. F., “Alcohol potently modulates climbing fiber-->Purkinje neuron synapses: role of metabotropic glutamate receptors,” J. Neurosci. 26(7), 1906–1912 (2006). 10.1523/JNEUROSCI.4430-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parnell S. E., et al. , “Maternal oral intake mouse model for fetal alcohol spectrum disorders: Ocular defects as a measure of effect,” Alcohol. Clin. Exp. Res. 30(10), 1791–1798 (2006). 10.1111/acer.2006.30.issue-10 [DOI] [PubMed] [Google Scholar]
- 6.Bake S., Tingling J. D., Miranda R. C., “Ethanol exposure during pregnancy persistently attenuates cranially directed blood flow in the developing fetus: evidence from ultrasound imaging in a murine second trimester equivalent model,” Alcohol. Clin. Exp. Res. 36(5), 748–758 (2012). 10.1111/acer.2012.36.issue-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang D., et al. , “Optical coherence tomography,” Science 254(5035), 1178–1181 (1991). 10.1126/science.1957169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larina I. V., et al. , “Live imaging of blood flow in mammalian embryos using Doppler swept-source optical coherence tomography,” J. Biomed. Opt. 13(6), 060506 (2008). 10.1117/1.3046716 [DOI] [PubMed] [Google Scholar]
- 9.Larina I. V., et al. , “Hemodynamic measurements from individual blood cells in early mammalian embryos with Doppler swept source OCT,” Opt. Lett. 34(7), 986–988 (2009). 10.1364/OL.34.000986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudheendran N., et al. , “Speckle variance OCT imaging of the vasculature in live mammalian embryos,” Laser Phys. Lett. 8(3), 247–252 (2011). 10.1002/lapl.v8.3 [DOI] [Google Scholar]
- 11.Syed S. H., et al. , “Optical coherence tomography for high-resolution imaging of mouse development in utero,” J. Biomed. Opt. 16(4), 046004 (2011). 10.1117/1.3560300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larina I. V., et al. , “Optical coherence tomography for live phenotypic analysis of embryonic ocular structures in mouse models,” J. Biomed. Opt. 17(8), 081410 (2012). 10.1117/1.JBO.17.8.081410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bystron I., Blakemore C., Rakic P., “Development of the human cerebral cortex: Boulder Committee revisited,” Nat. Rev. Neurosci. 9(2), 110–122 (2008). 10.1038/nrn2252 [DOI] [PubMed] [Google Scholar]
- 14.Blaas H. G., Eik-Nes S. H., “Sonoembryology and early prenatal diagnosis of neural anomalies,” Prenat. Diagn. 29(4), 312–325 (2009). 10.1002/pd.v29:4 [DOI] [PubMed] [Google Scholar]
- 15.Roebuck T. M., Mattson S. N., Riley E. P., “A review of the neuroanatomical findings in children with fetal alcohol syndrome or prenatal exposure to alcohol,” Alcohol. Clin. Exp. Res. 22(2), 339–344 (1998). 10.1111/acer.1998.22.issue-2 [DOI] [PubMed] [Google Scholar]
- 16.Clarren S. K., et al. , “Brain malformations related to prenatal exposure to ethanol,” J. Pediatr. 92(1), 64–67 (1978). 10.1016/S0022-3476(78)80072-9 [DOI] [PubMed] [Google Scholar]