Abstract
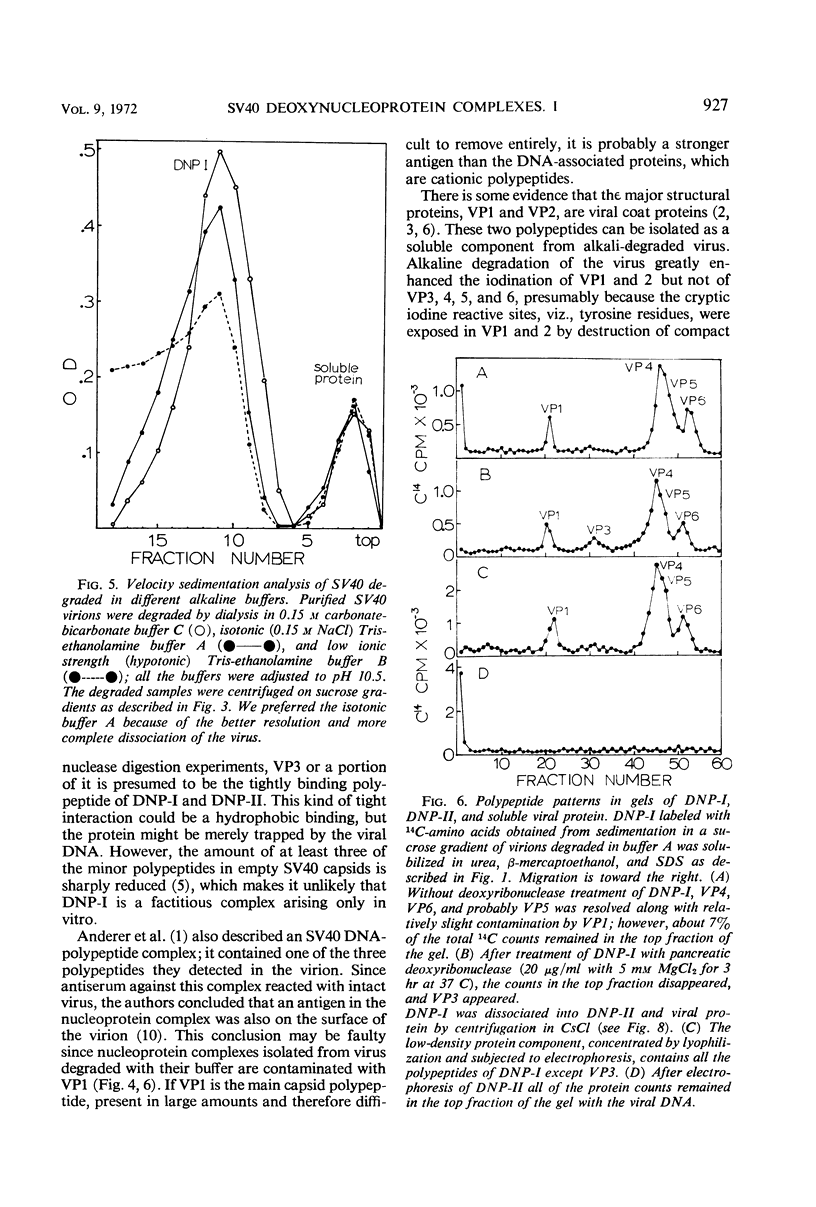
Velocity sedimentation analysis of simian virus 40 degraded in alkaline buffers, pH 10.5, yields two components: soluble protein containing the largest polypeptides, VP1 and VP2, of the virion, and a deoxynucleoprotein complex (DNP-I) containing the viral deoxyribonucleic acid (DNA) and the small polypeptides, VP4, 5, and 6, and all or part of VP3. Dissociation of DNP-I by equilibrium centrifugation in CsCl yields a complex (DNP-II) of the viral DNA and residual, tightly bound polypeptide; VP4, 5, and 6, but not VP3, are recovered after separation from DNP-II. Treatment of the virus with β-mercaptoethanol and iodination experiments suggest that VP1 and VP2 might exist as compact structures cross-linked with disulfide bonds, perhaps forming the capsid. VP4, 5, and 6 form a relatively stable complex with the viral DNA and are supposed to be the internal proteins. The location of VP3 is not well defined; at least a portion of it is tightly bound to the viral DNA.
Full text
PDF

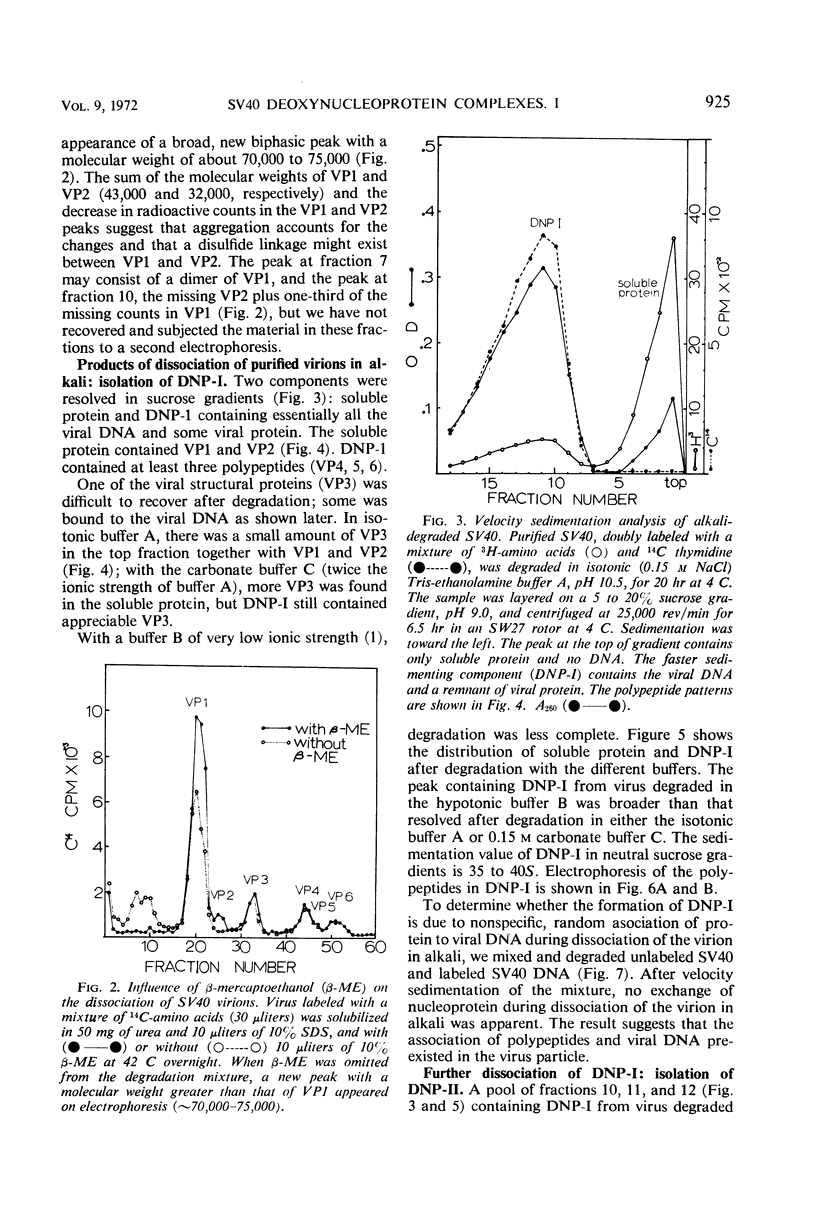



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderer F. A., Koch M. A., Schlumberger H. D. Structure of simian virus 40. 3. Alkaline degradation of the virus particle. Virology. 1968 Mar;34(3):452–458. doi: 10.1016/0042-6822(68)90065-2. [DOI] [PubMed] [Google Scholar]
- Anderer F. A., Schlumberger H. D., Koch M. A., Frank H., Eggers H. J. Structure of simian virus 40. II. Symmetry and components of the virus particle. Virology. 1967 Jul;32(3):511–523. doi: 10.1016/0042-6822(67)90303-0. [DOI] [PubMed] [Google Scholar]
- Barban S., Goor R. S. Structural proteins of simian virus 40. J Virol. 1971 Feb;7(2):198–203. doi: 10.1128/jvi.7.2.198-203.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M., Marty L., Suarez F. Capsid proteins of Simian virus 40. Biochem Biophys Res Commun. 1970 Jul 13;40(1):97–102. doi: 10.1016/0006-291x(70)91051-x. [DOI] [PubMed] [Google Scholar]
- Hare J. D., Chan J. C. Role of hydrogen and disulfide bonds in polyoma capsid structure. Virology. 1968 Mar;34(3):481–491. doi: 10.1016/0042-6822(68)90068-8. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Nonoyama M., Pagano J. S. Structure and function of the polypeptides in simian virus 40. II. Transcription of subviral deoxynucleoprotein complexes in vitro. J Virol. 1972 Jun;9(6):930–937. doi: 10.1128/jvi.9.6.930-937.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. A., Becht H., Anderer F. A. Structure of simian virus 40. V. Localization of the C-type polypeptide chains. Virology. 1971 Jan;43(1):235–242. doi: 10.1016/0042-6822(71)90241-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mattern C. F., Takemoto K. K., DeLeva A. M. Electron microscopic observations on multiple polyoma virus-related particles. Virology. 1967 Jul;32(3):378–392. doi: 10.1016/0042-6822(67)90288-7. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Perry J. L., To C. M., Consigli R. A. Alkaline degradation of polyoma virus. J Gen Virol. 1969 Apr;4(3):403–411. doi: 10.1099/0022-1317-4-3-403. [DOI] [PubMed] [Google Scholar]