Abstract
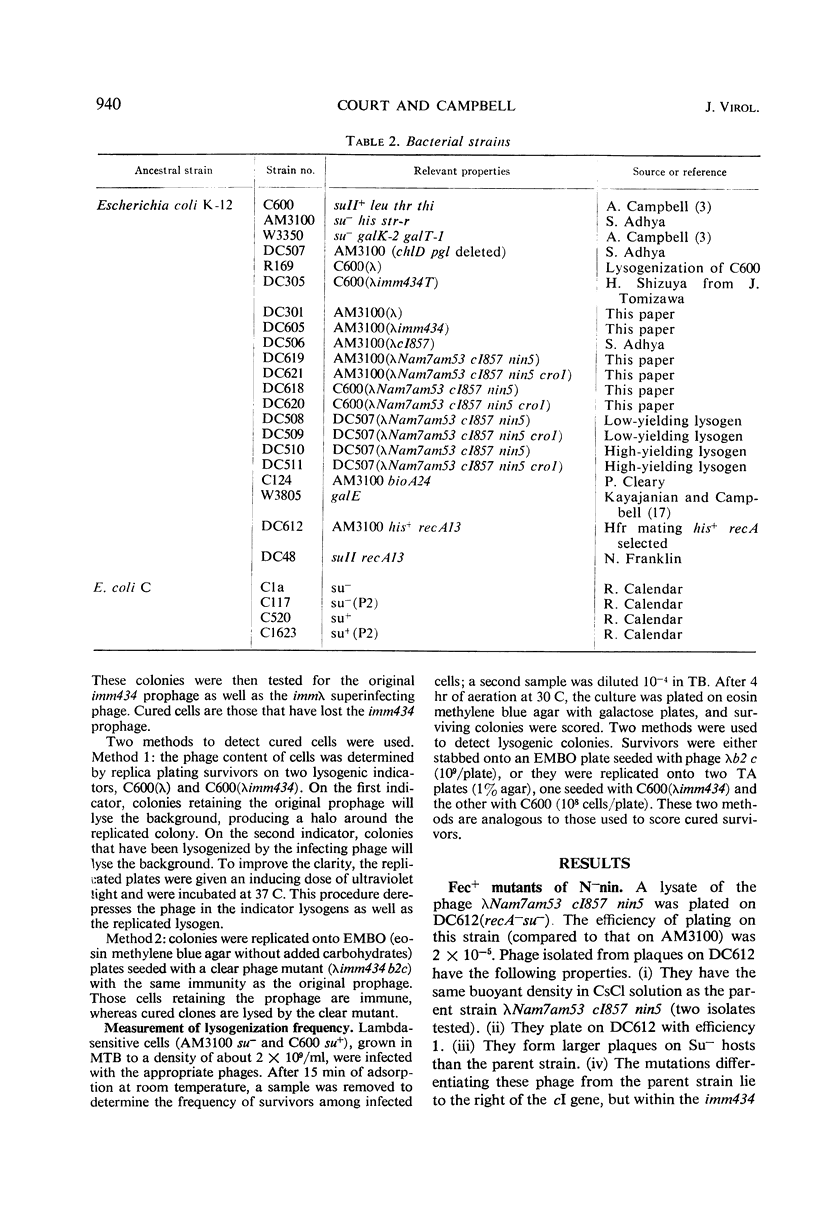
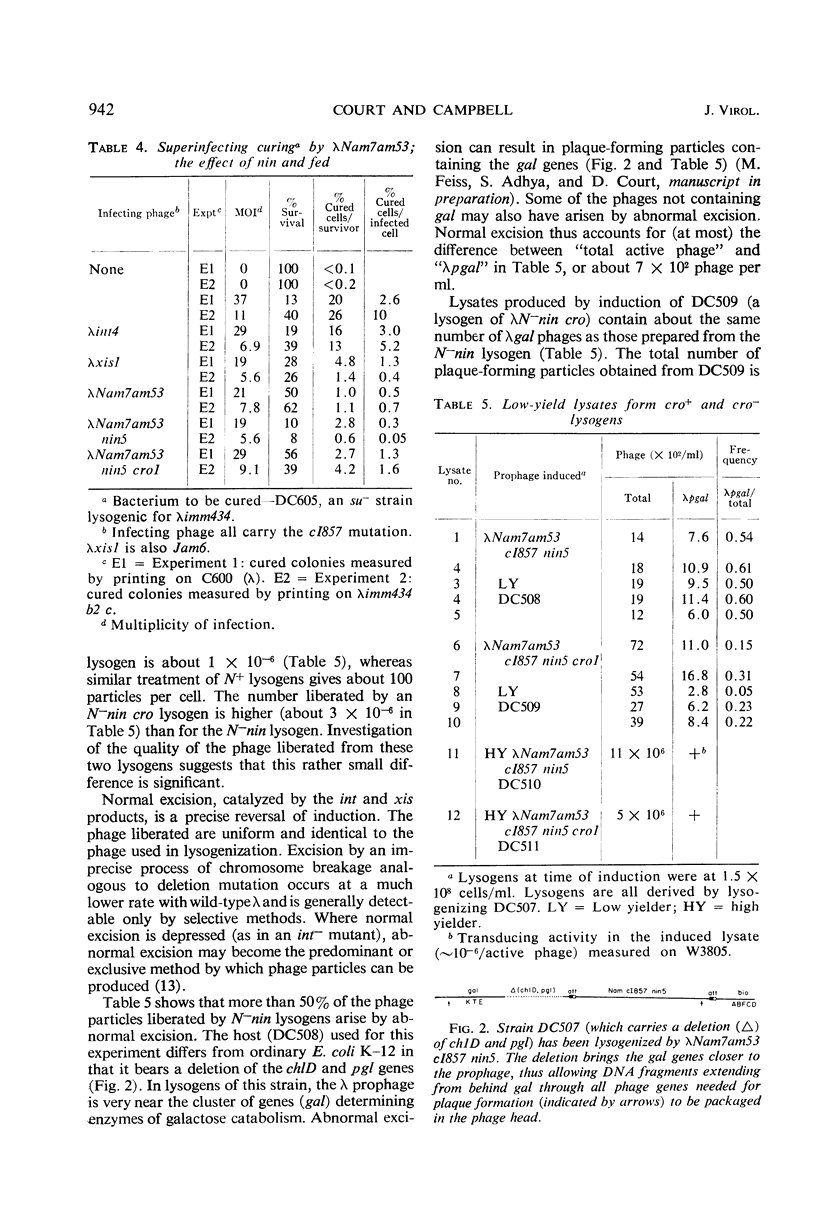
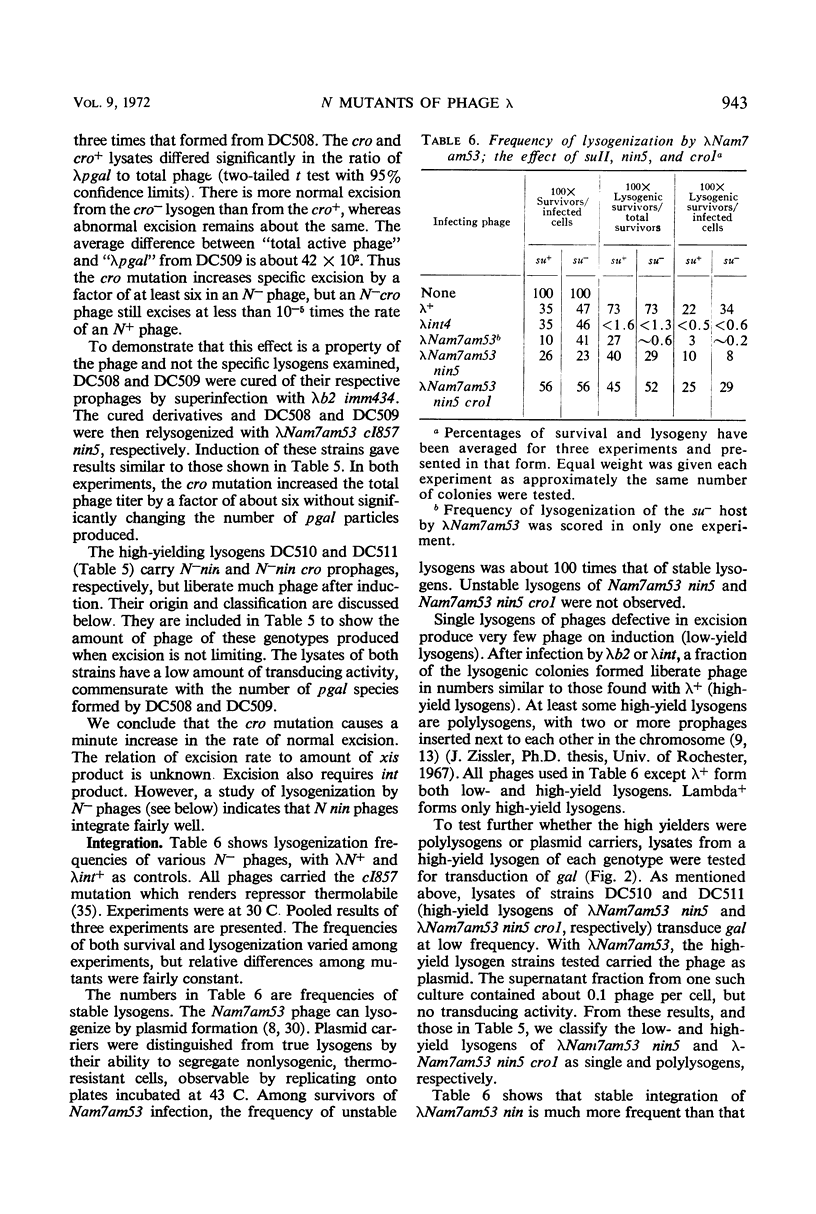
Mutants (N−nin) of bacteriophage λ in which the N gene product is not required for growth on wild-type Escherichia coli do not plate on recA bacterial mutants. Secondary mutants, selected for growth on recA, lie within the immunity region to the right of gene cI and appear identical to the cro mutants of Eisen et al. In an N+ phage, a cro mutation causes enhanced and prolonged production of λ exonuclease. N−cro phages make no detectable exonuclease, but show an increased rate of specific excision from lysogens and are excluded by P2 prophage. These properties, together with the ability to plate on recA, suggest that N−cro phages express genes to the left of N at a rate that is very low but higher than that for N−cro+ phages. N−nin phages can integrate at the normal site on the bacterial chromosome, but specific excision from lysogens is immeasurably low.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Cleary P., Campbell A. A deletion analysis of prophage lambda and adjacent genetic regions. Proc Natl Acad Sci U S A. 1968 Nov;61(3):956–962. doi: 10.1073/pnas.61.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKS K. STUDIES IN THE PHYSIOLOGICAL GENETICS OF SOME SUPPRESSOR-SENSITIVE MUTANTS OF BACTERIOPHAGE LAMBDA. Virology. 1965 Jul;26:489–499. doi: 10.1016/0042-6822(65)90011-5. [DOI] [PubMed] [Google Scholar]
- CAMPBELL A. Sensitive mutants of bacteriophage lambda. Virology. 1961 May;14:22–32. doi: 10.1016/0042-6822(61)90128-3. [DOI] [PubMed] [Google Scholar]
- Court D., Sato K. Studies of novel transducing variants of lambda: dispensability of genes N and Q. Virology. 1969 Oct;39(2):348–352. doi: 10.1016/0042-6822(69)90060-9. [DOI] [PubMed] [Google Scholar]
- Echols H., Green L. Establishment and maintenance of repression by bacteriophage lambda: the role of the cI, cII, and c3 proteins. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2190–2194. doi: 10.1073/pnas.68.9.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols H. Integrative and excisive recombination by bacteriophage lambda: evidence for an excision-specific recombination protein. J Mol Biol. 1970 Feb 14;47(3):575–583. doi: 10.1016/0022-2836(70)90324-4. [DOI] [PubMed] [Google Scholar]
- Eisen H., Brachet P., Pereira da Silva L., Jacob F. Regulation of repressor expression in lambda. Proc Natl Acad Sci U S A. 1970 Jul;66(3):855–862. doi: 10.1073/pnas.66.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Baron L. S. Plasmid formation after lambda bacteriophage infection of Escherichia coli-Salmonella typhosa hybrids. J Bacteriol. 1970 Apr;102(1):288–290. doi: 10.1128/jb.102.1.288-290.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingery R., Echols H. Mutants of bacteriophage lambda unable to integrate into the host chromosome. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1507–1514. doi: 10.1073/pnas.58.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. E., Yarmolinsky M. B. Integration-negative mutants of bacteriophage lambda. J Mol Biol. 1968 Feb 14;31(3):487–505. doi: 10.1016/0022-2836(68)90423-3. [DOI] [PubMed] [Google Scholar]
- Guarneros G., Echols H. New mutants of bacteriophage lambda with a specific defect in excision from the host chromosome. J Mol Biol. 1970 Feb 14;47(3):565–574. doi: 10.1016/0022-2836(70)90323-2. [DOI] [PubMed] [Google Scholar]
- Kaiser A. D., Masuda T. Evidence for a prophage excision gene in lambda. J Mol Biol. 1970 Feb 14;47(3):557–564. doi: 10.1016/0022-2836(70)90322-0. [DOI] [PubMed] [Google Scholar]
- Kayajanian G., Campbell A. The relationship between heritable physical and genetic properties of selected gal- and gal+ transducing lambda dg. Virology. 1966 Nov;30(3):482–492. doi: 10.1016/0042-6822(66)90124-3. [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Marcaud L., Sheldrick P., Luzzati D., Gros F. Studies of the messenger RNA of bacteriophage lambda, I. Various species synthesized early after induction of the prophage. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1013–1020. doi: 10.1073/pnas.61.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl G., Sironi G., Bialy H., Calendar R. Bacteriophage lambda; abortive infection of bacteria lysogenic for phage P2. Proc Natl Acad Sci U S A. 1970 Jul;66(3):587–594. doi: 10.1073/pnas.66.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzati D. Regulation of lambda exonuclease synthesis: role of the N gene product and lambda repressor. J Mol Biol. 1970 Apr 28;49(2):515–519. doi: 10.1016/0022-2836(70)90261-5. [DOI] [PubMed] [Google Scholar]
- Manly K. F., Signer E. R., Radding C. M. Nonessential functions of bacteriophage lambda. Virology. 1969 Feb;37(2):177–188. doi: 10.1016/0042-6822(69)90197-4. [DOI] [PubMed] [Google Scholar]
- Oda K. I., Sakakibara Y., Tomizawa J. I. Regulation of transcription of the lambda bacteriophage genome. Virology. 1969 Dec;39(4):901–918. doi: 10.1016/0042-6822(69)90026-9. [DOI] [PubMed] [Google Scholar]
- Pero J. Location of the phage lambda gene responsible for turning off lambda-exonuclease synthesis. Virology. 1970 Jan;40(1):65–71. doi: 10.1016/0042-6822(70)90379-x. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Regulation of lambda exonuclease. I. Properties of lambda exonuclease purified from lysogens of lambda T11 and wild type. J Mol Biol. 1966 Jul;18(2):235–250. doi: 10.1016/s0022-2836(66)80243-7. [DOI] [PubMed] [Google Scholar]
- Radding C. M., Shreffler D. C. Regulation of lambda exonuclease. II. Joint regulation of exonuclease and a new lambda antigen. J Mol Biol. 1966 Jul;18(2):251–261. doi: 10.1016/s0022-2836(66)80244-9. [DOI] [PubMed] [Google Scholar]
- Reichardt L., Kaiser A. D. Control of lambda repressor synthesis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2185–2189. doi: 10.1073/pnas.68.9.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- SUSSMAN R., JACOB F. [On a thermosensitive repression system in the Escherichia coli lambda bacteriophage]. C R Hebd Seances Acad Sci. 1962 Feb 19;254:1517–1519. [PubMed] [Google Scholar]
- Sato K., Campbell A. Specialized transduction of galactose by lambda phage from a deletion lysogen. Virology. 1970 Jul;41(3):474–487. doi: 10.1016/0042-6822(70)90169-8. [DOI] [PubMed] [Google Scholar]
- Signer E. R. On the control of lysogeny in phage lambda. Virology. 1970 Mar;40(3):624–633. doi: 10.1016/0042-6822(70)90207-2. [DOI] [PubMed] [Google Scholar]
- Signer E. R. Plasmid formation: a new mode of lysogeny by phase lambda. Nature. 1969 Jul 12;223(5202):158–160. doi: 10.1038/223158a0. [DOI] [PubMed] [Google Scholar]
- Signer E., Echols H., Weil J., Radding C., Shulman M., Moore L., Manly K. The general recombination system of bacteriophage lambda. Cold Spring Harb Symp Quant Biol. 1968;33:711–714. doi: 10.1101/sqb.1968.033.01.080. [DOI] [PubMed] [Google Scholar]
- Skalka A., Butler B., Echols H. Genetic control of transcription during development of phage gamma. Proc Natl Acad Sci U S A. 1967 Aug;58(2):576–583. doi: 10.1073/pnas.58.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szybalski W. Initiation and patterns of transcription during phage development. Proc Can Cancer Conf. 1969;8:183–215. [PubMed] [Google Scholar]
- Zissler J. Integration-negative (int) mutants of phage lambda. Virology. 1967 Jan;31(1):189–189. doi: 10.1016/0042-6822(67)90030-x. [DOI] [PubMed] [Google Scholar]



