Abstract
Transforming growth factor (TGF)-β has a dual role in liver, providing cytostatic effects during liver damage and regeneration, as well as carcinogenic functions in malignant transformation and hepatocellular cancer. In cultured hepatocytes, TGF-β can trigger apoptosis and epithelial-mesenchymal transition (EMT). Caveolin-1 is associated with progression of hepatocellular cancer and has been linked to TGF-β signaling. This study aimed at elucidating whether Caveolin-1 regulates TGF-β mediated hepatocyte fate. Knockdown of Caveolin-1 strongly reduced TGF-β mediated AKT phosphorylation, thus sensitized primary murine hepatocytes for proapoptotic TGF-β signaling. Restoration of AKT activity in Caveolin-1 knockdown cells via expression of a constitutive active AKT mutant did not completely blunt the apoptotic response to TGF-β, indicating an additional mechanism how Caveolin-1 primes hepatocytes for resistance to TGF-β triggered apoptosis. On the molecular level, Caveolin-1 interfered with TGF-β initiated expression of the proapoptotic mediator BIM. Additionally, RNAi for Caveolin-1 reduced (and its overexpression increased) expression of antiapoptotic mediators BCL-2 and BCL-xl. Noteworthy, reduced Caveolin-1 protein levels had no effect on collagen 1α1, E- and N-cadherin expression upon TGF-β challenge and thus no effect on hepatocyte EMT. Hence, via affecting TGF-β mediated non-Smad AKT signaling and regulation of pro- and antiapoptotic factors, Caveolin-1 is a crucial hepatocyte fate determinant for TGF-β effects.
Keywords: AKT, BCL2, BIM, EMT, liver
Transforming growth factor (TGF)-β is a pleiotropic cytokine that regulates many cellular events, such as proliferation, differentiation, migration and death. TGF-β binds to its cognate serine/threonine kinase receptors type I and II (TβRI/ALK5 and TβRII) to initiate signal transduction. Ligand binding leads to transactivation of TβRI/ALK5 by the type II receptor. Consequently, the type I receptor facilitates phosphorylation of R-Smads, namely Smad2 and Smad3. Activated R-Smads then heteromerize with Smad4, thereby increasing their nuclear presence to regulate gene expression. Besides the canonical Smad pathway, TGF-β can initiate the activation of other signaling molecules, among ERK, JNK, p38, PI3K/AKT. However, this non-canonical signaling is cell type dependent.1
In hepatocytes, TGF-β can exert an epithelial mesenchymal transition (EMT) as well as trigger apoptosis. The EMT process is accompanied by loss of cell polarity and cell-cell contacts (for example, via downregulation of E-cadherin and zonula occludens) and upregulation of mesenchymal markers such as Snai1, vimentin and N-cadherin that has been shown by us and other groups.2, 3, 4 Although solid evidences exist about hepatocyte EMT in vitro,5 in vivo transition is still under debate.6 Simultaneously to EMT, TGF-β induces apoptosis of a fraction of cultured hepatocytes. Moreover, TGF-β was shown to be a potent elicitor of hepatocyte apoptosis in vivo.7 The mechanisms how apoptosis is triggered by TGF-β are diverse. Amongst are cooperation of TGF-β with FasL or TNF-α and also activation of MAPK pathways like p38 and JNK.8 Even more, TGF-β can modulate NF-κB and therewith influence cell survival. In hepatocytes, TGF-β mediates its apoptotic function via induction of ROS and also of the proapoptotic protein BIM and thereby inducing the mitochondrial cell death pathway.9, 10 This then culminates in the activation of effector caspases like caspase 3. To date, it is unclear what factors regulate the decision for the cells' fate. Hints come from studies, which demonstrate that the Smad3/pAKT ratio might influence whether apoptosis is induced or not.11, 12, 13 Indeed, in cultured hepatocytes, TGF-β is capable of rapidly activating AKT which thus might direct the cells towards EMT.14
In this study, we report about Caveolin-1 being essential for TGF-β mediated activation of AKT in hepatocytes. Caveolin-1 is required for the formation of caveolae in lipid rafts on the plasma membrane, and TGF-β receptors were previously shown to internalize via caveolae.15 This internalization route was linked with receptor downregulation and abrogation of signaling. Caveolin-1 offers a scaffold for diverse signaling proteins and thus influences a variety of cellular events.16 Even more, an important function was shown in liver regeneration.17 In our system, the absence of Caveolin-1 led to a significant increase in apoptosis mediated by TGF-β in hepatocytes, which is tied with omitted AKT activation. Further, we show that EMT markers are not altered by Caveolin-1 knockdown, whereas significant differences were found for apoptosis-related genes. We therefore conclude that, Caveolin-1 primes hepatocytes for apoptotic triggers and offers an environment for non-Smad signaling, which might determine hepatocyte fate upon TGF-β challenge.
Results
TGF-β regulates genes involved in apoptosis and EMT in hepatocytes
Primary murine hepatocytes were stimulated with TGF-β1 for 24 h and a microarray analysis was performed to elucidate expression changes of genes related to apoptosis. Intriguingly, TGF-β is capable of regulating both pro and antiapoptotic genes (Table 1). This underlines that TGF-β mediated hepatocyte programmed death is tightly regulated. Therefore, players have to exist which decide about the hepatocyte fate in response to the availability of TGF-β.
Table 1. Apoptosis related genes regulated by TGF-β in primary hepatocytes.
| Full name | Symbol | Accession No. | Apoptosis | |
|---|---|---|---|---|
| 24 h down | Cell death activator CIDE-B | Cideb | NM_009894 | pro |
| Tumor necrosis factor receptor superfamily member 6 | Fas | NM_007987 | pro | |
| Mitogen-activated protein kinase kinase kinase 5 | ASK1 | NM_008580 | pro | |
| Phorbol-12-myristate-13-acetate-induced protein 1 | Pmaip1 | NM_021451 | pro | |
| 24 h up | Bcl-2-like protein 11 | BIM | AF032460 | pro |
| Beta-catenin-like protein 1 | Ctnnbl1 | NM_025680 | pro | |
| Lipopolysaccharide-induced tumor necrosis factor-alpha factor homolog | Litaf | AV360881 | pro | |
| Serine/threonine-protein kinase 17B | Stk17b | AV173139 | pro | |
| Endophilin-B1 | Sh3glb1 | BB221842 | pro | |
| Tumor necrosis factor receptor superfamily member 19L | Tnfrsf19l | NM_177073.6 | pro | |
| Insulin-like growth factor I | Igf1 | AF440694 | anti | |
| Astrocytic phosphoprotein PEA-15 | Pea15 | NM_011063.2 | anti | |
| Serum/glucocorticoid-regulated kinase 1 | Sgk | NM_011361 | anti | |
| Sphingosine kinase 1 | Sphk1 | AF068749 | anti |
TGF-β alters the expression of pro- and antiapoptotic genes (more than 1.5-fold change, assessed by microarray analysis).
Caveolin-1 knockdown increases canonical Smad signaling and abrogates TGF-β mediated AKT activation
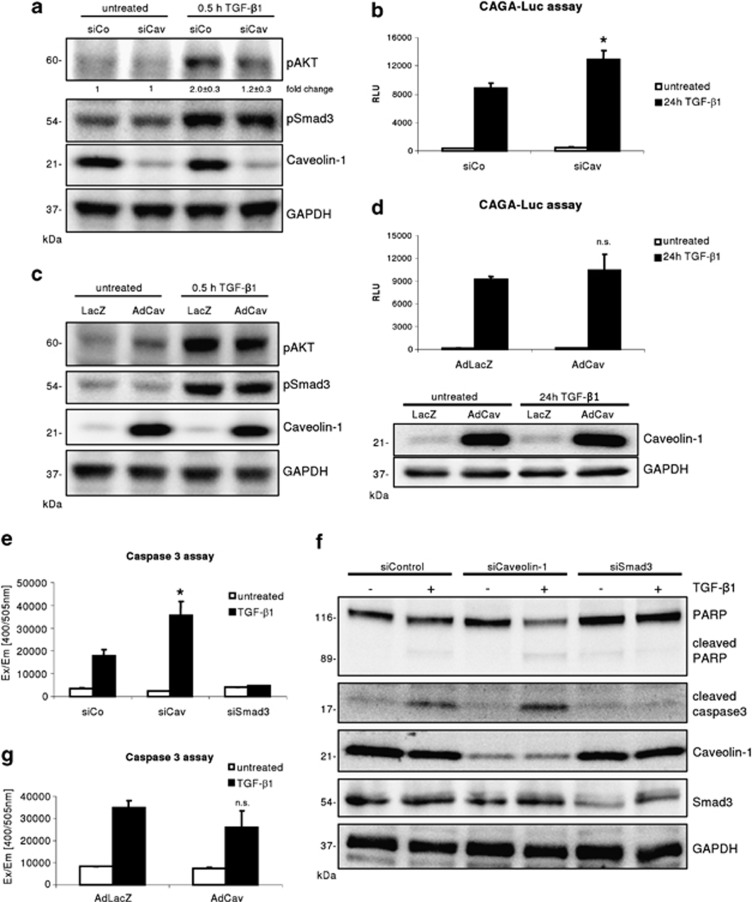
Caveolin-1 was shown to be a critical regulator of TGF-β receptor availability, which subsequently affects TGF-β signaling. Knocking down Caveolin-1 in hepatocytes did not change Smad3 activation, but significantly prevented phosphorylation of AKT (densitometry: siCo+TGF-β versus siCav+TGF-β: 2.0±0.3 versus 1.2±0.3, P=0.011, n=4) within 30 min of TGF-β1 treatment (Figure 1a) and.18 Total levels of Smad3 and AKT were not altered by the Caveolin-1 knockdown (not shown). However, applying a Smad luciferase reporter assay, TGF-β1 treated Caveolin-1 knockdown hepatocytes culminated in a significant enhancement of luciferase activity, when compared with controls (Figure 1b, P=0.0087). In contrast, elevated levels (via adenoviral Caveolin-1 gene transfer) of Caveolin-1 did not result in changes in AKT activation and total expression, although basal AKT activity was slightly enhanced in AdCav transduced hepatocytes. Also expression of Smad3 (not shown) and the Smad reporter assay (Figures 1c and d) remained unchanged. In dermal fibroblasts, Caveolin-1 overexpression was associated with elevated AKT phosphorylation.19 These findings imply that the physiological expression of Caveolin-1 in hepatocytes is mandatory and sufficient to enable TGF-β1 mediated activation of AKT. Furthermore, Caveolin-1 functions as a delimiter of the canonical Smad pathway.
Figure 1.
Reduced Caveolin-1 expression affects TGF-β mediated AKT phosphorylation, Smad3 signaling and hepatocyte apoptosis. (a) siControl and siCaveolin-1 transfected hepatocytes were stimulated for 30 min with 5 ng/ml TGF-β1. Knockdown of Caveolin-1 results in significant (densitometric normalized values, n=4, P=0.011) abrogation of AKT activation whereas canonical Smad3 activation is not altered. (b) Caveolin-1 knockdown significantly increased the CAGA9-MLP-Luc Smad3 reporter assay (24-h TGF-β1, 5 ng/ml; P=0.0087). (c) Adenoviral overexpression of Caveolin-1 does not alter Smad and AKT activation by TGF-β1 (5 ng/ml, 30 min). (d) Increased Caveolin-1 expression does not significantly alter the CAGA9-MLP-Luc reporter assay after 24-h treatment with 5 ng/ml TGF-β1. Western blot (reporter assay lysates) evidences the overexpression of Caveolin-1. (e) A caspase 3 assay reveals that knockdown of Caveolin-1 significantly increases hepatocyte apoptosis (n=3, P=0.010). As a control, knockdown of Smad3 completely abrogated TGF-β1 mediated apoptosis. (f) Western blotting of apoptosis relevant proteins displays increased caspase 3 activation (cleaved caspase 3) and PARP cleavage in siCaveolin-1 hepatocytes upon TGF-β1 (5 ng/ml, 48 h) treatment. (g) Overexpression of Caveolin-1 does not significantly reduce caspase 3 activity in primary hepatocytes (n=3, P=0.14)
Lack of Caveolin-1 facilitates TGF-β signaling towards apoptosis
In culture, TGF-β is inducing an EMT like process in a fraction of hepatocytes. However, as found in vivo, TGF-β can also instruct hepatocytes to undergo apoptosis. To delineate the response of Caveolin-1 deficient hepatocytes to TGF-β stimuli, a caspase 3 assay was applied. Compared with controls, knockdown of Caveolin-1 significantly increased the apoptotic response, as measured with a caspase 3 assay (Figure 1e; n=3, P=0.010). As a control, we additionally knocked down Smad3. Reduced levels of Smad3 completely abolished TGF-β1's capability to induce hepatocyte apoptosis. Furthermore, western blot analysis of control, Smad3 and Caveolin-1 siRNA transfected cells confirmed the above findings. PARP as well as caspase 3 cleavage (and therewith activation) were strongest in Caveolin-1 knockdown hepatocytes and absent in Smad3 siRNA samples (Figure 1f).
In line with the findings on TGF-β1 activated signaling cascades, AdCav-1 transduced cells do not show significant changes in the caspase 3 assay (Figure 1g; n=3, P=0.14). This highlights that physiologic expression of Caveolin-1 is critical for hepatocyte fate determination towards apoptosis or EMT.
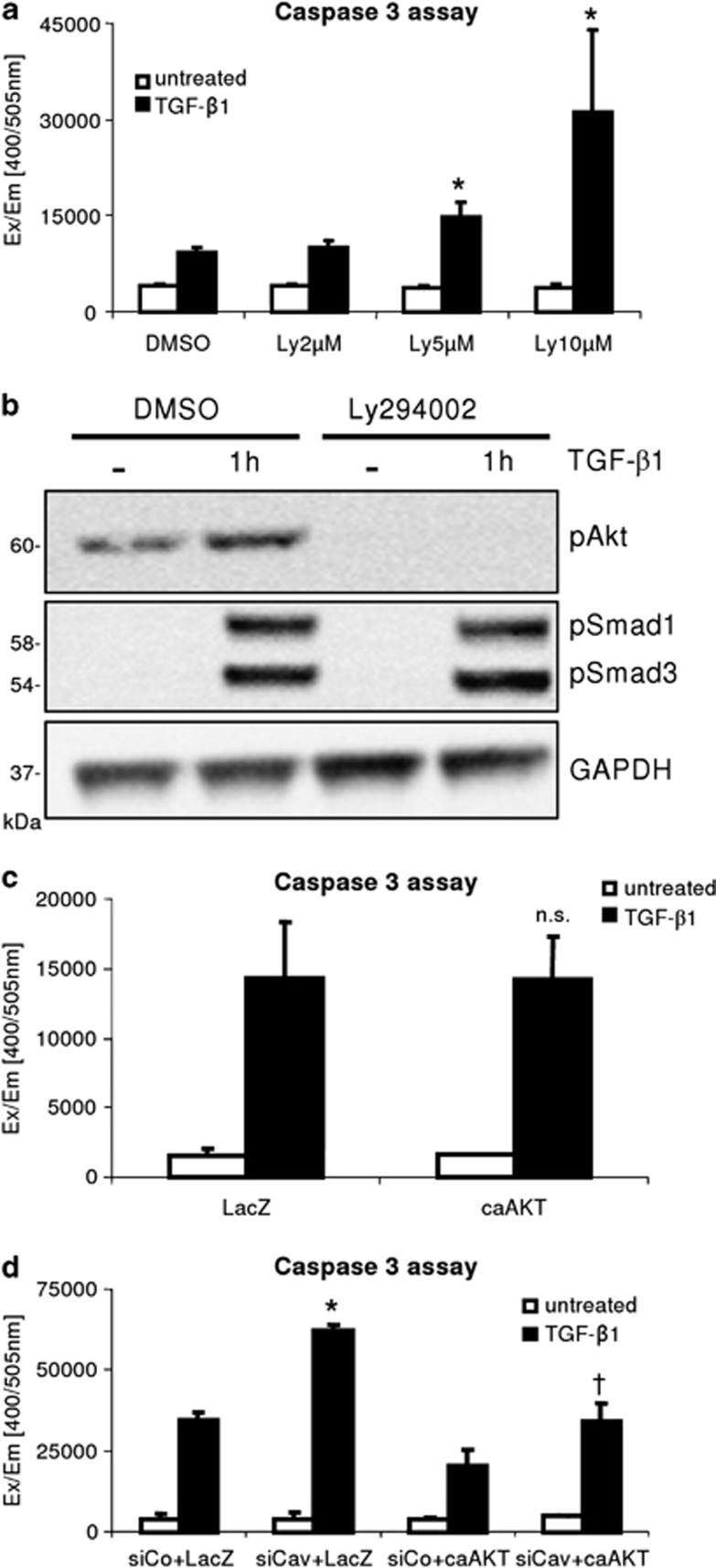
Abrogation of AKT activation enhances TGF-β mediated apoptosis
Although absence of Caveolin-1 increased the canonical Smad pathway in hepatocytes upon TGF-β stimulation, we hypothesized that the non-initiation of the AKT branch may be of relevance for the increased caspase 3 activity. This explanation is supported from the results of Caveolin-1 overexpression which did not reduce the CAGA-Luc reporter assay. To determine the effect of the AKT pathway on TGF-β mediated apoptosis, we used the PI3K/AKT inhibitor Ly294002 to blunt AKT activity. TGF-β induced apoptosis was strongly elevated in the absence of AKT signaling (Figure 2a), whereas basal caspase 3 activity was not elevated by Ly294002 and therewith toxicity of the inhibitor was excluded. Instead, this finding pronounces the relevance of intact AKT signaling for counteracting the TGF-β mediated apoptotic stimulus. We tested the efficiency of Ly294002 (10 μM) and found that both basal and TGF-β induced AKT phosphorylation were blunted (Figure 2b). No interference with Smad phosphorylation was detected. Next, we raised the question whether the effect of the Caveolin-1 knockdown towards TGF-β mediated apoptosis can be overruled by expression of the constitutive active form of AKT. Therefore, hepatocytes were transfected with siRNA for Caveolin-1 or a control oligo and subsequently transduced with AdcaAKT or AdLacZ as control (Figure 2c). Then, cells were stimulated with TGF-β1 for 48 h to trigger the apoptotic cascade. Expression of caAKT in siCaveolin-1 treated cells led to a reduction of caspase 3 activity to that of siControl/AdLacZ cells upon TGF-β stimulation (Figure 2d). This clearly demonstrates that the increase of apoptosis in cells lacking Caveolin-1 is reflecting the failure of AKT activation. Noteworthy, although caAKT reduced apoptosis also in siControl cells, its overexpression did not completely blunt TGF-β mediated apoptosis in hepatocytes.
Figure 2.
Lack of AKT activation determines hepatocyte apoptosis. (a) Ly294002 preincubation (2, 5 and 10 μM) dose-dependently increases TGF-β1 (5 ng/ml, 48 h) mediated hepatocytes apoptosis (significant for 5 and 10 μM, P=0.019 and P=0.014, respectively). Basal caspase 3 activity is not affected by inhibitor treatment. (b) 10 μM Ly294002 blocks basal and TGF-β stimulated AKT phosphorylation (1 h treatment). Smad3 phosphorylation was not. (c) Expression of a constitutive active form of AKT does not reduce basal TGF-β1 induced caspase 3 activity and apoptosis. (d) Hepatocytes, transfected with siControl and siCaveolin-1, were transduced with AdLacZ or AdcaAKT. TGF-β1 (5 ng/ml, 48 h) treatment results in a significant reduction in caspase 3 activity in siCav/AdcaAKT compared to siCav/AdLacZ cells (* siCav+LacZ versus siCo+LacZ, P=0.0001; †siCav+caAKT versus siCav+LacZ; P=0.0012)
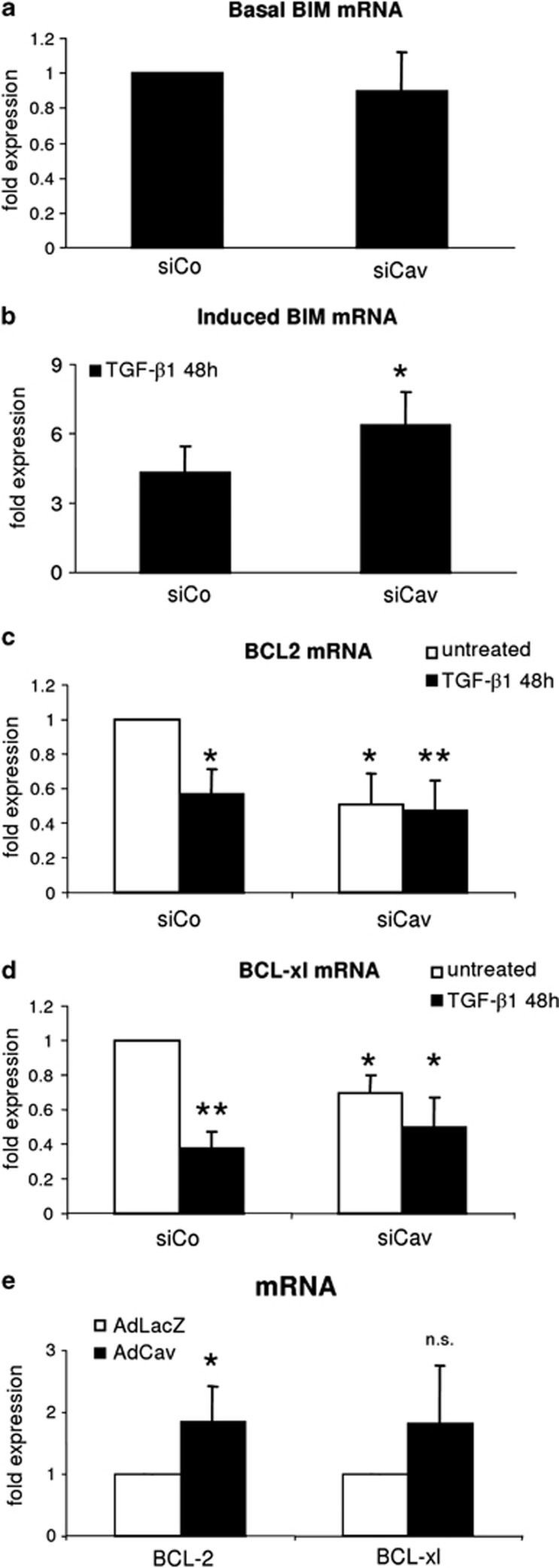
Caveolin-1 knockdown affects BCL2, BCL-xl and BIM expression
A main mechanism how TGF-β1 triggers the apoptotic cascade is the regulation of BCL family members that consist of pro- and antiapoptotic proteins. Hence, we first investigated the expression of the proapoptotic factor BIM, which has been previously linked to TGF-β mediated apoptosis in hepatocytes.20, 21, 22 Basal BIM expression was not altered in siCaveolin-1 samples compared with siControl (Figure 3a). Further, mRNA analysis of TGF-β treated siControl and siCaveolin-1 hepatocytes confirmed a significant elevation of BIM expression in the absence of Caveolin-1 (Figure 3b, n=3, P=0.043). In this context, the activation of the AKT pathway has been reported to negatively regulate BIM activity and expression.22, 23, 24
Figure 3.
Expression of apoptosis-related factors. (a) Knockdown of Caveolin-1 does not affect BIM mRNA expression (n=3). (b) Forty-eight hour stimulation with TGF-β1 yielded in an enhanced expression of BIM mRNA in siCaveolin-1 hepatocytes (n=3; P=0.043). (c) BCL2 (n=4, P=0.011) and (d) BCL-xl (n=3, P=0.037) mRNA expression in siControl and siCaveolin-1 cells. Basal expression is reduced in siCaveolin-1 samples. TGF-β1 affects expression only in siControl hepatocytes (*P<0.05; **P<0.01 compared with siControl untreated). (e) Overexpression of Caveolin-1 elevates basal BCL2 and BCL-xl expression in hepatocytes (BCL2: 1.84±0.26, n=5, P=0.013; BCL-xl: 1.81±0.43, n=5, P=0.093). Analyses were performed 72 h postadenoviral infection
As the balance between pro- and antiapoptotic proteins determines the fate of cells, we addressed whether the antiapoptotic proteins BCL2 and BCL-xl were affected by Caveolin-1 knockdown. Knockdown of Caveolin-1 correlated significantly with a reduction of BCL2 (n=4, P=0.011) and BCL-xl mRNA expression (n=3, P=0.037) (Figures 3c and d). On the contrary, overexpression of Caveolin-1 culminated in elevated basal expression of BCL2 and BCL-xl (BCL2: 1.845±0.26, n=5, P=0.013; BCL-xl: 1.81±0.43, n=5, P=0.093; Figure 3e). TGF-β potently downregulated mRNA levels of BCL2 (n=4, P=0.010) and BCL-xl (n=3, P=0.007) in siControl samples, but likely due to the low basal expression of these targets, no further significant reduction was identified in siCaveolin-1+TGF-β samples as compared with siCaveolin-1 untreated. However, BCL2 and BCL-xl levels are also significantly reduced when comparing siCaveolin-1+TGF-β1 versus siControl (BCL2: n=4, P=0.0092; BCL-xl: n=3, P=0.038). Taken together, our data argue for Caveolin-1 possessing priming effects towards an apoptotic response after a TGF-β challenge.
EMT markers are not altered by Caveolin-1 knockdown
A subsequent question to answer was whether the shift towards apoptosis affects the TGF-β mediated EMT process in the surviving hepatocytes. Therefore, Caveolin-1 was knocked down (>90% knockdown on mRNA level, not shown) and mRNA levels for Collagen 1α1 and Snai1 in TGF-β1 treated cells were analyzed. TGF-β potently induced expression of the EMT markers. When comparing the fold induction between siControl +TGF-β1 versus siCaveolin-1 +TGF-β1, no significant change could be identified. Collagen 1α1 was increased 1.2-fold (±0.24, n=3, P=0.25) in siCaveolin-1 +TGF-β samples and Snai1 expression was elevated 2.76-fold (±2.4, n=3, P=0.274), respectively (Figure 4a). For further validation, downregulation of E-Cadherin, upregulation of N-Cadherin and Pai1 by TGF-β1 in control and Caveolin-1 knockdown hepatocytes were assessed on protein level (Figure 4b). In both settings, no difference in repression of E-Cadherin and induction of N-Cadherin and Pai1 was found, as determined by densitometric analysis (Figures 4c–e). To conclude, the surviving hepatocytes demonstrate an unaltered EMT progress in the absence of Caveolin-1 expression.
Figure 4.
Analysis of EMT markers. (a) Collagen 1α1 and Snai1 mRNA levels are not significantly elevated after 48 h TGF-β1 treatment when comparing siCaveolin-1 +TGF-β versus siControl +TGF-β samples (Collagen 1α1: 1.2-fold ±0.24, n=3, P=0.25; Snai1: 2.76-fold ±2.4, n=3, P=0.274). (b) A representative western blot shows downregulation of E-Cadherin and induction of N-Cadherin and Pai1 by TGF-β1 (48 h) in siControl and siCaveolin-1 treated hepatocytes. (c–e) Densitometrical analysis of western blots (n=3) for N-Cadherin, E-Cadherin and Pai-1. Densitomeric values of the targets were normalized to GAPDH protein expression. No significant difference between control and Caveolin-1 knockdown samples was observed. (f) Illustration of Caveolin-1 effects in hepatocytes on TGF-β mediated apoptosis. Caveolin-1 knockdown leads to increased TGF-β1 mediated Smad signaling paralleled by absence of AKT activation. Antiapoptotic BCL2 and BCL-xl expression are affected by knockdown of Caveolin-1. As a consequence, hepatocyte apoptosis is elevated. The EMT branch is unaltered upon Caveolin-1 knockdown as illustrated by N-cadherin, E-cadherin and Collagen 1α1 expression
Discussion
Hepatocytes respond to TGF-β in a bivalent way. One fraction undergoes apoptosis, whereas the surviving cells obtain a mesenchymal phenotype (as they undergo EMT). Here, we define the activation of AKT being relevant for hepatocyte survival upon TGF-β challenge. Absence of Caveolin-1, an essential protein for caveolae formation on the plasma membrane, abrogated TGF-β mediated phosphorylation of AKT, which culminated in increased hepatocyte apoptosis. A recent study proposed that, TGF-β mediated activation of AKT is facilitated via EGFR (epidermal growth factor receptor) ligand secretion, EGFR activation and subsequent c-Src phosphorylation.25 However, Caveolin-1 was shown to counteract EGFR signaling.26, 27 Instead, EGFR propagate downstream signaling via clathrin-coated pit internalization and thus, the lack of Caveolin-1 should not affect TGF-β mediated activation of AKT. The general role of Caveolin-1 in regulating AKT activation is evident. PI3K/AKT signaling has recently been shown to require lipid raft compartments (where Caveolin-1 is usually located) to enable activation.28, 29, 30, 31 Further, a Caveolin-1 dependent signaling cascade involving RhoA/ROCK/PI3K/AKT has been described.32 Further research activities will shed light on how TGF-β induces AKT in a Caveolin-1 dependent manner.
Noteworthy, this study points out that endocytosis acts as a regulator of TGF-β signaling outcome in hepatocytes. Although it was proposed that TGF-β signaling is enhanced via clathrin-coated pit internalization and the caveolae/Caveolin-1 dependent route would serve for dampening of signaling,33 the presented work demonstrates that first, Caveolin-1 is required for activation of AKT by TGF-β and subsequently second, that reduced Caveolin-1 levels shift the physiological hepatocyte response towards apoptosis. Thus, Caveolin-1 regulates signaling not only by enhancing or dampening of the signal strength, but enables modulation which cellular program, EMT or apoptosis, is initiated. A further note against the general assumption of Caveolin-1 as a delimiter of TGF-β signaling, our data clearly show that the effects of altered Caveolin-1 expression levels can not be generalized. Knockdown did not result in a general enhancement of repression or elevation of TGF-β1 target genes, as exemplified on Collagen 1α1, E- and N-Cadherin. These observations further argue for Caveolin-1 being a modulator of the TGF-β pathway instead of being only responsible for the downregulation of surface receptors.33
Next, endogenous expression levels of Caveolin-1 are sufficient to facilitate AKT activation as its overexpression did neither affect the reporter, nor alter the caspase 3 assay significantly. However, AdCav transduction reduced enzyme activities moderately in the caspase 3 assay. This finding might be of relevance in the pathogenesis of hepatocellular carcinomas, as it was reported that Caveolin-1 is frequently overexpressed in tumor tissues.34, 35 However, the effects on pathogenesis are not fully solved and contradictory correlations between Caveolin-1 expression and disease progression have been discussed.34, 36 From the presented data, it is likely that the endogenous pool of Caveolin-1 is enough to generate an environment (for example, caveolae), in which activation of AKT by TGF-β can be accomplished. A further increase of caveolae formation thus would not affect physiologic AKT activation. Hence, the observed slight reduction of Smad signaling and caspase 3 activation in Caveolin-1 overexpressing cells might derive from the previously described function of caveolae with respect to TGF-β receptor degradation.22 Intriguingly, a hyperactivation of the AKT pathway did not further reduce the basal TGF-β mediated apoptotic process. This implies that a basal apoptotic rate is AKT signaling independent, but likely relies on Smad3-mediated signal transduction. Smad3 has previously been shown as essential for TGF-β mediated apoptosis in hepatocytes.14 Recently, a milestone paper about the metabolic effects of Caveolin-1 deficiency was published. Bosch et al. report that deficiency alters the mitochondrial membrane composition and subsequently affects the respiratory chain.37 Subsequently, more cell stress was measured. These findings offer an additional mechanism on the role of Caveolin-1 affecting the cell response to proapoptotic stimuli. Even more, our findings on basal BCL2 and BCL-xl expression might be explained by the above reported function that Caveolin-1 deficiency induces cell stress and subsequently shifts the balance to enhanced susceptibility for apoptosis. Confirming the influence of Caveolin-1 on basal apoptosis susceptibility, elevated Caveolin-1 expression levels affected expression of BCL2 and BCL-xl in a positive way. To conclude, our study proves that Caveolin-1 is a major modulator of the TGF-β pathway (illustrated in Figure 4f). Caveolin-1 is priming hepatocytes for proapoptotic stimuli and its expression is a prerequisite for TGF-β triggered AKT activation and hence sets the course for the hepatocyte fate.
Materials and Methods
Cell culture
Wild type primary hepatocytes were isolated from livers of male C57Bl/6 by collagenase perfusion.38 Hepatocytes then were plated on collagen coated plates and cultured as described14 in Williams E medium supplemented with l-glutamine, dexamethasone and foetal calf serum. After attachment (4-h), cells were kept in starvation medium without dexamethasone and foetal calf serum.
Reagents and antibodies
Recombinant TGF-β1 was purchased from Peprotech (Hamburg, Deutschland, Germany) and used at a final concentration of 5 ng/ml. siRNA targeting Caveolin-1 was from Dharmacon (Thermo Fisher Scientific, Waltham, MA, USA), a scrambled and the anti Smad3 siRNA were delivered by Qiagen (Hilden, Germany). AdCaveolin-1 was kindly provided by Dr. Soazig Le Lay-Peron (Paris, France) and described in ref. 39. AdcaAKT was described previously.14 siRNA was transfected over night using RNAiMAX (Invitrogen, Darmstadt, Germany). Antibodies used: pSmad3 (Epitomics, Abcam, Burlingame, CA, USA), pAKT, Smad3, GAPDH, PARP, cleaved caspase 3 (Cell Signaling, NEB, Frankfurt, Germany), Caveolin-1, Pai1 and horseradish peroxidase-linked secondary antibodies anti mouse and rabbit were from SantaCruz (Heidelberg, Germany). E-Cadherin was from BD Biosystems (Heidelberg, Germany), N-Cadherin was obtained from abcam (Burlingame, CA, USA).
Microarray analysis
RNA lysates from three different hepatocyte isolations were used for analysis. Cells were stimulated with 5 ng/ml TGF-β1 for 24 h. RNA was processed and data was analyzed as described in ref. 4. Data is freely available at ArrayExpress (http://www.ebi.ac.uk/arrayexpress/) at the European Bioinformatics Institute (Hinxton). Targets are shown with a regulation of >1.5-fold, however, most transcripts were changed >twofold.
Western blot analysis
Cell lysates were prepared using RIPA buffer (50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, Proteases Inhibitor Cocktail, Phosphatase Inhibitor Cocktail II (Roche, Mannheim, Germany)). Protein concentration was determined using the DC protein assay (Bio-Rad, Munich, Germany). Thirty-microgram protein per sample were separated by SDS–polyacrylamide gel electrophoresis (12%). Subsequently, proteins were transferred to a nitrocellulose membrane and blocked in 5% non-fat milk in TBST. Then, membranes were incubated with indicated antibodies and developed using Chemi-Smart (Vilber Lourmat, Eberhardzell, Germany). Densitometry was calculated with Aida densitometry software (Raytest, Straubenhardt, Germany).
Reporter gene assay
Ad(CAGA)9-MLP-Luc was used as a Smad3 luciferase reporter. Luciferase substrate was purchased from Promega (Mannheim, Germany). Luminometric measurements were performed using Tecan infinite M200 (Thermo Scientific, Waltham, MA, USA). Obtained values (Unit –relative light units, RLU) were normalized to protein content. siRNA transfection or AdCav-1 infection was carried out after reporter transduction. Experiments were performed in triplicates. Shown are the means of three experiments±S.E.
Caspase 3 assay
To assess caspase 3 activity, TGF-β treated hepatocytes were lysed in lysis buffer (50 mM HEPES, 100 mM NaCl, 0.1% CHAPS, 1 mM DTT, 0.1 mM EDTA, pH 7.4). Subsequently, 20-μg protein were incubated for 90 min at 37 °C in reaction buffer (50 mM HEPES, 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 0.1 mM EDTA, 10% (w/v) glycerol, pH 7.4) and AC-DEVD-AFC caspase 3 fluorimetric substrate (Biomol, Hamburg, Germany) to allow cleavage of substrate. Substrate turnover was obtained by fluorometric measurement using Tecan infinite M200 (excitation 400 nm; emission 505 nm). Each experiment was performed in triplicates. Shown are the means of three experiments±S.E.
RNA isolation, reverse transcription and qRT–PCR
Total RNA was extracted using the RNeasy Mini Kit (Qiagen). Subsequently, complementary DNA was synthesized from 1-μg RNA with the Quantitech Reverse Transcription kit (Qiagen). qRT–PCR was performed using Sybr green or taqman probe technology on a Stratagene Mx3005P. Snai1, PPIA and collagen 1α1 primer mixes (taqman) were purchased from Applied Biosystems. Primers for Bim, BCL2, BCL-xl and PPIA were from Qiagen (Sybr green).
Densitometry and statistics
For quantitative analysis of western blots (pAKT, GAPDH for normalization), Aida Image Analyzer v. 4.25 was used. Values were calculated from four independent experiments. Significance was calculated with the two-tailed Student's t-test. Values were pooled from at least three independent experiments or as indicated.
Acknowledgments
We are grateful for excellent technical assistance to Alexandra Müller. This work was funded by the BMBF program ‘Virtual liver' and the Deutsche Forschungsgemeinschaft DO 373/8-1 and TRR-SFB 77.
Glossary
- caAKT
constitutively active AKT
- EMT
epithelial mesenchymal transition
- PARP
poly-(ADP-ribose) polymerase
- TGF-β
transforming growth factor beta
The authors declare no conflict of interest.
Footnotes
Edited by A Stephanou
References
- Kang JS, Liu C, Derynck R. New regulatory mechanisms of TGF-beta receptor function. Trends Cell Biol. 2009;19:385–394. doi: 10.1016/j.tcb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Caja L, Bertran E, Campbell J, Fausto N, Fabregat I. The transforming growth factor-beta (TGF-beta) mediates acquisition of a mesenchymal stem cell-like phenotype in human liver cells. J Cell Physiol. 2011;226:1214–1223. doi: 10.1002/jcp.22439. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley S, Hamzavi J, Ciuclan L, Godoy P, Ilkavets I, Ehnert S, et al. Hepatocyte-specific Smad7 expression attenuates TGF-beta-mediated fibrogenesis and protects against liver damage. Gastroenterology. 2008;135:642–659. doi: 10.1053/j.gastro.2008.04.038. [DOI] [PubMed] [Google Scholar]
- Meyer C, Meindl-Beinker NM, Dooley S. TGF-beta signaling in alcohol induced hepatic injury. Front Biosci. 2010;15:740–749. doi: 10.2741/3643. [DOI] [PubMed] [Google Scholar]
- Taura K, Miura K, Iwaisako K, Osterreicher CH, Kodama Y, Penz-Osterreicher M, et al. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51:1027–1036. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793–d807. doi: 10.2741/A812. [DOI] [PubMed] [Google Scholar]
- Sanchez-Capelo A. Dual role for TGF-beta1 in apoptosis. Cytokine Growth Factor Rev. 2005;16:15–34. doi: 10.1016/j.cytogfr.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Carmona-Cuenca I, Roncero C, Sancho P, Caja L, Fausto N, Fernandez M, et al. Upregulation of the NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its pro-apoptotic activity. J Hepatol. 2008;49:965–976. doi: 10.1016/j.jhep.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Ramesh S, Qi XJ, Wildey GM, Robinson J, Molkentin J, Letterio J, et al. TGF beta-mediated BIM expression and apoptosis are regulated through SMAD3-dependent expression of the MAPK phosphatase MKP2. EMBO Rep. 2008;9:990–997. doi: 10.1038/embor.2008.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal A, Sjoblom T, Fedorova L, Imreh S, Beug H, Moustakas A. Sustained TGF beta exposure suppresses Smad and non-Smad signalling in mammary epithelial cells, leading to EMT and inhibition of growth arrest and apoptosis. Oncogene. 2008;27:1218–1230. doi: 10.1038/sj.onc.1210741. [DOI] [PubMed] [Google Scholar]
- Conery AR, Cao Y, Thompson EA, Townsend CM, Ko TC, Luo K. Akt interacts directly with Smad3 to regulate the sensitivity to TGF-beta induced apoptosis. Nat Cell Biol. 2004;6:366–372. doi: 10.1038/ncb1117. [DOI] [PubMed] [Google Scholar]
- Chen RH, Su YH, Chuang RL, Chang TY. Suppression of transforming growth factor-beta-induced apoptosis through a phosphatidylinositol 3-kinase/Akt-dependent pathway. Oncogene. 1998;17:1959–1968. doi: 10.1038/sj.onc.1202111. [DOI] [PubMed] [Google Scholar]
- Godoy P, Hengstler JG, Ilkavets I, Meyer C, Bachmann A, Muller A, et al. Extracellular matrix modulates sensitivity of hepatocytes to fibroblastoid dedifferentiation and transforming growth factor beta-induced apoptosis. Hepatology. 2009;49:2031–2043. doi: 10.1002/hep.22880. [DOI] [PubMed] [Google Scholar]
- Chen YG. Endocytic regulation of TGF-beta signaling. Cell Res. 2009;19:58–70. doi: 10.1038/cr.2008.315. [DOI] [PubMed] [Google Scholar]
- Lajoie P, Goetz JG, Dennis JW, Nabi IR. Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J Cell Biol. 2009;185:381–385. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alejandro Fernandez-Rojo M, Restall C, Ferguson C, Martel N, Martin S, Bosch M, et al. Caveolin-1 orchestrates the balance between glucose and lipid-dependent energy metabolism: Implications for liver regeneration. Hepatology. 2012;55:1574–1584. doi: 10.1002/hep.24810. [DOI] [PubMed] [Google Scholar]
- Meyer C, Godoy P, Bachmann A, Liu Y, Barzan D, Ilkavets I, et al. Distinct role of endocytosis for Smad and non-Smad TGF-beta signaling regulation in hepatocytes. J Hepatol. 2011;55:369–378. doi: 10.1016/j.jhep.2010.11.027. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee Y, Seo JE, Cho KH, Chung JH. Caveolin-1 increases basal and TGF-beta1-induced expression of type I procollagen through PI-3 kinase/Akt/mTOR pathway in human dermal fibroblasts. Cell Signal. 2008;20:1313–1319. doi: 10.1016/j.cellsig.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Ramjaun AR, Tomlinson S, Eddaoudi A, Downward J. Upregulation of two BH3-only proteins, Bmf and Bim, during TGF beta-induced apoptosis. Oncogene. 2007;26:970–981. doi: 10.1038/sj.onc.1209852. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang L, Chen A, Xiang G, Wang Y, Wu J, et al. Identification of the gene transcription and apoptosis mediated by TGF-beta-Smad2/3-Smad4 signaling. J Cell Physiol. 2008;215:422–433. doi: 10.1002/jcp.21325. [DOI] [PubMed] [Google Scholar]
- Ramesh S, Wildey GM, Howe PH. Transforming growth factor beta (TGFbeta)-induced apoptosis: the rise & fall of Bim. Cell Cycle. 2009;8:11–17. doi: 10.4161/cc.8.1.7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin KJ, Hsu CY, Hu XY, Chen H, Chen SW, Xu J, et al. Protein phosphatase 2A regulates bim expression via the Akt/FKHRL1 signaling pathway in amyloid-beta peptide-induced cerebrovascular endothelial cell death. J Neurosci. 2006;26:2290–2299. doi: 10.1523/JNEUROSCI.5103-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J Biol Chem. 2006;281:813–823. doi: 10.1074/jbc.M505546200. [DOI] [PubMed] [Google Scholar]
- Murillo MM, del Castillo G, Sanchez A, Fernandez M, Fabregat I. Involvement of EGF receptor and c-Src in the survival signals induced by TGF-beta1 in hepatocytes. Oncogene. 2005;24:4580–4587. doi: 10.1038/sj.onc.1208664. [DOI] [PubMed] [Google Scholar]
- Lajoie P, Partridge EA, Guay G, Goetz JG, Pawling J, Lagana A, et al. Plasma membrane domain organization regulates EGFR signaling in tumor cells. J Cell Biol. 2007;179:341–356. doi: 10.1083/jcb.200611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2008;314:3093–3106. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Lowry PR, Zhou X, Depry C, Wei Z, Wong GW, et al. PI3K/Akt signaling requires spatial compartmentalization in plasma membrane microdomains. Proc Natl Acad Sci USA. 2011;108:14509–14514. doi: 10.1073/pnas.1019386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Oh P, Horner T, Rogers RA, Schnitzer JE. Organized endothelial cell surface signal transduction in caveolae distinct from glycosylphosphatidylinositol-anchored protein microdomains. J Biol Chem. 1997;272:7211–7222. doi: 10.1074/jbc.272.11.7211. [DOI] [PubMed] [Google Scholar]
- Liu P, Ying Y, Ko YG, Anderson RG. Localization of platelet-derived growth factor-stimulated phosphorylation cascade to caveolae. J Biol Chem. 1996;271:10299–10303. doi: 10.1074/jbc.271.17.10299. [DOI] [PubMed] [Google Scholar]
- Chen L, Xiong S, She H, Lin SW, Wang J, Tsukamoto H. Iron causes interactions of TAK1, p21ras, and phosphatidylinositol 3-kinase in caveolae to activate IkappaB kinase in hepatic macrophages. J Biol Chem. 2007;282:5582–5588. doi: 10.1074/jbc.M609273200. [DOI] [PubMed] [Google Scholar]
- Park JH, Ryu JM, Han HJ. Involvement of caveolin-1 in fibronectin-induced mouse embryonic stem cell proliferation: role of FAK, RhoA, PI3K/Akt, and ERK 1/2 pathways. J Cell Physiol. 2011;226:267–275. doi: 10.1002/jcp.22338. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- Cokakli M, Erdal E, Nart D, Yilmaz F, Sagol O, Kilic M, et al. Differential expression of Caveolin-1 in hepatocellular carcinoma: correlation with differentiation state, motility and invasion. BMC Cancer. 2009;9:65. doi: 10.1186/1471-2407-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse EY, Ko FC, Tung EK, Chan LK, Lee TK, Ngan ES, et al. Caveolin-1 overexpression is associated with hepatocellular carcinoma tumorigenesis and metastasis. J Pathol. 2012;226:645–653. doi: 10.1002/path.3957. [DOI] [PubMed] [Google Scholar]
- Yang SF, Yang JY, Huang CH, Wang SN, Lu CP, Tsai CJ, et al. Increased caveolin-1 expression associated with prolonged overall survival rate in hepatocellular carcinoma. Pathology. 2010;42:438–445. doi: 10.3109/00313025.2010.494293. [DOI] [PubMed] [Google Scholar]
- Bosch M, Mari M, Herms A, Fernandez A, Fajardo A, Kassan A, et al. Caveolin-1 deficiency causes cholesterol-dependent mitochondrial dysfunction and apoptotic susceptibility. Curr Biol. 2011;21:681–686. doi: 10.1016/j.cub.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng HL, Ciuclan L, Liu Y, Hamzavi J, Godoy P, Gaitantzi H, et al. Profibrogenic transforming growth factor-beta/activin receptor-like kinase 5 signaling via connective tissue growth factor expression in hepatocytes. Hepatology. 2007;46:1257–1270. doi: 10.1002/hep.21806. [DOI] [PubMed] [Google Scholar]
- Le Lay S, Hajduch E, Lindsay MR, Le Liepvre X, Thiele C, Ferre P, et al. Cholesterol-induced caveolin targeting to lipid droplets in adipocytes: a role for caveolar endocytosis. Traffic. 2006;7:549–561. doi: 10.1111/j.1600-0854.2006.00406.x. [DOI] [PubMed] [Google Scholar]