Abstract
Mitochondrial dysfunction caused by protein aggregation has been shown to have an important role in neurological diseases, such as Parkinson's disease (PD). Mitochondria have evolved at least two levels of defence mechanisms that ensure their integrity and the viability of their host cell. First, molecular quality control, through the upregulation of mitochondrial chaperones and proteases, guarantees the clearance of damaged proteins. Second, organellar quality control ensures the clearance of defective mitochondria through their selective autophagy. Studies in Drosophila have highlighted mitochondrial dysfunction linked with the loss of the PTEN-induced putative kinase 1 (PINK1) as a mechanism of PD pathogenesis. The mitochondrial chaperone TNF receptor-associated protein 1 (TRAP1) was recently reported to be a cellular substrate for the PINK1 kinase. Here, we characterise Drosophila Trap1 null mutants and describe the genetic analysis of Trap1 function with Pink1 and parkin. We show that loss of Trap1 results in a decrease in mitochondrial function and increased sensitivity to stress, and that its upregulation in neurons of Pink1 mutant rescues mitochondrial impairment. Additionally, the expression of Trap1 was able to partially rescue mitochondrial impairment in parkin mutant flies; and conversely, expression of parkin rescued mitochondrial impairment in Trap1 mutants. We conclude that Trap1 works downstream of Pink1 and in parallel with parkin in Drosophila, and that enhancing its function may ameliorate mitochondrial dysfunction and rescue neurodegeneration in PD.
Keywords: Drosophila, mitochondria, Parkinson's disease, stress
Accumulating evidence suggests that the disruption of mitochondrial function and dynamics contributes to age-related neurodegenerative diseases, such as Parkinson's disease (PD).1 Accordingly, cells have developed complex quality control mechanisms to cope with the different challenges constantly imposed on the integrity of mitochondria.2, 3
Molecular quality control, the first level of the mitochondrial defence mechanism, involves the upregulation of nuclear genes that encode mitochondrial chaperones and proteases, ensuring the removal of misfolded and non-assembled polypeptides. This stress response is known as the mitochondrial unfolded-protein response (UPRmt).4
Furthermore, the highly dynamic nature of mitochondria facilitates quality control through fusion and fission.5, 6 Thus, damaged mitochondria can fuse with healthy organelles to replenish stores of deficient components. When the damage is excessive, such processes are overwhelmed; mitochondria become depolarised, and their components are targeted for recycling through a specific form of autophagy, termed mitophagy.7, 8
Recent findings have demonstrated that PTEN-induced putative kinase 1 (PINK1) is capable of directly modulating the activity of chaperones.9, 10 The TNF receptor-associated protein 1 (TRAP1) is a mitochondrial chaperone suggested to act as a cellular substrate of the PINK1 kinase. In cultured cells, PINK1 expression shows a protective effect against oxidative stress-induced apoptosis. Upon the induction of oxidative stress, TRAP1 is phosphorylated in a PINK1-dependent manner, and this phosphorylation is necessary to confer resistance to apoptosis.10
TRAP1 is a member of the HSP90 family, which has been shown to protect cells from oxidative stress and apoptosis caused by different agents,11, 12, 13, 14 and its upregulation in tumours has been associated with chemotherapy resistance.15, 16
The fruit fly, Drosophila melanogaster, has recently emerged as a powerful model system for studying the links between mitochondrial dysfunction and parkinsonian neurodegeneration.17 In Drosophila, the loss of Pink1 leads to mitochondrial dysfunction associated with muscle degeneration and the loss of dopaminergic neurons.18, 19 In addition, a decreased expression of Trap1 was found to enhance the loss of dopaminergic neurons in flies expressing a mutant form of human α-synuclein that causes PD.20
Here, we describe the consequences of Trap1 loss-of-function in Drosophila and its gain-of-function on mitochondrial integrity. We also genetically address the role of Trap1 in mitochondrial quality control through the Pink1/parkin pathway.
Results
Loss of Trap1 increases sensitivity to stress and causes a decline in mitochondrial function
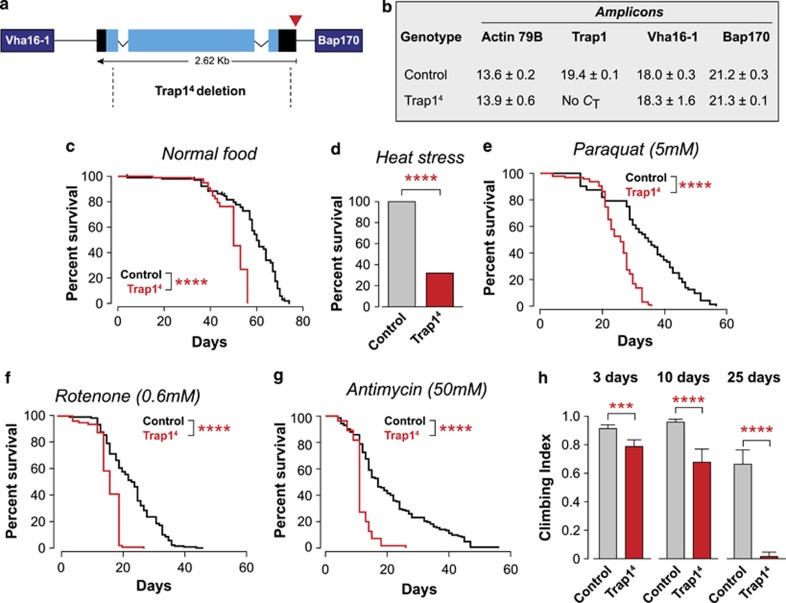
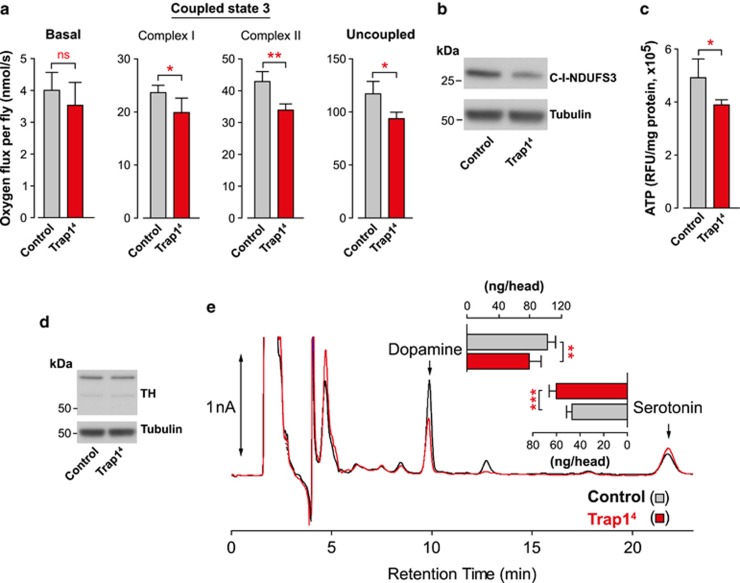
To investigate the in vivo role of Trap1 in Drosophila, we first determined the consequences of its loss. We obtained Trap14 mutant flies, generated by an imprecise excision of the P-element insertion line P(EPgy2)Trap1EY10238. The excision deleted the majority of the Trap1 gene (Figure 1a). We next performed quantitative real-time RT-PCR and confirmed that Trap14 mutants show a total loss of Trap1 mRNA, and that its neighbouring genes were not affected by the imprecise excision of the P-element (Figure 1b). The Trap1 mutant flies were viable and developed to adulthood; however, they presented a significantly shorter lifespan compared with the controls (Figure 1c). Given the proposed role of TRAP1 as a mitochondrial chaperone, we decided to investigate the consequences of exposing Trap14 mutants to increased levels of stress. We observed a decreased viability of Trap14 mutants subjected to heat stress (Figure 1d). The Trap14 mutants were also more sensitive to paraquat, a pesticide linked to PD by epidemiological studies21 (Figure 1e), and to mitochondrial poisons such as rotenone (Figure 1f) and antimycin (Figure 1g). The Trap14 mutants showed an age-dependent impaired climbing ability, suggesting a locomotor deficit (Figure 1h). In Pink1 mutant flies, locomotor defects result from mitochondrial impairment in the Drosophila skeletal muscles, including the indirect flight muscle.18, 19 To determine if the loss of Trap1 lead to mitochondrial impairment, we compared the respiration rates in the controls and mutants. This analysis revealed a significant decrease in the respiratory function of the Trap1 mutants (Figure 2a). Trap14 mutants also showed a decrease in the levels of the mitochondrial complex I (Figure 2b). Mitochondria are responsible for the production of the majority of cellular energy in the form of ATP. The measurement of ATP levels in Trap14 mutants revealed a significant decrease when compared with controls (Figure 2c). Taken together, these results suggest that the loss of the mitochondrial chaperone Trap1 results in a decrease in mitochondrial function, which is associated with a loss of ATP in adult flies. The loss of dopaminergic neurons can be indirectly assessed through the analysis of the expression levels of tyrosine hydroxylase (TH), an enzyme expressed in dopaminergic neurons.22 We failed to detect any differences in the TH levels of Trap14 mutants (Figure 2d); however measuring neurotransmitter levels in the heads of Trap14 mutants revealed a significant decrease in the dopamine content, compared to the controls (Figure 2e).
Figure 1.
The loss of Trap1 in Drosophila causes motor impairment and an increased sensitivity to stress. (a) Genomic map of Trap1 (cytological location 42B2). Black, untranslated regions; light blue, exons. The P-element insertion (EY10238) is indicated by the red triangle. The neighbouring genes (Vha16-1 and Bap170) are indicated in dark blue. Trap14 deletion, delimited by the dashed lines, removes most of the Trap1 gene. (b) Analysis of the expression levels of Trap1 and its neighbouring genes. Expression levels were measured by real-time PCR in 3-day-old flies with the indicated genotypes (mean Ct±S.D., n=4 per genotype). Expression of actin was used as a control. No Ct for the Trap1 transcript was detected in Trap14 mutants. (c) Trap14 mutants (red) have a reduced lifespan compared with the controls (black). Fly viability was scored over a period of 75 days, using a minimum of 100 flies per genotype. The statistical significance is indicated (log-rank, Mantel–Cox test). (d–g) Trap1 mutant flies show enhanced sensitivity to stress. (d) Flies were subjected to heat stress, and viability was assessed after 24 h. A total of 100 males (4 days old) were assayed for each genotype. The asterisks indicate significant values (Fisher's exact test, two-sided, alpha<0.05). (e–g) Flies were maintained on food supplemented with the indicated drugs, and the viability was scored over a period of 60 days, using a minimum of 100 flies per genotype. The statistical significance is indicated by the asterisks (log-rank, Mantel–Cox test). (h) Trap14 mutants show a decrease in motor performance. Flies with the indicated genotypes and ages were tested using a standard climbing assay (mean±S.D., n≥80 flies for each genotype). The asterisks indicate significant values (two-way ANOVA with the Bonferroni multiple comparison test)
Figure 2.
The loss of Trap1 in Drosophila results in a reduction of mitochondrial function and brain dopamine levels. (a) Decreased respiration in Trap14 mutant flies. Activity was measured by high-resolution respirometry in 20-day-old flies. Data are shown as the means±S.D. (n≥4 in each genotype). Significant values relative to the control are indicated by asterisks (two-tailed unpaired t test). (b) Decreased complex I protein levels in Trap14 mutant flies. Whole-fly (20 days old) protein lysates were subjected to western blot analysis with the indicated antibodies. (c) Trap14 mutants have lower levels of ATP compared with the controls. The ATP levels were measured using a bioluminescent assay. Data are shown as the means±S.D. (n=4 for each genotype). The statistical significance is indicated by asterisks (two-tailed unpaired t-test). (d) Analysis of TH levels in Trap1 mutant flies. Fly-head protein lysates from 20-day-old flies were used for western blotting analysis with the indicated antibodies. (e) Trap1 mutant flies have decreased dopamine and increased serotonin levels. Neurotransmitter levels were assessed by HPLC with electrochemical detection. Data are shown as the means±S.D. (n≥5 for each genotype). The statistical significance is indicated by asterisks (two-tailed unpaired t-test)
Trap1 expression enhances motor performance and protects against oxidative stress
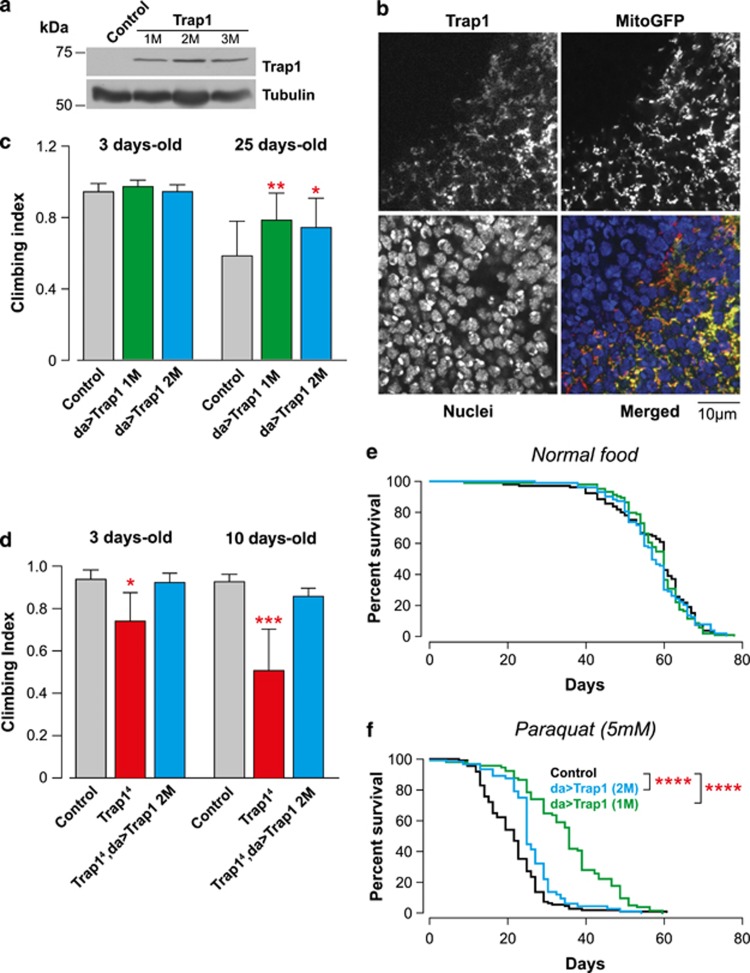
Recently, TRAP1 was suggested to work downstream of PINK1 in the prevention of mitochondrial dysfunction associated with PD pathogenesis.10 We therefore decided to test the epistatic relationship between Trap1 and Pink1 in flies. We generated transgenic flies expressing full-length Trap1, and using UAS-GAL4 system23 we confirmed the expression of the transgene by western blot analysis (Figure 3a). To investigate its intracellular localisation, we crossed UAS-Trap1 flies with a transgenic line expressing mitochondria-targeted green-fluorescent protein (mitoGFP). This analysis revealed that Trap1 localises to the mitochondria (Figure 3b). To further investigate the phenotypic consequences of Trap1 expression, we compared the locomotor performance of Trap1 flies with that of controls using a climbing assay. This analysis revealed that Trap1 expression leads to a significantly enhanced climbing performance in aged flies (Figure 3c) and suppressed the climbing defects of Trap14 mutants (Figure 3d). We previously showed that treating flies with paraquat leads to the upregulation of mitochondrial chaperones as a means to protect mitochondria from the oxidative stress triggered by this pesticide.24 Additionally, TRAP1 phosphorylation was reported to be enhanced in response to oxidative stress.10 Flies expressing Trap1 show a normal lifespan compared with the controls (Figure 3e). However, Trap1 expression significantly increased the lifespan of paraquat-treated flies (Figure 3f), indicating that the expression of this mitochondrial chaperone protects against oxidative stress in vivo.
Figure 3.
Expression of Drosophila Trap1 confers resistance to stress. (a) Analysis of the Trap1 expression levels in transgenic flies. Whole-fly lysates were analysed by western blotting with the indicated antibodies. (b) Trap1 localises to the mitochondria. Confocal analysis of the posterior compartment of larval wing discs coexpressing Trap1 and a mitoGFP under the control of the enGAL4 driver. In the merged colour image, red corresponds to Trap1, blue to nuclei and green to mitoGFP. (c) The expression of Trap1 results in increased motor performance. Flies with the indicated genotypes and ages were tested using a standard climbing assay (mean±S.D., n≥120 flies for each genotype). The asterisks indicate significant values relative to the control (two-way ANOVA with the Bonferroni multiple comparison test). (d) The expression of Trap1 reversed the decrease in motor performance in Trap14 mutants. Flies with the indicated genotypes and ages were tested using a standard climbing assay (mean±S.D., n≥80 flies for each genotype). The asterisks indicate significant values relative to the control (two-way ANOVA with the Bonferroni multiple comparison test). (e) Trap1 expression does not affect the total lifespan. A total of 100 flies per genotype were maintained on normal food for a period of 80 days. (f) Trap1-expressing flies (green and blue) show enhanced resistance to paraquat toxicity compared with the controls (black). Fly viability was scored over a period of 60 days using a minimum of 100 flies per genotype. The statistical significance is indicated by the asterisks (log-rank, Mantel–Cox test)
Expression of Trap1 suppresses mitochondrial dysfunction in Pink1 mutants
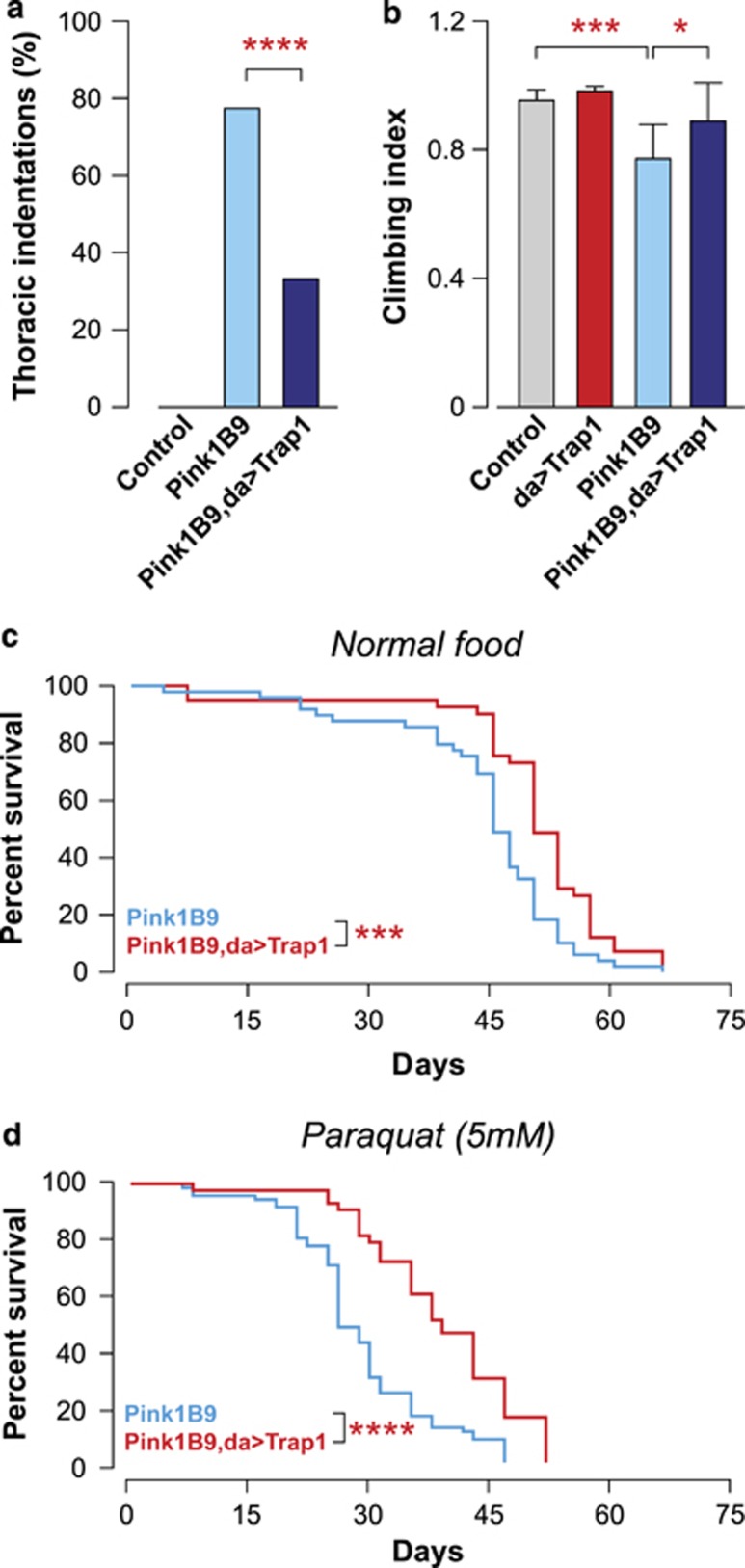
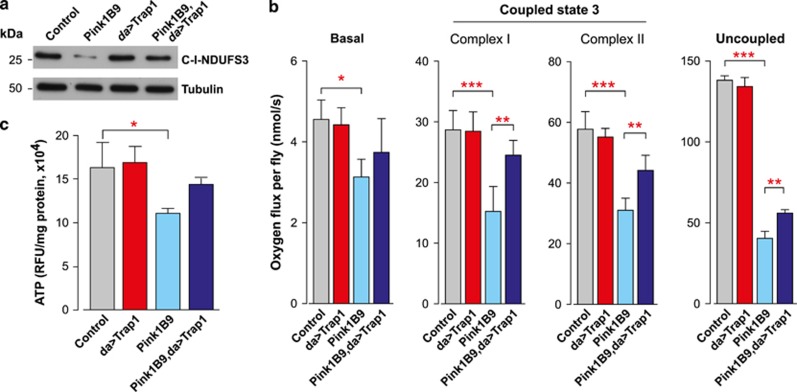
The expression of TRAP1 in cultured cells was shown to be required for PINK1-dependent inhibition of oxidative stress-induced cytochrome c release and cell death.10 The mitochondrial dysfunction in Pink1 mutants is associated with muscle pathology, affecting the indirect flight muscles, leading to the appearance of thoracic indentations and impaired motor performance.18, 19 The expression of Trap1 partially rescued the degree of thoracic indentations (Figure 4a) and the impaired motor performance in the Pink1B9 mutants (Figure 4b). Mitochondrial defects present in the Pink1 mutants are also associated with a decreased lifespan.18 We noted that the expression of Trap1 increased the lifespan of the Pink1 mutants (Figure 4c) and decreased their sensitivity to paraquat (Figure 4d). Taken together, these observations suggest that the ubiquitous expression of Trap1 protects against the phenotypic consequences of Pink1 mutations, and that Trap1 acts genetically downstream of Pink1. The mitochondrial dysfunction in the Pink1 mutant flies is reflected by a loss of mitochondrial proteins and decreased respiration. By expressing Trap1 in Pink1B9 mutants, we observed a recovery of the mitochondrial protein content, indirectly assessed through the analysis of the levels of a complex I subunit (Figure 5a); a partial recovery of the respiration rates (Figure 5b) and ATP levels (Figure 5c). Taken together, these observations indicate that Trap1 expression results in a recovery of the mitochondrial function in Pink1 mutants.
Figure 4.
Trap1 gain-of-function rescues Pink1 mutant flies. (a) Expression of Trap1 rescues the thoracic defects of Pink1B9 mutants. Thoracic indentations were counted up to 24 h after eclosion (n>900 for each genotype). The asterisks indicate significance (χ2, two-sided, alpha<0.05). (b) Trap1 expression suppresses motor impairment in the Pink1B9 mutants. 10-day-old flies with the indicated genotypes were tested using a standard climbing assay (mean±S.D., n=120 flies per genotype). The statistical significance relative to Pink1B9 is indicated by asterisks (one-way ANOVA with Dunnett's multiple comparison test). (c and d) Trap1 expression enhances the lifespan of Pink1B9 mutants. A minimum of 80 flies per genotype were maintained on either normal (c) or paraquat-containing food (d). Fly viability was scored over a period of 70 days. The statistical significance is indicated by the asterisks (log-rank, Mantel–Cox test)
Figure 5.
Drosophila Trap1 reverses mitochondrial dysfunction in Pink1 mutants. (a) Trap1 expression restores the mitochondrial complex I protein levels in the Pink1B9 mutant flies. Whole-fly protein lysates were used for western blotting analysis with the indicated antibodies. (b) Trap1 expression partially rescues the mitochondrial respiratory defects in the Pink1B9 mutants. Measurements of state three respiration of complex I and complex II, and complex II uncoupled respiration by high-resolution respirometry. The data are shown as the means±S.D. (n=6 for each genotype). The statistical significance is indicated by the asterisks (one-way ANOVA with Dunnett's multiple comparison test). (c) Expression of Trap1 increased the ATP levels in the Pink1B9 mutants. ATP levels were measured using a bioluminescent assay. Data are shown as the means±S.D. (n=4 for each genotype). The statistical significance is indicated by the asterisks (one-way ANOVA with Dunnett's multiple comparison test relative to the control)
Neuronal expression of Trap1 is sufficient to revert the phenotype of Pink1 mutants
The genetic removal of the function of Drosophila Pink1 results in apoptotic muscle degeneration and a decrease in the brain dopamine levels as a consequence of the degeneration of dopaminergic neurons.19
The loss of mitochondria at neuronal synapses causes defects in neurotransmission of the Drosophila neuromuscular junction (NMJ).25 Given that one of the most important features of Pink1 mutant flies is the degeneration of both the indirect flight muscle and dopaminergic neurons, we next tested whether the degeneration of the indirect flight muscle involves a presynaptic component, and is therefore preceded by neuronal loss.
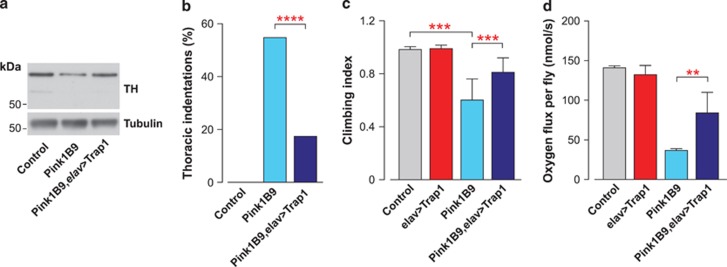
We detected a decrease in the TH levels in Pink1B9 mutants that was reversed upon the expression of Trap1 using a pan-neural elav-GAL4 driver (Figure 6a). This result indicates that the neuronal expression of Trap1 can suppress neurodegeneration in Pink1 mutant flies. Next, we evaluated the consequence of the neuronal expression of Trap1 on phenotypes associated with muscle degeneration. The neuronal expression of Trap1 was sufficient to suppress muscle degeneration in Pink1B9 mutants, which was reflected in a decrease in the degree of thoracic indentations (Figure 6b) and an improved climbing performance (Figure 6c). Finally, the neuronal expression of Trap1 significantly reversed the respiration deficit present in Pink1 mutant flies (Figure 6d). Taken together, these results indicate that the neuronal rescue of mitochondrial function in Pink1 mutants by Trap1 is sufficient to suppress muscle degeneration and motor impairment.
Figure 6.
Targeted neuronal expression of Trap1 rescues mitochondrial dysfunction in Pink1 mutants. (a) Decreased TH levels observed in the Pink1B9 mutants can be partially restored by the expression of Trap1 in neurons. Fly-head protein lysates from 20-day-old flies were used for western blotting analysis with the indicated antibodies. (b) Neuronal expression of Trap1 is capable of diminishing the degree of thoracic indentations in the Pink1B9 mutants. Thoracic indentations were counted up to 24 h after eclosion (n>300 for each genotype). The asterisks indicate the significance relative to Pink1B9 (χ2, two-sided, alpha<0.05). (c) Targeted neuronal expression of Trap1 improves the climbing performance of Pink1B9 mutants. 10-day-old flies with the indicated genotypes were tested using a standard climbing assay (mean±S.D., n=120 flies per genotype). The statistical significance relative to Pink1B9 is indicated by the asterisks (one-way ANOVA with Dunnett's multiple comparison test). (d) Expression of Trap1 specifically in neurons is sufficient to improve mitochondrial respiration in the Pink1B9 mutants. Complex II-uncoupled respiration was measured by high-resolution respirometry. Data are shown as the means±S.D. (n=6 for each genotype). The statistical significance is indicated by the asterisks (one-way ANOVA with Dunnett's multiple comparison test relative to the Pink1B9 mutant)
Genetic epistasis analysis of parkin and Trap1
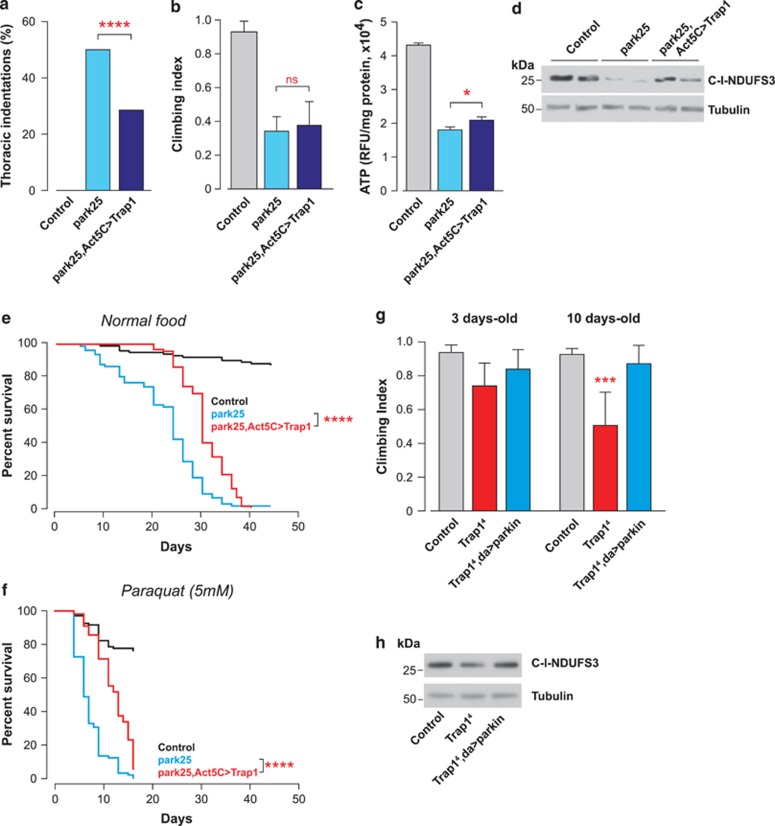
Upon mitochondrial damage, PINK1 has been shown to recruit Parkin, a cytosolic E3 ubiquitin ligase, to damaged mitochondria.26, 27 Parkin recruitment to damaged mitochondria has been proposed to cause the ubiquitination of the profusion factor Mfn to label terminally damaged mitochondria for autophagic degradation,28 a process that has been defined as organellar quality control.3, 29 The importance of Parkin as a downstream effector of PINK1 was confirmed in a series of genetic studies in Drosophila, which showed that the expression of parkin could rescue the phenotype of Pink1 mutant flies.18, 19 To address the epistatic relationship between parkin and Trap1, we first tested whether Trap1 expression could also rescue the parkin mutant phenotype. The expression of Trap1 partially rescued the degree of thoracic indentations (Figure 7a) but not the impaired motor performance in the parkin mutants (Figure 7b). We also observed that the expression of Trap1 had a modest effect on the suppression of the ATP loss present in parkin mutants (Figure 7c) and caused a recovery in the mitochondrial protein content, indirectly assessed through the analysis of the levels of a complex I subunit (Figure 7d). Moreover, Trap1 expression in parkin mutants led to an increased level of survival in flies maintained on normal food (Figure 7e) and on paraquat-containing food (Figure 7f). We next tested whether parkin expression could also rescue the Trap1 mutant phenotype. When we expressed parkin in Trap14 mutant flies, we observed a significant suppression of their climbing defects (Figure 7g), as well as a marked recovery of the levels of mitochondrial complex I (Figure 7h). Taken together, these results show that the enhanced expression of Trap1 can partially compensate for the loss of parkin, and reciprocally, the enhanced expression of parkin can also ameliorate some of the Trap1 mutant-associated phenotypes. This suggests that both Trap1 and parkin act in a parallel pathway that modulates mitochondrial competence.
Figure 7.
Epistatic relationship between Trap1 and parkin. (a) Trap1 expression decreases the degree of thoracic indentations in parkin mutant flies, park25. Thoracic indentations were counted up to 24 h after eclosion (n>250 for each genotype). The asterisks indicate the significance (χ2, two-sided, alpha<0.05). (b) The expression of Trap1 fails to rescue the climbing defect of the park25 mutants. 3-day-old flies with the indicated genotypes were tested using a standard climbing assay (mean±S.D., n>120 flies per genotype). (c) Trap1 expression increases the levels of ATP in the park25 mutants. The ATP levels were measured using a bioluminescent assay. Data are shown as the means±S.D. (n=4 for each genotype). The statistical significance is indicated by the asterisks (one-way ANOVA with Dunnett's multiple comparison test relative to park25). (d) Trap1 expression restores the mitochondrial complex I protein levels in the park25 mutant flies. Whole-fly protein lysates were used for western blotting analysis with the indicated antibodies. (e) Trap1 expression enhances the lifespan of the park25 mutants. Fly viability was scored over a period of 40 days using a minimum of 100 flies per genotype. The statistical significance is indicated by the asterisks (log-rank, Mantel–Cox test). (f) Expression of Trap1 increases the resistance of park25 mutants to paraquat-induced stress. Fly viability was scored over a period of 15 days using a minimum of 80 flies per genotype. The statistical significance is indicated by the asterisks (log-rank, Mantel–Cox test). (g) The expression of parkin rescues the climbing defects of Trap14 mutants. Flies with the indicated genotypes and ages were tested using a standard climbing assay (mean±S.D., n>60 flies per genotype). The asterisks indicate significant values (two-way ANOVA with the Bonferroni multiple comparison test). (h) parkin expression restores the mitochondrial complex I protein levels in Trap14 mutant flies. Whole-fly protein lysates from 20-day-old flies were used for western blotting analysis with the indicated antibodies
Discussion
We have characterised the Drosophila orthologue of the TRAP1 chaperone and found that, similar to its mammalian counterpart, it localises to the mitochondria. Using a loss-of-function approach, through the generation of Trap1 mutant flies that lack Trap1 expression, we determined that its loss compromises mitochondrial function. Mutations in either Pink1 or parkin influence mitochondrial dynamics and function, causing an impairment of fission, which affects male fertility by compromising the normal development of mitochondrial derivatives (also known as nebenkern).18, 19, 30, 31 During spermatogenesis, Drosophila mitochondria undergo significant morphological changes that require fusion to form a large spherical nebenkern and subsequent fission when the nebenkern unfurls to yield two mitochondrial derivatives.32 The Trap1 mutants developed normally to adulthood and were both viable and fertile, suggesting that their mitochondrial defects do not compromise the mitochondrial fission and fusion processes required for normal spermatogenesis.
Although Trap1 mutants show no defects in development, they had a decreased lifespan, progressive locomotor defects and a decrease in the brain dopamine levels. Taken together, these results suggest that this mitochondrial chaperone is important to minimise the age-dependent decline of mitochondrial function in post-mitotic cells, such as muscle cells and neurons, which rely heavily on mitochondrial function. The milder phenotype of Trap1 mutants compared with the Pink1 mutants suggest that other effectors of molecular quality control acting downstream of Pink1 can compensate for the potential defects caused by the loss of Trap1. In this respect, the serine protease HtrA2, a downstream target of PINK19, 33 involved in mitochondrial molecular quality control,34 could potentially partially compensate for any disturbances in protein folding in the intermembrane space of mitochondria.
The expression of Trap1 was sufficient to enhance the climbing performance of aged flies and promote resistance to oxidative stress (Figure 3). Mitochondria in skeletal muscles are highly specialised towards energy production for contractile activity,35 and therefore, the expression of this chaperone might contribute to the maintenance of mitochondrial performance in tissues with high-energy demand, such as skeletal muscles, thereby enhancing the motor performance.
Our results indicate that the neuronal expression of Trap1 in Pink1 mutants is sufficient to suppress muscle degeneration. Mitochondria have been shown to be concentrated in both the presynaptic nerve terminal and the postsynaptic endplates in muscle,36 indicating that a decline in mitochondrial bioenergetic function may affect synaptic transmission and result in a loss of NMJ innervation. Our data suggest that mitochondrial dysfunction at the presynaptic terminal of the NMJ is a major determinant of the pathologies of the skeletal muscle present in the Pink1 mutants, and that the compromise of the motor neuron function in the Pink1 mutants causes muscle dysfunction; therefore, is likely to precede muscle degeneration.
In Drosophila, the expression of parkin can rescue the phenotype of Pink1 mutants, presumably by enhancing the organellar quality control through autophagic disposal of defective mitochondria.29 Our data suggest that Trap1 expression can partially compensate for the mitochondrial defects present in parkin mutant flies. Given that Trap1 is likely to act as a molecular chaperone in the clearance of misfolded proteins, its expression may lead to enhanced molecular quality control in mitochondria, and therefore decrease the demand for organellar quality control as a clearance mechanism for mitochondria overwhelmed by excessive protein misfolding. Enhanced expression of Trap1 may in turn decrease the demand on organellar quality control in parkin mutants, leading to a decrease in the degree of dysfunctional mitochondria in these insects. We also observed that the expression of parkin was capable of suppressing some of the phenotypic consequences associated with Trap1 mutations. This suggests that both the molecular and organellar pathways for mitochondrial quality control work in parallel, and the enhancement of either one of these can relieve pressure on the other and contribute to a better mitochondrial health.
Materials and Methods
Antibodies
The following primary antibodies were used in this study: anti-TRAP1 mouse IgG (clone 30) (612342), BD Transduction Laboratories, Franklin Lakes, NJ, USA; anti-NDUFS3 mouse monoclonal (ab14711), Mitosciences, Eugene, OR, USA; anti-TH rabbit polyclonal (AB152), Millipore (Billerica, MA, USA); and anti-α-tubulin IgG1 clone B-5-1-2, Sigma-Aldrich (St Louis, MO, USA).
Genetics and Drosophila strains
Transgenic strains with UAS-Trap1 were generated by P-element-mediated transformation. Transgenic flies were generated at BestGene Inc. (Chino Hills, CA, USA). Three independent transgenic lines were obtained, and all were tested for protein expression. Pink1B9, park25, UAS parkin62B and da-GAL4 flies were a gift from A Withworth (MRC, Centre for Developmental and Biomedical Genetics, University of Sheffield, Sheffield, UK). Trap14 line was kindly provided by Dr. JE Treisman (New York University School of Medicine, NY, USA). w1118, Act5C-GAL4, enGAL4 and elav-GAL4 lines were obtained from Bloomington Stock Centre (Indiana University, Bloomington, IN, USA). Fly stocks and crosses were maintained on standard cornmeal agar media at 25 °C. The genotypes of the flies used in each individual figure are listed in Supplementary Table S1.
Molecular biology
Expression constructs encoding Drosophila Trap1 sequence were generated by polymerase chain reaction using appropriate adaptor primers. The PCR fragments were cloned into the GAL4-responsive pUAST expression vector for the generation of transgenic fly strains.
HPLC measurement of dopamine and serotonin
For sample preparation, 15 male fly heads (20 days old) were dissected under mild CO2 anaesthesia, and quickly frozen in dry ice. The heads were then homogenised in 50 μl chilled 0.1 M perchloric acid using a motorised, hand-held tissue homogeniser. The chilled homogenates of fly heads were filtered through a low-binding Durapore (0.22 μm) PVDF membrane using Ultrafree-MC centrifugal devices. The dopamine and serotonin measurements were performed immediately after sample preparation. Supernatant fluid (30 μl) was eluted at a flow rate of 50 μl/min through a 150 × 1.0 mm C18 column. The mobile phase contained 50 mM phosphoric acid, 8 mM NaCl, 0.1 mM EDTA, 12.5% (v/v) methanol, 500 mg/l octanesulfonic acid (OSA) (pH 6.0). Analysis was performed using an Alexys LC-EC system equipped with a DECADE II electrochemical detector.
Immunofluorescence and confocal microscopy
Third instar larvae were dissected, fixed in 4% paraformaldehyde, stained with the anti-TRAP1 antibody and incubated with Hoechst 33342. Wing imaginal discs were mounted on glass slides with Vectashield (Vector Laboratories, Burlingame, CA, USA) and imaged on a Zeiss LSM510 confocal microscope.
Statistical analysis
The data are presented as mean values, and the error bars indicate±S.D. Inferential statistical analysis was performed using the Prism and StatMate software packages (www.graphpad.com). The significance level is indicated as **** for P<0.0001, *** for P<0.001, ** for P<0.01, * for P<0.05 and ns for P>0.05.
ATP assays
Five male flies (10 or 20 days old) were homogenised in 100 μl of 6 M guanidine-HCl in extraction buffer (100 mM Tris and 4 mM EDTA, pH 7.8) to inhibit ATPases.
Homogenised samples were subjected to rapid freezing in liquid nitrogen, followed by boiling for 5 min. Samples were cleared by centrifugation, and supernatant was diluted (1/50) with extraction buffer and mixed with a luminescent solution (CellTiter-Glo Luminescent Cell Viability Assay, Promega, Fitchburg, WI, USA). Luminescence was measured on an Infinite M200Pro multifunction reader (TECAN, Männedorf, Switzerland). The relative ATP levels were calculated by dividing the luminescence by the total protein concentration, which was determined by the Bradford method.
Climbing assays
The climbing assays were performed as previously described,37 using a countercurrent apparatus developed initially for phototaxis experiments. A total of 20 male flies were placed into the first chamber, taped to the bottom and then given 20 s to climb a distance of 10 cm. Those flies that successfully climbed 10 cm or beyond in 20 s were then shifted to a new chamber, and both sets of flies were given another opportunity to climb the 10-cm distance. This procedure was repeated a total of five times. After five trials, the number of flies in each chamber was counted. At least 120 flies were used for each genotype tested.
Respirometry analysis
Mitochondrial respiration was assayed at 37 °C by high-resolution respirometry using an OROBOROS Oxygraph. DatLab software package (OROBOROS, Innsbruck, Austria) was used for the data acquisition (2-s-time intervals) and analysis, including the calculation of the time derivative of the oxygen concentration, signal deconvolution dependent on the response time of the oxygen sensor and correction for instrumental background oxygen flux. Respiration was assayed by homogenising two flies using a pestle in MiR05 respiration buffer (20 mM HEPES, 10 mM KH2PO4, 110 mM sucrose, 20 mM taurine, 60 mM K-lactobionate, 0.5 mM EGTA, 3 mM MgCl2, 1 g/l fatty acid-free BSA). The basal respiration rates were assayed in the absence of exogenous respiration substrates. For coupled (state 3) assays, complex I activity was assayed in MiR05 respiration buffer in the presence of 2 mM malate, 10 mM glutamate and 5 mM ADP. Complex II was assayed in respiration buffer supplemented with 1 mM rotenone, 10 mM succinate and 5 mM ADP. ADP was not added for the measurement of respiration in uncoupled mitochondria obtained by one freeze (liquid nitrogen)–thaw cycle of the fly tissue.
Lifespan analysis
Groups of 10 newly eclosed males of each genotype were placed into separate vials with food and maintained at 25 °C. The flies were transferred to vials containing fresh food every 2–3 days, and the number of dead flies was recorded. The data are presented as Kaplan–Meier survival distributions, and the significance was determined by log-rank tests.
Drug treatments
All the drugs used in this study were incorporated into the fly food. The concentrations of each of the drugs in the food were the following: 5 mM for paraquat, 600 μM for rotenone and 50 mM for antimycin A. The newly eclosed males were placed with normal food in groups of 10 for 1 day prior to their transfer to vials containing drug. The flies were transferred to new vials every 2–3 days, and the number of dead flies was recorded. The assay was completed after all the flies died. A minimum of 80 flies per genotype were analysed. The results are presented as Kaplan–Meier survival distributions, and the significance was determined by log-rank tests.
Preparation of Drosophila protein lysates and western blot analysis
Single adult flies (3, 10 or 20 days old) were homogenised using a motorised pestle in lysis buffer (0.1% Triton-X100, 10 mM EDTA, 1 mM DTT, 100 mM KCl, 20 mM HEPES (pH 7.5), 5% glycerol, 1 μg/ml chymostatin, 1 μg/ml leupeptin, 1 μg/ml pepstatin A and 1 μg/ml antipain). The lysates were subsequently cleared by centrifugation. The proteins were separated on 12 or 10% SDS-PAGE gels and transferred to Immobilon PVDF membranes (Millipore). The membranes were blocked in 5% milk and then incubated with the indicated primary antibody prior to incubation with the appropriate HRP-conjugated secondary antibody. The antibody complexes were visualised by enhanced chemiluminescence (ECL). The results are representative of at least three biological replicates per genotype.
RNA extraction and quantitative real-time RT-PCR
The isolation of total RNA from three adult flies per sample was performed using the RNeasy Mini Kit (Qiagen, Hilden, Germany). Quantitative real-time RT-PCR was performed on an M × 4000 (Stratagene, La Jolla, CA, USA) real-time cycler using the QuantiTect SYBR Green RT-PCR system (Qiagen). Primers used (QuantiTect Primer Assay, Qiagen) were Cat. No. QT00967393 (actin 79B, used as a housekeeping gene), Cat. No. QT00940520 (Trap1), Cat. No. QT00501130, (Vha16-1) and Cat. No. QT00940527, (Bap170). The quantification was performed using the comparative Ct method.38
Heat stress
For heat sensitivity, 4-day-old male flies were divided into groups of 15 and placed in empty vials (no food or water). The flies were maintained at 25 °C for 30 min, after which the thermo stress was applied by placing the vials in a water bath at 37 °C for 1 h. This step was followed by a 30-min recovery at 25 °C and a new heat stress for 1 h at 37 °C. The flies were then transferred to vials containing fresh food and maintained at 25 °C. The number of living flies was recorded 24 h later. A total of 100 flies per genotype were tested for thermo stress.
Acknowledgments
We would like to thank Alex Whitworth, Dr. JE Treisman and the Bloomington Drosophila Stock Centre for the fly stocks, and Jalpa Parmar for the fly food preparation. We thank Inês P Castro, Nicoleta Moisoi and Roberta Tufi for reagents and advice. We also thank Dr. Aaron Voigt for sharing unpublished data. This work is funded by the Medical Research Council, UK.
Glossary
- ATP
adenosine triphosphate
- PD
Parkinson's disease
- ROS
reactive oxygen species
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by G Melino.
Supplementary Material
References
- de Castro IP, Martins LM, Tufi R. Mitochondrial quality control and neurological disease: an emerging connection. Expert Rev Mol Med. 2010;12:e12. doi: 10.1017/S1462399410001456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MJ, Tatsuta T, Langer T. Quality control of mitochondrial proteostasis. Cold Spring Harb Perspect Biol. 2011;3:a007559. doi: 10.1101/cshperspect.a007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsuta T, Langer T. Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J. 2008;27:306–314. doi: 10.1038/sj.emboj.7601972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes CM, Ron D. The mitochondrial UPR - protecting organelle protein homeostasis. J Cell Sci. 2010;123:3849–3855. doi: 10.1242/jcs.075119. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Klionsky DJ. Mitochondria removal by autophagy. Autophagy. 2011;7:297–300. doi: 10.4161/auto.7.3.14502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plun-Favreau H, Klupsch K, Moisoi N, Gandhi S, Kjaer S, Frith D, et al. The mitochondrial protease HtrA2 is regulated by Parkinson's disease-associated kinase PINK1. Nat Cell Biol. 2007;9:1243–1252. doi: 10.1038/ncb1644. [DOI] [PubMed] [Google Scholar]
- Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5:e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua G, Zhang Q, Fan Z. Heat shock protein 75 (TRAP1) antagonizes reactive oxygen species generation and protects cells from granzyme M-mediated apoptosis. J Biol Chem. 2007;282:20553–20560. doi: 10.1074/jbc.M703196200. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Shima G, Aiuchi T, Horie M, Hori K, Nakajo S, et al. Involvement of tumor necrosis factor receptor-associated protein 1 (TRAP1) in apoptosis induced by beta-hydroxyisovalerylshikonin. J Biol Chem. 2004;279:42503–42515. doi: 10.1074/jbc.M404256200. [DOI] [PubMed] [Google Scholar]
- Montesano Gesualdi N, Chirico G, Pirozzi G, Costantino E, Landriscina M, Esposito F. Tumor necrosis factor-associated protein 1 (TRAP-1) protects cells from oxidative stress and apoptosis. Stress. 2007;10:342–350. doi: 10.1080/10253890701314863. [DOI] [PubMed] [Google Scholar]
- Xiang F, Huang YS, Shi XH, Zhang Q. Mitochondrial chaperone tumour necrosis factor receptor-associated protein 1 protects cardiomyocytes from hypoxic injury by regulating mitochondrial permeability transition pore opening. FEBS J. 2010;277:1929–1938. doi: 10.1111/j.1742-4658.2010.07615.x. [DOI] [PubMed] [Google Scholar]
- Costantino E, Maddalena F, Calise S, Piscazzi A, Tirino V, Fersini A, et al. TRAP1, a novel mitochondrial chaperone responsible for multi-drug resistance and protection from apoptotis in human colorectal carcinoma cells. Cancer Lett. 2009;279:39–46. doi: 10.1016/j.canlet.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Landriscina M, Laudiero G, Maddalena F, Amoroso MR, Piscazzi A, Cozzolino F, et al. Mitochondrial chaperone Trap1 and the calcium binding protein Sorcin interact and protect cells against apoptosis induced by antiblastic agents. Cancer Res. 2010;70:6577–6586. doi: 10.1158/0008-5472.CAN-10-1256. [DOI] [PubMed] [Google Scholar]
- Lu B, Vogel H. Drosophila models of neurodegenerative diseases. Annu Rev Pathol. 2009;4:315–342. doi: 10.1146/annurev.pathol.3.121806.151529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- Butler EK, Voigt A, Lutz AK, Toegel JP, Gerhardt E, Karsten P, et al. The mitochondrial chaperone protein TRAP1 mitigates alpha-Synuclein toxicity. Plos Genet. 2012;8:e1002488. doi: 10.1371/journal.pgen.1002488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C, La Vecchia C, Nicotera P. Paraquat and Parkinson's disease. Cell Death Differ. 2010;17:1115–1125. doi: 10.1038/cdd.2009.217. [DOI] [PubMed] [Google Scholar]
- Wills J, Jones J, Haggerty T, Duka V, Joyce JN, Sidhu A. Elevated tauopathy and alpha-synuclein pathology in postmortem Parkinson's disease brains with and without dementia. Exp Neurol. 2010;225:210–218. doi: 10.1016/j.expneurol.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Pimenta de Castro I, Costa AC, Lam D, Tufi R, Fedele V, Moisoi N, et al. Genetic analysis of mitochondrial protein misfolding in Drosophila melanogaster. Cell Death Differ. 2012;19:1308–1316. doi: 10.1038/cdd.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziviani E, Tao RN, Whitworth AJ. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc Natl Acad Sci USA. 2010;107:5018–5023. doi: 10.1073/pnas.0913485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro IP, Martins LM, Loh SH. Mitochondrial quality control and Parkinson's disease: a pathway unfolds. Mol Neurobiol. 2011;43:80–86. doi: 10.1007/s12035-010-8150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riparbelli MG, Callaini G. The Drosophila parkin homologue is required for normal mitochondrial dynamics during spermiogenesis. Dev Biol. 2007;303:108–120. doi: 10.1016/j.ydbio.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Deng H, Dodson MW, Huang H, Guo M. The Parkinson's disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci USA. 2008;105:14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MT.SpermatogenesisIn: Arias MBaAM, (ed)The Development of Drosophila melanogaster 71–147.1993
- Tain LS, Chowdhury RB, Tao RN, Plun-Favreau H, Moisoi N, Martins LM, et al. Drosophila HtrA2 is dispensable for apoptosis but acts downstream of PINK1 independently from Parkin. Cell Death Differ. 2009;16:1118–1125. doi: 10.1038/cdd.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisoi N, Klupsch K, Fedele V, East P, Sharma S, Renton A, et al. Mitochondrial dysfunction triggered by loss of HtrA2 results in the activation of a brain-specific transcriptional stress response. Cell Death Differ. 2009;16:449–464. doi: 10.1038/cdd.2008.166. [DOI] [PubMed] [Google Scholar]
- Powers SK, Wiggs MP, Duarte JA, Zergeroglu AM, Demirel HA. Mitochondrial signaling contributes to disuse muscle atrophy. Am J Physiol Endocrinol Metab. 2012;303:E31–E39. doi: 10.1152/ajpendo.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Figueras H, Price SD, Peng YY. Dense-cored vesicles, smooth endoplasmic reticulum, and mitochondria are closely associated with non-specialized parts of plasma membrane of nerve terminals: implications for exocytosis and calcium buffering by intraterminal organelles. J Comp Neurol. 1999;403:378–390. doi: 10.1002/(sici)1096-9861(19990118)403:3<378::aid-cne7>3.0.co;2-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci USA. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.