Abstract
Background
IgE cross-linking triggers many cellular processes that drive allergic disease. While the role of IgE in mediating allergic responses is best described on basophils and mast cells, expression of the high-affinity IgE receptor on other innate immune cells, including monocytes, suggests that it may impact the function of these cells in allergic environments.
Objectives
To determine the effect of IgE cross-linking on the function of human monocytes.
Methods
Monocytes purified from healthy donor blood samples were cultured for 4–96 hr with media alone, a cross-linking anti-IgE antibody, or control IgG. Surface CD14 and CD64 expression and secreted cytokine concentrations were determined. Monocyte function was determined by assessing: 1) phagocytosis of E. coli or apoptotic HEp2 cells and 2) killing of intracellular E. coli. Select experiments were performed on monocytes obtained from participants with elevated versus normal serum IgE concentrations.
Results
IgE cross-linking on monocytes increased CD14 expression and induced secretion of TNF-á, IL-6, and autoregulatory IL-10. These effects were greatest in individuals with elevated serum IgE concentrations. In contrast, IgE cross-linking reduced CD64 expression and significantly impaired phagocytic function without disrupting the capacity of monocytes to kill bacteria.
Conclusion
IgE cross-linking drives monocyte pro-inflammatory processes and autoregulatory IL-10 in a serum IgE-dependent manner. In contrast, monocyte phagocytic function is critically impaired by IgE cross-linking. Our findings suggest that IgE cross-linking on monocytes may contribute to allergic disease by both enhancing detrimental inflammatory responses and concomitantly crippling phagocytosis, a primary mechanism utilized by these cells to resolve inflammation.
Keywords: Monocyte, IgE, FcεRI, IgE cross-linking, Allergy, Pro-inflammatory, Autoregulatory, Phagocytosis, Apoptotic debris
Introduction
Despite the significant healthcare burden of atopic disease in the United States1–3, the mechanisms underlying pathogenesis are incompletely understood. IgE plays a critical role in mediating atopic disease: significant correlations between serum IgE concentration and disease have been demonstrated in allergic asthma and atopic dermatitis4, 5. Indeed, serum IgE concentration represents a diagnostic criterion for these conditions6, 7. Therapies that reduce serum IgE concentration, such as omalizumab, result in clinical improvement in patients with severe atopic disease8, 9.
IgE exerts its effect on atopic disease via the high-affinity IgE receptor, FcεRI10. Upon cross-linking of allergen-specific IgE by a multivalent allergen the receptor is activated, resulting in intracellular signaling and cell-type specific effects10. Surface FcεRI expression on several immune cells, including basophils and dendritic cells, is increased in individuals with atopic disease and correlates with serum IgE concentration11, 12.
FcεRI mediates IgE-dependent pathways in several cell types, and its role is best characterized in basophils and mast cells. In these cells, IgE cross-linking induces release of inflammatory mediators including histamine, prostaglandins, and cytokines13, 14. FcεRI plays an important role on myeloid and plasmacytoid dendritic cells (mDC and pDC, respectively) as well12; IgE cross-linking on these cells induces pro-inflammatory cytokine secretion15, 16. In pDCs, IgE- and toll-like receptor(TLR)9-mediated pathways have been shown to oppose one other16; allergic stimulation via this pathway also interferes with in vitro pDC antiviral responses17.
FcεRI is also expressed on monocytes and is increased in individuals with atopic diseases11, 18, 19. Present in high numbers at mucosal surfaces and in the skin both during steady state and inflammatory conditions, such as allergen exposure, monocytes and their progeny are poised to influence allergic responses20–25. Monocytes play many important roles during inflammatory processes, including regulating immune responses through the release of cytokines26, and resolving inflammation though phagocytosis of cellular debris27–29. Expression of specific surface molecules can also reflect functional properties of monocytes. CD14 contributes to TLR4 signaling and is thus important for immune responses to lipopolysaccharide (LPS)30. CD64, the high affinity IgG receptor, contributes to phagocytosis; its expression reflects monocyte phagocytic function31, 32.
Despite the expression of FcεRI on monocytes from both atopic and non-atopic individuals11, 18, 19 and the importance of these cells in inflammatory processes, the consequences of FcεRI activation on monocytes remain incompletely characterized. Stimulation of FcεRI has been shown to induce activation of NF-kB and secretion of TNFα, IL-6, and MCP-1 in human monocytes33, 34. In addition, FcεRI cross-linking of GM-CSF and IL-4 treated monocytes in vitro has been shown to promote IL-10 secretion and differentiation into macrophages35.
We set out to define the impact of IgE cross-linking on the function of human monocytes and to determine whether serum IgE concentration impacts the magnitude of these responses. Monocytes, by virtue of their expression of FcεRI, inflammatory capacity, and prevalence in mucosal tissues, have the potential to significantly influence allergic inflammation. Determining how IgE cross-linking impacts monocyte function will lead to a better understanding of the role of this important cell type in allergic processes and may reveal critical pathways that contribute to the pathogenesis of allergic disease.
Methods
Monocyte Purification
Leukocyte-enriched blood samples were obtained from a local blood bank and diluted 1:1 (vol/vol) with PBS (GIBCO, Grand Island, NY; supplemented with 2% heat-inactivated FCS and 2 mM EDTA). For select experiments, blood was drawn from human donors into tubes containing acid citrate dextrose. Peripheral blood mononuclear cells (PBMC) were isolated by centrifugation with Ficoll-Paque (GE Healthcare, Uppsala, Sweden) and monocytes were purified using the EasySep Negative Selection Human Monocyte Enrichment Kit (Stemcell Technologies, Vancouver, Canada). Purity ranged from 85%–95%.
Monocyte culture
Isolated monocytes were cultured in complete RPMI 1640 media (GIBCO; supplemented with 10% heat-inactivated FCS, 1% penicillin-streptomycin, 1% Na pyruvate, 1% glutamate, 1% HEPES buffer solution, 1% non-essential amino acids, and 100 mM β-mercaptoethanol) at a concentration of 1×106 monocytes/ml. Rabbit anti-human-IgE (αIgE) or rabbit IgG (IgG) (1 or 10 µg/ml; Bethyl Laboratories, Montgomery, TX) was added to monocyte cultures as indicated. For select experiments, F(ab)’2 fragments derived from αIgE and IgG antibodies (GenScript, Piscataway, NJ) were added at 10 µg/ml. For cytokine neutralization experiments, mouse anti-human-IL-10, -IL-6, -TNFα, -IL-10Rα, -IL-6R, -TNFRI or IgG1 or IgG2b isotype controls (R&D Systems, Minneapolis, MN) were added to monocyte cultures at 10 µg/ml (anti-TNFα, IgG1) or 5 µg/ml (others). Time points reflect distinct cultures for indicated times, with no removal or replacement of media or antibodies.
Flow cytometry
The following fluorochrome-conjugated anti-human antibodies were used: CD14-V450, CD64-FITC, CD64-PE, FcεRI-PE (BD Biosciences, San Diego, CA). Cells were rinsed with PBS and stored in Streck Cell Preservative (Streck, Omaha, NE) at 4° C prior to staining. Preserved samples we re washed, resuspended in 100 µl PBS and incubated with 2.5 µl of each antibody for 30 min at 4° C. Cells were then washed and resuspended in 1% paraformaldehyde. Samples were subsequently acquired on a BD LSR II flow cytometer (BD Biosciences, San Diego, CA) and analyzed with FlowJo software (Tree Star, Ashland, OR). Mean fluorescence intensity for CD14+ cells was determined and subsequently converted to mean equivalent standard fluorescence (MESF), using Ultra Rainbow Calibration Particles (Spherotech, Lake Forest, IL) and FlowJo.
Cytokine analysis
Supernatants were harvested and stored at −80° Cels ius (C) until use. Concentrations of TNFα and IL-10 in monocyte culture supernatants were measured by ELISA using Legend Max Human ELISA kits (BioLegend, San Diego, CA). IL-6 concentration was determined using READY-SET-GO! Human IL-6 ELISA kit (eBioscience, San Diego, CA).
Phagocytosis assays
BODIPY FL-conjugated E. coli BioParticles (Molecular Probes, Eugene, OR) were opsonized with E. coli Opsonizing Reagent (Molecular Probes) according to manufacturer instructions, added to monocyte cultures at 10 bacteria/monocyte and incubated at 37° C for 2 hr. For microscopy, mo nocytes were washed and mounted onto slides with a Cytospin 4 Centrifuge (Thermo Scientific, Waltham, MA). Slides were fixed with methanol and coverslipped with Vectashield Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA). Images were acquired on a Deltavision Deconvolution Microscope (Applied Precision, Issaquah, WA) for 50 cells per sample. Internalized particles were counted in ImageJ using a macro written by D Pyle. For flow cytometry, monocytes were washed, stained for CD14, and acquired on the LSR II.
For selected experiments, HEp2 cells were grown in complete DMEM media (GIBCO; supplemented as above). Cells were incubated with 5 µM Carboxyfluorescein Succinimidyl Ester (CFSE) (Molecular Probes) for 10 minutes, washed extensively with media, and incubated for 24 hr with 1 µg/ml Actinomycin D (Sigma, St. Louis, MO) to induce apoptosis. Apoptotic cells were washed, opsonized with 100 µg/ml whole human IgG (Bethyl Laboratories, Montgomery, TX) and added to monocyte cultures at a 1:1 ratio. After 4 hr, cells were washed and stained for CD14 for flow cytometry analysis. In some experiments, CD14+ monocytes were sorted on a BD FACSAria flow cytometer (BD Biosciences, San Diego, CA) based on CFSE fluorescence and imaged as above.
Bacterial killing assays
E. coli (DH5α strain, a kind gift from David Farrar) were grown in LB media (Sigma) and added to monocyte cultures at 10 CFU/monocyte for 45 min. Gentamicin (Amresco, Solon, OH) was added (100 µg/ml) and monocytes were harvested immediately (0 hr) or after 16 hr. Monocytes were washed extensively, counted, and lysed in sterile deionized H2O. Lysates were plated on LB agar (Sigma) overnight and colonies were counted. CFU/Cell was determined for each harvest and % of bacteria killed was calculated as %Killed = (CFU/Cell0hr − CFU/Cell16hr) ÷ CFU/Cell0hr × 100. This calculation reflects bacterial killing regardless of the amount of phagocytosis.
Patient recruitment
Individuals with a history of serum IgE concentration >100 U/ml were recruited for select experiments. Individuals with lower IgE were recruited as controls. All participants had positive skin test to ≥1 indoor allergen. Skin tests were performed36 and serum IgE levels were determined17 as previously described. This study was approved by the University of Texas Southwestern institutional review board. Written informed consent and assent were obtained.
Data analysis and Statistics
Data are presented as means ± SEM. For all data sets with N>8, Grubb’s test for outliers was applied with α=0.0001 and outliers were removed from analysis. Where indicated, data were normalized to the media condition for each experiment. For three or more conditions, one-way repeated measures ANOVA and pairwise Tukey’s post hoc comparisons were performed for each time point. For comparison of two conditions, paired or unpaired t tests were performed with Holm-Sidak correction where appropriate. For experiments comparing high vs. low IgE individuals, Pearson correlations between experimental results and log of serum IgE concentration were performed. p<.05 was considered significant. All statistical analyses were performed with GraphPad Prism versions 5 and 6.
Results
The majority of the assays reported hereafter were performed on monocytes from healthy human blood donors. To establish potential clinical impact, we performed certain assays on monocytes obtained from participants with either low (<100 U/ml) or high (>100 U/ml) serum IgE levels. Participant information is reported in Table I. The majority of participants in both groups had a history of asthma and all tested positive for at least one environmental allergen by skin test. There was no statistically significant difference in age or demographic characteristics between groups. As we have reported previously for pDCs from individuals with elevated IgE levels17, monocyte expression of FcεRI was significantly elevated in the high IgE group. Data from these experiments were compared for correlation with serum IgE concentration; these analyses are summarized in Table EI in the Online Repository.
Table I.
Participant Information
| Low IgE | High IgE | P value | |
|---|---|---|---|
| Number | 6 | 6 | n/a |
| Age | 23.0 (18–27) | 21.2 (14–44) | 0.71 |
| Gender | 3 Male, 3 Female | 4 Male, 2 Female | 1.00 |
| Ethnicity | 2 Black, 2 White, 2 Hispanic | 5 Black, 1 White | 0.16 |
| Atopy | |||
| -Asthma | 4 | 6 | 0.45 |
| -Allergic Rhinitis | 3 | 6 | 0.18 |
| -Atopic Dermatitis | 0 | 1 | 1.00 |
| -Skin Test (≥1 Positive) | 6 | 6 | n/a |
| -Skin Test (# of Positive) | 3.5 (1–7) | 5.8 (3–9) | 0.11 |
| Serum IgE (U/mL) | 50.2 (25–99) | 477.0 (134–1017) | 0.01 |
| Monocyte FcεRI (MESF) | 2951 (1592–4582) | 5171 (2011–8065) | 0.04 |
Demographic information, atopic status, serum IgE concentration, and monocyte FcεRI expression are presented for enrolled participants. P values for categorical values were calculated by Chi-square or Fisher’s Exact Test. Mean (range) and t test are shown for quantitative values.
IgE cross-linking alters monocyte surface marker expression
To determine the effect of IgE-mediated stimulation on functionally relevant monocyte surface markers, we analyzed the impact of IgE cross-linking on the surface expression of CD14 and CD64.
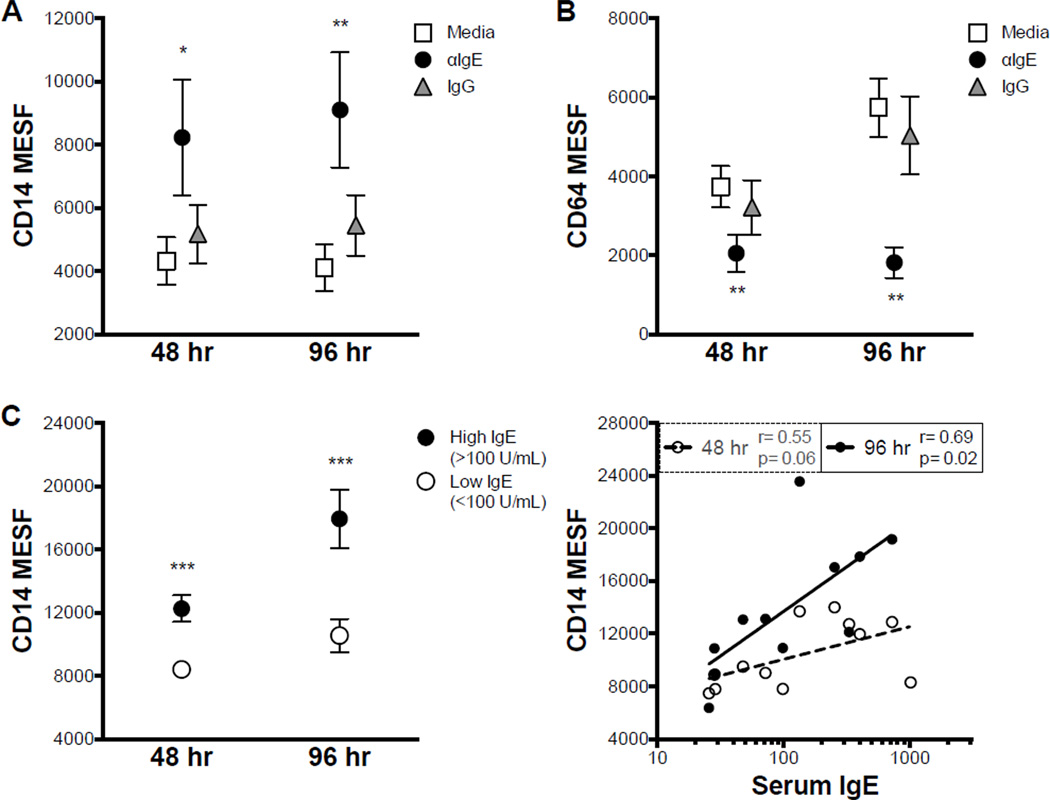
IgE cross-linking resulted in significant up regulation of CD14 expression at both time points measured (Fig. 1 A). In contrast, surface expression of CD64 was significantly diminished by IgE cross-linking (Fig. 1 B). An F(ab)’2 fragment of the IgE cross-linking antibody induced similar up regulation of CD14, indicating that the effects of the whole antibody were not mediated by Fcγ receptors (Online Repository Fig. E1 A).
Figure 1. IgE cross-linking up regulates surface CD14 and down regulates CD64.
A,B. Monocyte CD14 (A) and CD64 (B) expression after 48 or 96 hr culture in indicated conditions (N≥9). C. CD14 expression after IgE cross-linking on monocytes from individuals with low or high serum IgE (left); Pearson correlation between CD14 expression and serum IgE (right; N≥11). * p<.05, ** p<.01, *** p<.001 for αIgE vs. Media and IgG within time points (A,B) or High IgE vs. Low IgE (C).
Additionally, CD14 up regulation after IgE cross-linking was greater in individuals with elevated serum IgE (Fig. 1 C) at both time points. Moreover, the expression of CD14 after IgE cross-linking was positively correlated with serum IgE levels (Fig. 1 C) at 96 hr.
IgE cross-linking induces secretion of pro-inflammatory cytokines and autoregulatory IL-10
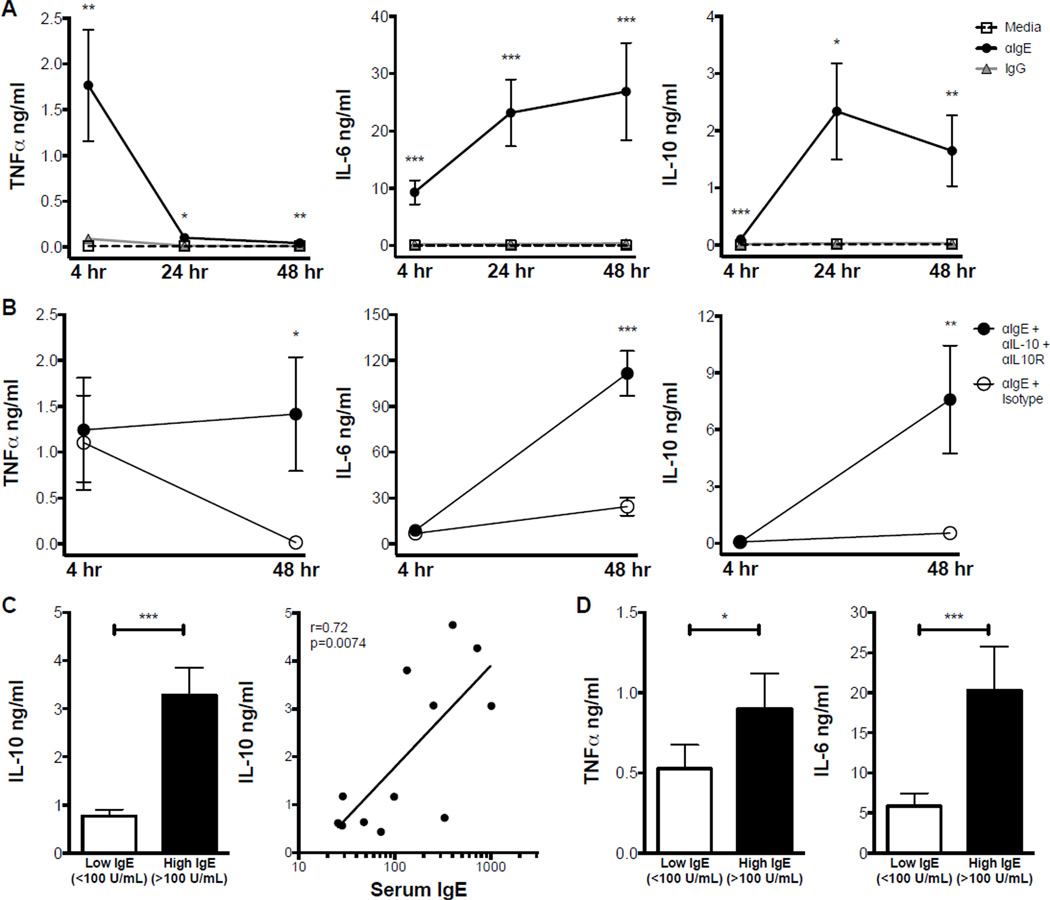
To determine the temporal patterns and interactions of cytokines induced by IgE cross-linking, we analyzed three cytokines commonly secreted by monocytes: TNFα, IL-6, and IL-10.
TNFα secretion was significantly increased by IgE cross-linking at all time points measured (Fig. 2 A, left). Interestingly, the concentration of TNFα induced by IgE cross-linking was greatest after 4 hr and diminished significantly by 24 hr. This reduction could reflect degradation of TNFα between 4 and 24 hr. IgE cross-linking induced robust IL-6 secretion at 4 and 24 hr; in contrast to TNFα, IL-6 levels were maintained at 48 hr (Fig. 2 A, middle). The F(ab)’2 fragment of the IgE cross-linking antibody induced similar secretion of IL-6 after 48 hr (Online Repository Fig. E1 B), confirming that effects of αIgE on monocytes are not Fcγ-mediated.
Figure 2. IgE cross-linking induces monocyte secretion of TNFá, IL-6, and autoregulatory IL-10.
A. TNFα (left), IL-6 (middle), and IL-10 (right) concentrations in indicated monocyte culture conditions after 4, 24, or 48 hr (N≥9). B. Similar data for monocytes cultured with αIgE in the presence of IL-10 and IL-10R neutralizing antibodies or isotype control (N=3). C. IL-10 concentration after 48 hr of IgE cross-linking on monocytes from individuals with low (<100 U/ml) or high (>100 U/ml) serum IgE (left) and corresponding Pearson correlation (right; N=12). D. Concentration of TNFα after 4 hr (left); IL-6 after 48 hr (right) of IgE cross-linking on monocytes from individuals with low vs. high serum IgE (N≥10). * p<.05, ** p<.01, *** p<.001 for αIgE vs. Media and IgG (A), Blocking Antibodies vs. Isotype (B), or High IgE vs. Low IgE (C,D).
IgE cross-linking also induced significant IL-10 secretion (Fig. 2 A, right). Notably, the greatest IL-10 concentrations were observed after 24 hr of IgE cross-linking and corresponded with lower TNFα concentrations. To evaluate a potential regulatory role of IL-10 on TNFα production, we used neutralizing antibodies against both IL-10 and its receptor in the presence of IgE cross-linking. The neutralizing antibodies were chosen such that they did not interfere with cytokine detection by ELISA. IL-10 blockade prevented the reduction in IgE-mediated TNFα secretion over time (Fig. 2 B, left), suggesting an autoregulatory role for IL-10. Interestingly, IL-10 blockade also led to a dramatic increase in IgE-mediated secretion of IL-6, as well as IL-10 itself (Fig. 2 B, middle and right). However, neutralization of TNFα and IL-6 did not affect IL-10 levels (Online Repository Fig. E2), suggesting that the induction of IL-10 by IgE cross-linking is not mediated by TNFα or IL-6.
Interestingly, this autoregulatory IL-10 secretion was increased in monocytes from individuals with elevated serum IgE (Fig. 2 C, left); in fact, IL-10 secretion after IgE cross-linking significantly correlated with serum IgE concentration (Fig. 2 C, right). While the increased autoregulatory IL-10 response in individuals with elevated serum IgE might predict reduced pro-inflammatory cytokine secretion, this was not the case. TNFα secretion upon IgE cross-linking was actually increased and IL-6 secretion was 4-fold higher in participants with elevated IgE (Fig. 2 D).
We next examined the effects of αIgE concentration on IgE-mediated surface marker expression and cytokine secretion. IgE cross-linking induced concentration dependent up regulation of CD14, down regulation of CD64, and secretion of IL-6, and IL-10, while TNFα secretion was similar at both concentrations (Online Repository Fig. E3). Subsequent experiments were performed using 10 µg/ml, as this concentration of anti-IgE induced the maximum effect.
IgE cross-linking impairs monocyte phagocytosis
Given the inflammatory nature of IgE-mediated monocyte cytokine secretion, we next explored the impact of IgE cross-linking on a critical monocyte function: phagocytosis.
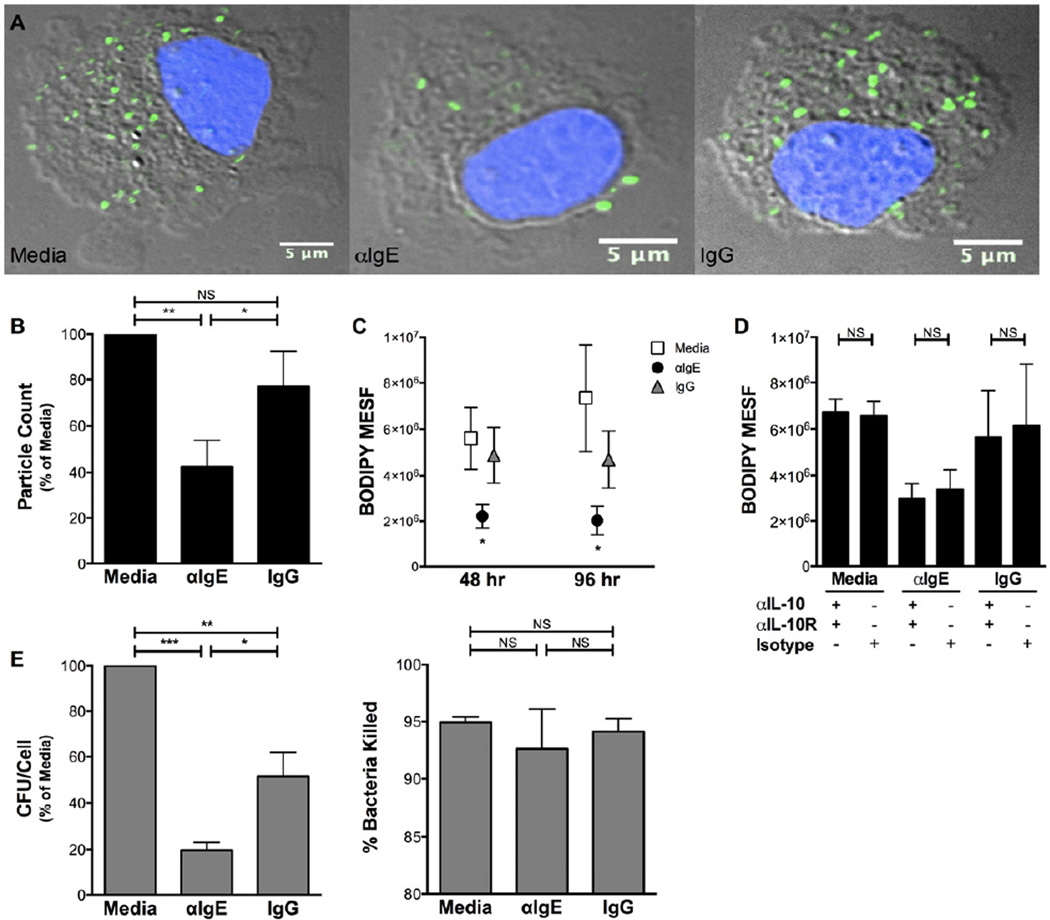
Utilizing microscopy to quantitate internalized bacteria, we determined that IgE cross-linking significantly impairs monocyte phagocytosis. Monocytes exposed to IgE cross-linking internalized fewer killed, opsonized bacteria compared to monocytes cultured in control conditions (Fig. 3 A). Quantitation of internalized bacteria revealed a significant reduction in phagocytosis after IgE cross-linking (Fig. 3 B). To extend these findings to multiple time points and assess the role of specific cytokines in phagocytosis, we utilized a higher throughput flow cytometry assay to similarly measure phagocytosis; this revealed a significant reduction in monocyte phagocytosis at both 48 and 96 hr after IgE cross-linking (Fig. 3 C). Interestingly, the impairment of phagocytosis induced by IgE cross-linking was not altered by neutralization of TNFα, IL-6, or IL-10 (Fig. 3 D, Online Repository Fig. E4), suggesting that this effect of IgE cross-linking is independent of IgE-mediated cytokine secretion and not subject to autoregulation by IL-10.
Figure 3. IgE cross-linking impairs monocyte phagocytic function.
A,B. Photomicrographs (A, grey=DIC, blue=DAPI, green=BODIPY-FL) and particle count per cell (B) of BODIPY-labeled bacteria phagocytosed by monocytes after 96 hr culture in indicated conditions (N=5). C. Fluorescence of internalized bacteria determined by flow cytometry for indicated conditions (N≥9). D. Effect of IL-10 neutralization on phagocytosis in indicated conditions (N=3). E. Internalization (CFU/Cell, left) of live bacteria after 48 hr culture; percent of internalized bacteria killed (right) after additional 16 hr incubation (N=4). * p<.05, ** p<.01, *** p<.001, NS p>.05 for αIgE vs. Media and IgG (C) or indicated comparisons (B,D,E).
To assess the extent of the functional impairment resulting from IgE cross-linking, we next investigated whether bacterial killing was altered in monocytes that had already engulfed bacteria. By comparing the number of live, internalized bacteria immediately after phagocytosis and after a 16 hr period, we were able to determine the ability of monocytes to kill internalized bacteria even when different numbers of bacteria were initially engulfed. Confirming our findings with killed, opsonized bacteria (Fig. 3 A–C), monocytes exposed to 48 hr of IgE cross-linking internalized fewer live, unopsonized bacteria than monocytes in control conditions (Fig. 3 E, left). Surprisingly, IgE cross-linking did not affect the killing of internalized bacteria after a further 16 hr incubation (Fig. 3 E, right), suggesting that the IgE-mediated functional deficit on monocytes is specific to phagocytosis.
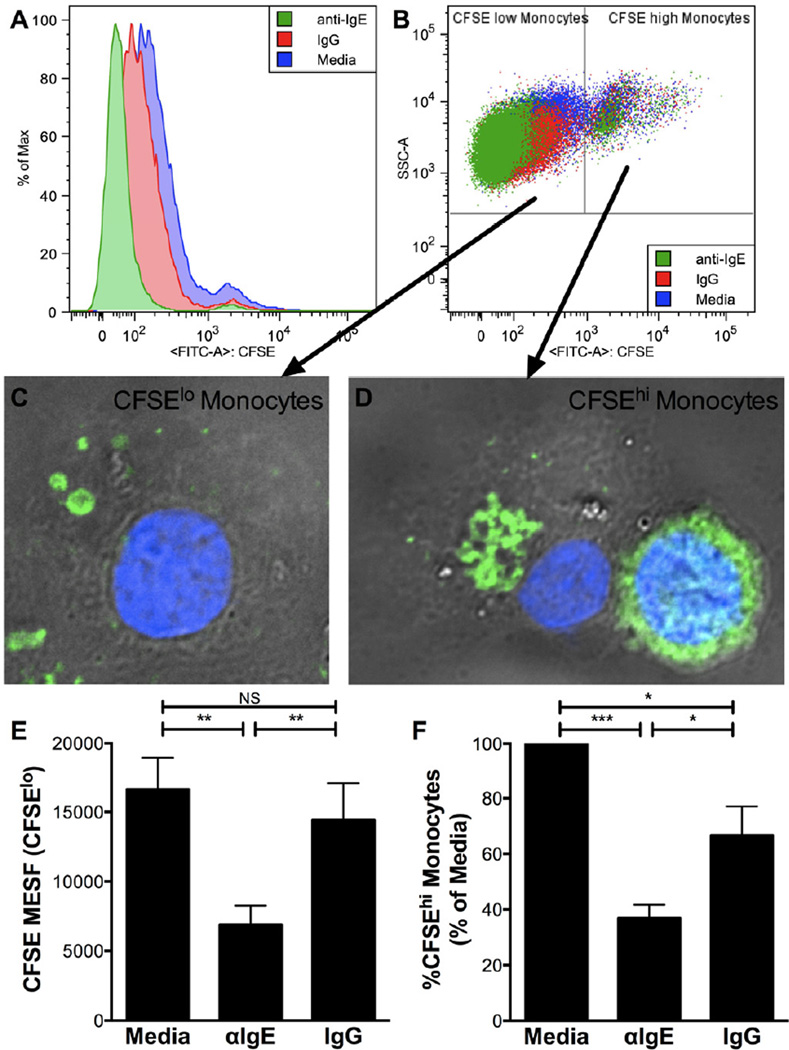
One important function of monocytes and their progeny is the clearing of apoptotic debris after infection or inflammation27–29. We next determined the effect of IgE cross-linking on phagocytosis of apoptotic cells. After exposure of monocytes to CFSE-labeled apoptotic cells, monocyte CFSE fluorescence was diminished in the IgE cross-linking condition, indicating diminished phagocytosis of apoptotic cells (Fig. 4 A). Interestingly, two distinct populations of monocytes were observed: CFSElowwhich contained small apoptotic debris (Fig. 4 B,C), and CFSEhighwhich contained large apoptotic cell remnants (Fig. 4 B,D). IgE cross-linking significantly reduced monocyte phagocytosis of small debris (CFSElow monocytes; Fig. 4 E) as well as the percentage of monocytes that phagocytosed large apoptotic cells (CFSEhigh monocytes; Fig. 4 F). In combination, these two measures reflect diminished phagocytosis of apoptotic cells by monocytes exposed to IgE cross-linking.
Figure 4. IgE cross-linking inhibits phagocytosis of apoptotic cells.
A,B. CFSE fluorescence in monocytes (gated on CD14+) after phagocytosing CFSE-labeled apoptotic cells (A); gating strategy for CFSElow and CFSEhigh monocytes(B). C,D. Photomicrographs (grey=DIC, blue=DAPI, green=CFSE) of sorted CFSElow monocytes(C) and CFSEhigh monocytes(D). E,F. CFSE fluorescence of CFSElow monocytes (E) and percent CFSEhigh of total monocytes (F) for indicated culture conditions (N=4). * p<.05, ** p<.01, *** p<.001, NS p>.05 for indicated comparisons (E,F).
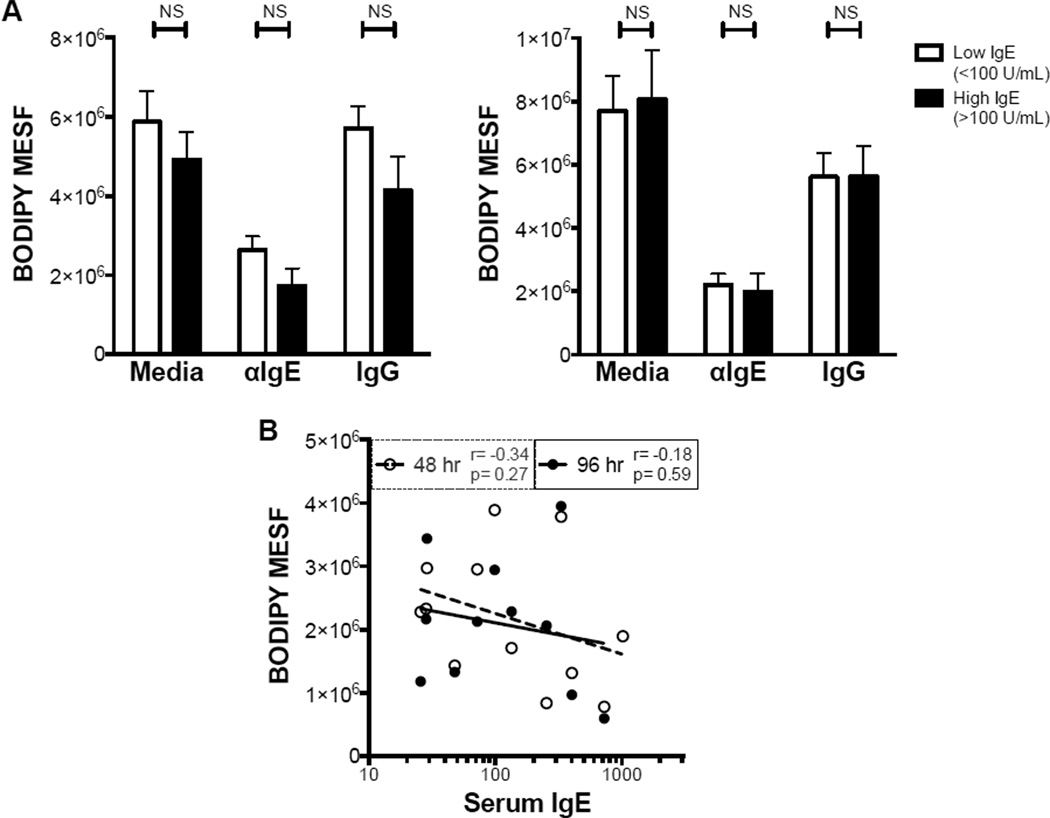
Unlike the pro-inflammatory effects of IgE cross-linking, the impairment of monocyte phagocytosis was not dependent on serum IgE concentration. Monocytes from participants with elevated IgE levels showed similar levels of phagocytosis after 48 and 96 hr of IgE cross-linking (Fig. 5 A). Moreover, there was no significant correlation between serum IgE and phagocytosis after IgE cross-linking (Fig. 5 B), again suggesting that IgE-mediated inhibition of phagocytosis is independent of the pro-inflammatory effects of IgE cross-linking.
Figure 5. Inhibition of phagocytosis by IgE cross-linking is independent of serum IgE concentration.
A,B. Fluorescence of internalized bacteria in monocytes from individuals with low (<100 U/ml) or high (>100 U/ml) serum IgE, cultured for 48 (A, left) or 96 (A, right) hr in indicated conditions; corresponding Pearson correlation (B) for αIgE condition (N=12). NS p>.05 for indicated comparisons.
The effects of IgE cross-linking on monocytes are not mediated by contaminating basophils
Because basophils express high levels of FcεRI and secrete immunomodulatory mediators upon IgE cross-linking14, we examined possible basophil contamination by determining the percentages of CD14− HLA-DR− FcεRI+ cells in each experiment. While most purified monocyte preparations contained <1% CD14− HLA-DR− FcεRI+ cells, some contained more (1–4.8%). To rule out potential basophil contribution to the above results, we compared the magnitude of IgE-mediated effects to the % CD14− HLA-DR− FcεRI+ cells in each experiment and no relationships were observed (data not shown).
Discussion
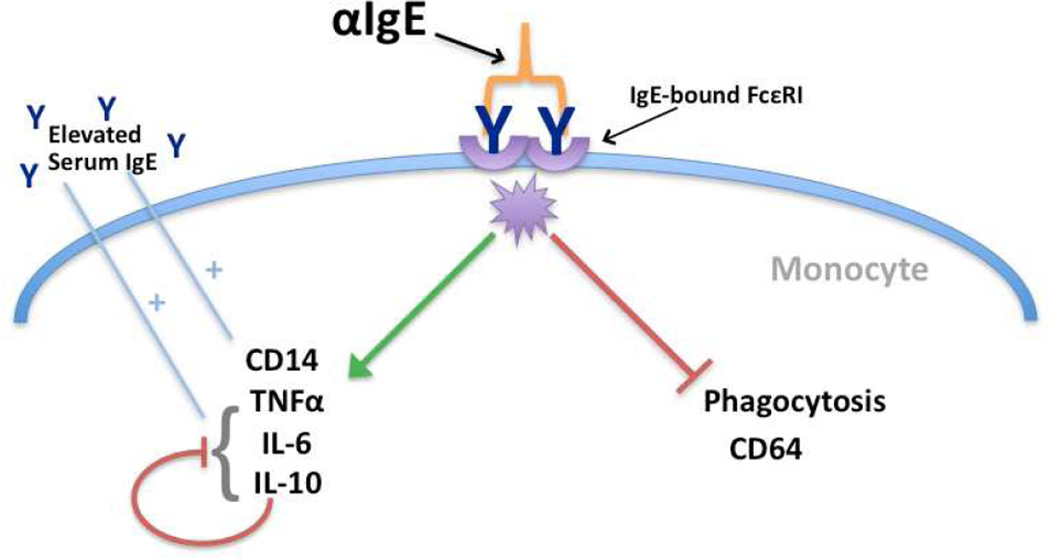
In this report, we demonstrate for the first time that IgE cross-linking impairs the function of human monocytes. Despite the inflammatory phenotype induced by IgE cross-linking, the phagocytic function of these cells is concomitantly crippled (Fig. 6). In addition, this study is the first to demonstrate the impact of serum IgE concentration, a biomarker of allergic disease, on the magnitude of IgE-mediated monocyte responses.
Figure 6. Proposed model of IgE cross-linking on human monocytes.
IgE cross-linking drives (green arrow) increased CD14 expression and secretion of both pro-inflammatory cytokines (TNFα and IL-6) and autoregulatory IL-10. These effects are enhanced in individuals with elevated serum IgE (blue lines). In contrast, phagocytosis and CD64 expression are suppressed by IgE cross-linking (red line). The IgE-mediated impairment of phagocytosis is independent of regulation by IL-10, suggesting that divergent pathways are activated by IgE cross-linking on monocytes.
IgE-mediated induction of CD14 represents one potential mechanism by which monocytes may contribute to allergic inflammation. Our finding that IgE-mediated CD14 expression correlates with serum IgE concentration may explain the clinical observation that allergen exposure up regulates CD14 on monocytes from sensitized individuals37. Since CD14 is essential for LPS responses, one potential consequence of increased CD14 expression is enhancement of this response. This is relevant to allergic disease considering that individuals with allergic asthma have increased bronchial reactivity to inhaled LPS38, which itself contributes to airway inflammation in mouse models of allergic asthma39. Our results suggest a potential link between increased CD14 expression and allergic airway disease and a role for IgE in this process.
Another prominent finding in our study was the rapid and robust secretion of the inflammatory cytokines TNFα and IL-6. Several studies have implicated TNFα in the pathogenesis of allergic disease, where it has been shown to impact airway inflammation40–42. Additionally, IL-6 sputum concentrations correlate inversely with respiratory function in asthma patients43. The results of our study suggest that IgE cross-linking on monocytes could thus contribute to allergic disease via the induction of TNFα and IL-6 secretion.
The gradual rise in IL-10 secretion was in marked contrast to the kinetics of TNFα induced by IgE cross-linking. The ability of IL-10 neutralization to reverse the TNFα decline and dramatically augment secretion of IL-6, and even IL-10 itself, suggests that IL-10 acts in an autocrine fashion to limit IgE-mediated cytokine secretion and possibly induce degradation of existing TNFα. In fact, IL-10 is proposed to play a suppressive role in allergic asthma44. Since IL-10 has been shown to suppress T cell and monocyte/macrophage responses to pathogens45, 46, excess IL-10 could potentially disrupt these immune responses. Given the importance of pathogen-associated exacerbations of allergic diseases47, this potential effect of IgE-mediated monocyte IL-10 secretion represents an exciting direction for future studies.
We report for the first time that IgE cross-linking specifically disrupts monocyte phagocytosis without affecting bacterial killing. The apparent discrepancy between IgE-mediated impairment of phagocytosis and induction of a pro-inflammatory program – including TNFα, a cytokine known to promote phagocytosis48 – suggests potential activation of divergent pathways by IgE cross-linking. One possible mechanism is impairment of TNFα responsiveness after IgE cross-linking, as increased TNFα concentration after IL-10 neutralization did not rescue the IgE-mediated repression of phagocytosis. However, TNFα unresponsiveness cannot completely account for IgE-mediated effects on monocyte function, as bacterial killing, another TNFα-responsive process48, remained intact. CD64 is also involved in phagocytosis and its expression is known to reflect monocyte phagocytic ability31, 32. The down regulation of CD64 induced by IgE cross-linking represents another potential mechanism contributing to impaired phagocytosis.
Another key regulator of phagocytosis is SH2-domain-containing inositol 5’ phosphatase 1 (SHIP), which inhibits macrophage phagocytosis through its action on membrane phospholipids49. Interestingly, SHIP has also been reported to augment TLR-induced pro-inflammatory cytokine secretion50 and to mediate formation of reactive oxygen intermediates, which promote killing after phagocytosis51. In addition to its roles in macrophages, SHIP is a negative regulator of allergic signaling in basophils; upon activation by IgE cross-linking it limits degranulation52. Activation of SHIP by IgE cross-linking in monocytes could potentially explain our observation of impaired phagocytosis despite secretion of pro-inflammatory cytokines and intact bacterial killing.
The relevance of IgE-mediated disruption of phagocytosis to allergic disease is evidenced by studies demonstrating that alveolar macrophage phagocytosis of bacteria and apoptotic cells is impaired in individuals with severe allergic asthma53, 54. Indeed, reduced macrophage phagocytosis has been correlated with increased sputum eosinophils and reduced respiratory function in individuals with allergic asthma32. Furthermore, macrophage ingestion of apoptotic granulocytes has been shown to reflect the resolution of asthma symptoms55, underscoring the importance of this process in allergic disease. IgE-mediated impairment of monocyte and macrophage phagocytosis in vivo could thus lead to reduced clearance of apoptotic debris and delayed resolution of inflammation.
In summary, we demonstrate that IgE cross-linking drives select functions in human monocytes including the secretion of both pro-inflammatory and autoregulatory cytokines, which is enhanced in monocytes from individuals with elevated serum IgE levels. In contrast, the ability of monocytes to engulf bacteria or cellular debris is significantly impaired by IgE cross-linking. This suggests that while allergic stimulation of monocytes promotes some aspects of inflammation, it concomitantly impairs the ability of these cells to resolve inflammation via phagocytosis. For individuals with elevated serum IgE concentration, the inflammation induced by allergic stimulation may be more pronounced considering their enhanced secretion of TNFα and IL-6. Yet, their ability to resolve such inflammation is blocked by IgE-mediated signaling. The ability of IL-10 to modulate IgE-mediated pro-inflammatory cytokine secretion without impacting the impairment of phagocytosis suggests that divergent pathways are induced by IgE cross-linking (Fig. 6). Further, the discordant regulation of cytokine secretion and phagocytosis may perpetuate inflammation that occurs during infection in the context of allergic stimulation. Future studies designed to delineate how IgE-mediated signaling leads to these disparate functional effects will further elucidate the consequences of IgE cross-linking on human monocytes and provide a foundation for understanding the role of this important cell type in IgE-mediated allergic disease.
Supplementary Material
Key Messages.
IgE cross-linking on monocytes increases expression of CD14 and the inflammatory cytokines TNFα and IL-6; these effects are enhanced in individuals with elevated serum IgE concentration.
IgE cross-linking induces IL-10 secretion in a serum IgE dependent manner, which acts in an autocrine fashion to limit TNFα and IL-6 secretion.
In contrast to the inflammatory phenotype, IgE cross-linking specifically impairs a critical monocyte function – phagocytosis; this effect is not subject to regulation by IL-10.
Acknowledgments
We thank Deborah Gonzales, Dolores Santoyo, Brenda Lewis, and Rebecca Hardy for assistance with the enrollment of the study participants. We thank Angela Mobley and the UT Southwestern Flow Cytometry Facility for assistance with flow-based assays. We also thank Dr. Andrew Koh for lending reagents.
Funding Sources:
William A. and Joyce M. Sellars Distinguished Chair in Allergy and Immunology
NIH NIAID, R01-AI098077
NIH NIAID, R01-AI056222
NIH NRSA, T32-AI005284
NIH NIGMS, T32-GM008014
Abbreviations
- αIgE
cross-linking anti-IgE antibody
- IgG
control rabbit IgG antibody
- mDC
myeloid dendritic cell
- pDC
plasmacytoid dendritic cell
- FcεRI
high-affinity IgE receptor
- MESF
mean equivalent standard fluorescence
- LPS
lipopolysaccharide
- PBMC
peripheral blood mononuclear cells
- TLR
toll-like receptor
- SHIP
SH2-domain-containing inositol 5’ phosphatase 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(Suppl 3):S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 2.Meltzer EO, Blaiss MS, Derebery MJ, Mahr TA, Gordon BR, Sheth KK, et al. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol. 2009;124:S43–S70. doi: 10.1016/j.jaci.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Mancini AJ, Kaulback K, Chamlin SL. The socioeconomic impact of atopic dermatitis in the United States: a systematic review. Pediatr Dermatol. 2008;25:1–6. doi: 10.1111/j.1525-1470.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 4.Laske N, Niggemann B. Does the severity of atopic dermatitis correlate with serum IgE levels? Pediatr Allergy Immunol. 2004;15:86–88. doi: 10.1046/j.0905-6157.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- 5.Kovac K, Dodig S, Tjesic-Drinkovic D, Raos M. Correlation between asthma severity and serum IgE in asthmatic children sensitized to Dermatophagoides pteronyssinus. Arch Med Res. 2007;38:99–105. doi: 10.1016/j.arcmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Kjaer HF, Eller E, Andersen KE, Host A, Bindslev-Jensen C. The association between early sensitization patterns and subsequent allergic disease. The DARC birth cohort study. Pediatr Allergy Immunol. 2009;20:726–734. doi: 10.1111/j.1399-3038.2009.00862.x. [DOI] [PubMed] [Google Scholar]
- 7.Matsui EC, Sampson HA, Bahnson HT, Gruchalla RS, Pongracic JA, Teach SJ, et al. Allergen-specific IgE as a biomarker of exposure plus sensitization in inner-city adolescents with asthma. Allergy. 2010;65:1414–1422. doi: 10.1111/j.1398-9995.2010.02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thaiwat S, Sangasapaviliya A. Omalizumab treatment in severe adult atopic dermatitis. Asian Pac J Allergy Immunol. 2011;29:357–360. [PubMed] [Google Scholar]
- 9.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu LC. Immunoglobulin E receptor signaling and asthma. J Biol Chem. 2011;286:32891–32897. doi: 10.1074/jbc.R110.205104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sihra BS, Kon OM, Grant JA, Kay AB. Expression of high-affinity IgE receptors (Fc epsilon RI) on peripheral blood basophils, monocytes, and eosinophils in atopic and nonatopic subjects: relationship to total serum IgE concentrations. J Allergy Clin Immunol. 1997;99:699–706. doi: 10.1016/s0091-6749(97)70033-2. [DOI] [PubMed] [Google Scholar]
- 12.Foster B, Metcalfe DD, Prussin C. Human dendritic cell 1 and dendritic cell 2 subsets express FcepsilonRI: correlation with serum IgE and allergic asthma. J Allergy Clin Immunol. 2003;112:1132–1138. doi: 10.1016/j.jaci.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Ugajin T, Satoh T, Kanamori T, Aritake K, Urade Y, Yokozeki H. FcepsilonRI, but not FcgammaR, signals induce prostaglandin D2 and E2 production from basophils. Am J Pathol. 2011;179:775–782. doi: 10.1016/j.ajpath.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawaguchi M, Tanaka S, Nakatani Y, Harada Y, Mukai K, Matsunaga Y, et al. Role of mast cells and basophils in IgE responses and in allergic airway hyperresponsiveness. J Immunol. 2012;188:1809–1818. doi: 10.4049/jimmunol.1101746. [DOI] [PubMed] [Google Scholar]
- 15.Le T, Tversky J, Chichester KL, Bieneman AP, Huang SK, Wood RA, et al. Interferons modulate Fc epsilon RI-dependent production of autoregulatory IL-10 by circulating human monocytoid dendritic cells. J Allergy Clin Immunol. 2009;123:217–223. doi: 10.1016/j.jaci.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroeder JT, Chichester KL, Bieneman AP. Toll-like receptor 9 suppression in plasmacytoid dendritic cells after IgE-dependent activation is mediated by autocrine TNF-alpha. J Allergy Clin Immunol. 2008;121:486–491. doi: 10.1016/j.jaci.2007.09.049. [DOI] [PubMed] [Google Scholar]
- 17.Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, et al. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. J Immunol. 2010;184:5999–6006. doi: 10.4049/jimmunol.0901194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng YX, Foster B, Holland SM, Klion AD, Nutman TB, Casale TB, et al. CD2 identifies a monocyte subpopulation with immunoglobulin E- dependent, high-level expression of Fc epsilon RI. Clin Exp Allergy. 2006;36:1436–1445. doi: 10.1111/j.1365-2222.2006.02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang Y, Wang P, Zhao M, Liang G, Yin H, Zhang G, et al. Demethylation of the FCER1G promoter leads to FcepsilonRI overexpression on monocytes of patients with atopic dermatitis. Allergy. 2012;67:424–430. doi: 10.1111/j.1398-9995.2011.02760.x. [DOI] [PubMed] [Google Scholar]
- 20.Lenzo JC, Turner AL, Cook AD, Vlahos R, Anderson GP, Reynolds EC, et al. Control of macrophage lineage populations by CSF-1 receptor and GM-CSF in homeostasis and inflammation. Immunol Cell Biol. 2012;90:429–440. doi: 10.1038/icb.2011.58. [DOI] [PubMed] [Google Scholar]
- 21.Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178:2000–2007. doi: 10.4049/jimmunol.178.4.2000. [DOI] [PubMed] [Google Scholar]
- 22.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gill MA, Long K, Kwon T, Muniz L, Mejias A, Connolly J, et al. Differential recruitment of dendritic cells and monocytes to respiratory mucosal sites in children with influenza virus or respiratory syncytial virus infection. J Infect Dis. 2008;198:1667–1676. doi: 10.1086/593018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lensmar C, Katchar K, Eklund A, Grunewald J, Wahlstrom J. Phenotypic analysis of alveolar macrophages and lymphocytes following allergen inhalation by atopic subjects with mild asthma. Respir Med. 2006;100:918–925. doi: 10.1016/j.rmed.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Leon B, Ardavin C. Monocyte migration to inflamed skin and lymph nodes is differentially controlled by L-selectin and PSGL-1. Blood. 2008;111:3126–3130. doi: 10.1182/blood-2007-07-100610. [DOI] [PubMed] [Google Scholar]
- 26.Makela SM, Strengell M, Pietila TE, Osterlund P, Julkunen I. Multiple signaling pathways contribute to synergistic TLR ligand- dependent cytokine gene expression in human monocyte-derived macrophages and dendritic cells. J Leukoc Biol. 2009;85:664–672. doi: 10.1189/jlb.0808503. [DOI] [PubMed] [Google Scholar]
- 27.Narasaraju T, Ng HH, Phoon MC, Chow VT. MCP-1 antibody treatment enhances damage and impedes repair of the alveolar epithelium in influenza pneumonitis. Am J Respir Cell Mol Biol. 2010;42:732–743. doi: 10.1165/rcmb.2008-0423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology. 2011;216:753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Reed JL, Brewah YA, Delaney T, Welliver T, Burwell T, Benjamin E, et al. Macrophage impairment underlies airway occlusion in primary respiratory syncytial virus bronchiolitis. J Infect Dis. 2008;198:1783–1793. doi: 10.1086/593173. [DOI] [PubMed] [Google Scholar]
- 30.Brekke OL, Christiansen D, Fure H, Pharo A, Fung M, Riesenfeld J, et al. Combined inhibition of complement and CD14 abolish E. coli-induced cytokine-, chemokine- and growth factor-synthesis in human whole blood. Mol Immunol. 2008;45:3804–3813. doi: 10.1016/j.molimm.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Grage-Griebenow E, Flad HD, Ernst M, Bzowska M, Skrzeczynska J, Pryjma J. Human MO subsets as defined by expression of CD64 and CD16 differ in phagocytic activity and generation of oxygen intermediates. Immunobiology. 2000;202:42–50. doi: 10.1016/S0171-2985(00)80051-0. [DOI] [PubMed] [Google Scholar]
- 32.Alexis NE, Soukup J, Nierkens S, Becker S. Association between airway hyperreactivity and bronchial macrophage dysfunction in individuals with mild asthma. Am J Physiol Lung Cell Mol Physiol. 2001;280:L369–L375. doi: 10.1152/ajplung.2001.280.2.L369. [DOI] [PubMed] [Google Scholar]
- 33.Kraft S, Novak N, Katoh N, Bieber T, Rupec RA. Aggregation of the high-affinity IgE receptor Fc(epsilon)RI on human monocytes and dendritic cells induces NF-kappaB activation. J Invest Dermatol. 2002;118:830–837. doi: 10.1046/j.1523-1747.2002.01757.x. [DOI] [PubMed] [Google Scholar]
- 34.Von Bubnoff D, Matz H, Cazenave JP, Hanau D, Bieber T, De La Salle H. Kinetics of gene induction after FcepsilonRI ligation of atopic monocytes identified by suppression subtractive hybridization. J Immunol. 2002;169:6170–6177. doi: 10.4049/jimmunol.169.11.6170. [DOI] [PubMed] [Google Scholar]
- 35.Novak N, Bieber T, Katoh N. Engagement of Fc epsilon RI on human monocytes induces the production of IL-10 and prevents their differentiation in dendritic cells. J Immunol. 2001;167:797–804. doi: 10.4049/jimmunol.167.2.797. [DOI] [PubMed] [Google Scholar]
- 36.Gruchalla RS, Pongracic J, Plaut M, Evans R, 3rd, Visness CM, Walter M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Monteseirin J, Bonilla I, Chacon P, Vega A, Camacho MJ, Guardia P, et al. Allergen-dependent CD14 modulation and apoptosis in monocytes from allergic patients. Allergy. 2003;58:1027–1032. doi: 10.1034/j.1398-9995.2003.00249.x. [DOI] [PubMed] [Google Scholar]
- 38.Michel O, Duchateau J, Sergysels R. Effect of inhaled endotoxin on bronchial reactivity in asthmatic and normal subjects. J Appl Physiol. 1989;66:1059–1064. doi: 10.1152/jappl.1989.66.3.1059. [DOI] [PubMed] [Google Scholar]
- 39.Tsuchiya K, Siddiqui S, Risse PA, Hirota N, Martin JG. The presence of LPS in OVA inhalations affects airway inflammation and AHR but not remodeling in a rodent model of asthma. Am J Physiol Lung Cell Mol Physiol. 2012;303:L54–L63. doi: 10.1152/ajplung.00208.2011. [DOI] [PubMed] [Google Scholar]
- 40.Lee WI, Yao TC, Yeh KW, Chen LC, Ou LS, Huang JL. Stronger Toll-like receptor 1/2, 4, and 7/8 but less 9 responses in peripheral blood mononuclear cells in non-infectious exacerbated asthmatic children. Immunobiology. 2012 doi: 10.1016/j.imbio.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Widegren H, Erjefalt J, Korsgren M, Andersson M, Greiff L. Effects of intranasal TNFalpha on granulocyte recruitment and activity in healthy subjects and patients with allergic rhinitis. Respir Res. 2008;9:15. doi: 10.1186/1465-9921-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hutchison S, Choo-Kang BS, Bundick RV, Leishman AJ, Brewer JM, McInnes IB, et al. Tumour necrosis factor-alpha blockade suppresses murine allergic airways inflammation. Clin Exp Immunol. 2008;151:114–122. doi: 10.1111/j.1365-2249.2007.03509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morjaria JB, Babu KS, Vijayanand P, Chauhan AJ, Davies DE, Holgate ST. Sputum IL-6 concentrations in severe asthma and its relationship with FEV1. Thorax. 2011;66:537. doi: 10.1136/thx.2010.136523. [DOI] [PubMed] [Google Scholar]
- 44.Nayyar A, Dawicki W, Huang H, Lu M, Zhang X, Gordon JR. Induction of Prolonged Asthma Tolerance by IL-10-Differentiated Dendritic Cells: Differential Impact on Airway Hyperresponsiveness and the Th2 Immunoinflammatory Response. J Immunol. 2012;189:72–79. doi: 10.4049/jimmunol.1103286. [DOI] [PubMed] [Google Scholar]
- 45.Lee KS, Jeong ES, Heo SH, Seo JH, Jeong DG, Choi YK. IL-10 suppresses bactericidal response of macrophages against Salmonella Typhimurium. J Microbiol. 2011;49:1050–1053. doi: 10.1007/s12275-011-1043-z. [DOI] [PubMed] [Google Scholar]
- 46.Brooks DG, Walsh KB, Elsaesser H, Oldstone MB. IL-10 directly suppresses CD4 but not CD8 T cell effector and memory responses following acute viral infection. Proc Natl Acad Sci U S A. 2010;107:3018–3023. doi: 10.1073/pnas.0914500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackson DJ, Johnston SL. The role of viruses in acute exacerbations of asthma. J Allergy Clin Immunol. 2010;125:1178–1187. doi: 10.1016/j.jaci.2010.04.021. quiz 1188–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamura N, Hazeki K, Okazaki N, Kametani Y, Murakami H, Takaba Y, et al. Specific role of phosphoinositide 3-kinase p110alpha in the regulation of phagocytosis and pinocytosis in macrophages. Biochem J. 2009;423:99–108. doi: 10.1042/BJ20090687. [DOI] [PubMed] [Google Scholar]
- 50.Fang H, Pengal RA, Cao X, Ganesan LP, Wewers MD, Marsh CB, et al. Lipopolysaccharide-induced macrophage inflammatory response is regulated by SHIP. J Immunol. 2004;173:360–366. doi: 10.4049/jimmunol.173.1.360. [DOI] [PubMed] [Google Scholar]
- 51.Kamen LA, Levinsohn J, Cadwallader A, Tridandapani S, Swanson JA. SHIP-1 increases early oxidative burst and regulates phagosome maturation in macrophages. J Immunol. 2008;180:7497–7505. doi: 10.4049/jimmunol.180.11.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gibbs BF, Rathling A, Zillikens D, Huber M, Haas H. Initial Fc epsilon RI-mediated signal strength plays a key role in regulating basophil signaling and deactivation. J Allergy Clin Immunol. 2006;118:1060–1067. doi: 10.1016/j.jaci.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 53.Fitzpatrick AM, Holguin F, Teague WG, Brown LA. Alveolar macrophage phagocytosis is impaired in children with poorly controlled asthma. J Allergy Clin Immunol. 2008;121:1372–1378. 1378, e1371–e1373. doi: 10.1016/j.jaci.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huynh ML, Malcolm KC, Kotaru C, Tilstra JA, Westcott JY, Fadok VA, et al. Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am J Respir Crit Care Med. 2005;172:972–979. doi: 10.1164/rccm.200501-035OC. [DOI] [PubMed] [Google Scholar]
- 55.Kulkarni NS, Hollins F, Sutcliffe A, Saunders R, Shah S, Siddiqui S, et al. Eosinophil protein in airway macrophages: a novel biomarker of eosinophilic inflammation in patients with asthma. J Allergy Clin Immunol. 2010;126:61–69. e63. doi: 10.1016/j.jaci.2010.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.