Abstract
Sensitization remains a major barrier to kidney transplantation. Sensitized patients comprise 30% of the kidney transplant waiting list but fewer than 15% of highly sensitized patients are transplanted each year. Options for highly sensitized patients with an immunologically incompatible live donor include desensitization or kidney paired donation (KPD). However, these options when used alone may still not be sufficient to allow a compatible transplant for recipients who are broadly sensitized with cumulative calculated panel reactive antibody (cPRA) > 95%. We describe in this report the combined use of both desensitization and KPD to maximize the likelihood of finding a compatible match with a more immunologically favorable donor through a kidney exchange program. This combined approach was used in five very highly sensitized patients, all with cPRA 100%, who ultimately received compatible living and deceased donor kidney transplants. We conclude that early enrollment in paired kidney donor exchange and tailored desensitization protocols are key strategies to improve care and rates of kidney transplantation in highly sensitized patients.
Keywords: desensitization, kidney exchanges, kidney transplantation, HLA antibodies, donor-specific antibodies
Introduction
Kidney transplantation is the treatment of choice for patients with end-stage renal disease (ESRD) (1). Sensitization is a major barrier to successful kidney transplantation. Sensitized patients comprise approximately 30% of the deceased donor waiting list and have the longest waiting times because of difficulty in finding a compatible donor (2). Despite priority status in the organ allocation algorithm, fewer than 15% of highly sensitized patients are transplanted per year (3). Based on Organ Procurement and Transplantation Network (OPTN) data as of the end of 2010, although patients with cumulative calculated panel reactive antibody (cPRA) 80–95% have benefited with increased transplantation rates, those patients with cPRA > 95% remain difficult to transplant especially those fully sensitized with cPRA 100%. Current options for highly sensitized patients with an immunologically incompatible live donor include desensitization, kidney paired donation (KPD), or a combination (4).
For the past decade, desensitization has been successful in select patients. Current agents for desensitization include intravenous immunoglobulin (IVIG), rituximab, plasmapheresis, as well as newer agents such as bortezomib and eculizumab (5–7). Recent studies show a survival benefit that more than doubled by eight years for patients undergoing desensitization and transplantation compared with remaining on dialysis (8). Unfortunately, many patients do not respond to desensitization, especially highly sensitized patients with broad and strong human leukocyte antigen (HLA) antibody reactivity. Furthermore, long-term outcomes in positive crossmatch kidney transplant recipients may be inferior compared to immunologically compatible transplants (9). KPD is a creative option that matches a more immunologically compatible donor with a recipient through a registry. However, difficult-to-match donor-recipient combinations, including broadly sensitized patients, continue to pose a challenge.
Combining desensitization and KPD for patients who have strong antibody reactivity to a proposed but willing donor, while also keeping them on the deceased donor waiting list, increases the pool of potential donors. We hypothesized that this combined approach would enable our highly sensitized patients to be transplanted. Other centers, including Johns Hopkins, have combined KPD and desensitization to increase transplant rates (10–12). Both desensitization and KPD programs are expensive and require significant resources. Therefore, we sought to determine how best to utilize these strategies in highly sensitized patients.
We report our experience of five highly sensitized patients, all with cumulative cPRA 100%, who underwent desensitization in combination with KPD and successfully received kidney transplants. By carefully analyzing donor and recipient HLA typing and antibodies, our objective is to create a strategy on how to enroll donor-recipient pairs in KPD and prescribe desensitization therapy to enable successful transplantation in the very highly sensitized patients.
Case Histories
The recipient demographic information, HLA typing, histocompatibility data, and clinical outcomes are depicted (Tables 1–4). Desensitization therapy consisted of monthly high-dose intravenous immunoglobulin (IVIG) (2 gm/kg), rituximab (375 mg/m2) after four doses of IVIG, and plasmapheresis followed by bortezomib at 1.3 mg/m2 in one patient who did not respond to IVIG and rituximab. Immunosuppression was anti-thymocyte globulin induction, IVIG (2 gm/kg) at the time of transplant followed by another dose three weeks post-transplant, and mycophenolate mofetil (MMF), tacrolimus, and prednisone for maintenance immunosuppression. The patients and their incompatible donors were entered into the National Kidney Registry (NKR).
Table 1.
Patient demographics
| Parameters | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Age at time of transplant (yr) | 33 | 46 | 30 | 33 | 26 |
| Sex | M | M | M | F | M |
| Cause of ESRD | FSGS | IgA | SLE | Cortical necrosis | Obstructive nephropathy |
| Prior kidney transplantation (n) | 1 | 1 | 1 | 1 | 1 |
| Waiting time for a transplant (yr) | 3 | 5.5 | 8 | 8.2 | 5.2 |
| Blood Type | O | O | A | O | O |
FSGS = focal segmental glomerulosclerosis
IgA = IgA nephropathy
SLE = systemic lupus erythematosus
Table 4.
Clinical outcomes
| Parameters | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
|---|---|---|---|---|---|
| Post-operative (mo) | 24 | 23 | 22 | 22 | 3 |
| Acute rejection | |||||
| Borderline | 1 | 0 | 1 | 0 | 0 |
| Cell-mediated | 0 | 0 | 0 | 0 | 0 |
| Antibody-mediated | 0 | 0 | 0 | 0 | 0 |
| Creatinine (mg/dl) 1 mo | 1.5 | 1.4 | 1.6 | 1 | 2.5 |
| Creatinine (mg/dl) 3 mo | 1.6 | 1.4 | 1.5 | 1 | 2.1 |
| Creatinine (mg/dl) 6 mo | 1.8 | 1.6 | 1.3 | 1 | N/A |
| Creatinine (mg/dl) 1 yr | 1.4 | 1.4 | 1.5 | 0.9 | N/A |
| Last creatinine (mg/dl) post-transplant (mo) | 1.5 (24) | 1.5 (23) | 1.1 (22) | 0.9 (22) | 2.1 (3) |
| Graft loss (%) | 0 | 0 | 0 | 0 | 0 |
An acceptable crossmatch to proceed with transplantation was T-cell and B-cell flow crossmatch (FXM) with a median flow-channel shift (MCS) of ≤ 200 after adjusting for presence of autoantibodies (normal range MCS T-cell FXM ≤ 88 and B-cell FXM ≤ 100). HLA antibodies are considered positive with normalized mean fluorescence intensity (MFI) of ≥ 1000 and “possible” with MFI 500–999. All patients had post-transplant donor specific antibody (DSA) monitoring and protocol kidney biopsies at implantation and 3 and 12 months post-transplant. We performed retrospective T-cell and B-cell FXM and DSA analyses for recipients against both their original intended donors and KPD donors to elucidate the effect of desensitization and matching on ultimately finding a compatible donor (Table 3).
Table 3.
Histocompatibility data.
| I. Original intended donor | |||||||
|---|---|---|---|---|---|---|---|
| Flow Cross match | DSA | ||||||
| Patient | Cum cPRA | pre- desensitization T cell XM auto/allo | pre- desensitization B cell XM auto/allo | post- desensitization T cell XM auto/allo | post- desensitization B cell XM auto/allo | pre- desensitization DSA (MFI) | post- desensitization DSA (MFI) |
| Case 1 | 100% | NA/475 | NA/477 | 112/250 | 134/320 | A2 (913–1780) A29 (613–758) B44 (8667–9262) |
A2 (571–922) A29 (501–561) B44 (6882–7972) |
| Case 2 | 100% | NA/8 | NA/288 | 50/63 | 27/338 | A24 (1154–1582) DQ2 (15799–19749) |
A24 (Neg) DQ2 (19304–21311) |
| Case 3 | 100% | 98/383 | 177/434 | NA | NA | A3 (13068) A68 (9535–9931) DR1 (5855–6021) DQ5 (8761–14004) DQ8 (849–1290) |
A3 (5696) A68 (5960–9204) DR1 (16942–17523) DQ5 (13911–15814) DQ8 (175–1319) |
| Case 4 | 100% | NA/399 | NA/361 | 30/327 | 24/325 | A68 (5445–6209) B44 (15137–15455) |
A68 (5220–5652) B44 (9750–9790) |
| Case 5 | 100% | 17/439 | 50/464 | NA | NA | A2 (528–1467) A26 (1165) B62 (2044) DR53 (7181–9685) DQ9 (669) |
A2 (1543–2209) A26 (757) B62 (556) DR53 (7493–9862) DQ9 (Neg) |
| II. KPD donor or Deceased donor | |||||||
|---|---|---|---|---|---|---|---|
| Flow Cross match | DSA | ||||||
| Patient | Cum cPRA | pre- desensitization T cell XM auto/allo | pre- desensitization B cell XM auto/allo | post- desensitization T cell XM auto/allo | post- desensitization B cell XM auto/allo | pre- desensitization DSA (MFI) | post- desensitization DSA (MFI) |
| Case 1 | 100% | NA | NA | 111/148 | 124/245 | A2 (913–1780) | A2 (571–922) |
| Case 2 | 100% | NA | NA | 48/39 | 47/228 | DR13 (591–1755) DQ6 (805) |
DR13 (Neg) DQ6 (Neg) |
| Case 3 | 100% | 243/325 | 257/324 | 130/171 | 148/254 | A29 (3314–3967) B45 (899) DR4 (441–965) DR53 (1435–2373) DQ2 (473–1935) |
A29 (1279–1498) B45 (Neg) DR4 (Neg) DR53 (552–706) DQ2 (Neg) |
| Case 4 | 100% | NA | NA | 46/0 | 30/14 | None | None |
| Case 5 | 100% | NA | NA | 0/40 | 1/2 | B62 (2044) | B62 (556) |
Case 1
The patient is a 33-year-old man with ESRD secondary to biopsy-proven primary focal segmental glomerulosclerosis (FSGS) (Table 1). He received a living kidney transplant with his mother as donor in 1995. He experienced acute cellular rejection in 1997, and his graft failed secondary to biopsy-proven recurrent FSGS and transplant glomerulopathy. He started peritoneal dialysis in 2008. He underwent transplant nephrectomy in 2009. In 2010 his friend (56-years-old, blood type O) came forward as a potential donor. Their FXM was strongly positive: T-cell 475 MCS and B-cell 477 MCS with several DSA (Table 3).
In an attempt to undergo a living kidney transplant with his friend as the intended donor, in March 2010, he started desensitization therapy with monthly high-dose IVIG infusions. In June 2010 he had partial response to therapy with T-cell 250 MCS and B-cell 320 MCS. Because he was still above the FXM cutoff to proceed with transplantation, the patient and his potential donor enrolled in KPD while continuing desensitization. In July 2010 a match from a 45-year-old, blood type O potential donor was identified in the KPD pool. The FXM against the donor in the exchange was T-cell 148 MCS and B-cell 245 MCS with a “possible” DSA to A2 (Table 3). He received a kidney transplant from this donor in July 2010. His protocol biopsy at 3 months post-transplant showed borderline acute cellular rejection (C4d negative) treated with prednisone (Table 4). He has not developed post-transplant DSA and has stable allograft function without proteinuria 24 months post-transplant.
Case 2
The patient is a 46-year-old man with ESRD secondary to biopsy-proven IgA nephropathy (Table 1). He received a deceased donor kidney transplant in 1993. He suffered two episodes of acute cellular rejection and subsequently started dialysis in 2007. His friend (48-years-old, blood type O) came forward as a potential donor. Their FXM was negative for T-cells (8 MCS) and positive for B-cells (288 MCS) mainly driven by a high-strength DSA to DQ2 (Table 3).
He began desensitization with monthly high-dose IVIG infusions in December 2009 and rituximab in March 2010. After four months of desensitization, the B-cell FXM remained strongly positive with a persistent antibody to DQ2 (Table 3). In June 2010 the patient and his potential donor entered into KPD while continuing desensitization. In August 2010 a match from a 64-year-old, blood type O, DQ2 negative, potential donor was identified in the KPD pool. The FXM against the donor in the exchange was T-cell 39 MCS and B-cell 228 MCS. He received a kidney transplant from this donor in August 2010. He has stable allograft function without DSA 23 months post-transplant (Table 4).
Case 3
The patient is a 30-year-old man with ESRD secondary to biopsy-proven lupus nephritis. He received a deceased donor kidney transplant in 1996 that failed in 2001 secondary to chronic rejection. He underwent a transplant nephrectomy in 2002. He also suffered from difficulties with dialysis access and, eventually, was dialyzing through a right thigh arteriovenous fistula.
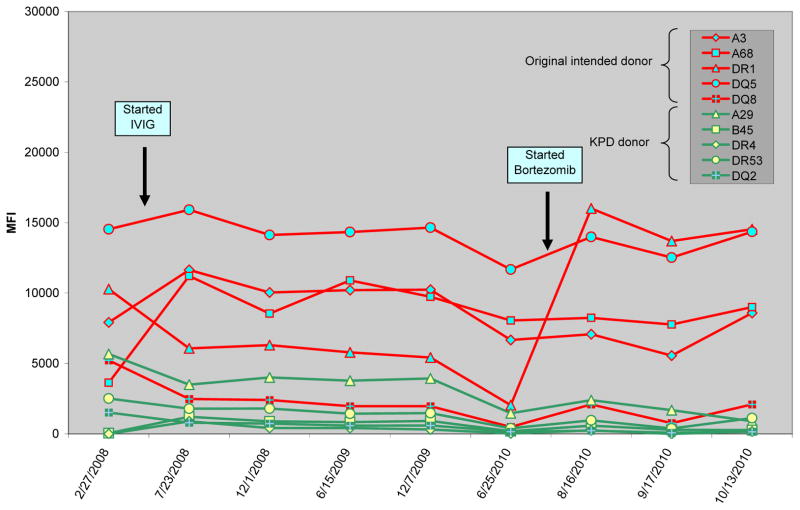
In March 2008 he started desensitization in an attempt to receive a compatible transplant from the deceased donor waiting list. He received monthly high-dose IVIG, rituximab (two doses), and plasmapheresis. In June 2010 his friend (24-year-old, blood type A) came forward as a potential donor. Their FXM was strongly positive: T-cell 383 MCS and B-cell 434 MCS with multiple high level DSA (Table 3). Because of strong reactivity against the intended donor, the patient and donor entered into KPD. In addition, he began therapy with plasmapheresis and bortezomib in combination with IVIG in an attempt to lower high-strength HLA antibodies (Figure 1). In August 2010, after three cycles of plasmapheresis and bortezomib, a match from a 33-year-old, blood type A potential donor was identified in the KPD pool. The FXM against the donor in the exchange was T-cell 171 MCS and B-flow 254 MCS with only one low positive DSA. He subsequently received a kidney transplant from this donor in September 2010. Post-transplant he developed a de novo DSA against Cw6. His protocol 3-month kidney biopsy showed borderline acute cellular rejection (C4d negative) treated with prednisone. He remains with excellent graft function 22 months post-transplant (Table 4).
Figure 1.
Effect of intravenous immunoglobulin (IVIG) and bortezomib on the donor specific antibodies (DSA) in both the original intended donor (red) and the KPD donor (green) in case 3.
Case 4
The patient is a 33-year-old woman with ESRD secondary to cortical necrosis after meningococcal sepsis (Table 1). She received a pediatric en bloc deceased donor kidney transplant in 1999. She experienced acute cellular rejection in 2001 and vesiculoureteral reflux requiring ureter reimplantation. She underwent transplant nephrectomy 2002 at which time she returned to dialysis. In 2009 her mother (54 years-old, blood type O) came forward as a potential donor. Their FXM was positive: T-cell 399 MCS and B-cell 361 MCS.
She started monthly IVIG infusions in March 2010 with one dose of rituximab at that time. In June 2010 she had partial response to therapy with T-cell 327 MCS and B-cell 325 MCS. At that time the patient and her potential donor enrolled in KPD while continuing desensitization. In September 2010 she received a zero-mismatched deceased donor kidney transplant. She has stable allograft function without DSA 22 months post-transplant (Table 4).
Case 5
The patient is a 26-year-old man with ESRD secondary to obstructive uropathy from posterior urethral valves. He underwent a living related kidney transplant with his mother as a donor in 1998. He had several episodes of acute rejection and his transplant failed in 2004. He underwent transplant nephrectomy in 2009. In December 2010 his friend (22 years-old, blood type A) came forward as a potential donor. Their FXM was positive with T-cell 439 MCS and B-cell 464 MCS.
He began desensitization therapy with monthly IVIG infusions in May 2011 and rituximab October 2011. He had partial response to therapy. In an attempt to further decrease HLA antibodies, he proceeded with plasmapheresis in April 2012. After one session of plasmapheresis and prior to receiving bortezomib, he received an offer for a deceased donor transplant in April 2012. He has not developed post-transplant DSA after 3 months.
Discussion
We identified five highly sensitized kidney transplant recipients, all with cPRA 100%, who underwent desensitization prior to participating in KPD. We enrolled two patients in KPD after desensitization failed to lower high-strength HLA antibodies against the intended donors. KPD enabled them to find compatible donors for whom they had low-strength or no HLA antibodies prior to desensitization. For patient 1, we found a donor lacking B44 for which the patient had strong reactivity. Although the patient had an antibody against A2, the MFI was low enough after desensitization therapy to proceed with transplantation. For patient 2, because the patient had strong reactivity to DQ2, which did not decrease with desensitization, we attempted to find a donor lacking DQ2. In addition, after desensitization, two low-strength DSA decreased. This approach was possible because these two patients were not broadly sensitized to most of the common HLA genotypes.
For patient 3, who had high-strength antibodies against common HLA antigens, desensitization was necessary to enable us to lower HLA antibodies sufficiently to find a compatible match from the KPD pool. We tested his serum prior to desensitization against the matched donor. Notably, the initial FXM showed strong reactivity to the donor in the KPD pool that diminished after desensitization. In this case, bortezomib seemed to have the most significant effect on lowering HLA antibodies (Figure 1) in contrast to published experience that did not find a beneficial desensitization effect with bortezomib (13). Perhaps the difference may have been that we used bortezomib in conjunction with IVIG, rituximab, and plasmapheresis in a manner similar to investigators who used bortezomib successfully in the setting of desensitization in heart transplantation (14).
Highly sensitized patients (cPRA ≥ 80%) have low match rates in KPD, often below 15% (15, 16). Most of these patients are sensitized to common HLA antigens and are seeking to match a donor with a rare HLA genotype. By computer simulations, we can predict who can find a match in a KPD database (15, 16). Predicting who responds to desensitization therapy is more challenging. Patients 4 and 5 are two highly sensitized patients, both blood type O, who underwent desensitization, enrolled in KPD, and subsequently received deceased donor kidney transplants. One intended donor was over 50 years old (blood type O) and the other intended donor was blood type A. In these two recipients who did not find a match in the KPD pool, desensitization enabled them to receive deceased donor kidney transplants. One patient received a zero-mismatched kidney from a deceased donor, and one patient received a compatible deceased donor.
Another strategy we and other centers, including Johns Hopkins, have implemented is to raise the threshold for listing “unacceptable antigens” in highly sensitized patients undergoing desensitization and, thereby, allow the presence of low-strength DSA (11). It is unclear whether low-strength HLA antibodies lead to allograft injury (17). Our center threshold for listing unacceptable HLA antigens is 1000 MFI. However, in patients undergoing desensitization, we list HLA antibodies with MFI strength 3000 or higher. Other centers have expanded the KPD pool by encouraging compatible pairs to participate, especially pairs with blood group O donors (18–20).
In these five highly sensitized patients, we used multiple strategies to increase their chances of undergoing transplantation. In each case, the patient’s potential living donor pool was expanded by enrolling them in a KPD program while increasing the number of potential compatible matches on the deceased donor list through desensitization. All five patients were successfully transplanted: three with living donors through KPD and two with deceased donors. Upon review of the changing levels of recipient HLA antibodies and the actual donor HLA genotypes, one recipient was able to find a donor though KPD only because he also underwent desensitization. Although the other recipients were transplanted through KPD or with a compatible deceased donor that would not have required them to be desensitized, the recipient HLA antibody profile against these grafts improved by desensitization. Therefore, early enrollment in KPD, participation in desensitization protocols, and implementation of less strict criteria to allow for presence of low-strength DSA are viable strategies aimed to improve care and rates of transplantation in highly sensitized patients.
Table 2.
HLA Typing
| HLA-A | HLA-B | HLA-Cw | HLA-DR | HLA-DQ | |
|---|---|---|---|---|---|
| Case 1 | A3, X | B65, 57 | Cw8, 17 | DR7, X | DQ2, 9 |
| Original donor | A2, 29 | B44, X | Cw16, 5 | DR11, 15 | DQ7, 6 |
| KPD donor | A2, 26 | B62, 38 | Cw9, 12 | DR4, 13 | DQ8, 6 |
| HLA-A | HLA-B | HLA-Cw | HLA-DR | HLA-DQ | |
| Case 2 | A11, 26 | B27, 35 | Cw1, 4 | DR1, 1 | DQ5, X |
| Original donor | A1, 24 | B8, 46 | Cw1, 7 | DR17, 14 | DQ2, 5 |
| KPD donor | A3, 30 | B71, 55 | Cw7, 9 | DR13, 15 | DQ6, 6 |
| HLA-A | HLA-B | HLA-Cw | HLA-DR | HLA-DQ | |
| Case 3 | A23, 26 | B65, 35 | Cw4, 8 | DR8, 13 | DQ4, 7 |
| Original donor | A3, 68 | B35, 39 | Cw4, 7 | DR1, 4 | DQ5, 8 |
| KPD donor | A23, 29 | B44, 45 | Cw4, 6 | DR4, 7 | DQ2, X |
| HLA-A | HLA-B | HLA-Cw | HLA-DR | HLA-DQ | |
| Case 4 | A3, 29 | B7, 7 | Cw7, 15 | DR13, 15 | DQ6, 6 |
| Original donor | A3, 68 | B7, 44 | Cw7, 7 | DR13, 15 | DQ6, 6 |
| Deceased donor | A3, X | B7, X | Cw7, X | DR15, X | DQ6, X |
| HLA-A | HLA-B | HLA-Cw | HLA-DR | HLA-DQ | |
| Case 5 | A3, 11 | B8, 48 | Cw7, 8 | DR1, 11 | DQ5, 7 |
| Original donor | A2, 26 | B62, X | Cw4, 9 | DR9, 13 | DQ6, 9 |
| Deceased donor | A1, 30 | B8, 62 | Cw7, 10 | DR1, 17 | DQ2, 5 |
Acknowledgments
The authors thank Katie Burke for excellent HLA database assistance and Glenn Chertow for review and critique of the manuscript. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K23AI104401. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
J. M. Yabu, Email: jyabu@stanford.edu.
M. J. Pando, Email: marpan@stanford.edu.
S. Busque, Email: sbusque@stanford.edu.
M.L. Melcher, Email: melcherm@stanford.edu.
References
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. The New England journal of medicine. 1999;341 (23):1725. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 2.Vo AA, Lukovsky M, Toyoda M, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. The New England journal of medicine. 2008;359 (3):242. doi: 10.1056/NEJMoa0707894. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed July 2012];Network OPTN. Scientific registry of transplant recipients: OPTN: data. 2011 Available at: http://optn.transplant.hrsa.gov/
- 4.Montgomery RA, Simpkins CE, Segev DL. New options for patients with donor incompatibilities. Transplantation. 2006;82 (2):164. doi: 10.1097/01.tp.0000226105.42713.37. [DOI] [PubMed] [Google Scholar]
- 5.Wahrmann M, Haidinger M, Kormoczi GF, et al. Effect of the proteasome inhibitor bortezomib on humoral immunity in two presensitized renal transplant candidates. Transplantation. 2010;89 (11):1385. doi: 10.1097/TP.0b013e3181d9e1c0. [DOI] [PubMed] [Google Scholar]
- 6.Stegall MD, Diwan T, Raghavaiah S, et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11 (11):2405. doi: 10.1111/j.1600-6143.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- 7.Vo AA, Peng A, Toyoda M, et al. Use of intravenous immune globulin and rituximab for desensitization of highly HLA-sensitized patients awaiting kidney transplantation. Transplantation. 2010;89 (9):1095. doi: 10.1097/TP.0b013e3181d21e7f. [DOI] [PubMed] [Google Scholar]
- 8.Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA-incompatible kidney recipients and survival. The New England journal of medicine. 2011;365 (4):318. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 9.Haririan A, Nogueira J, Kukuruga D, et al. Positive cross-match living donor kidney transplantation: longer-term outcomes. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9 (3):536. doi: 10.1111/j.1600-6143.2008.02524.x. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery RA, Zachary AA, Ratner LE, et al. Clinical results from transplanting incompatible live kidney donor/recipient pairs using kidney paired donation. JAMA: the journal of the American Medical Association. 2005;294 (13):1655. doi: 10.1001/jama.294.13.1655. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery RA. Renal transplantation across HLA and ABO antibody barriers: integrating paired donation into desensitization protocols. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10 (3):449. doi: 10.1111/j.1600-6143.2009.03001.x. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery RA, Lonze BE, Jackson AM. Using donor exchange paradigms with desensitization to enhance transplant rates among highly sensitized patients. Current opinion in organ transplantation. 2011;16 (4):439. doi: 10.1097/MOT.0b013e32834897c1. [DOI] [PubMed] [Google Scholar]
- 13.Guthoff M, Schmid-Horch B, Weisel KC, Haring HU, Konigsrainer A, Heyne N. Proteasome inhibition by bortezomib: Effect on HLA-antibody levels and specificity in sensitized patients awaiting renal allograft transplantation. Transpl Immunol. 2012 doi: 10.1016/j.trim.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Patel J, Everly M, Chang D, Kittleson M, Reed E, Kobashigawa J. Reduction of alloantibodies via proteosome inhibition in cardiac transplantation. J Heart Lung Transplant. 2011;30 (12):1320. doi: 10.1016/j.healun.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Segev DL, Gentry SE, Warren DS, Reeb B, Montgomery RA. Kidney paired donation and optimizing the use of live donor organs. JAMA: the journal of the American Medical Association. 2005;293 (15):1883. doi: 10.1001/jama.293.15.1883. [DOI] [PubMed] [Google Scholar]
- 16.Gentry SE, Segev DL, Montgomery RA. A comparison of populations served by kidney paired donation and list paired donation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5 (8):1914. doi: 10.1111/j.1600-6143.2005.00964.x. [DOI] [PubMed] [Google Scholar]
- 17.van den Berg-Loonen EM, Billen EV, Voorter CE, et al. Clinical relevance of pretransplant donor-directed antibodies detected by single antigen beads in highly sensitized renal transplant patients. Transplantation. 2008;85 (8):1086. doi: 10.1097/TP.0b013e31816b3ed1. [DOI] [PubMed] [Google Scholar]
- 18.Bingaman AW, Wright FH, Murphey CL. Kidney paired donation in live-donor kidney transplantation. The New England journal of medicine. 2010;363 (11):1091. doi: 10.1056/NEJMc1004959. [DOI] [PubMed] [Google Scholar]
- 19.Bingaman AW, Wright FH, Jr, Kapturczak M, Shen L, Vick S, Murphey CL. Single-Center Kidney Paired Donation: The Methodist San Antonio Experience. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012 doi: 10.1111/j.1600-6143.2012.04070.x. [DOI] [PubMed] [Google Scholar]
- 20.Gentry SE, Segev DL, Simmerling M, Montgomery RA. Expanding kidney paired donation through participation by compatible pairs. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7 (10):2361. doi: 10.1111/j.1600-6143.2007.01935.x. [DOI] [PubMed] [Google Scholar]