Abstract
Objectives
To investigate the effects of the selective Rho-kinase (ROCK) inhibitor azaindole-1 on erectile function under physiologic and pathophysiologic conditions in the rat.
Methods
The effect of intracavernosal (i.c.) injections of azaindole-1 on change in ICP, ICP/MAP, AUC, and response duration were investigated in the anesthetized rat under control conditions and when NANC neurotransmission and cholinergic function or sGC were inhibited or after cavernosal nerve crush injury.
Results
The i.c. injections of azaindole-1 produced dose-related increases in ICP/MAP and AUC that were long lasting at the highest doses studied when compared with the prototypical ROCK-inhibitor fasudil. Erectile responses were not altered by 7-NI and atropine in doses that reduced the response to cavernosal nerve stimulation by 86%, indicating that they were independent of NO release by cavernosal nerves or activation of muscarinic receptors in the corpora cavernosa. Erectile responses to azaindole-1 were not altered by the sGC inhibitor ODQ in a dose that attenuated responses to the NO donor SNP indicating that they were independent of an action on sGC. The erectile response to ic injections of azaindole-1 or Y-27632 which was reported to be NO/cGMP- dependent were not attenuated after cavernosal nerve crush injury.
Conclusions
The present studies indicate azaindole-1 has long lasting erectile activity that is independent of NO release, muscarinic receptor, or sGC activation or the integrity of the cavernosal nerves.
Keywords: Azaindole-1, selective Rho-kinase inhibitor, erectile dysfunction, oxidative stress, impaired cavernosal nerve function
Introduction
The small GTPase RhoA is a member of the Rho family of proteins that regulate cellular function including vascular smooth muscle contraction[1]. Rho kinases (ROCKs) are downstream effectors for RhoA that regulate calcium sensitivity of vascular and corporal smooth muscle. Activation of ROCK promotes vasoconstriction and it has been hypothesized that ROCK has an important role in maintaining the penis in a detumescent state by promoting vasoconstriction in small penile arteries and the corpora cavernosa[2]. It has been reported that ROCK-inhibitors induce penile erection by relaxing vascular smooth muscle in penile tissue by an NO independent mechanism and that these agents could be used in the treatment of erectile dysfunction (ED). Although prototypical ROCK-inhibitors such as Y-27632 or fasudil and analogs have been reported to have potent erectile activity in the rat, these agents also have inhibitory effects on a number of protein kinases including PKA that can alter vascular smooth muscle function[2–5]. In the present study we investigated erectile responses to azaindole-1, a highly selective ROCK-inhibitor with good pharmacokinetic properties that has little if any inhibitory effect on a large number of kinases[6, 7]. The results of these studies show that azaindole-1 produces potent, long lasting increases in erectile activity that is independent of NO released from cavernosal nerves, activation of muscarinic receptors or activation of sGC in the corpora cavernosa and that erectile responses to i.c. injection of azaindole-1 are not attenuated by acute cavernosal nerve crush injury in the rat.
Materials and Methods
The Institutional Animal Care and Use Committee of Tulane University School of Medicine approved the experimental protocol used in these studies, and all procedures were conducted in accordance with institutional guidelines. For these experiments, adult male Sprague–Dawley rats, weighing 334–447 g, were anesthetized with Inactin (thiobutabarbital), 100 mg/kg i.p. Supplemental doses of Inactin were given i.p. as needed to maintain a uniform level of anesthesia. Body temperature was maintained with a heating lamp. The trachea was cannulated with a short segment of PE-240 tubing to maintain a patent airway, and the left carotid artery was catheterized with PE-50 tubing for measurement of systemic arterial pressure. ICP was measured with a 25-gauge needle inserted into the left crura of the penis connected to PE-50 tubing filled with heparin. Systemic arterial pressure and ICP were measured with Namic Perceptor DT pressure transducers and a data acquisition system (Biopac MP 100A-CE, Santa Barbara, USA). ICP, systemic arterial pressure and MAP obtained by electronic averaging were continuously recorded and were displayed and stored on a Dell PC. The left jugular vein was catheterized with PE-50 tubing for the systemic administration of drugs and fluids. A 25-gauge needle connected to PE-50 tubing was placed in the right crura of the penis for administration of azaindole-1, fasudil, SNP, ODQ and Y-27632. Maximal ICP in response to i.c. injection of the vasodilator agents or in response to cavernosal nerve stimulation was measured at the peak of the pressure increase. The AUC and duration of the increase in ICP were measured to characterize the total erectile response.
Cavernosal nerve stimulation was carried out as previously described in the literature[3]. For nerve stimulation, the bladder and prostate were exposed through a midline abdominal incision. The cavernosal nerve was identified posterolateral to the prostate on one side, and a stainless steel bipolar stimulating electrode was placed on the nerve. The cavernosal nerve was stimulated with square wave pulses at a frequency of 10 Hz at 5V with a pulse width of 5 ms and a duration of 60 seconds with a Grass Instruments SD9 Stimulator. A rest period of at least 15 minutes was allowed between nerve stimulation trials.
The experiments in this study were designed to (i) characterize increases in ICP in response to i.c. injection of a wide range of doses of the ROCK-inhibitor azaindole-1, (ii) to compare responses to i.c. injections of azaindole-1 with responses to the prototypical ROCK-inhibitor fasudil and the NO donor SNP, (iii) to investigate the role of muscarinic receptor activation and nNOS in mediating increases in ICP in response to i.c. injection of azaindole-1 and fasudil, (iv) to investigate the response to the ROCK-inhibitors when sGC was inhibited with ODQ and (v) to investigate the effect of azaindole-1 and Y-27632 on the response to cavernosal nerve stimulation after acute cavernosal nerve crush injury.
In the first set of experiments responses to i.c. injections of azaindole-1 (1–100 μg/kg), fasudil (1–100 μg/kg) and SNP (0.1–10 μg/kg) were compared and changes in ICP, the ratio of ICP/MAP at maximum ICP, the duration of the increase in ICP and AUC were measured. I.c. injections of the vehicle for azaindole-1, fasudil, or SNP into the right crura had no significant effect on ICP.
In the second set of experiments, the effects of the nNOS inhibitor 7-NI and the muscarinic receptor antagonist atropine on the response to cavernosal nerve stimulation at 10 Hz and the ROCK-inhibitors were investigated. The cavernosal nerve was stimulated before and 15 minutes after administration of 7-NI, 10 mg/kg i.v., and then atropine, 1 mg/kg i.v., was administered and the nerve was again stimulated to evaluate the combined effect of muscarinic receptor antagonism and nNOS inhibition on the response to cavernosal nerve stimulation at 10 Hz. Responses to i.c. injections of azaindole-1 and fasudil 30 μg/kg were investigated after treatment with 7-NI and atropine.
In the third set of experiments the effects of the sGC inhibitor ODQ on responses to the ROCK-inhibitors and SNP were investigated. In these experiments i.c. injection of SNP 1 μg/kg was given before and after i.c. administration of ODQ 2 mg/kg to assess the effect of the sGC inhibitor on the response to the NO donor. The effects of i.c. injection of azaindole-1 30 μg/kg and fasudil 30 μg/kg were determined in these animals before and after treatment with ODQ.
In the last set of experiments the effects of azaindole-1 and Y-27632 on the erectile response after acute cavernosal nerve crush injury was investigated. The nerve was crushed with three 15 second applications of a 3 inch forcep 5 mm distal to the major pelvic ganglion.
The use of a chronic model of cavernosal nerve injury may be more relevant to the clinical situation where structural changes in the corpra cavernosum may occur. However the use of an acute model can provide information on the effect of removal of tonic nerve activity on the response to the Rho kinase inhibitor.
Azaindole-1 (6-chloro-N4-{3,5-difluoro-4-[(3-methyl-1H-pyrrolo[2,3-b]pyridin-4-yl)oxy]-phenyl}pyrimidine-2,4-diamine) was obtained from Dr. Johannes-Peter Stasch of the Institute of Cardiovascular Research, Pharma Research Centre, Bayer, Wuppertal, Germany, and was dissolved in Transcutol-Cremophor EL-0.9% NaCl solution (10:10:80). ODQ was also dissolved in the Transcutol-Cremophor vehicle. Fasudil and Y-27632 were dissolved in 0.9% NaCl solution. 7-Nitroindazole was dissolved in DMSO (Sigma-Aldrich). For i.c. injections the doses of agents were prepared in a 200 μl volume and were injected through the 25 gauge needle in the right crura. The data are expressed as mean ± SE and were analyzed using a one-way ANOVA followed by a Dunnett’s post hoc test or a two-tailed student’s t-test for paired data. A p value of less than 0.05 was the criterion for statistical significance.
Results
Erectile responses to azaindole-1 and fasudil
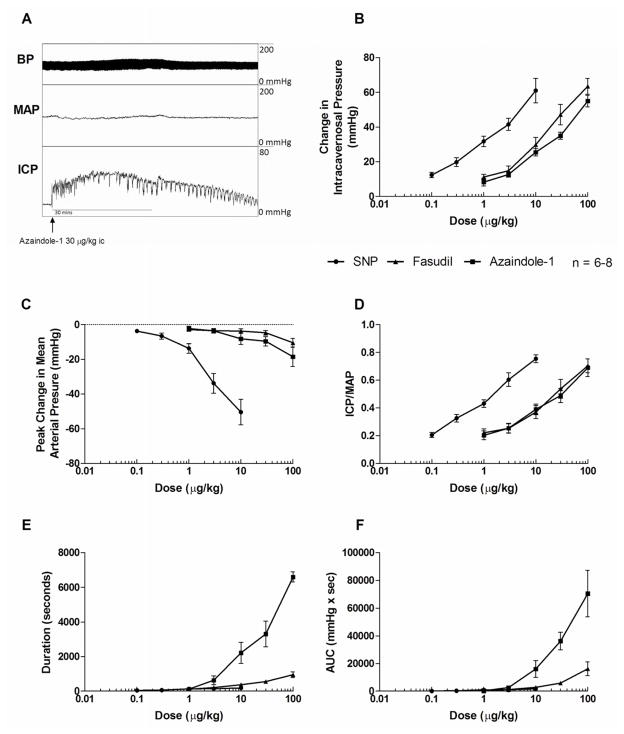
Erectile responses to the ROCK-inhibitor azaindole-1 were investigated in the anesthetized rat and i.c. injections of azaindole-1 and fasudil in doses of 1–100 μg/kg produced dose-related increases in ICP, ICP/MAP, AUC, duration and decreases in MAP (Fig. 1). Responses to i.c. injections of azaindole-1 were rapid in onset (20–40 seconds) and long in duration (6598 seconds at the highest dose studied). Representative tracings showing an increases in ICP in response to i.c. injection of azaindole-1 30 μg/kg are shown in figure 1A and erectile responses to azaindole-1 and the prototypical ROCK-inhibitor fasudil are compared (Fig 1). The increases in ICP/MAP in response to i.c. injections of azaindole-1 and fasudil were similar whereas the AUC and duration of the ICP change in response to azaindole-1 were significantly greater when compared to fasudil (Fig. 1). Both ROCK-inhibitors produced similar decreases in MAP when injected i.c. (Fig. 1B).
Figure 1.
Representative tracing showing changes in ICP, MAP, systolic and diastolic BP following intracavernosal injection of azaindole-1 30 μg/kg (A). Line graphs comparing changes in ICP (B), peak changes in MAP (C), absolute values for ICP/MAP (D), AUC (E) and response duration (F) in response to intracavernosal injection of a wide range of doses of azaindole-1, fasudil and sodium nitroprusside (SNP). n indicates number of experiments.
Responses when nNOS is inhibited and muscarinic receptors are blocked
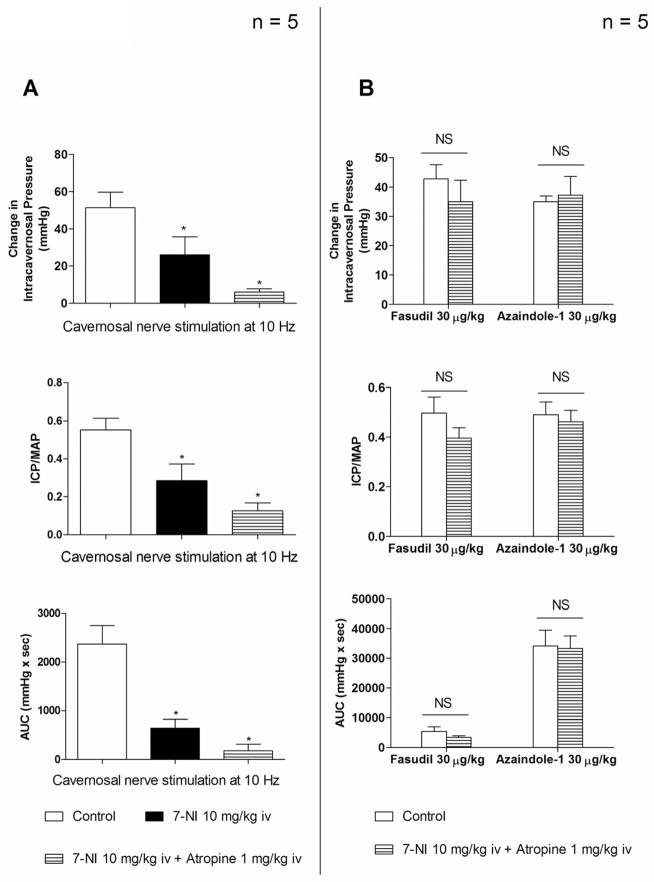
Responses to i.c. injections of azaindole-1 and fasudil were investigated in experiments in which nNOS was inhibited by 7-NI and muscarinic receptors were blocked with atropine. The i.v. injection of 7-NI 10 mg/kg and atropine 1 mg/kg reduced the response to cavernosal nerve stimulation at 10 Hz by 86 % (Fig. 2A). After the response to cavernosal nerve stimulation was significantly reduced by treatment with 7-NI and atropine the i.c. injection of azaindole-1 or fasudil at a dose of 30 μg/kg produced increases in ICP, ICP/MAP and AUC that were not significantly different than responses to the two ROCK-inhibitors under control conditions (Fig. 2B).
Figure 2.
Bar graphs comparing changes in ICP, absolute values for ICP/MAP and AUC (A) in response to cavernosal nerve stimulation at 10 Hz before and after administration of 7-NI 10 mg/kg iv and atropine 1 mg/kg iv. Bar graphs comparing changes in ICP, absolute values for ICP/MAP and AUC (B) in response to intracavernosal injection of azaindole-1 and fasudil 30 μg/kg before and after treatment with 7-NI 10 mg/kg iv and atropine 1 mg/kg iv. n indicates number of experiments, * indicates p < 0.05 when compared to control using a two-tailed Student t test. NS indicates no significant difference using a two-tailed student t test.
Effect of ODQ
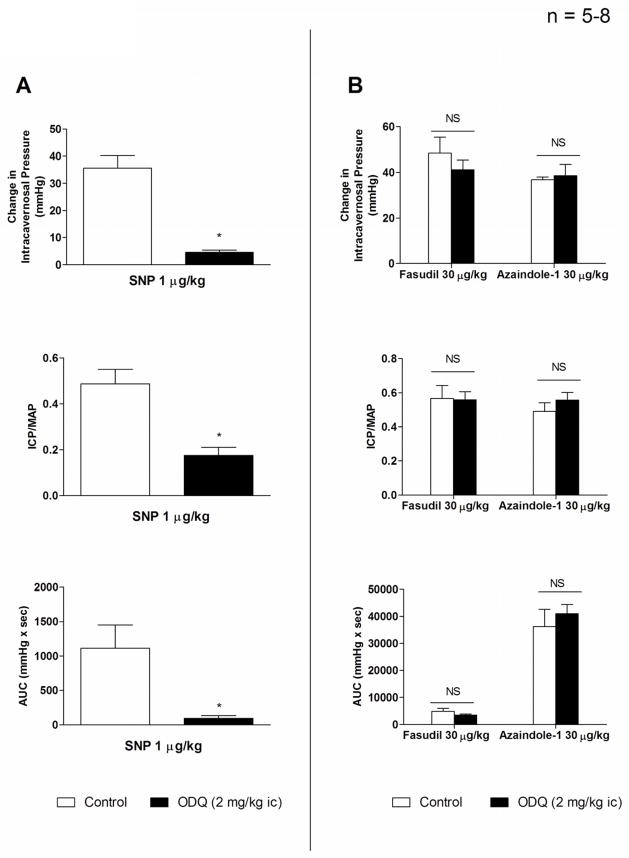
The effect of the sGC inhibitor ODQ on erectile responses to SNP, azaindole-1 and fasudil was investigated in the anesthetized rat. The i.c. injection of SNP 1 μg/kg produced a significant increase in ICP, ICP/MAP, and AUC (Fig. 3A). Following treatment with ODQ in a dose of 2 mg/kg i.c. the increases in ICP, ICP/MAP and AUC in response to i.c. injection of SNP are significantly decreased (Fig. 3A). Following administration of ODQ the increases in ICP, ICP/MAP and AUC in response to i.c. injections of azaindole-1 or fasudil 30 μg/kg were not different than responses obtained under control conditions (Fig. 3B).
Figure 3.
Bar graphs comparing changes in ICP, absolute values for ICP/MAP and AUC (A) in response to intracavernosal injection of SNP 1 μg/kg before and after treatment with ODQ 2 mg/kg i.c. Bar graphs comparing changes in ICP, absolute values for ICP/MAP and AUC (B) in response to intracavernosal injection of azaindole-1 30 μg/kg and fasudil 30 μg/kg before and after treatment with ODQ 2 mg/kg i.c. n indicates number of experiments, * indicates p < 0.05 when compared to response under control conditions using a two tailed student t test, NS indicates no significant difference with two tailed student t test.
Response to azaindole-1 after cavernosal nerve crush injury
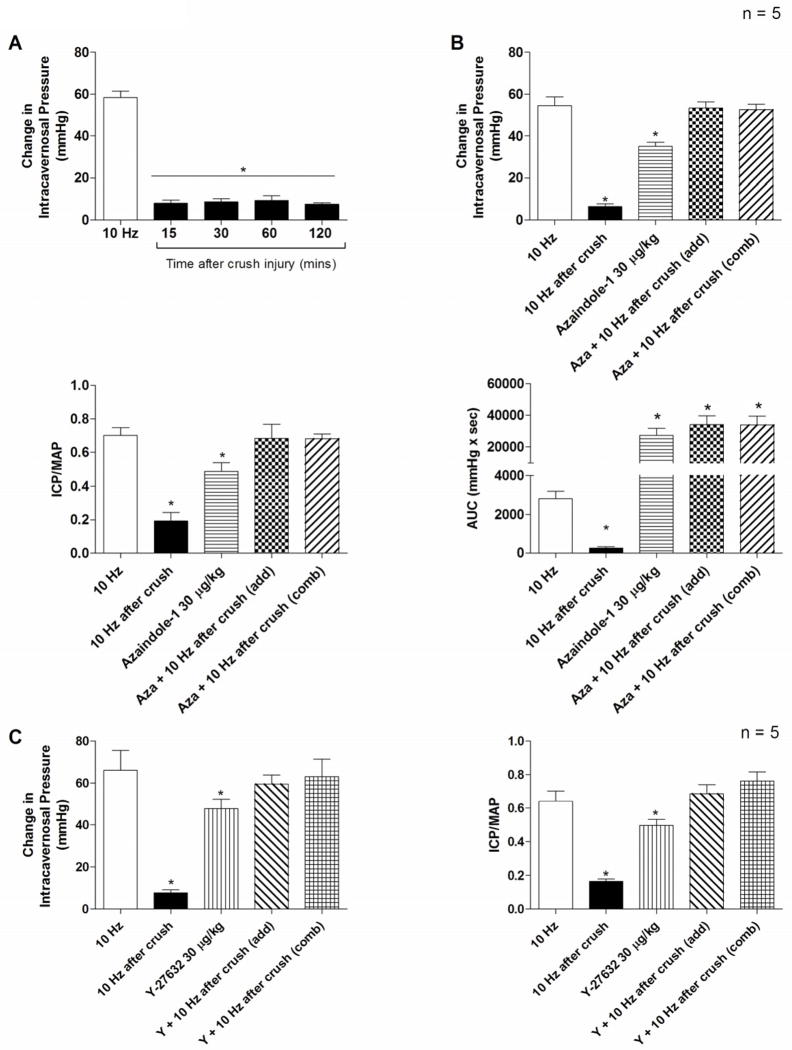
The effect of azaindole-1 and Y-27632 on erectile function after acute cavernosal nerve injury was investigated and these data are summarized in figure 4. Acute nerve injury induced by forcep compression of the cavernosal nerve decreased the response to electrical stimulation of the cavernosal nerve. The increase in ICP in response to cavernosal nerve stimulation at 10 Hz was reduced by 89 % and the response to cavernosal nerve stimulation did not return to control value over the 2 hour period experiments were carried out (Fig. 4A). The i.c. injection of azaindole-1 30 μg/kg or Y-27632 30 μg/kg produced an increase in ICP that was not significantly different from the response observed in control animals (Figs. 1 and 4). The increase in response to i.c. injection of azaindole-1 30 μg/kg or Y-27632 30 μg/kg during cavernosal nerve stimulation at 10 Hz following nerve crush injury produced an increase in the erectile response that was not significantly different than the algebraic sum of the responses to nerve stimulation at 10 Hz and i.c. injection of azaindole-1 30 μg/kg or Y-27632 30 μg/kg separately (Fig. 4B and 4C). The observation that the algebraic sum of the erectile response to i.c. injection of azaindole-1 or Y-27632 and cavernosal nerve stimulation at 10 Hz are similar indicate that the increases in ICP are additive and that the Rho-kinase inhibitors do not enhance the nerve mediated erectile response.
Figure 4.
Bar graphs comparing increases in ICP in response to cavernosal nerve stimulation at 10 Hz before (open bar) and at 15, 30, 60 and 120 mins after acute nerve crush injury (black bar) (A), * indicates p < 0.05 using a One way ANOVA with a Dunnett’s post hoc test comparing all values to control, n indicates number of experiments.
Bar graphs comparing the changes in ICP, absolute values for ICP/MAP, and AUC in response to cavernosal nerve stimulation (10Hz) in the control period (open bar), following nerve crush injury (black bar), i.c. injection of azaindole-1 (30 μg/kg) (hatched bar), the algebraic sum of response to azaindole-1 and nerve stimulation at 10 Hz following crush injury (checked bar) and the combined response to simultaneous nerve stimulation following crush injury at 10 Hz and i.c. injection of azaindole-1 (30 μg/kg) (slanted bar) (B), n indicates number of experiments, * indicates p < 0.05, using a One way ANOVA with a Dunnett’s post hoc test comparing all values to control.
Bar graphs comparing changes in ICP and absolute values for ICP/MAP in response to cavernosal nerve stimulation (10 Hz) in the control period (open bar), following nerve crush injury (solid bar), i.c. injection of Y-27632 (30 μg/kg) (vertical hatched bar), the algebraic sum of response to Y-27632 and nerve stimulation at 10 Hz following crush injury (slanted bar), and the combined response to simultaneous nerve stimulation following crush injury at 10 Hz and i.c. injection of Y-27632 (30 μg/kg) (squared bar) (C), n indicates number of experiments, * indicates p < 0.05, using a One way ANOVA with a Dunnett’s post hoc test comparing all values to control.
Comment
New findings in this study are that the selective ROCK-inhibitor, azaindole-1, had potent long lasting erectile activity that is independent of NANC neurotransmission, muscarinic receptor or sGC activation and that the response to i.c. injection of azaindole-1 was not attenuated after acute cavernosal nerve injury. The present results show that i.c. injections of azaindole-1 produced dose-related increases in ICP in the anesthetized rat that were similar to responses to the prototypical ROCK-inhibitor fasudil when doses were expressed on a μg/kg basis, however the AUC and duration of the increase in ICP were greater for azaindole-1 than for fasudil. Both ROCK-inhibitors were less potent than the NO donor SNP and had a longer duration of action than SNP.
The role of NO release from cavernosal nerves and of muscarinic receptors in mediating the increase in ICP in response to the ROCK-inhibitors were investigated and responses were not altered by treatment with the nNOS inhibitor 7-NI or atropine which attenuated the increase in ICP in response to cavernosal nerve stimulation by 86%. These data indicate that the increases in ICP in response to azaindole-1 and fasudil are independent of an effect on NO released from the cavernosal nerves or activation of muscarinic receptors in the corpora cavernosa. These data are consistent with studies in the literature showing that the response to Y-27632, the first widely studied ROCK-inhibitor, are not modified by treatment with the nonselective NOS inhibitor L-NAME[4].
The present results show that increases in ICP in response to azaindole-1 and to Y-27632 are not altered by acute cavernosal nerve crush injury. These results are consistent with a previous study in which responses to Y-27632 were not modified by inhibition of NANC function but are not in agreement with studies in which the response to Y-27632 was reduced in in-vitro preparations from diabetic or L-NAME treated animals[8].
The effect of inhibition of sGC with ODQ on erectile responses was investigated and following treatment with ODQ, the increase in ICP in response to i.c. injection of SNP was reduced by 87 %. ODQ had no significant effect on the increase in ICP/MAP or AUC in response to i.c. injection of azaindole-1 or fasudil. These data provide support for the hypothesis that erectile responses to azaindole-1 and fasudil are independent of activation of sGC in rat penile tissue. These data are consistent with results showing that ODQ did not alter relaxation responses to the ROCK-inhibitors H-1152 and Y-27632 in phenylephrine precontracted rat corpora cavernosal strips[9]. ODQ has been reported to inhibit the response to NO by oxidizing the heme iron on the β subunit sGC[10]. ODQ has been shown to inhibit the activation of sGC by NO in a large number of isolated tissue studies and to inhibit responses to NO donors in a few in vivo studies[11–14]. NO binds to the reduced form of sGC and increases the conversion of GTP to cGMP promoting vascular and cavernosal smooth muscle relaxation and penile erection. When sGC is oxidized by ODQ the response to NO is decreased and vasorelaxation and the erectile response are inhibited.
The present results and previous studies provide support for the concept that ROCK-inhibitors would be useful in the treatment of ED caused by impaired NO release, impaired muscarinic receptor activation or impaired activation of sGC by NO[15–18]. Penile erection involves a complex interaction between the central nervous system and local mediators[19]. Although it has been hypothesized that peptides such as CGRP and VIP play a role in erection NO is now considered to be the main mediator of penile erection[20, 21]. The release of NO from the nerves innervating penile tissue and the endothelium mediated by muscarinic receptors activates sGC, increasing cGMP levels. This results in vascular and cavernosal smooth muscle relaxation and erection[20]. Agents or disease processes that impair NO formation, release, or bioavailability and NO-sGC-cGMP signaling induce ED and agents that improve NO-sGC-cGMP signaling improve erectile function [22, 23]. The ability of the ROCK-inhibitors to improve erectile responses when NO formation, release or signaling are impaired or cavernosal nerve injury has occurred in disease states such as hypertension, diabetes or atherosclerosis or after surgical procedures suggest that these agents may be useful in the treatment of ED[3, 24, 25].
A number of studies suggest that ROCK is involved in the pathogenesis of a variety of cardiovascular diseases including those associated with ED[4, 26, 27]. Much information has been learned from studies with Y-27632 or fasudil which has been approved for the treatment of cerebral vasospasm in subarachnoid hemorrhage[28, 29]. Although fasudil and Y-27632 have been useful in defining the role of ROCK in physiologic and pathophysiologic processes, these agents have nonspecific inhibitory effects on other kinases such as PKA which can alter vascular smooth muscle function[5]. Azaindole-1 is a highly selective, orally effective ROCK-inhibitor which has little if any effect on a number of kinases involved in smooth muscle function and has been shown to have a favorable effect in a number of cardiovascular disorders[7, 30]. The results of the present study indicate that azaindole-1 has potent, long lasting erectile activity in the rat and suggest that it may be useful in the treatment of ED.
The results with azaindole-1 are similar to data with SAR407899 another novel Rho-kinase inhibitor without NO-cGMP dependence that has recently been shown to exert long lasting erectile responses in animals after oral administration and did not lose efficiacy in diabetic or hypertensive animals[8]. The results with potent long lasting Rho-kinase inhibitors such as azaindole-1 or SAR407899 suggest that these agents may be useful in the treatment of patients with severe ED. However the potential to induce priapism with the use of long acting ROCK inhibitors should be considered.
The interaction of ROCK and endogenous NO was also investigated in experiments in which the response to cavernosal nerve stimulation and i.c. injection of the ROCK-inhibitors were compared when the interventions were administered separately or together. These results suggest that responses to the ROCK-inhibitors and cavernosal nerve stimulation are additive. These results are consistent with studies in which Y-27632 was injected i.c. and L-NAME was used to inhibit NOS in the rat [4]. The present data are not in agreement with experiments in which the fasudil analog H-1152 was injected i.p. and the response to cavernosal nerve stimulation was enhanced at stimulus frequencies of 1–4 Hz[9]. The reason for the difference is uncertain but may involve differences in experimental design, route of administration or the ROCK-inhibitor used in the study[9].
Conclusions
In summary, the results of the present study show that azaindole-1, a selective ROCK-inhibitor, had potent long lasting erectile activity in the anesthetized rat. Erectile responses to azaindole-1 were independent of NO released from nerves innervating penile tissue or the effect of muscarinic receptor stimulation or sGC activation in the corpora cavernosa. These data show that the erectile response to azaindole-1 is not impaired after acute cavernosal nerve crush injury. These data suggest that azaindole-1 may be useful in the treatment of ED when NANC or cholinergic innervation is impaired, the NO-sGC-cGMP signaling pathway is inhibited or cavernosal nerve injury has occurred.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, et al. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275(28):21722–9. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- 2.Mills TM, Chitaley K, Wingard CJ, Lewis RW, Webb RC. Effect of Rho-kinase inhibition on vasoconstriction in the penile circulation. J Appl Physiol. 2001;91(3):1269–73. doi: 10.1152/jappl.2001.91.3.1269. [DOI] [PubMed] [Google Scholar]
- 3.Bivalacqua TJ, Champion HC, Usta MF, et al. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2004;101(24):9121–6. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chitaley K, Wingard CJ, Clinton Webb R, et al. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med. 2001;7(1):119–22. doi: 10.1038/83258. [DOI] [PubMed] [Google Scholar]
- 5.Breitenlechner C, Gassel M, Hidaka H, et al. Protein kinase A in complex with Rho-kinase inhibitors Y-27632, Fasudil, and H-1152P. structural basis of selectivity. Structure. 2003;11(12):1595–607. doi: 10.1016/j.str.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Kast R, Schirok H, Figueroa-Perez S, et al. Cardiovascular effects of a novel potent and highly selective azaindole-based inhibitor of Rho-kinase. Br J Pharmacol. 2007;152(7):1070–80. doi: 10.1038/sj.bjp.0707484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pankey EA, Byun RJ, Smith WB, 2nd, et al. The Rho kinase inhibitor azaindole-1 has long-acting vasodilator activity in the pulmonary vascular bed of the intact chest rat. Can J Physiol Pharmacol. 90(7):825–35. doi: 10.1139/y2012-061. [DOI] [PubMed] [Google Scholar]
- 8.Guagnini F, Ferazzini M, Grasso M, Blanco S, Croci T. Erectile properties of the Rho-kinase inhibitor SAR407899 in diabetic animals and human isolated corpora cavernosa. J Transl Med. 10:59. doi: 10.1186/1479-5876-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teixeira CE, Ying Z, Webb RC. Proerectile effects of the Rho-kinase inhibitor (S)-(+)- 2-methyl-1-[(4-methyl-5-isoquinolinyl)sulfonyl]homopiperazine (H-1152) in the rat penis. J Pharmacol Exp Ther. 2005;315(1):155–62. doi: 10.1124/jpet.105.086041. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y, Brandish PE, DiValentin M, Schelvis JP, Babcock GT, Marletta MA. Inhibition of soluble guanylate cyclase by ODQ. Biochemistry. 2000;39(35):10848–54. doi: 10.1021/bi9929296. [DOI] [PubMed] [Google Scholar]
- 11.Pankey EA, Bhartiya M, Badejo AM, Jr, et al. Pulmonary and systemic vasodilator responses to the soluble guanylyl cyclase activator, BAY 60–2770, are not dependent on endogenous nitric oxide or reduced heme. Am J Physiol Heart Circ Physiol. 2011;300(3):H792–802. doi: 10.1152/ajpheart.00953.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh GC, O’Neill AB, Moreland RB, Sullivan JP, Brioni JD. YC-1 potentiates the nitric oxide/cyclic GMP pathway in corpus cavernosum and facilitates penile erection in rats. Eur J Pharmacol. 2003;458(1–2):183–9. doi: 10.1016/s0014-2999(02)02730-9. [DOI] [PubMed] [Google Scholar]
- 13.Stasch JP, Schmidt PM, Nedvetsky PI, et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest. 2006;116(9):2552–61. doi: 10.1172/JCI28371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt HH, Schmidt PM, Stasch JP. NO- and haem-independent soluble guanylate cyclase activators. Handb Exp Pharmacol. 2009;(191):309–39. doi: 10.1007/978-3-540-68964-5_14. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan ME, Thompson CS, Dashwood MR, et al. Nitric oxide and penile erection: is erectile dysfunction another manifestation of vascular disease? Cardiovasc Res. 1999;43(3):658–65. doi: 10.1016/s0008-6363(99)00135-2. [DOI] [PubMed] [Google Scholar]
- 16.Bivalacqua TJ, Usta MF, Champion HC, Kadowitz PJ, Hellstrom WJ. Endothelial dysfunction in erectile dysfunction: role of the endothelium in erectile physiology and disease. J Androl. 2003;24(6 Suppl):S17–37. doi: 10.1002/j.1939-4640.2003.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 17.Park K, Kim SW, Rhu KS, Paick JS. Chronic administration of an oral Rho kinase inhibitor prevents the development of vasculogenic erectile dysfunction in a rat model. J Sex Med. 2006;3(6):996–1003. doi: 10.1111/j.1743-6109.2006.00327.x. [DOI] [PubMed] [Google Scholar]
- 18.Murat N, Soner BC, Demir O, Esen A, Gidener S. Contractility of diabetic human corpus cavernosum smooth muscle in response to serotonin mediated via Rho-kinase. Pharmacology. 2009;84(1):24–8. doi: 10.1159/000221380. [DOI] [PubMed] [Google Scholar]
- 19.Priviero FB, Leite R, Webb RC, Teixeira CE. Neurophysiological basis of penile erection. Acta Pharmacol Sin. 2007;28(6):751–5. doi: 10.1111/j.1745-7254.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 20.Rajfer J, Aronson WJ, Bush PA, Dorey FJ, Ignarro LJ. Nitric oxide as a mediator of relaxation of the corpus cavernosum in response to nonadrenergic, noncholinergic neurotransmission. N Engl J Med. 1992;326(2):90–4. doi: 10.1056/NEJM199201093260203. [DOI] [PubMed] [Google Scholar]
- 21.Trigo-Rocha F, Aronson WJ, Hohenfellner M, Ignarro LJ, Rajfer J, Lue TF. Nitric oxide and cGMP: mediators of pelvic nerve-stimulated erection in dogs. Am J Physiol. 1993;264(2 Pt 2):H419–22. doi: 10.1152/ajpheart.1993.264.2.H419. [DOI] [PubMed] [Google Scholar]
- 22.Maas R, Schwedhelm E, Albsmeier J, Boger RH. The pathophysiology of erectile dysfunction related to endothelial dysfunction and mediators of vascular function. Vasc Med. 2002;7(3):213–25. doi: 10.1191/1358863x02vm429ra. [DOI] [PubMed] [Google Scholar]
- 23.Jeremy JY, Ballard SA, Naylor AM, Miller MA, Angelini GD. Effects of sildenafil, a type-5 cGMP phosphodiesterase inhibitor, and papaverine on cyclic GMP and cyclic AMP levels in the rabbit corpus cavernosum in vitro. Br J Urol. 1997;79(6):958–63. doi: 10.1046/j.1464-410x.1997.00206.x. [DOI] [PubMed] [Google Scholar]
- 24.Gratzke C, Strong TD, Gebska MA, et al. Activated RhoA/Rho kinase impairs erectile function after cavernous nerve injury in rats. J Urol. 2010;184(5):2197–204. doi: 10.1016/j.juro.2010.06.094. [DOI] [PubMed] [Google Scholar]
- 25.Bivalacqua TJ, Deng W, Champion HC, Hellstrom WJ, Kadowitz PJ. Gene therapy techniques for the delivery of endothelial nitric oxide synthase to the corpora cavernosa for erectile dysfunction. Methods Mol Biol. 2004;279:173–85. doi: 10.1385/1-59259-807-2:173. [DOI] [PubMed] [Google Scholar]
- 26.Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005;25(9):1767–75. doi: 10.1161/01.ATV.0000176193.83629.c8. [DOI] [PubMed] [Google Scholar]
- 27.Usta MF, Bivalacqua TJ, Koksal IT, Toptas B, Surmen S, Hellstrom WJ. The protective effect of aminoguanidine on erectile function in diabetic rats is not related to the timing of treatment. BJU Int. 2004;94(3):429–32. doi: 10.1111/j.1464-410X.2004.04937.x. [DOI] [PubMed] [Google Scholar]
- 28.Rikitake Y, Kim HH, Huang Z, et al. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36(10):2251–7. doi: 10.1161/01.STR.0000181077.84981.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Usta MF, Bivalacqua TJ, Tokatli Z, et al. Stratification of penile vascular pathologies in patients with Peyronie’s disease and in men with erectile dysfunction according to age: a comparative study. J Urol. 2004;172(1):259–62. doi: 10.1097/01.ju.0000132154.38285.c7. [DOI] [PubMed] [Google Scholar]
- 30.Dahal BK, Kosanovic D, Pamarthi PK, et al. Therapeutic efficacy of azaindole-1 in experimental pulmonary hypertension. Eur Respir J. 2010;36(4):808–18. doi: 10.1183/09031936.00140309. [DOI] [PubMed] [Google Scholar]