Abstract
Objective
The purpose of the present study is to compare efficacy and safety of buccal midazolam with intravenous diazepam in control of seizures in Iranian children.
Methods
This is a randomized clinical trial. 92 patients with acute seizures, ranging from 6 months to 14 years were randomly assigned to receive either buccal midazolam (32 cases) or intravenous diazepam (60 cases) at the emergency department of a children's hospital. The primary outcome of this study was cessation of visible seizure activity within 5 minutes from administration of the first dosage. The second dosage was used in case the seizure remained uncontrolled 5 minutes after the first one.
Findings
In the midazolam group, 22 (68.8%) patients were relieved from seizures in 10 minutes. Meanwhile, diazepam controlled the episodes of 42 (70%) patients within 10 minutes. The difference was, however, not statistically significant (P=0.9). The mean time required to control the convulsive episodes after administration of medications was not statistically significant (P=0.09). No significant side effects were observed in either group. Nevertheless, the risk of respiratory failure in intravenous diazepam is greater than in buccal midazolam.
Conclusion
Buccal midazolam is as effective as and safer than intravenous diazepam in control of seizures.
Keywords: Midazolam, Diazepam, Seizure, Buccal Drug Administration, Intravenous Injections, Childhood
Introduction
Recurrent paroxysmal events in children involve a wide range of differential diagnoses. Neurological and cardiac disorders are the most important contributing factors. Seizures lie among prevalent diseases of childhood and occur in 10% of children[1]. In the emergency setting, the intravenous route is considered as the most suitable method, delivering adequate quantities of benzodiazepines in a short time[2]. However, when intravenous administration is not available, other forms of benzodiazepine administration such as rectal diazepam or buccal midazolam may offer an alternative way of drug administration[3].
Midazolam, a potent anticonvulsant, is commonly used intravenously and at times intramuscularly. Benzodiazepines contain an imidazole ring which is highly water soluble and is rapidly absorbed from rectal, nasal, and buccal mucosa. The ring is also highly lipophilic at physiologic pH, a characteristic that facilitates its rapid effect on the central nervous system[4]. A few studies have confirmed both efficacy and safety of buccal midazolam as well as rectal diazepam[6–10]. Meanwhile, few studies have reported severe respiratory depression following buccal midazolam administration; a fact that might have resulted from using high doses of the medication[6, 8]. In the study conducted by Thomas Marshal[11], buccal midazolam was found effective in seizure attacks but it was mentioned that the medication was unlicensed for such a purpose.
These findings shed light on the necessity to perform further studies to evaluate the efficacy and potential side effects of buccal midazolam. The purpose of this study was to determine whether buccal midazolam is efficient in control of convulsive episodes in children irrespective of the etiology of the seizure in comparison with intravenous diazepam, namely, the best accepted way of acute seizure episodes therapy[1, 2, 9].
Subjects and Methods
Patients
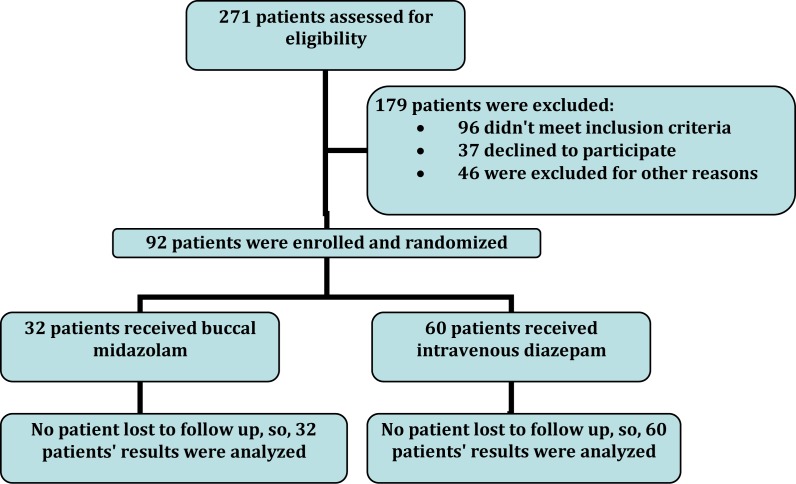
This study was approved by the Clinical Ethics Committee of Shahid Beheshti University of Medical Sciences. 271 children, aged 6 months to 14 years, admitted to the pediatric emergency ward of Mofid Children's Hospital due to seizure diagnosis between October 2007 and September 2008. Mofid Children's Hospital has full-time medical and nursing staff on site. The inclusion criteria dictated that the patients needed to fulfill the following criteria: (1) documented seizure persisting at the time of administration of anticonvulsant; (2) types of atonic, tonic and tonic-clonic seizures; (3) seizure lasting for more than 5 min. The exclusion criteria were: (1) patients who received intravenous diazepam or other benzodiazepines within 24 hours prior to presentation of the seizure; (2) previous history of narrow angle acute glaucoma; (3) doubt about the historical information given by patient's family. After history taking and physical examination, 46 patients had conditions that mimicked seizure like drugs side effects (e.g. extrapyramidal movement due to metoclopramide), movement disorders (like myoclonic jerks), etc. 96 of 271 patients did not meet inclusion criteria and 37 declined to participate. So, 92 children (51 boys, 41 girls) were enrolled in the study (Fig 1).
Fig. 1.
Flow diagram of patients
Consecutive patients were enrolled and were randomized to receive either buccal midazolam or intravenous diazepam. A random number table was used for randomization.
Evaluation
The primary outcome variable was clinical cessation of overt seizure activity[12]. If the seizure was not controlled within five minutes of administration of buccal midazolam or intravenous diazepam, the second dose of the same drug was given to patient. In the event that the seizure remained uncontrolled within ten minutes after the first buccal midazolam or intravenous diazepam administration, either phenobarbital or phenytoin was used as the second line antiepileptic.
Dosages of the medications administered
Buccal midazolam (Epistatus, Midazolam Buccal liquid, and Midazolam Maleate) was used with following doses: 2.5 mg for children aged 6-12 months, 5 mg for 1-4 years, 7.5 mg for 5-9 years, and 10 mg for 10 years or older. Buccal midazolam in the appropriate dose was drawn into a syringe. Children received buccal midazolam by placing the syringe between their teeth and cheek, and after drug administration the cheek was gently massaged. Intravenous diazepam was administered in a dosage of 0.3 mg/kg/dose and through an intravenous line as usual.
Study procedures
Screening and subsequent enrollment of the patients was performed consecutively. All the nurses and doctors of the emergency ward were aware of the study and helped our team for administration of drugs as well as follow up of the patients and completing the information sheet. In case the patient fulfilled the inclusion criteria, an informed consent was provided after a parent or legal guardian was briefed about the study purpose and procedures. In the next step, the patient was randomly assigned into one of the two treatment groups, either buccal midazolam (first group) or intravenous diazepam (second group). In order to record the underlying causes of convulsions, patient's history, physical examination, and laboratory evaluations including determination of electrolytes and glucose were considered for all patients. Neuroimaging was performed whenever required. Heart rate, respiratory rate, blood pressure, and hemoglobin oxygen saturation were monitored continuously all through the procedure. During the seizure, oxygen was administered by nasal prongs. Patients were followed up for 24 hours after drug administration. Any side effects including apnea, hypotension, bradycardia,… due to drug administration were also recorded.
The duration of seizures before buccal midazolam or intravenous diazepam therapy was approximate, based on the history obtained from the patient's attendants and other family members. For each patient, the time of noticing the first convulsion at the arrival in emergency, the time of administrating the drugs and the time of cessation of all motor seizure activity were recorded. The drug administration was considered as “FAILED” in case the convulsive motor activities did not stop within 10 minutes of administration. Nobody was lost to follow up in both groups, because the results of intervention were recorded on site and all patients were admitted for at least 24 hours.
Statistical analysis
This study was a prospective randomized controlled clinical trial (interventional). Every patient included in the study, was assigned by a number in series and the numbering was based upon ‘Table of random sampling’ for grouping them in case and control groups. Differences between proportions were statically tested by Chi-square or Fisher's exact test. All other numerical or quantitative comparisons were performed using Student's unpaired t-test. A P-value of 0.05 or less was considered significant. SPSS version 17.0 was used for most analyses.
Findings
Fifty-one (55.4%) out of 92 participants were male and 41 (44.6%) female. In the buccal midazolam group, 14 patients were male and 18 were female while in the intravenous diazepam group, 37 were male and 23 were female (P=0.1). The mean age was 17.5±10.1 months (6 to 60 months). The median age in buccal midazolam group was 18.4±10.3 and in the other group was 17.1±10.1. This difference was not statistically significant (P=0.6). 82% of patients’ age was equal or less than 24 months with mean weight of 10.6±2.73kg (6 to 18kg). Twenty (21.8%) patients had tonic seizure, 58 (63%) had tonic-clonic seizures and others (15.2%) had atonic seizure. In the buccal midazolam group, 9 patients had tonic seizures, 18 had tonic-clonic and 5 had atonic seizures. In intravenous diazepam group, 11 had tonc seizures, 40 had tonic-clonic and 9 had atonic seizures. There was no statistically significant difference in the seizure types between the two groups (P=0.5). The etiologies included idiopathic epilepsy 64 (70%), febrile seizure 9 (10%), and cryptogenic or symptomatic epilepsy 19 (20%). Underlying disorders associated with the symptomatic or cryptogenic seizure episodes has been shown in table 1. In our study, 32 (34.8%) of patients received buccal midazolam and 60 (65.2%) intravenous diazepam. None of the patients had received any treatment before admission. Comparison of the two treatment responses is illustrated in Table 2 and Fig 2. In the first group, the first administration was effective in controlling the seizures in 13 (40%) whereas in the second, seizures were completely controlled after the first dosage in 24 (40%) (P=0.9). Overall, 22 (68.7%) of the patients in the first group were relieved from seizures after the first or the second dosage whereas the applied medication controlled seizures in 42 (70%) patients of the second group (P=0.9).
Table 1.
Underlying disorders associated with the symptomatic or cryptogenic seizure episodes
| Cause | Number (%) |
|---|---|
| Developmental delay | 5 (5.4) |
| Mental retardation | 4 (4.3) |
| Metabolic disease | 1 (1.1) |
| Structural abnormality of the brain | 3 (3.3) |
| Others | 6 (6.5) |
Table 2.
Control of seizures and seizure control time in two groups
| Parameter | Buccal midazolam | IV diazepam | P-value |
|---|---|---|---|
| Seizure control time after first dose (mean±SD) | 3.76 (0.39) | 2.25 (0.40) | <0.001 |
| Seizure control time after 2 nd dose (mean±SD) | 8.44 (0.39) | 7.54 (0.60) | <0.001 |
| Seizure control time after first or 2nd dose (mean±SD) | 5.68 (2.39) | 4.52 (2.68) | 0.09 |
| Controlled seizure in F.C. | 3/3 | 5/6 | 0.5 |
| Controlled seizure in cryptogenic and symptomatic epilepsy | 4/7 | 6/12 | 0.8 |
| Controlled seizure in idiopathic epilepsy | 18/22 | 36/42 | 0.8 |
| Overall Controlled seizure | 22/32 | 42/60 | 0.9 |
| Controlled seizure in age of ≤24 months | 19/25 | 34/50 | 0.5 |
| Controlled seizure in age of >24 months | 3/7 | 8/10 | 0.1 |
| Controlled seizure in male | 8/14 | 28/37 | 0.2 |
| Controlled seizure in female | 14/18 | 14/23 | 0.2 |
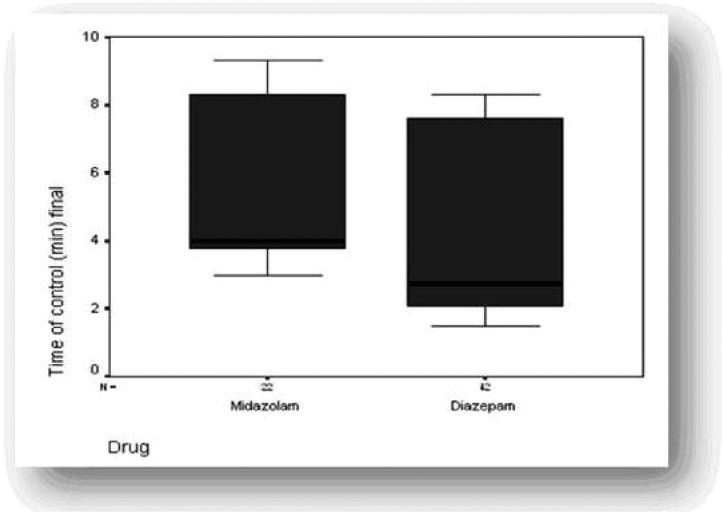
Fig. 2.
Box and whisker plots of time from drug administration up to the end of seizure
Significant adverse effects included agitation observed in 11 and 25 patients, and mild hypotension in 7 and 9 patients in the first and second group, respectively. In the second group, 4 patients experienced apnea; such an adverse effect was not reported from the first group.
All the vital parameters of other children remained within normal limits. There was no mortality overall. There was no statistically significant difference in the side effects between the two groups (agitation P=0.5, hypotention P=0.4, apnea P=0.2). The mean time for medication effect was not significantly shorter with intravenous diazepam as compared to buccal midazolam (P=0.09), but the mean time for control of patients in the first or the second administration drug dose was significantly shorter with intravenous diazepam than with buccal midazolam (Fig. 2 and Table 2).
Discussion
According to our results, administration of intravenous diazepam or buccal midazolam resulted in cessation of convulsions similarly. The overall rate of response in both groups was similar and this suggests that buccal midazolam is effective in controlling acute convulsions of generalized tonic, tonic-clonic, or atonic type. So far there has been a few study comparing intravenous diazepam and buccal midazolam[4, 13]. In that study the rate of controlled seizures was similar in both groups, but the overall frequency of control of convulsive episodes by buccal midazolam was 85%. In Ashrafi's et al study, midazolam ceased all of the seizures with 5 min and diazepam ceased 82% of patient's seizures with 5 min of drug administration[5]. In another study from Iran Javadzadeh et al showed the time needed to control seizure by intranasal midazolam was shorter than intravenous diazepam[13]. In other researches such as our study, efficacy of buccal midazolam was confirmed[3, 5, 8–10]. Our results showed the time of seizure control in both groups of patients in the first five minutes or the second five minutes after administration of drugs was not significantly different. In the previous study, the results of the mean time for controlled seizure in the two groups was similar to our study, and the time for seizure cessation with intravenous diazepam was less than buccal midazolam[4]. In another study, mean time for seizure cessation with buccal midazolam was reported to be 3.89±2.22 minutes[3]. These differences of overall control and the time required to complete the control should be subject to further investigation.
There is increasing evidence that the longer seizures persist, the more difficult they will be to stop. A previous study showed that first line therapy stopped seizures in 80% of patients when administered within 2 hours after the onset of the seizures, but less than 40% of patients were controlled if the treatment started after 2 hours[14]. The results of the present study manifest that buccal midazolam efficacy is similar to intravenous diazepam for seizure cessation and because buccal midazolam administration is very easy and fast, it can prevent status epilepticus. So far, no serious adverse effects have been reported in most cases of buccal midazolam administration[4, 7, 15, 16], but in some of the studies respiratory depression has been observed[6, 8]. Probably, this complication is a result of high dosage of buccal midazolam (0.5 mg/kg/dose). Administration of buccal midazolam with a dose of 0.3 mg/kg/dose does not seem to cause side effects. In our study, respiratory depression with buccal midazolam was not seen; some patients experienced mild hypotension and agitation whereas patients who took intravenous diazepam showed respiratory depression, mild hypotension, and agitation.
The results of our study must be interpreted in the face of certain limitations. This study was not blinded and placebo was not administered, although placebo administration in these situations may not be ethical. Another limitation of our study was small numbers of patients, so we suggest this study must be repeated with more patients.
Conclusion
Our results suggest that buccal midazolam at a dose of 0.3 mg/kg may be as effective as intravenous diazepam for the treatment of acute convulsive seizures in children and it is safer than intravenous diazepam, so buccal midazolam can be used at home very easy, safe and effective.
Acknowledgment
We would like to thank all the participants of this study for their cooperation. We are also grateful to the Mofid Hospital personnel and the research section of Shahid Beheshti University of Medical Sciences.
Conflict of Interest
None
References
- 1.Narchi H. Infantile masturbation mimicking paroxysmal disorders. J Pediatr Neurol. 2003;1(1):43–54. [Google Scholar]
- 2.Rey E, Treluyer JM, Pons G. Pharmacokinetic optimization of benzodiazepine therapy for acute seizures. Clin Pharmacokint. 1999;36(6):409–24. doi: 10.2165/00003088-199936060-00003. [DOI] [PubMed] [Google Scholar]
- 3.Onur Kutlu N, Dogrul M, Yakinci C, et al. Buccal midazolam for treatment of prolonged seizures in children. Brain Dev. 2003;25(4):275–8. doi: 10.1016/s0387-7604(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 4.Talukdar B, Chakrabarty B. Efficacy of buccal midazolam compared to intravenous diazepam in controlling convulsion in children: A randomized controlled trial. Brain Dev. 2009;31(10):744–9. doi: 10.1016/j.braindev.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Ashrafi MR, Khosroshahi N, Karimi P, et al. Efficacy and usability of buccal midazolam in controlling acute prolonged convulsive seizures in children. Eur J Child Neurol. 2010;14(5):434–8. doi: 10.1016/j.ejpn.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Mpimbaza A, Ndeezi G, Staedke S, et al. Comparison of buccal midazolam with rectal diazepam in the treatment of prolonged seizures in Uganda children: a randomized clinical trial. Pediatrics. 2008;121(1):58–64. doi: 10.1542/peds.2007-0930. [DOI] [PubMed] [Google Scholar]
- 7.Baysun S, Aydin OF, Atmaca E, et al. A comparison of buccal midazolam and rectal midazolam for the acute treatment of seizures. Clin Pediatr. 2005;44(9):771–6. doi: 10.1177/000992280504400904. [DOI] [PubMed] [Google Scholar]
- 8.Mcintyre J, Robertson S, Norris E, Appleton R. Safety and efficacy of buccal midazolam versus rectal diazpam for emergency treatment of seizures in children. Lancet. 2005;366(9481):205–10. doi: 10.1016/S0140-6736(05)66909-7. [DOI] [PubMed] [Google Scholar]
- 9.Scott DC, Besag FMC, Neville BGR. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood andadolescence: a randomised trial. Lancet. 1999;353(9153):623–6. doi: 10.1016/S0140-6736(98)06425-3. [DOI] [PubMed] [Google Scholar]
- 10.Baysun S, Aydin OF, Atmaca E, et al. A Comparison of Buccal midazolam and rectal diazpam for the acute treatment of seizure. Clin Pediatr. 2005;44(9):771–6. doi: 10.1177/000992280504400904. [DOI] [PubMed] [Google Scholar]
- 11.Marshall T. Community resource team: a systematic review of the use of buccal midazolam in the emergency treatment of prolonged seizure in adults with learning disabilities. Br J Learn Disab. 2007;35(2):99–101. [Google Scholar]
- 12.Lahat E, Goldman M, Barr J, et al. Intranasal midazolam as a treatment of autonomic crisis in patients with familial dysautonomia. Pediatr Neurol. 2000;22(1):19–22. doi: 10.1016/s0887-8994(99)00109-5. [DOI] [PubMed] [Google Scholar]
- 13.Javadzadeh M, Sheibani K, Hashemieh M, et al. Intranasal midazolam compared with intravenous diazepam in patients suffering from acute seizure: a randomized clinical trial. Iran J Pediatr. 2012;22(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Lowenstein DH, Alldredge BK. Status epilepticus. N Engl J Med. 1998;338:970–6. doi: 10.1056/NEJM199804023381407. [DOI] [PubMed] [Google Scholar]
- 15.Kutlu NO, Yakinci C, Dogrul M, et al. Intranasal midazolam for prolonged convulsive seizures. Brain Dev. 2000;22(6):359–61. doi: 10.1016/s0387-7604(00)00155-8. [DOI] [PubMed] [Google Scholar]
- 16.Fukuta O, Braham RL, Yanase H, et al. Intranasal administration of midazolam: pharmacokinetic and pharmacodynamic properties and sedative potential. ASDC J Dent Child. 1997;64(2):89–98. [PubMed] [Google Scholar]