Abstract
Objective
Since a new epidemic (third wave) of retinopathy of prematurity (ROP) sensed throughout the world in recent years, we aimed to assess newer risk factors for advanced ROP which needs treatment in Iranian neonates as a new target output of various neonatal care for this serious disease of newborn infants especially those born prematurely.
Methods
In an analytic cross-sectional study all neonates <1500 g birth weight and/or <32 weeks gestational age admitted to our NICU as a tertiary level intensive care unit in Milad Hospital, Tehran, Iran during June 2006-June 2007 were included. All data were extracted from medical records and compared in two groups with or without treatment.
Findings
Seventy one neonate infants entered our study. Twelve neonates (16.9%) progressed to advanced ROP. Final multivariate analysis model revealed that mean leukocyte counts during first 14 days of life (P=0.04), transfusions number (P=0.01) and hypocapnic episodes during first 14 days of life (P=0.02) were significantly different between the two groups of infants independently, even after simultaneous adjustment.
Conclusion
Based on our findings, more amenable risk factors should be approached regarding more careful modulation of such overlooked risk factors which may lessen the burden of prematurity.
Keywords: Neonate, Retinal Surgery, Retinopathy of Prematurity, Risk Factor, Very Low Birth Weight
Introduction
Retinopathy of prematurity (ROP), formerly known as retrolental fibroplasia vascular proliferative disorder, is a topic of concern in the premature infants that may lead to blindness[1, 2]. The more recently emerging treatment modalities combined with more careful control improved survival rates in very premature infants in developing countries and increased the rate of ROP severity[3, 4]. With better treatment modalities and higher survival rates among very low birth weight (VLBW) infants as we anticipated, clinicians involved with neonatal care encounter on a more frequent basis, even predictable sequences of prematurity, which sometimes place a very heavy financial burden on the health care system. To make the suggested risk factors such as sepsis[5], severe fungal infection (SFI)[6–8], high number of transfusions[9], perinatal steroids exposure[10], hypo and hypercapnea[11], hyperglycemia[12, 13], apnea and bag/mask ventilation after 2nd day, male gender[14], lipid within total parenteral nutrition (TPN) solutions[15], twins and triplets[16–18], intra ventricular hemorrhage (IVH) and Respiratory Distress Syndrome (RDS)[19] more explicit, we conducted this study in Milad Hospital as one of the largest hospitals with level III NICU in Iran. Our aim was to analyze the data set to identify significant risk factors for advanced-type ROP.
Subjects and Methods
Patients
We designed our study as a retrospective longitudinal one collecting data from medical records of Milad Hospital during June 2006-May 2007. All infants with gestational age (GA) of <32 wk and/or birth wieght (BW) of <1500 g, who survived beyond 4 weeks and/or completed ophthalmic exams for ROP, entered our study (n=71). GA was assessed from obstetric ultrasound and confirmed by clinical assessment of infants based on New Ballard Score (NBS). We distributed the infants into two groups:Advanced or Surgical group (n=12) which included the infants who underwent retinal surgery by either photocoagulation or cryotherapy and the other group, non-surgical (n=59), included the infants without ROP or ophthalmologic findings severe enough to meet the criteria for retinal surgery. Based upon the policy of the hospital all parents or guardians had been approached regarding informed consent and declaration as permission to use the contents of the medical records for research purpose. All participants in this study were eligible for our research. This study has been accepted by the ethics committee of Tehran University of Medical Sciences and Milad Hospital and all researchers undertook Helsinki's treaty.
Definitions, terminology and medical procedures
Severe hypercapnea was considered as PaCO2 >80 mmHg, hypocapnea as PaCO2 <30 mmHg, hyperglycemia as BS >150 mg/dl, bacterial sepsis by either positive blood culture or cerebro-spinal fluid culture for any pathogenic bacteria during first 14 days of life, chronic lung disease (CLD) as described and staged by NIH workshop[20]. Further we described necrotizing enterocolitis (NEC) as clinical diagnosis of staff neonatologist with abdominal X-ray consistent with NEC based on Modified Bell's stage in which stages IIIA, and IIIB were separated as advanced stages[21] and IVH using Papile staging in which stage III and IV are classified as severe IVH[22]. Also, we defined advanced ROP or treatment group regarding any location (zone), severity (stage), plus disease, extent and prethreshold or threshold ROP which needs treatments according to the latest AAP guidelines[23]. Severe RDS was our institutional liberal definition for a clinical and radiographic diagnosis of RDS being severe enough with at least 3 days of first intubation and mechanical ventilation. In the TPN group we provided the infants with admixture of lipid, protein and glucose in parenteral solution. We usually started TPN from day 2 with glucose and protein, and lipid was added to this regimen using peripherally inserted venous catheter or via central vein catheter in prolonged cases that were still mechanically ventilated. Granulocyte-colony stimulating factor (G-CSF) was started when the neonates were septic and leukopenic (absolute neutrophil count <1500/µl). Only those administered during first 10 days of life were recorded. Dexamethasone was also used to treat CLD with short course regimen started at 0.1 mg/kg q12h on day 1, 0.075 mg/kg q 12h on day 2 and 0.05 mg/kg q12h on day 3 to lessen corticosteroids side effects and facilitate extubation and weaning from ventilator.
Assessments
The first ophthalmologic examination was conducted by expert ophthalmologists at postmenstural age (PMA) of 31 to 36 weeks by means of indirect funduscopy. Reports and subsequent examinations were performed at the discretion of ophthalmologist mostly adhered to the International Classifications of Retinopathy of Premturity (ICROP) and the American Academy of Pediatrics (AAP) guidelines[23, 24]. Pupil dilation was achieved by twice instilling one drop of phenylephrine 1% and one drop of tropicamide 0.5% within a 5-minute interval. We used a glucometer for checking bedside blood sugar (BS) at least 3 times a day and serum BS was checked simultaneously if the device indicated high or low BS. Testing frequency was based on test findings:For glucose <40 mg/dl, every 2 hours and for stable glucose >40 mg/dl, every 8 hours. We added 10% to the BS value for data recorded by the glucometer in order to equalize it to serum BS as hemocells within the whole blood may consume the glucose and lower BS. All preterm infants had cerebral ultrasound examinations for IVH at the third and seventh day of life (DOL) and were followed up based on our institutional protocols.
Blood samples were extracted either through umbilical catheters or direct peripheral arteriotomy or venotomy in order to evaluate leukocyte counts during first 10 days of life, BS and bilirubin during first 2 weeks. Moreover, initial arterial blood gas (ABG) and electrolytes were recorded for all infants. Other laboratory tests were done if clinically indicated.
Statistical Analysis
In descriptive analysis, the parameters such as frequency, mean, and standard deviation (SD) were reported. The analytical procedures were performed using statistical tests. To test the differences between parametric and non-parametric variable means in the two groups of study, the Independent t-test and Mann-Whitney U-test were used, respectively. A Chi2 statistical test was also used to evaluate the possible statistical differences in the distribution of qualitative variables among different groups of the study. In addition, Cochran's test was used to evaluate the confounding effects of qualitative variables.
Receiver operating characteristics (ROC) curve analysis was also performed to assess the predictability of advanced ROP with quantitative variables of the study, and then to compare the area under curve (AUC) of these variables. The cutoff points were determined in each ROC analysis based on Youden's index in order to determine highest value from summation of (sensitivity) and (specificity-1). Finally, binary logistic regression analysis was performed in order to evaluate the potential role of different risk factors to predict advanced ROP as a binary outcome. For this purpose, a maximum number of 6 variables (with clinical or statistical significance in previous univariate analysis) were included in the model each time (less than 10% of the cases). Backward stepwise (Likelihood Ratio) model was performed in logistic regression analysis. Thereafter, the Nagelkerke R Square index was used in order to compare the strength of each predicting model.
A 5 percent probability of a type I error (two-tailed), and a power of 80 percent were considered in the analysis. All reported P-values are two-tailed and a P-value of <0.05 was considered significant.
Findings
Baseline Characteristics
After exclusion of 67 (Death=21, incomplete follow up=20, digital files number and file name mismatch=7, unavailable files=19), seventy one infants met the criteria for inclusion in the study (out of the 138 VLBW infants born). There were 38 (53.5%) boys and 33 (46.5%) girls with the mean gestational age and birth weight of 30.17 (SD=2.47) wk and 1234 (SD=232) g, respectively. The mean Apgar scores of these neonates were 5.94 (SD=1.83) and 7.55 (SD=1.57) in the first and fifth minutes, respectively.
Regarding the results of subsequent examinations, advanced ROP occurred in 12 (16.9%) of VLBW infants requiring further intervention; while this in the other 59 neonates did not occur. Six infants out of 15 (40%) with GA ≤28 wk had advanced ROP, while this frequency decreased to 10.7% (6 out of 56) in neonates with GA >28 wk. We started G-CSF for 5 infants in surgical group vs 6 infants in a non-surgical ROP group. Two septic infants out of total 12 infants in surgical group and one infant in non-surgical ROP group were provided with domapine ±dubotamine due to hypotension during severe sepsis. Our study displayed that elevated leukocyte count during first few days of life in premature neonates and especially mean WBC > 11.100/µl possesses high risk for advanced-ROP [sensitivity: 69%, specificity: 77%, positive predictive value (PPV): 42.8% and negative predictive value (NPV): 92%].
Univariate analysis of risk factors
Different gestational and neonatal factors were recorded and compared between the two groups of infants (Table 1). As shown in Table 1, gestational age, birth weight, gender distribution, Apgar scores in both the first and fifth minutes, massive transfusion, serum WBC counts, both hypercapnic and hypocapnic episodes, administration of G-CSF, sepsis, severe fungal infection, postnatal steroid administration, IVH, severe RDS, TPN with lipid administration and hyperglycemic episodes were significantly different between the infants without advanced ROP and the neonates with advanced ROP.
Table 1.
Demographic and follow-up variables of two groups
| Variable | Non-ROP or ROP I/II (n=59) | ROP III/IV (n=12) | P-value | |
|---|---|---|---|---|
| Gestational age (wk) | 30.63 (2.33) | 27.92 (1.88) | <0.001 | |
| Gestational age groups | ≤28 weeks | 9 (15.3%) | 6 (50%) | 0.007 |
| >28 weeks | 50 (84.7%) | 6 (50%) | ||
| Birth weight (g) | 1278 (215) | 1014 (185) | <0.001 | |
| Gender% | Boy | 28 (47.5%) | 10 (83.3%) | 0.03 |
| Girl | 31 (52.5%) | 2 (16.7%) | ||
| Delivery% | Normal Vaginal Delivery | 48 (81.4%) | 8 (66.7%) | 0.3 |
| Cesarean Section | 11 (18.6%) | 4 (33.3%) | ||
| Perinatal Problems% | Pre-eclampsia | 13 (22%) | 3 (25%) | 0.8 |
| Twin | 13 (22%) | 2 (16.7%) | 1 | |
| Triplet | 4 (6.8%) | 1 (8.3%) | 0.8 | |
| Apgar score [mean(SD)] | First minute | 6.30 (1.53) | 4.18 (2.23) | <0.001 |
| Fifth minute | 7.80 (1.43) | 6.36 (1.75) | 0.005 | |
| Transfusion status | Mean number | 2.39 (2.88) | 10.83 (7.67) | 0.003 |
| 0-3 times% | 43 (72.9%) | 1 (8.3%) | <0.001 | |
| ≥4 times% | 16 (27.1%) | 11 (91.7%) | ||
| Mean leukocyte count (/µL) | 8510 (2710) | 12268 (4554) | 0.02 | |
| Hypercapnea (Episodes/patient) | 0.69 (2.37) | 2.42 (3.82) | 0.04 | |
| Hypocapnea (Episodes/patient) | 0.73 (1.58) | 4.58 (5.30) | 0.03 | |
| Use of G-CSF ††† % | 6 (10.2%) | 5 (41.7%) | 0.006 | |
| Sepsis% | 11 (18.6%) | 6 (50%) | 0.02 | |
| Severe Fungal Infection% | 0 | 2 (16.7%) | 0.03 | |
| Steroids administration% | Prenatal | 12 (20.3%) | 3 (25%) | 0.7 |
| Postnatal | 5 (8.5%) | 4 (33.3%) | 0.04 | |
| Apnea leading to bag/mask ventilation% | 5 (8.5%) | 3 (25%) | 0.1 | |
| Intraventricular Hemorrhage% | Grade 0-2 | 59 (100%) | 10 (83.3%) | 0.03 |
| Grade ≥3 | 0 | 2 (16.7%) | ||
| Necrotizing Enterocolitis% | 2 (3.4%) | 2 (16.7%) | 0.07 | |
| Total Parenteral Nutrition with lipids% | 23 (39%) | 11 (91.7%) | 0.001 | |
| Vasopressor infusion% | 1 (1.7%) | 2 (16.7%) | 0.07 | |
| Glycemic status [Mean glucose (SD)] (mg/dl) | During 14 days | 105.06 (39.60) | 104.38 (39.32) | 0.9 |
| On 3rd day | 93.39 (51.72) | 133.08 (64.90) | 0.03 | |
| On 12th day | 140.61 (61.10) | 89.71 (32.66) | 0.06 | |
| Hyperglycemia (Episodes/patient) | 1.44 (1.91) | 5.58 (4.94) | 0.01 |
ROP: Retinopathy of prematurity; SD: Standard Deviation; G-CSF: Granulocyte Colony Stimulating Factor
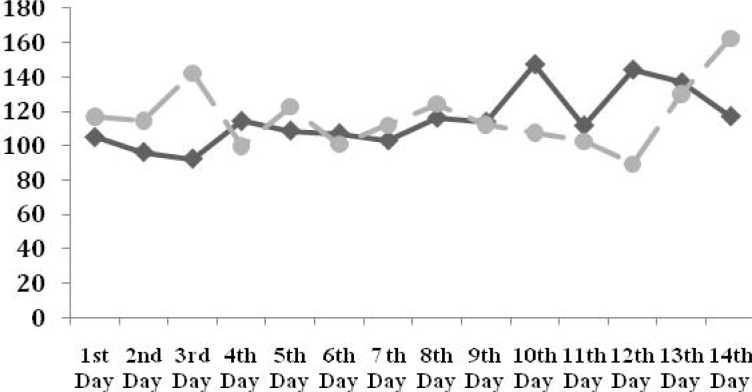
Longitudinal evaluation of blood sugar within 14 days of follow-up is illustrated in Fig. 1. The mean BS was significantly higher on the 3rd day in infants with advanced ROP (133.08 vs. 93.39 mg/dl, P=0.03).
Fig. 1.
Evolution of blood sugar during 14 days of follow-up in neonates without ROP or ROP stage I/II (black line) and ROP stage III/IV (gray dotted line) (* P<0.05)
Univariate analysis displayed risks for advanced ROP related to male gender (OR=5.54, 95% CI:1.12-27.47), transfusion more than 4 times or >60 cc/kg (OR=30.80, 95%CI:3.66-259.26), receiving postnatal steroid for CLD treatment (OR=5.40, 95%CI:1.19-24.44), early introduction of lipids to parenteral nutrition package (≤6 days after birth) (OR=17.22, 95%CI:2.08-142.43), bacterial sepsis (OR=4.36, 95%CI:1.18-16.13), use of G-CSF (OR=6.31, 95%CI:1.52-26.23) and developing severe RDS (OR=4.5, 95%CI:1.23-16.23).
However, the variables including route of delivery, perinatal problems, total mean glucose during the complete first 2 weeks of life, twins, multiple births, NEC, apnea leading to bag/mask ventilation and prenatal steroids were not significantly related to advanced ROP in univariate analysis (P>0.05).
Interaction analysis
After controlling for the confounding effects of sepsis (P=0.002) and G-CSF administration (P=0.001) by Cochran's test, mean WBC >11.100/µl is still an independent risk factor for advanced ROP. On the other hand, while evaluating the association between G-CSF administration and advanced ROP in patients with WBC >11.100/µl no significant relationship was observed (P=0.2).
ROC curve analysis
ROC curve analysis revealed that gestational age (P=0.003), birth weight (P=0.001), Apgar scores in both the first (P=0.009) and fifth minutes (P=0.03), number of transfusion (P<0.001), serum WBC counts (P=0.006), hypercapnic (P=0.02) and hypocapnic episodes (P=0.001), level of blood sugar on 3rd day of life (P=0.01) and the number of hyperglycemic episodes (P=0.006) had significant diagnostic values to differentiate inadvanced from advanced cases (Table 2).
Table 2.
ROC analysis of different demographic and follow-up quantitative variables to differentiate non-ROP or ROP I/II from infants with ROP III/IV
| Variable | Cut-Point value | Sensitivity | Specificity | Area Under Curve (AUC) | P-value | |
|---|---|---|---|---|---|---|
| Gestational age (wk) | 28 | 90.7% | 54.5% | 0.794 | 0.003 | |
| Birth weight (g) | 1060 | 83.3% | 63.6% | 0.823 | 0.001 | |
| Apgar score | First minute | 4 | 98.1% | 54.5% | 0.759 | 0.009 |
| Fifth minute | 7 | 87% | 72.7% | 0.718 | 0.028 | |
| Transfusion numbers | 4 | 90.9% | 73.8% | 0.867 | <0.001 | |
| Mean leukocyte count (/µL) | 8375 | 90.9% | 57.1% | 0.773 | 0.006 | |
| 11100 | 63.6% | 78.6% | ||||
| 13050 | 54.5% | 92.9% | ||||
| Hypercapnea (Episodes/patient) | 2 | 54.5% | 88.1% | 0.728 | 0.021 | |
| Hypocapnea (Episodes/patient) | 2 | 63.6% | 88.1% | 0.825 | 0.001 | |
| Glycemic status | 83.5 | 90.9% | 54.8% | 0.742 | 0.014 | |
| Mean glucose on 3 rd day (mg/dl) | 145.5 | 54.5% | 85.7% | |||
| Hyperglycemia (Episodes/patient) | 3 | 63.6% | 90.5% | 0.773 | 0.006* |
ROP: Retinopathy of prematurity
Multivariate binary logistic regression analysis
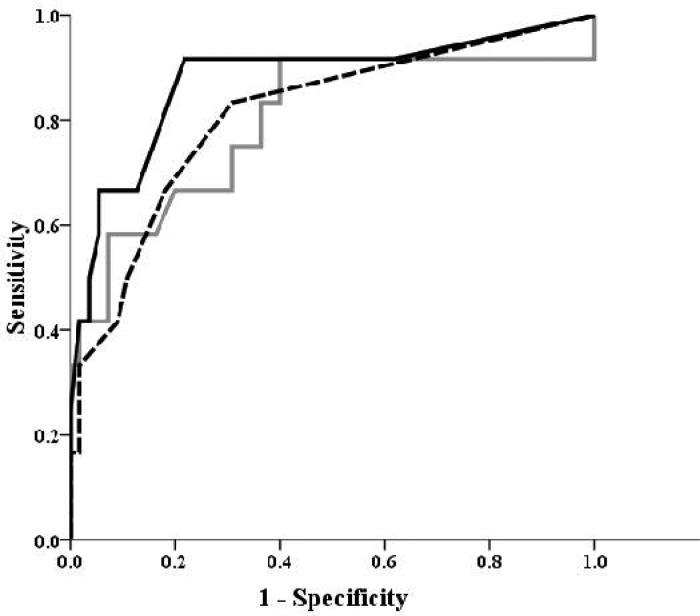
After the enrolment of different significant variables of univariate analysis in Backwardstepwise (Likelihood Ratio) model, the final multivariate regression analysis (R2=0.769, P<0.001) retained the variables including mean leukocyte counts (P=0.04), massive transfusion (P=0.01) and hypocapnic episodes (P=0.02); which were significant after simultaneous adjustment and independently predictedadvanced ROP (Table 3). The AUC of these risk factors are also compared with each other in Fig. 2.
Table 3.
Multivariate binary logistic regression analysis of different variables to predict the occurrence of retinopathy of prematurity III/IV in very low birth weight infants
| Variable | Exp (B) | 95% CI for Exp (B) | P-value |
|---|---|---|---|
| Mean leukocyte count | 1.00 | 1.000-1.001 | 0.04 |
| Transfusion | 1.54 | 1.103-2.149 | 0.01 |
| Hypocapnea | 1.84 | 1.097-3.096 | 0.02 |
| Constant | 0.000 | 0.001 |
R2=0.769, P<0.001 / CI: Confidence Interval
Fig. 2.
Comparison of the area under curve (AUC) of the number of transfusions (black line), serum leukocyte counts (gray line) and episodes of hypocapnea (black dash) to distinguish non- retinopathy of prematurity (ROP) or ROP I/II infants from the ones with ROP III/IV (AUCTransfusion >AUCHypocapnea >AUCWBC)
Discussion
In this study we entered a relative large number of variables regarding advanced ROP occurrence. We tried to find out the independent predictor variables and the dependent predictor variables as well. To the best of our knowledge, it is the first article to review risk factors regarding requiring treatment of ROP in premature infants by either cryotherapy or laser manipulation. The prevalence of advanced ROP was 16.9% in our patients which is somehow lower than in a previous study from Iran by Karkhaneh et al (22.6%)[25]. Previously, prevalence rates of 8% to 29% was reported from Iran. This incontinency may be best explained by diverse inclusion criteria with different GA and BW thresholds[26–29].
Another study by Mutlu et al in Turkey showed that the frequency of ROP was 37.1% for any stage and 7.2% for stage 3 or greater in VLBW infants[30]. Despite the lower frequency of severe ROP in their study, the cut-point value for GA to include premature cases was 34 wks. Considering exclusion of a relative large number of infants (n=67) may have biased our final information and some causal relationships inevitably may be either overestimated or underestimated.
As previous studies showed, ours too reveals that low GA, low birth weight and male gender are inevitable risk factors for advanced ROP progression among very premature infants [14, 24, 25].
Low Apgar scores especially <4 in first minute and <6 in fifth minute which may lead to resuscitation with ambobag, chest compression and medications are the first red flags for more careful attention to ROP follow up as these factors influence intra-thoracic pressure and whole body and especially retinal and brain blood circulation.
We found out the mean leukocyte count during the first 10 DOL was significantly higher in infants with advanced ROP compared to the remaining infants without advanced ROP. This retinal damages may be due to inflammatory responses by WBC.
Our results displayed that SFI and bacterial sepsis may predispose to advanced ROP. One of the infants with SFI received postnatal dexamethasone. However, more research is needed to explore the real correlation between SFI and advanced ROP and postnatal steroids exposure. The exact mechanism by which C. albicans increases the ROP is not clear and yet to be understood[6].
The administration of G-CSF was significantly higher in surgical ROP group (5/12 infants vs 6/59). This product increases total count with stimulation of bone marrow to release these cells. Immature WBCs which could encompass toxic granules due to immaturity may cascade a systemic and especially local inflammatory responses in retina and ultimately lead to cell damage in the eyes. Conclusively, according to the significant difference in advanced ROP occurrence between two groups, we hypothesized that this adjunct therapy for septic leucopenic infants may be harmful for the retina by abrupt increase in WBC count and probably increase risk for advanced ROP progression.
Early initiation of TPN adding multipurpose 10% lipid vials showed a higher incidence of ROP in our institute. This may be due to a greater number of free radicals after lipid introduction. Some studies showed that protection of TPN solutions from light may lessen free radicals, augment stability of the vitamins added to solutions[31–33].
We also compared a group of infants that had at least one apneic episode leading to bag/mask ventilation after the first DOL, with infants without any apneic episode during hospital stay. Cerebral blood flow fluctuation or drugs used for neonatal resuscitation may negatively affect retinal blood flow with increased risks for ROP.
The number of transfusions including whole blood and packed cells were also assessed for risk estimation. However, usage of fresh frozen plasma (FFP), platelets and intravenous immunoglobins (IVIG) were excluded from our analysis. There was a significant increased incidence of advanced ROP in infants with ≥4 transfusions; this may be controlled by reducing unnecessary phlebotomies and implementation of some new smaller volume analysis methods.
Hypocapnea and hypercapnea are frequent events in ventilated infants during remedial course. As blood carbon dioxide tension (PaCO2) may influence blood flow regulations within tiny vessels of the retina and cerebrum, we recorded PaCO2 of the first 14 DOL according to ABG analysis. Frequency of ordered ABG was based upon clinical status of infants and the changes in ventilator settings. The number of hypocapnic and hypercapnic episodes were compared during the first 2 weeks of life in the two groups with and without advanced ROP. If they were off ventilator, clinically stable and had no ABG, it was considered to be within normal ranges. As higher numbers of hypercapnic and hypocapnic episodes were related to increased incidence of advanced ROP, ventilation strategies which permit PaCO2 to reach 80 mmHg or higher are not recommended.
Hyperglycemia is a common occurrence in VLBW infants. The important role of hyperglycemia induced vasoproliferative retinopathy has been widely studied in adults[34, 35]. Some newer studies proposed sweet blindness in neonates as well as adults[12]. Measuring average serum blood glucose for the first 2 weeks of life displayed no significant results in our study. However, the calculated power was low (45%) and this may be due to low sample size of infants with advanced ROP to demonstrate the significance of the difference in the mean blood glucose of the first 2 weeks between the two groups of study. On the other hand, mean glucose in 3rd DOL was significantly higher in surgical group; while the mean BS of the 12th day was significantly lower in this group. This may emphasize that in comparison with the mean BS of first 14 DOL, the fluctuation in serial blood sugar measurements may be a more important risk factor.
It seems that sweet blindness, which is widely studied in adult diabetic patients, may need to be reviewed more carefully in neonatal population. When hyperglycemia occurred, the glucose concentration was first lowered to no less than 5% in the ultimate solution, and then insulin infusion was started. According to the above findings, tight glucose control and insulin infusion may prevent further retinal neovascularisation[36].
Prenatal administration of steroids (dexamethasone or betamethasone) did not increase risk of ROP, but postnatally, for chronic lung disease (CLD) treatment had some important side effects on the retina as more infants progressed to advanced ROP.
During recent years, an increasing number of multiple pregnancies was observed due to the use of assisted reproductive techniques (ART) which increased premature births, low BW and low GA deliveries[37]. In our study we did not find a significant correlation between multiple births and ROP in agreement with previous studies concluding that multiple pregnancies did not seem to be an independent risk factor for ROP[18]. Finally, since early reports of the toxicity of oxygen, most frequently administered drug in NICUs, many modifications to optimizing oxygen therapy in premature infants has been made in recent decades. Since early sixties, liberal use of oxygen had been condemned by many authorities[38] and also many studies introduced oxygen as a main risk factor to progress to severe ROP even in the case of progression of laser-treated retinopathy of prematurity to retinal detachment[5]. Our study displayed disparate results from Bourla et al research. They concluded that premature infants with the history of sepsis, oxygen therapy, mechanical ventilation, RDS, and PDA have a higher risk for failure of laser therapy and consequent retinal detachment; in which blood transfusion had minimal effect and RDS was introduced as a significant risk factor. Surprisingly our study failed to clarify RDS, mechanical ventilation and concomitant oxygen toxicity as one major and independent predictor. Considering reports of severe ROP cases with minimal oxygen exposure[39], such dogmatic over-emphasis on superior importance of oxygen toxicity saluted by all other predictor risk factors may better be retestified. Also, we did not enter separately oxygen requirement and mechanical ventilation as two well-known potent risk factors and there may be some statistically selection bias in final multivariate binary logistic regression analyses reports. Neonates were divided by either requiring treatment or not. As another limitation of the research, no comparison accomplished for other stages of ROP due to inaccurate and imprecise recorded description and staging of the neonates for whom medical care and follow-ups other than treatment were presumed. Moreover, considering excluding a relative large number of infants (n=67) mainly for death may have biased our final information and some causal relationships inevitably may be either overestimated or underestimated. Currently no proven strategies and methods are available to prevent ROP. Various interventions to prevent or limit the progression of ROP have been introduced without clear benefits, although further evaluation may be needed. Since oxidative injury has been investigated, more potent antioxidants besides vitamin E, D-penicillamine and others may be preventive and attenuate serious ROP side effects[40, 41]. Specifically, augmentative role of N-acetyl cyteine on antioxidative system and nitric oxide[42] and anti-venous endothelia growth factor (VEGF) medication preventing the progression to severe ROP may be an interesting topic for future researches. In our study, in addition to many other previously well-known risk factors, we found a strong relation between mean leukocyte count, massive transfusion, administration of G-CSF, hypercapnic and hypocapnic episodes with advanced ROP.
Conclusion
In conclusion, controlling for more suitable ventilator strategies, judicious use of G-CSF, better TPN solutions, careful glucose control, more suitable CLD treatment protocols and less phlebotomies may warrant a better prognosis of ROP in VLBW infants. More extended studies in future by other researchers may determine the exact role of hyperglycemia and also hypoglycemia as two common events during first few days of life, regarding ROP. A better understanding of possible impact of systemic inflammation and especially local inflammatory responses either humoral or cell mediated in retina would be obtained following bacterial and fungal infections or G-CSF administration by conducting animal retinal pathology studies.
Acknowledgment
This study took place in Milad Hospital, Tehran, Iran. We especially thank Milad Hospital NICU nurses and clinicians for their full-time care of these lovely tiny creatures. In particular, we thank Dr. Behzad Moghadam for his cooperation. In addition we thank Mrs. Tafreshi and Fardad, Head nurses of Milad Hospital NICU's.
Conflict of Interest
None
References
- 1.Gibson Dl, Sheps SB, Uh SH, et al. Retinopathy of prematurity-induced blindness:birth-wight-specific survival and the new epidemic. Peidtarics. 1990;86(3):405–412. [PubMed] [Google Scholar]
- 2.Gilbert C, Foster A. Childhood blindness in the context of vision 2020: the right to sight. Bull World Health Organ. 2001;79(3):227–32. [PMC free article] [PubMed] [Google Scholar]
- 3.Valentine P, Jackson C, Kalina R, et al. Increased survival of low birth weight infant: impact on the incidence of retinopathy of prematurity. Pediatrics. 1989;84(3):442–5. [PubMed] [Google Scholar]
- 4.Gilbert C, Rahi J, Eckstein M, et al. Retinopathy of prematurity in middle-income countries. Lancet. 1997;350(9070):12–14. doi: 10.1016/S0140-6736(97)01107-0. [DOI] [PubMed] [Google Scholar]
- 5.Bourla DH, Gonzales CR, Valijan S, et al. Association of systemic risk factors with the progression of laser-treated retinopathy of prematurity to retinal detachment. Retina. 2008;28(3 Suppl):S58–64. doi: 10.1097/IAE.0b013e31815075b0. [DOI] [PubMed] [Google Scholar]
- 6.Mittal M, Dhanireddy R, Higgins RD. Candida sepsis and association with retionopathy of prematurity. Pediatrics. 1998;101(4 Pt 1):654–7. doi: 10.1542/peds.101.4.654. [DOI] [PubMed] [Google Scholar]
- 7.Bharwani SK, Dhanireddy R. Systemic fungal infection is associated with the development of retinopathy of prematurity in very low birth weight infants: a meta-review. J Perinatol. 2008;28(1):61–6. doi: 10.1038/sj.jp.7211878. [DOI] [PubMed] [Google Scholar]
- 8.Haroon Parupia MF, Dhanireddy R. Association of postnatal dexamethasone use and fungal sepsis in the development of severe retinopathy of prematurity and progression to laser therapy in extemely low-birth-weight infants. J Perinatol. 2001;21(4):242–7. doi: 10.1038/sj.jp.7200531. [DOI] [PubMed] [Google Scholar]
- 9.Cooke RW, Clark D, Hickey-Dwyer M, et al. The apparent role of blood transfusions in the development of retinopathy of prematurity. Eur J Pediatr. 1993;152(10):833–6. doi: 10.1007/BF02073381. [DOI] [PubMed] [Google Scholar]
- 10.Batton DG, Roberts C, Trese M, et al. Severe retinopathy of prematurity and steroid exposure. Pediatrics. 1992;90(4):534–6. [PubMed] [Google Scholar]
- 11.Holmes JM, Zhang S, Leske DA, et al. The effect of carbon dioxide on oxygen-induced retinopathy in the neonatal rat. Curr Eye Res. 1997;16(7):725–32. doi: 10.1076/ceyr.16.7.725.5054. [DOI] [PubMed] [Google Scholar]
- 12.Garg R, Agthe AG, Donohue PK, et al. Hyperglycemia and retinopathy of prematurity in very low birth weight infants. J Perinatol. 2003;23(3):186–94. doi: 10.1038/sj.jp.7210879. [DOI] [PubMed] [Google Scholar]
- 13.Heimann K, Peschgens T, Kwiecien R, et al. Are recurrent hyperglycemic episodes and median blood glucose level a prognostic factor for increased morbidity and mortality in premature infants ≤1500 g? J Perinat Med. 2007;35(3):245–8. doi: 10.1515/JPM.2007.057. [DOI] [PubMed] [Google Scholar]
- 14.Darlow BA, Hutchinson JL, Henderson-Smart DJ, et al. Prenatal risk factors for severe retinopathy of prematurity among very preterm infants of the Australian and New Zealand neonatal network. Pediatrics. 2005;115(4):990–6. doi: 10.1542/peds.2004-1309. [DOI] [PubMed] [Google Scholar]
- 15.Simmer K, Rao SC. Early introduction of lipids to parenterally-fed preterm infants. Cochrane Database Syst Rev. 2005;18(2):CD005256. doi: 10.1002/14651858.CD005256. [DOI] [PubMed] [Google Scholar]
- 16.Friling R, Axer Siegel R, Hersocovici Z, et al. Retinopathy of prematurity in assisted vs natural conception and singleton vs multiple births. Ophthalmology. 2007;114(2):321–4. doi: 10.1016/j.ophtha.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Friling R, Rosen SD, Monost T, et al. Retinopathy of prematurity in multiple gestation, very low birth weight infants. J Pediatr Ophthalmol Strabismus. 1997;34(2):96–100. doi: 10.3928/0191-3913-19970301-08. [DOI] [PubMed] [Google Scholar]
- 18.Riazi-Esfahani M, Alizadeh Y, Karkhaneh R, et al. Retinopathy of prematurity: single vs multiple-birth pregnancies. J Ophthalmic Vis Res. 2008;3(1):47–51. [PMC free article] [PubMed] [Google Scholar]
- 19.Hungerford J, Stewart A, Hope P. Ocular sequelae of preterm birth and their relation to ultrasound evidence of cerebral damage. Br J Ophthalmol. 1986;70(6):463–8. doi: 10.1136/bjo.70.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 21.Walsh MC, Kliegman RM. Necrotizing enterocolitis:treatment based on staging criteria. Pediatr Clin North Am. 1986;33(1):179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volpe JJ. Neurology of the newborn. 5th ed. Philadelphia: WB Saunders; 2008. Intracranial Hemorrhage: Subdural, primary subarachnoid, cerebellar, intraventricular (term infant) and miscellaneous; p. 483. [Google Scholar]
- 23.American Academy of Pediatrics, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus. Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117(2):572–6. doi: 10.1542/peds.2005-2749. [DOI] [PubMed] [Google Scholar]
- 24.International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity. Arch Ophthalmol. 2005;123(7):991–9. doi: 10.1001/archopht.123.7.991. [DOI] [PubMed] [Google Scholar]
- 25.Karkhaneh R, Mousavi SZ, Riazi-Esfahani M, et al. Incidence and risk factors of retinopathy of prematurity in a tertiary eye hospital in Tehran. Br J Ophthalmol. 2008;92(11):1446–9. doi: 10.1136/bjo.2008.145136. [DOI] [PubMed] [Google Scholar]
- 26.Ghaseminejad A, Niknafs P. Distribution of retinopathy of prematurity and its risk factors. Iran J Pediatr. 2011;21(2):209–14. [PMC free article] [PubMed] [Google Scholar]
- 27.Bayat-Mokhtari M, Pishva N, Attarzadeh A, et al. Incidence and risk factors of retinopathy of prematurity among preterm infants in Shiraz/ Iran. Iran J Pediatr. 2010;20(3):303–7. [PMC free article] [PubMed] [Google Scholar]
- 28.Saeidi R, Hashemzadeh A, Ahmadi S, Rahmani Sh. Prevalence and predisposing factors of retinopathy of prematurity in very low-birth-weight infants discharged from NICU. Iran J Pediatr. 2009;19(1):59–63. [Google Scholar]
- 29.Khatami SF, Yousefi Ah, Fatahi Bayat Gh, Mamuri Gh. Retinopathy of prematurity among 1000-2000 gram birth weight newborn infants. Iran J Pediatr. 2008;18(2):137–42. [Google Scholar]
- 30.Mutlu FM, Altinsoy HI, Mumcuoglu T, et al. Screening for retinopathy of prematurity in a tertiary care newborn unit in Turkey: frequency, outcomes and risk factor analysis. J Pediatr Ophthalmol Strabismus. 2008;45(5):291–8. doi: 10.3928/01913913-20080901-12. [DOI] [PubMed] [Google Scholar]
- 31.Laborie S, Lavoie JC, Pineault M, et al. Contribution of multivitamins, air, and light in the generation of peroxides in adult and neonatal parenteral nutrition solutions. Ann Pharmacother. 2000;34(4):440–5. doi: 10.1345/aph.19182. [DOI] [PubMed] [Google Scholar]
- 32.Sargent KM, Harrison A, Lalari V, et al. Photo-protection of TPN in neonates is associated with enhanced nitric oxide production, mesenteric vasodilatation and improved feeding tolerance during initiation of enteral nutrition; CPS Abstracts; 2005. [Google Scholar]
- 33.Silvers KM, Sluis KB, Darlow BA, et al. Limiting light induced lipid peroxidation and vitamin loss in infant parenteral nutrition by adding multivitamin preparations to Intralipid. Acta Pediatr. 2001;90(3):242–9. doi: 10.1111/j.1651-2227.2001.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 34.Atherton A, Hill DW, Keen H, et al. The effect of acute hyperglycaemia on the retinal circulation of the normal cat. Diabetologia. 1980;18(3):233–7. doi: 10.1007/BF00251922. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan PM, Davies GE, Caldwell G, et al. Retinal blood flow during hyperglycemia. A laser Doppler velocimetry study. Invest Ophthalmol Vis Sci. 1990;31(10):2041–5. [PubMed] [Google Scholar]
- 36.Kondo T, Vicent D, Suzuma K, et al. Knockout of insulin and IGF-1 receptors on vascular endothelial cells protects against retinal neovascularisation. J Clin Invest. 2003;111(12):1835–42. doi: 10.1172/JCI17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luke B. The changing pattern of multiple births in the United States: maternal and infant characteristics 1973 & 1990. Obstet Gynecol. l994;84(1):101–6. [PubMed] [Google Scholar]
- 38.Silverman WA. A cautionary tale about supplemental oxygen: the albatross of neonatal medicine. Pediatrics. 2004;113(2):394–6. doi: 10.1542/peds.113.2.394. [DOI] [PubMed] [Google Scholar]
- 39.Buksh MJ, Dai SH, Kuschel CA. AP-ROP in an infant with minimal oxygen exposure. J Peadiatr Child Health. 2008;44(4):228–30. doi: 10.1111/j.1440-1754.2008.01287.x. [DOI] [PubMed] [Google Scholar]
- 40.Münzel T, Daiber A, Ullrich V, Mülsch A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cgmp-dependent protein kinase. Arterioscler Thromb Vasc Biol. 2005;25(8):1551–7. doi: 10.1161/01.ATV.0000168896.64927.bb. [DOI] [PubMed] [Google Scholar]
- 41.Tomida M, Ishimaru J, Hayashi T, et al. The redox states of serum and synovial fluid of patients with temporomandibular joint disorder. Jpn J Physiol. 2003;53(5):351–5. doi: 10.2170/jjphysiol.53.351. [DOI] [PubMed] [Google Scholar]
- 42.Failli P, Palmieri L, D'Alfonso C, et al. Effect of N-acetyl-L L-cysteine on peroxynitrite and superoxide anion production of lung alveolar macrophages in systemic sclerosis. Nitric Oxide. 2002;7(4):277–82. doi: 10.1016/s1089-8603(02)00120-9. [DOI] [PubMed] [Google Scholar]