Abstract
Background
Malignant infantile osteopetrosis (MIOP) presents early in life with extreme sclerosis of the skeleton and reduction of bone marrow spaces. Since there is a defect in the bone marrow, the disease can cause anemia, extramedullary hematopoiesis secondary to anemia leading to hepatosplenomegaly, cranial nerves compression and severe growth failure. This disorder is often lethal within the first decade of life because of secondary infections. Stem cell transplantation (SCT) remains the only curative therapy.
Case Presentation
We report a two-month old male infant, diagnosed as MIOP while investigating the cause of hepatosplenomegaly. The patient was referred for stem cell transplantation.
Conclusion
Malignant infantile osteopetrosis should be kept in mind as a rare cause of hepatosplenomegaly and the patient should be referred for stem cell transplantation before neurologic or visual impairment develops.
Keywords: Osteopetrosis, Infant, Hepatomegaly, Splenomegaly
Introduction
Malignant infantile osteopetrosis (MIOP) is an autosomal recessive disorder characterized by reduced activity of osteoclasts, resulting in generalized bone osteosclerosis. Abnormal osteoclast activity paired with normal bone formation by osteoblasts leads to the development of densely sclerotic fragile bones[1]. The disease presents in the first few months of life with the manifestations relating to underlying defect in osteoclastic bone resorption[2]. Overgrowth of cranial nerve foramina results in nerve compression, which frequently affects the optic, auditory, and facial nerves. Increased bone density also obliterates the medullary cavity, leading to extramedullary hematopoiesis, hepatospleno-megaly, anemia, and thrombocytopenia. Growth retardation and recurrent infections are also common[1]. The disease is fatal in infancy and is cured with hematopoietic stem cell transplantation, with a rate of success by 50% and unsatisfactory rescue of growth and visual deterioration[3].
In this article, we report a two-month old boy, who presented with hepatosplenomegaly and thrombocytopenia in newborn period and is diagnosed as infantile malignant osteopetrosis which is a rare cause of hepatosplenomegaly and thrombocytopenia.
Case Presentation
A two month-old male infant was referred to the department of pediatric gastroenterology of Sisli Etfal Education and Research Hospital because of hepatosplenomegaly and thrombocytopenia. Past medical history of the patient revealed that he was hospitalized in the first day of his life in the neonatal unit because of poor feeding. Sepsis work up was done. He received ampicilin-gentamycin therapy empirically. In the follow up hypocalcemia and intracranial hemorrhage were observed. The patient was referred to our clinic for further investigation of hepatosplenomegaly and thrombocytopenia.
He was born after an uncomplicated pregnancy and delivery in the 38th gestational week according to Ballard. Birth weight was 2600 gr. Apgar score was 7/9. Parental consanguinity was significant in the past medical history. One son and one daughter of the family died in infancy period with unknown etiologies.
Systemic examination revealed hepatomegaly with the liver noted to be 4/3 cm, splenomegaly with the spleen noted to be 3 cm. The patient had a bulging anterior fontanel with 4×4 cm in size. His weight was 3.4 kg (<3rd percentile), his height was 44 cm (<3rd percentile) and head circumference of the patient was 38 cm (50-75 percentile). He had pale appearance and fundoscopic examination showed optic atrophy bilaterally.
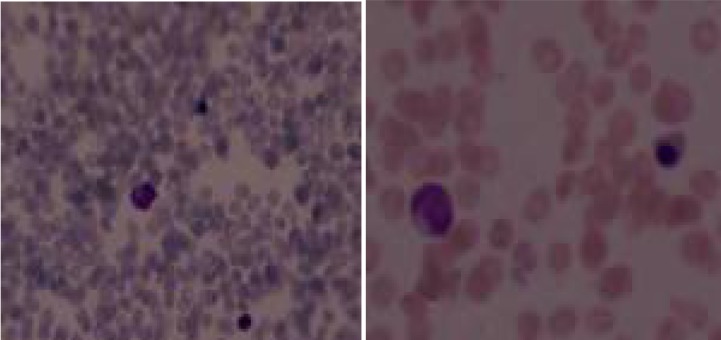
Laboratory examination yielded the following: hemoglobin 11.4 g/dl; white blood cell count 4400 cells/mm3 and platelet count 24.000/mm3. Biochemical analysis was normal except for AST 118 U/L, LDH 1150 U/L, parathyroid hormone (PTH) 349 pg/mL, alkaline phosphatase (ALP) 847 IU/L, acid phosphatase 16.4 U/L. Peripheral blood smear was significant for leukoerytroblastosis (Fig. 1). Arterial blood gas analysis was normal. Serology for toxoplasma, rubella, cytomegalovirus, herpes, and hepatitis virus was negative. Immunoglobulin (G, M, A) levels, lymphocyte subsets, urine and serum amino acid and urine organic acid analyses were also normal. Tandem mass spectrophotometry showed no abnormality.
Fig. 1.
Leukoerytroblastosis is significant in peripheral blood smear of the patient
Abdominal ultrasonography detected a marked hepatosplenomegaly. Cranial ultrasonography showed ventriculomegaly of the lateral and third ventricles. A computed axial tomographic scan of orbits demonstrated the narrowing of the optic foramina bilaterally. Bone marrow aspiration showed hypocellularity. Bone marrow culture was negative. Liver biopsy demonstrated extra-medullary hematopoiesis, and ballooning of parancymal cells were significant. Skeletal survey revealed diffuse sclerosis of all bones. Visual evoked potentialisation and brain-stem evoked response examination were planned.
Our initial diagnosis was congenitally acquired infection (TORCH) or a kind of storage disease (like lipid storage or lysosomal diseases) but physical examination and laboratory results confirmed the diagnosis of osteopetrosis. The disease was presented in newborn period and two children of the family had died. The absence of any metabolic acidosis with an alkaline urine pH and the absence of any cerebral calcifications excluded a diagnosis of carbonic anhydrase II deficiency syndrome. Familial erytrophagocytic lympho-histiocytosis and familial histoplasmosis were also considered in the differential diagnosis. Peripheral blood smear, bone marrow aspiration and culture findings and liver biopsy findings did not support the diseases.
The patient was referred for hematologic stem cell transplantation (SCT). He had two available HLA matched donors in his family who were patient's older sisters. He was taken to the stem cell transplantation program, but unfortunately blindness developed in the patient. He was still in follow-up 10 months after the diagnosis.
Discussion
Osteopetrosis is a family of bone diseases characterized by osteoclast failure and impaired bone resorption[4]. It has three forms which are: autosomal recessive, autosomal dominant and X-linked inheritance. Autosomal Recessive Osteopetrosis (ARO) may have the most severe course. Having an incidence of 1:250,000 in general population, ARO is more frequent in certain ethnic groups, including inhabitants of Costa-Rica in whom the incidence is much higher than elsewhere (3.4:100,000)[5].
Infantile, or malignant, osteopetrosis is present at birth or develops within the first months of infancy[5]. Our patient had the signs and symptoms from the first day of his life.
Abnormal bone formation and fibrous tissue replace the bone marrow space and finally hematopoesis is decreased. Extramedullar hematopoesis occurs resulting in leukoerythro-blastic anemia and thrombocytopenia. Liver and spleen enlarge progressively. Hemolysis resulting from hypersplenism worsens the anemia and thrombocytopenia[1, 6]. Our case was referred for hepatosplenomegaly and peripheral blood smear was significant for leukoerytroblastosis and he needed transfusions for anemia also for thrombocytopenia before liver biopsy. Liver biopsy of the patient demonstrated signs of extramedullary hematopoiesis.
Visual impairment results from bony encroachment on the optic foramina. It is a common initial symptom[7]. Optic atrophy is also present in a significant number of cases. In the series reported by Phadke et al[8], optic atrophy was present in three of six cases. In our case optic atrophy was found bilaterally in the early days of his life.
Radiologic findings showed increased bone density with defective metaphyseal remodeling. The “bone within-bone” appearance is characteristic and diagnostic[5]. Computerized tomography scan can be used for diagnosis and to determine the effect of the treatment. It is also used to assess auditory and optic canal[9]. The skeletal survey and CT scan of the patient was specific for radiologic findings of osteopetrosis.
Differential diagnoses include congenital disorders (e.g., pseudohypoparathyroidism, pyknodysostosis, and hypoparathyroidism), chemical poisoning (e.g., lead, fluoride, and beryllium), malignancies (myeloproliferative diseases and leukemia), and sickle cell disease[5]. Based on clinical history and radiographic findings, our case was accepted as the infantile or malignant type, with autosomal recessive inheritance. Familial histoplasmosis was excluded because bone marrow culture was negative and peripheral blood smear and liver biopsy findings did not support the disease.
Stem cell transplantation (SCT) is the only curative therapy for patients with MIOP, and it should be performed as soon as the diagnosis is made because neurologic impairment occurs in early infancy and will become irreversible even after successful SCT. Successful results have been achieved in patients transplanted with HLA matched sibling donor stem cells[10]. Two children in the patient's family were found available as HLA-matched donors. Infantile osteopetrosis warrants treatment because of the adverse outcome associated with the disease. The goals of pharmacotherapy are to reduce morbidity and to prevent complications. Appropriate medications include vitamin D supplements, corticosteroids, interferon, and erythropoietin. The definitive treatment is bone marrow transplantation, and the 5-year survival for recipients of HLA-identical bone marrow transplants is 79%[7].
Genetic consultation is important. Prenatal diagnosis of osteopetrosis early in pregnancy is an indication for termination of the pregnancy[11]. Our patient was also referred for genetic consultation and mutation analysis but because of financial problems mutation analysis was not performed.
Conclusion
MIOP is a rare disease of infancy. It should be considered in the differential diagnosis of hepatosplenomegaly. After the diagnosis the patient must be referred immediately for SCT before neurological impairment and blindness develop.
References
- 1.Gerritsen EJA, Vossen JM, Van Loo IHG, et al. Autosomal recessive osteopetrosis: variability of findings at diagnosis and during the natural course. Pediatrics. 1994;93(2):247–53. [PubMed] [Google Scholar]
- 2.Felix R, Hofstetter W, Cecchini MG. Recent developments in the understanding of the pathophysiology of osteopetrosis. Eur J Endocrinol. 1996;134(2):143–56. doi: 10.1530/eje.0.1340143. [DOI] [PubMed] [Google Scholar]
- 3.Del Fattore A, Cappariello A, Teti A. Genetics, pathogenesis and complications of osteopetrosis. Bone. 2008;42(1):19–29. doi: 10.1016/j.bone.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 4.Whyte MP. Osteopetrosis. In: Royce PM, Steinman B, editors. Connective Tissue and its Heritable Disorders: Medical, Genetic, and Molecular Aspects. 2nd ed. New York: Wiley-Liss, Inc.; 2002. pp. 753–70. [Google Scholar]
- 5.Subramaniam A, Singh A, Chavan M, et al. Autosomal recessive osteopetrosis: case report of two siblings. Oral Radiol. 2008;24(2):80–4. [Google Scholar]
- 6.Ozsoylu S. Malignant osteopetrosis and juvenile chronic myeloid leukemia. Pediatr Hematol Oncol. 1994;11(3):337–8. doi: 10.3109/08880019409141678. [DOI] [PubMed] [Google Scholar]
- 7.Venkateshwar V, Voidya A, Roy P, et al. Osteopetrosis. Med J Armed Forces India. 2003;59(4):344–6. doi: 10.1016/S0377-1237(03)80153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phadke SR, Gupta A, Pahi J, Pandey A, Gautam P, Agarwal SS. Malignant recessive osteopetrosis. Indian Paediatr. 1999;36(1):69–74. [PubMed] [Google Scholar]
- 9.Shapiro F. Osteopetrosis. Current clinical considerations. Clin Orthop Relat Res. 1993;294:34–44. [PubMed] [Google Scholar]
- 10.Tsuji Y, Ito S, Isoda T, et al. Successful nonmyeloablative cord blood transplantation for an infant with malignant infantile osteopetrosis. J Pediatr Hematol Oncol. 2005;27(9):495–8. doi: 10.1097/01.mph.0000179961.72889.bf. [DOI] [PubMed] [Google Scholar]
- 11.Besbas N, Draaken M, Ludwig M, et al. A novel CLCN7 mutation resulting in a most severe form of autosomal recessive osteopetrosis. Eur J Pediatr. 2009;168(12):1449–54. doi: 10.1007/s00431-009-0945-9. [DOI] [PubMed] [Google Scholar]