Abstract
Arenavirus particles are enveloped and contain two single-strand RNA genomic segments with ambisense coding. Genetic plasticity of the arenaviruses comes from transcription errors, segment reassortment, and permissive genomic packaging, and results in their remarkable ability, as a group, to infect a wide variety of hosts. In this review, we discuss some in vitro studies of virus genetic and phenotypic variation after exposure to selective pressures such as high viral dose, mutagens and antivirals. Additionally, we discuss the variation in vivo of selected isolates of Old World arenaviruses, particularly after infection of different animal species. We also discuss the recent emergence of new arenaviruses in the context of our observations of sequence variations that appear to be host-specific.
Keywords: arenavirus, LCMV-Clone13, LCMV-Armstrong, Mopeia/Lassa reassortant ML29, mutation, viral population, co-evolution
1. Background
Here we review genetic and phenotypic variations of arenaviruses at the level of virus families, at the level of genera, at the level of species, and finally within those entities known as “strains” or “isolates”. Sequence analysis reveals that the Arenaviridae, the Filoviridae and the Bunyaviridae share some structural motifs that could be derived from common ancestry or functions. Classical studies on the prototypic arenavirus species, lymphocytic choriomeningitis virus (LCMV), have followed the sequence and phenotypic variation of the LCMV-Clone 13 and LCMV-Armstrong strains in vivo and in vitro. Such studies shed light on our own analyses of the Mopeia/Lassa reassortant virus, ML29 as it varies after passage through a variety of host species.
1.1. Arenavirus Structure
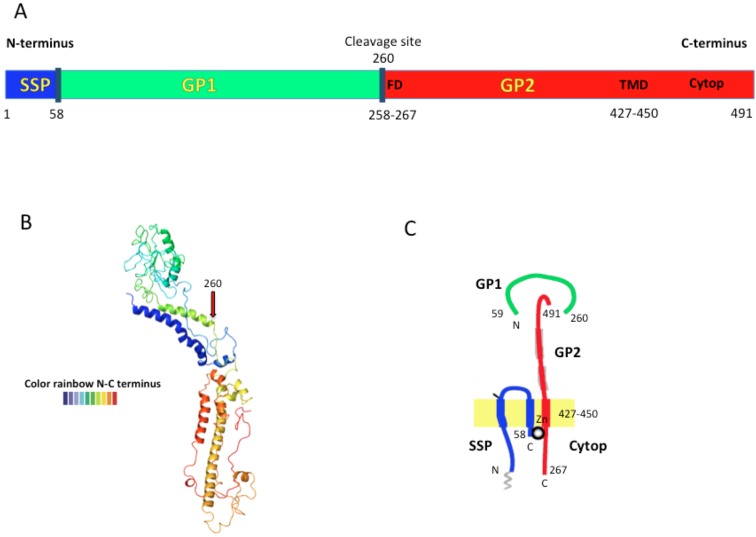
Arenaviruses have enveloped particles containing bi-segmented genomes of single-stranded RNA encoding four viral proteins in an ambisense manner. The small segment called S (~3.4kb), encodes the glycoprotein precursor (GPC) and the nucleocapsid protein (NP) that are the most important immunogens of the virus (Figure 1 and Figure 2). The NP and GPC sequences are separated by a noncoding intergenic region (IGR) [1]. The GPC is processed into a signal peptide (SSP), and into portions GP1 and GP2 (Figure 1 and Figure 2), which function to mediate viral assembly, entry, and uncoating and to determine cell tropism. NP has multiple functions: it encapsidates the arenavirus genome segments, interacts with L protein to form the RNP core for RNA replication and transcription, associates with Z protein for viral assembly, plays an important role in suppressing the innate immune response, and has exonuclease and nucleotide binding activity [2,3,4,5,6,7,8,9].
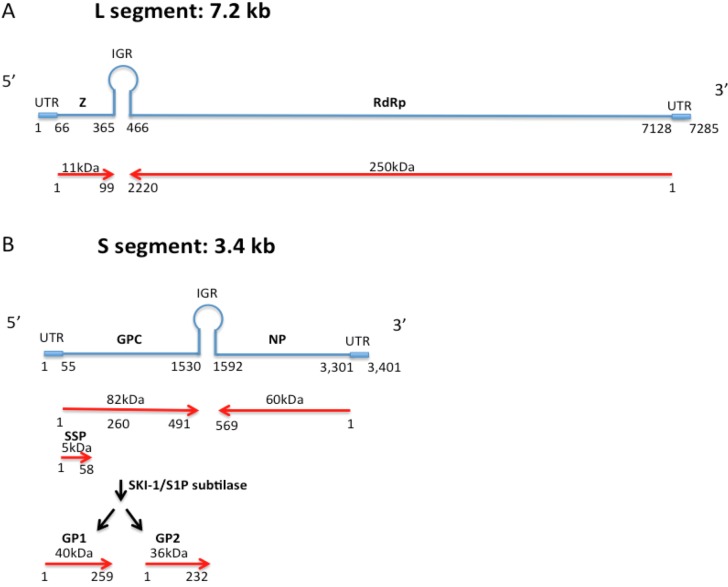
Figure 1.
Arenavirus genome structure from 5’ to 3’ end. A) The LASV L genome segment (7.2 kb) is represented in blue and is composed of an untranslated region (UTR) from nucleotide 1 to 66 and from 7129 to 7285, the gene encoding the zinc-binding protein (Z) from nucleotide 67 to 365, the intergenic region IGR from nucleotide 365 to 466, and the RNA-dependent RNA polymerase protein encoding gene (RdRp) from nucleotide 466 to 7128. B) The LASV S segment genome (blue lines) contains the untranslated region (UTR) from nucleotide 1 to 55 and from 3302 to 3401, the gene encoding the glycoprotein precursor protein (GPC) from nucleotide 57 to 1530, the intergenic region IGR from nucleotide 1531 to 1592, and the nucleocapsid protein encoding gene (NP) from nucleotide 1593 to 3301. The red arrows represent the 491 amino acid long GPC with its stable signal peptide (SSP) and glycoproteins 1 and 2 (GP1 and GP2) produced after maturation cleavage. The 569 amino acid long NP is shown encoded in the antisense orientation.
Figure 2.
The LASV envelope glycoprotein precursor (GPC) structure is shown in A) from N- to C-terminus containing SSP, GP1 and GP2 proteins. The dark blue lines represent the cleavage points. The fusion domain (FD); transmembrane domain (TMD), and cytoplasmic domain (Cytop) are shown in brown letters (modified from [19]). B) The predicted Lassa-Josiah GPC structure obtained by open-source software (Phyre2). The structure goes from N-terminus (blue) to C-terminus (red). C) Schematic representation of the trimeric GPC subunit assembled in the cell membrane. GP1 is the most external protein bound to GP2 that is embedded in the lipid membrane. GP2 is thought to interact with SSP through an inter-subunit zinc finger (ball) (modified from [20]).
The Large (L) segment (~7.2Kb), encodes the small zinc-binding protein (Z) that functions as a matrix protein, interacts with L and NP and other host proteins, plays a role in viral transcription and replication, has pro-apoptotic activity and is essential for virus budding [3,5,10,11,12,13,14]. The L segment also encodes the L protein that is an RNA-dependent RNA polymerase (RdRp). L and NP are the minimal trans-acting virus factors required for replication and transcription [2,15,16]. L and Z are also separated by an IGR [17]. Both RNA segments are flanked by noncoding regions (UTR) that function with the IGR as cis-acting elements for RNA replication and transcription [15,17,18] (Figure 1).
Arenavirus molecular biology so far has been focused on characterizing the role of each domain of the viral proteins in vitro using reverse genetics platforms. However, since single mutations can affect the functions of several proteins in a complex, an additional approach has been to isolate viruses with different biological properties in vivo, and then to determine which mutations contribute most to the functions related to the in vivo phenotype. New molecular and crystal structures now elucidate the role of each viral component and guide the production of better vaccine candidates and antiviral compounds.
1.2. Arenavirus Taxonomy
The International Committee on Taxonomy of Viruses (ICTV) established the family Arenaviridae, based on morphological, physiochemical, and serological parameters [21,22]. This family now includes 25 species of viruses (insider update by MSS, a member of the International Committee on Taxonomy of Viruses or ICTV-arenavirus group). All the currently-classified arenaviruses are carried by rodents, except for Tacaribe virus that is carried by bats (see virus abbreviations in Supplementary Table 1). At least 10 of these viruses occasionally infect human beings causing zoonotic diseases [22,23]. The arenaviruses can be divided into two serocomplexes: The Old World (OW) and the New World (NW), also known as the LASV-LCMV complex and the Tacaribe complex, respectively. The OW group includes the globally-distributed LCMV and the African arenaviruses that infect rodents of the family Muridae, subfamily Murinae. The NW viruses include South and North American arenaviruses infecting rodents of the family Muridae, subfamily Sigmodontinae [24]. It is highly possible that long-term co-evolution with the Sigmodontine rodents drove the evolution of distinct New World viral species [25].
In addition to the criteria that divide the Arenaviridae into NW and OW groups, species demarcations are determined by the following criteria of their member viruses: significant differences in antigenic cross-reactivity and cross-neutralization; role as an etiological agent of disease (or not) in humans; presence in a defined geographical area, host species or group of species, and significant protein sequence differences compared to other viruses in the genus (i.e., showing a divergence between viruses of different species of at least 12% in the nucleoprotein sequence). However, there are still some poorly-defined criteria for classification. For example, consider the criterion that the amino acid sequence of the NP has less than 88% homology to the closest arenavirus. It is interesting that some Lassa isolates vary more than 12% from each other, and should be classified in different species but they are not. Another critical point is the possibility of reassortment among viruses assigned to the same species. It is odd that Lassa and Mopeia were able to reassort, even though they are assigned to different species, perhaps due to compatibility between the Lassa and Mopeia terminal sequences. In addition, there are several reports of arenaviruses detected by molecular techniques from human or animal samples, however some of them are not yet associated with human diseases or failed to replicate in cell cultures (see Supplementary Table 1). With non-isolated viruses, it is impossible to study serological and morphological parameters to give an accurate characterization. Although, they can not be classified as new Arenavirus species until infectious isolates become available [26] (Supplementary Table 1), it is important to capture and record their characteristics because they shed light on the evolution of the arenaviruses.
1.3. Antigenic Characteristics
Broad serological cross-reaction and neutralizing or complement-fixing antibodies are used to identify the OW and NW arenavirus complexes [27,28,29,30,31,32]. Monoclonal antibodies (MAbs) produced against the GP2 of two African arenaviruses reacted broadly against American arenaviruses, demonstrating highly conserved epitopes in this family [33,34]. In a more narrow way, Mabs against JUNV NP reacted only with NW arenaviruses [35] or reacted only with isolates from local foci, suggesting a strong antigenic stability of those viruses in local areas [34]. Despite those results, several attempts failed to define clearer serological differences between arenaviruses useful for species classification [34,36]. However, the close antigenic relationship could be useful to design immuno-prevention systems to induce cross-protection against related strains. For example, guinea pigs and marmosets inoculated with TCRV are protected against JUNV disease [23,37]. Candid #1, a vaccine for JUNV, cross-protected rhesus monkeys from disease after challenge with MACV; both viruses belong to clade B of NW viruses [38]. Similarly, guinea pigs, marmosets or rhesus monkeys are protected from LASV disease after inoculation with MOPV and endemic regions for this virus have no reported Lassa Fever (LF) cases even though LASV and MOPV share the same rodent host [39,40]. In conclusion, the antigenic relationships between members of the family Arenaviridae suggests that viruses assigned to a given species are very stable in small geographical areas, and that OW and NW groups diverged a long time ago.
1.4. Virus-Host Coevolution
Arenaviruses normally produce persistent infections in rodents, with chronic viremia and viruria, spreading virus through urine, feces, and saliva to other rodents and accidentally infecting humans or other mammals [41,42,43]. Although it is generally thought that infections in rodents are asymptomatic, some studies showed that infection can cause weight changes, widespread viral dissemination, increased mortality, and reduction in size and fecundity of survivors. All those changes depended on viral loads, viral genomic sequences, and the genetic backgrounds of the mice [44,45,46]. A strong example of arenavirus infection affecting mouse breeding are the experimental infections of large vesper mouse (Calomys callosus) females with MACV resulting in female sterility [47]. In other studies, adult white-throated wood rat (Neotoma albigula) pregnant females were seronegative for WWAV in a population with 60% seropositivity, indicating that little vertical transmission occurred. Similar observations are reported for the drylands vesper mouse (Calomys musculinus)-Junin virus disease model, where transmission is more common between adult males and associated with the presence of scars, implying violent transmission during aggressive encounters [48,49,50]. In contrast, the infection of rodents classified as Mastomys sp. with LASV seems to have no sex or age bias suggesting more frequent vertical transmission from dam to pups [51]. From this evidence, it can be inferred that vertical transmission has a greater impact in reducing mouse populations than horizontal transmission; exerting strong selective pressures on mice infected early in life.
In general, diseases produced by arenaviruses are located in endemic foci determined by the presence of the natural host. However, the geographic localization of a specific arenavirus can be more restricted than the distribution of its reservoir. LCMV is an exception with worldwide distribution through common house mice from the family Muridae, or hamsters from the family Cricetidae. African viruses circulate in endemic areas between rodents from the family Muridae, genus Mastomys, Praomys, or Arvicanthis. The American arenaviruses circulate in specific areas between rodents from the family Cricetidae, genera Oryzomys, Sigmodon, Neotoma, Nephelomys, Oecomys, Calomys, Zygodontomys, Neacomys, or Akodon (Supplementary Table 1). TACV is an exception believed to circulate in bats (Artibeus sp.) (Supplementary Table 1). The fact that each arenavirus is usually associated with a single type of rodent or closely related rodents [25], together with phylogenetic studies in both virus and host, indicates an apparent codivergence between them [52]. The current rodent radiation hypothesis [53] states that holoartic cricetids (ancestor of murids) spread to the Americas from North to South in different waves evolving into ancestors of the Neotominae and Sigmodontinae sub-families, probably carrying the NW arenavirus ancestors. Additionally, cricetids spread to the Asian continent following different migration waves; from there, they continued to Europe and then to North Africa evolving toward the Muridae sub-family and carrying the OW arenavirus ancestors. The sub-Saharan conditions of radiation, weather, human migration, influenced the high diversification and isolation of those rodents that populated South Africa and carried the OW African arenaviruses [53]. In the case of the newly discovered Lujo virus, the sequence analysis showed that it is most strongly related to OW viruses but shares GP sequences with the NW viruses [54]. Similarly, the characteristics of other OW viruses coalesce into distinct species, suggesting that, within the African continent, signatures of co-evolution might have been obliterated by multiple evolutionary events in which viruses jumped from one host to another [55,56].
Arenavirus-rodent interactions exemplify virus-host codivergence, in which one rodent species diverges from another so much, that the viruses of each host are no longer able to infect the same hosts equally well. Codivergence and cospeciation are similar: if two species are closely associated, as in viruses and their hosts, they might speciate or evolve in parallel. Thus it could eventually be possible to assign host species by the population of parasites (including viruses) they carry [52]. An alternative hypothesis suggests a combination of co-speciation events and virus transfer among rodent species that may explain the codivergence incongruities seen in some members of this family [57,58]. One study in NW arenaviruses found that both events occurred during arenaviruses evolution suggesting not only codivergence but also evolution via host switching, implying an arenaviral potential for becoming panzootic [59].
Ten arenaviruses are known to cause natural (LCMV, LASV, LUJV, MACV, JUNV, GTOV, CHPV, and WWAV) or laboratory acquired (TCRV, FLEV, and SABV) human disease in specific geographical regions (Supplementary Table 1). Those viruses do not appear to be monophyletic, suggesting that the pathogenic phenotype has risen in multiple independent events during virus evolution [57].
There is also evidence of selective pressures on human populations by arenaviruses. Two genes related to LASV infectivity and immunity (LARGE and interleukin 21) had been associated with a possible positive selection after viral infection leading to LF resistance. Those genetic traits are evidence that the human incidental host co-evolved with LASV. In addition, LASV infection in pregnant women results in nearly 100% fetal lethality exerting a strong selective pressure in the endemic regions. Thus, arenaviruses exert selective pressures on both human and carrier host populations [60,61].
During the codivergence process, OW and NW arenaviruses evolved to use different cell receptors (alpha-dystroglycan vs. transferrin receptor 1), different entry mechanism (delivery to endosomes via vesicular trafficking vs. endocytosis), and different protective immune responses in rodent and in primate incidental hosts (innate vs. acquired immunity) [62,63,64,65,66,67,68]. All those co-evolution findings raise interesting questions such as: Why are arenaviruses so species-specific despite high mutation rates that could increase host range? What mouse or monkey genes are controlling resistance to arenavirus infections? Did non-pathogenic arenaviruses and mice or human populations co-evolve to co-exist without disease? Those answers could help us understand arenavirus co-divergence and identify target genes for disease treatment.
2. Arenavirus Plasticity
The arenaviruses, like other RNA viruses, are highly divergent due to high mutation rates from a low-fidelity viral RdRp and due to reassortment and possibly recombination events that contributed to viral diversification during arenavirus evolution [25,69,70]. Although some reassortants were produced in vitro, those experiments indicated that there are restrictions that prevent recovery of all possible combinations [71,72,73,74,75,76]. So far, no reassortant arenavirus has been isolated from nature and recombination appears to be rare and occurs only between phylogenetically close strains [52,70,77,78]. Therefore, the high frequency of transcription errors appears to be the main driver of arenavirus evolution. The estimated mutation rate of RNA viruses ranges from 10-3 to 10-5 per nucleotide incorporated during replication [79,80]. Lethal mutagenesis studies in vitro estimate that the error frequencies of the LCMV RdRp are between 1 × 10-4 and 5.7 × 10-4 substitutions per nucleotide [81,82]. Those results are consistent with the genetic heterogeneity seen in other arenaviruses [83]. Comparison of nucleic acid and protein sequences within specific arenaviruses showed identities ranging from 90–95% [84], even if they were isolated in the same region [85,86]; and viruses isolated from different regions ranged from 78 to 86% [85,86]. Sequence comparison of all proteins of seven pathogenic arenaviruses showed identities ranging from 44 to 63% [84]. LASV isolate variation is the highest among this family with nucleotide and amino acid variations of 27% and 15% respectively, followed by PIRV with 26% and 16% variations isolated in very close geographical regions [87,88]. The genetic diversity within and between isolated arenavirus groups, suggests that the spatial heterogeneity may be reflected in host range and pathogenicity. Consequently, sequence analysis of new virus isolates could be useful for tracking the source of arenaviruses outbreaks [86].
2.1 Arenavirus Variation in vitro
When referring to a virus strain we are describing the most abundant variant from a closely-related virus swarm containing individual particles with broadly-distributed mutations. A viral isolate containing many variants, called quasi-species, could act as a unit of selection through a continuous dynamic process of genetic variation, competition, and selection [89]. However, it is important to emphasize that the whole “virus swarm” contributes to the characteristics of the virus strain and will be the target of selection instead of individual variants. For example, some variants carrying lethal mutations not only compete with the fittest replicating unit but also cooperate with other mutants complementing each other, thus assuring the survival of the units containing "lethal mutations" [90]. Several studies describe the effect of the mutant spectra in the virus population phenotype making it more virulent or more attenuated according to the predominant quasi-species. For example, defective interfering particles could play a role in attenuating the virulence of a viral swarm by interfering with virus replication [91,92,93,94,95]. Therefore it is important to take into account that serial passages of arenaviruses in cell lines or animals could lead to the accumulation of mutations changing the viral phenotype from the natural reservoirs or from clinical samples [14,84,96]. In fact, consecutive passage of arenavirus through cell lines has been used to detect viral strains with pathogenicity differing from the original viral stock [72,97,98]. However, in vitro changes should occur at a different rate than those seen in vivo, due to the absence of several selective forces (e.g., immune system, a variety of receptors, different tissue compartments).
Several studies explore arenavirus variability under specific selective pressures as a model of viral evolution. For instance, serial passages of LCMV in the presence of a mutagen such as 5-fluorouracil (5-FU), and an anti-viral like ribavirin leads the viral population into extinction in an event known as “error catastrophe” or “lethal mutagenesis” [81,99,100]. This phenomenon is explained as enhanced mutation rates that produce accumulation of many non-viable mutants resulting in increased sensitivity to anti-virals and an abortive infection. In other words, genome flexibility allows the adaptation of viral populations to environmental changes; however there is an “error threshold” beyond which viral fitness is considerably reduced and the virus population may face “viral extinction” [83,101,102]. On the other hand, with low genomic variation (increased fidelity of replication) the viral population does not have the capacity to adapt to new conditions due to low quasi-species diversity. Under those circumstances, viral survival can also be compromised [103,104].
Cell culture manipulations of Lassa and Mopeia viruses revealed that these African viruses species were so closely related as to be able to reassort with one another [72]. Initially, Vero cell cultures were co-infected with a Lassa strain (Josiah) and a Mopeia strain (AN20410). Both small plaque (Mopeia phenotype) and large plaque (Lassa phenotype) progeny viruses were observed and some proved to be reassortants between Lassa and Mopeia [72]. The progeny isolate that grew best, ML29, was a small-plaque isolate with its large genomic segment (L) from Mopeia and its small segment from Lassa [105]. This reassortant had 18 mutations with respect to the parental viruses [106]. These mutations included 3 amino acid changes (Mopeia to ML29) in the non-conserved regions of the polymerase (Y851N, R1233G and D2136N), one amino acid change in GP2 (Lassa to ML29; K272E), and two amino acid changes in NP (A485D within the DEDD exonuclease of the NP C-terminus [9], and N173S). Two mutations in the panhandle (complementary termini) region and several mutations that did not impact the proteome were hypothesized to stabilize the RNA structure of the reassortant. Since ML29 is a candidate attenuated vaccine for Lassa fever, the stability of its phenotype and genome were assessed after 12 passages in Vero cell culture. The small plaque phenotype and the ability to protect mice from lethal intracerebral inoculation was retained through all 12 passages, as was the consensus sequence of the open reading frames. However, deep sequence analysis revealed the gradual increase in single nucleotide polymorphisms (SNPs) with in vitro passage, though none of the SNPs exceeded 25% frequency within the viral population [107]. This agrees with the idea that one viral preparation can maintain a swarm of quasi-species with a variety of sequences; yet retain its phenotypic characteristics [102].
Thus, changes in virus diversity in vitro, increasing or decreasing mutation rates, can affect virus fitness and viral tropism and produce a spectrum of outcomes depending on particular conditions of the virus-host interaction. Those findings were explored with the goal of using “lethal mutagenesis” as an anti-viral treatment, and viral clones with high fidelity polymerases as attenuated vaccines [103,108].
2.2. Arenavirus Variation within a Single Host
Chronic infections are the best models for studying the structure and evolution of a virus population within a single host since viruses have a chance to evolve after extensive replication, genomic changes and selection. The prototypic virus LCMV is able to produce acute or persistent infections in mice and has been used as a model to study viral population dynamics in vivo [109,110]. The intra-host viral variability and the existence of tissue-specific virus populations had been studied in plant and mammalian hosts, suggesting that viral compartmentalization is caused by selective pressure exerted by each target tissue [109,111,112,113,114,115,116,117,118,119,120].
During the peak of an arenavirus acute infection in monkeys, the number of viral particles in blood is around 1 × 107 PFU/ml [39,121,122,123]. With mutation rates approximately 1 × 10-4 substitutions per nucleotide and a virus of approximately 104 nucleotides in size, by the time maximum viremia is reached (after several days of replication) every possible mutation had multiple chances to occur. In fact there is a close correlation between arenavirus concentration and severity of disease. Analysis of cytotoxic T lymphocyte or neutralizing antibody escape mutants in mice, showed mutation rates around 3 × 10-4 substitutions per nucleotide and suggested that those mutations are present and are selected from the virus stock instead of appearing spontaneously [124,125,126]. This process is limited and guided by the selective pressure exerted by the host and its different conditions. For instance, laboratory mice infected at the time of birth with LCMV-Armstrong develop persistent infection, and after 8–6 weeks, virus isolated from the nervous system (CNS) was similar to the parental virus, causing acute infections and strong immune responses in adult mice; whereas isolates from spleen (such as LCMV-Docile and LCMV-Clone 13) produced chronic infections with suppressed T-cell responses and susceptibility to opportunistic infections [109,110,127,128].
Sequence comparison of viral isolates from those two tissues (brain or lymphoid/spleen) revealed five nucleotide changes but only two amino acid differences: one at residue 260 of GP1, phenylalanine (F) to leucine (L); and the other at residue 1079, lysine (K) to glutamine (Q) in the L protein [128,129,130,131] (Table 1A and B). For lymphoid isolates the predominant change was from phenylalanine to leucine (F260L) and the predicted GPC structures showed significant changes (Figure 3) that could affect GPC processing. 43 of the 47 spleen isolates (~91%) had L and 4 (~8%) had F. Meanwhile in variants selected in brain the F260 parental sequences were more abundant. 48 out of 59 had L260 (~81%) and 11 had F260 (~18%). There was no phenotype correlated with the RdRp K1079Q mutation alone [128,130,132]. However, another study showed that this mutation contributes to persistence and immunosuppressive phenotype [131]. Although the F260 mutation predominated in each tissue-specific isolate, there were still some F260 or L260 remaining quasi-species in each viral sample. Additionally, some viruses reverted to parental phenotype without changing back to the F260 residue [130]. Recent pyro-sequencing analysis of LCMV-Armstrong and -Clone 13 showed diversity in each viral preparation (Table 1A and B). The new sequencing of old isolates concluded that the original amino acid changes were indeed the major changes, with additional changes detectable but not dominant in the population. Researchers at Scripps and Geneva discovered an additional amino acid difference between LCMV isolates Clone 13 and Armstrong that were not corroborated by recent pyrosequencing studies of older isolates from the Salvato and Ahmed laboratories; however, reverse genetic studies found that the new amino acid changes did not affect the Clone 13 phenotype of persistence and immunosuppression [131,133]. The Clone 13 phenotype depended on contributions from both of the major mutations in the virus stock (the mutation in GP favored entry into dendritic cells and the mutation in the polymerase favored replication in dendritic cells resulting in increased antigen presentation and death of virus-specific T cells) [131,133].
Table 1.
LCMV sequence diversity. Comparison between different isolates of LCMV-Armstrong and LCMV-Clone 13. (A) Nucleotide and protein single nucleotide polymorphisms (SNPs) from the S segment. (B) Nucleotide and protein SNPs from the L segment. Black color letters represent the consensus sequence. Green, blue, and red letters represent abundance of that mutation at nearly 20%, 40% and 100%, respectively. Blue dark squares are the reported differences between both virus strains as follows: In the S segment nucleotide mutation 603 corresponds to amino acid 177, nucleotide mutation 855 corresponds to amino acid 260, nucleotide mutation 1298 corresponds to amino acid 407; in the L segment nucleotide mutation 3797 corresponds to amino acid 1079, nucleotide mutation 1798 corresponds to amino acid 412. The light blue square holds the previously reported mutation and confirmation of its presence in our laboratory strain. The Salvato 2012 pyro-sequencing shows diversity in those viral clones even within the same clone from the same laboratory.
(A) S segmet
| S segment | |||||||
|---|---|---|---|---|---|---|---|
| LCMV strains | UTR | GP1 | GP2 | NP | |||
| nt/aa | nt/aa | nt/aa | nt/aa | nt/aa | nt/aa | nt | |
| 5’ end | 467 | 603 | 606-7 | 855 | 1015-16 | 1298 | 2290 |
| LCMV Armstrong | T/N | A/N | GC/A | T/F | AA/E | T/D | G/N |
| Grande-Perez et al. [101] | |||||||
| LCMV Armstrong | C/N | G/D | CG/R | T/F | CC/A | T/D | G/N |
| Salvato et al. [17,129] | |||||||
| LCMV Armstrong | C/N | A/D | GC/D | T/F | AA/E | T/D | A/N |
| Zapata et al. [135] | |||||||
| LCMV C13 | C/N | G/D | GC/D | C/L | AA/E | C/D | A/N |
| Flatz et al. [134] | |||||||
| LCMV Cl 13 | C/N | G/D | GC/D | C/L | AA/E | C/D | G/N |
| Zapata et al. [135] | |||||||
(B) L Segment
| L Segment | ||||||||||||||||
| LCMV strains | IGR | L protein | UTR | |||||||||||||
| nt | nt | nt | nt/aa | nt/aa | nt/aa | nt/aa | nt/aa | nt/aa | nt/aa | nt/aa | nt/aa | nt/aa | nt/aa | nt | aa | |
| 5’ end | 415 | 447 | 453 | 884 | 886 | 1201 | 1435 | 1798 | 2491 | 3797 | 5100 | 5795 | 7116 | 7165 | 7197 | 7200 |
| LCMV Armstrong | G | G | G | A/T | T/T | T/H | G/V | G/A | C/F | A/K | A/K | T/L | C/T | G/L | T | C |
| Grande-Perez et al. [101] | ||||||||||||||||
| LCMV Armstrong | G | G | T/S | A/T | C/H | C/V | G/A | T/F | A/K | C/T | C/L | T/I | A/L | - | G | |
| Salvato et al. [17,129] | ||||||||||||||||
| LCMV Armstrong | G | G | G | A/T | T/T | C/H | C/V | G/A | T/F | A/K | A/K | C/L | C/T | G/L | - | G |
| Zapata et al. [135] | ||||||||||||||||
| LCMV C13 | A | G | G | A/T | T/T | C/H | C/V | A/A | T/F | C/Q | A/K | C/L | C/T | G/L | - | G |
| Flatz et al. [134] | ||||||||||||||||
| LCMV Cl 13 | A | C | A | A/T | T/T | C/H | C/V | A/A | T/F | C/Q | A/K | C/L | C/T | G/L | - | G |
| Zapata et al. [135] | ||||||||||||||||
Figure 3.
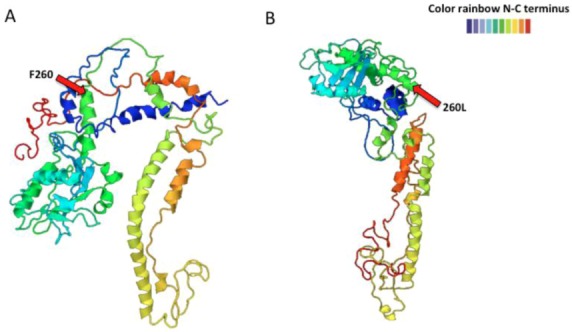
LCMV-ARM53b (A) and -Clone 13 GPC (B) predicted structure using the Phyre2 program. One amino acid change from F to L at position 260 alters the predicted GPC structure. The structure goes from N-terminus in blue color to the C-terminus in red color.
In the Oldstone laboratory, a stock of the LCMV-WE strain was found to harbor viruses that did or did not cause growth hormone deficiency syndrome (GHDS) in persistently-infected C3H/st mice. A close analysis of plaque-purified viral clones illustrated that the virus strain contained variants of different phenotype. Less than 5% of the viral isolates were able to replicate to high titer in GH-producing cells and cause GHDS in C3H/st mice. The GHDS phenotype was due to a G to A nucleotide substitution at position 535 (serine 153 to phenylalanine at the protein level) of the LCMV-WE GP. However, the stable presence of GHDS-phenotypically silent variants in the LCMV-WE viral inoculum was not enough to induce disease, indicating that pathogenic variants can be maintained within a non-pathogenic viral population. Experimental mixtures of LCMV WEc54 (serine) and WEc2.5 (phenylalanine) showed that the proportion of the latter has to be between 10% and 50% in order to induce GHDS. In addition, this study found variability even inside each cloned virus [92,94].
In order to evaluate the role of the immune system in selecting variants of LCMV, different isolates from tissues of immunocompetent and immunocompromised animals were tested in vivo. After inoculation of adult mice, all animals showed the original pathogenic pattern. Viruses from lymphoid organs (Clone13-like) caused persistent infections and immunosuppression, in contrast to virus isolated from CNS. Those findings suggested that the immune system is not the main selective force and sequence analysis showed again that the mutation in the position 260 was tissue specific (F260L or F260I associated with lymphoid organs). Additionally, LCMV-Clone13-like viruses were found only in cells in the marginal zone and white pulp of the spleen, specifically in CD11c (+) and DEC-205 (+) splenic dendritic cells; while LCMV-Armstrong-like were found in the red pulp in CD11c (-) and DEC-205 (-) splenic dendritic cells. The specific tropism of those mutants was associated with increased receptor GP1/α–DG affinity. LCMV- Clone 13 (F260L or F260I) and LCMV WEc54 (S153) showed around 2–3 logs higher affinity for α–DG than their parental virus LCMV ARM and LCMV WEc2.2 (S153F) [132,136]. Those results indicated that receptor-virus interaction in target cells are selecting viral clones in vivo, defining the disease outcome. Additional selective pressures include enzyme processing rates, different nucleotide concentrations, codon bias, dose and route of infection and other microbes in the host. For example, there is some contribution from the arenavirus polymerase to the induction of persistence by LCMV-Clone 13, in that its mutation K1079Q gives it preferential ability to replicate in plasmacytoid dendritic cells over LCMV-Armstrong [131].
In conclusion, the analysis of specific mutations focuses on the function of each viral protein or genome component and its biological significance. However, the analysis of complex viral populations is in its infancy, and it could give a broader picture of how mixtures of particles, or quasi-species, impact pathogenesis and immunity. Therefore, the use of new tools such as deep sequencing and mathematical models can approach the pharmacological and vaccine research from a more holistic angle.
2.3. Arenavirus Variation after Passage in Multiple Hosts
In addition to their natural hosts, arenaviruses can infect other animals including hamsters, rabbits, squirrels, guinea pigs, dogs, chickens, bats, and primates. Some of them are refractory to disease [14,86,137,138,139,140,141,142,143,144]. Arenaviruses co-evolved with their natural host but little is known about those processes in other animals and the implication for pathogenesis. It has been demonstrated that the same virus induces different outcomes depending on the host. For instance, C3H/st, BALB/WEHI, and SWR/J mice infected at birth with LCMV-Armstrong, E-350, or Pasteur strains develop persistent infection but only C3H/st mice develop Growth Hormone Deficiency Syndrome. In contrast, LCMV strains Traub and WE failed to induce disease in those animals [145]. Examples can also be taken from the South American Guanarito virus (GTOV). GTOV virus isolated from rodents in Venezuela, showed higher sequence variation than human isolates suggesting differential host-specific selection of GTOV strains [146].
Since the Lassa vaccine candidate, ML29 was passaged in many different animals (mice, guinea pigs, marmosets, and rhesus macaques), and we took the opportunity to examine sequence variation with the goal of finding host-specific variants. In order to study the complexity and stability of ML29 in vivo, rhesus monkeys, marmosets, and mice were vaccinated and followed for several weeks by isolation of serum virus. Isolates were characterized by pyro-sequencing and showed several SNPs indicating the heterogeneity within cloned viral populations. As mentioned previously, the variation after 12 passages in vitro was less than 20% and within the expected mutation rates of arenaviruses. Some animals developed a very low viremia during the first two weeks after vaccination. ML29 virus isolated during these first few weeks was subjected to pyro-sequencing and proved to have a lower number of SNPs (less variation) than virus obtained from in vitro passage, and surprisingly, some of the variations were host-specific (Table 2). The virus-host adaptations also seemed to accumulate with time in the primate host [107]. Protein prediction analysis showed that those mutations, at the amino acid level, induced structural changes in GPC and NP (Figure 4 and Figure 5).
Table 2.
Host-specific SNPs found after ML29 vaccination of different animals. Those specific mutations were found in rhesus macaque monkeys (Blue shade), marmosets (Pink shade), mouse (Orange shade) or common to marmoset and mouse (Green shade). Letters and numbers represent the amino acid change and the protein position. Those sites with the same amino acid represent synonymous changes.
| Animal | Glycoprotein | Nucleoprotein | RdRp | |||
|---|---|---|---|---|---|---|
| Monkey | I 252 | M 179 L | L 266 L | |||
| D 341 G | L 494 L | |||||
| R 551 K | H 1572 Y | |||||
| Marmoset | I 252 L | , | I 252 M | |||
| Mouse | I 252 M | R 59 R | ||||
| T 223 A | ||||||
Figure 4.
Predicted ML29 host-specific changes in GPC. The left-most structure shows the ML29 GPC predicted structure after passage in Vero cells. After inoculation into marmosets, the recovered viruses showed an isoleucine (I) to leucine (L) change at position 252 that affects the predicted GPC structure (middle structure). Another mutation at the same position, I to M, also changed GPC structure (figure on the right). The structure goes from N-terminus (blue) to C-terminus (red).
Figure 5.
Predicted ML29 host-specific changes in NP. The left-most structure shows the ML29 NP predicted structure after passage in Vero cells. The middle structure represents the predicted NP changes (M179L, D341G, R551K) occurring in virus recovered from rhesus macaques only. The structure on the left shows two changes (R59R, and T223A) seen in mouse and marmosets. All host-specific mutations induced conformational changes in the NP predicted structure. The structure goes from N-terminus (blue) to C-terminus (red).
Viral infection starts with binding to cellular receptors on the host cell. The virus:receptor interaction plays a role in tropism, host-range and pathogenesis. As discussed above, one amino acid change in the arenaviral GP or RdRp gives the virus the capacity to infect different cells and alters viral pathogenicity [128,130,147]. On the other hand, arenavirus receptors are highly conserved in vertebrates [148], though not all infected animals become productively infected. In fact, not all cells in the same individual expressing those receptors are infected, suggesting that differential receptor-processing, viral diversity, or other cellular factors are involved in viral tropism and virulence [148,149]. For instance, SKI-1/S1P cleavage of GPC from different OW arenaviruses, occurs at different rates suggesting that this protein acts as a selective factor, shaping the specific co-evolution of the arenaviruses and their hosts [150]. Additionally, GP, RdRp and NP sequences from LCMV-Armstrong, LCMV-C13, LASV, and ML29 showed synonymous mutations [128,129,130] (Table 1A and B) that could be also be giving advantage to some viral particles either by allowing the virus to use cells with specific pools of tRNA [151] or by changing the viral RNA structure, therefore changing the viral fitness and the virus phenotype. Evidence that synonymous mutations play a role in viral selection has been seen in long term HIV infection in which the viral population evolves towards the usage of host-preferred codons [152]. This is a field that needs further exploration and arenaviruses are suitable for such studies.
2.4. Examples of Arenavirus Variation due to Co-Evolution with other Viruses
Sequence analysis of recently emerging viruses suggested that genes from arenaviruses share some homology with other negative-strand virus such as filoviruses and bunyaviruses [153,154]. The tick-borne Crimean Congo Hemorrhagic Fever Virus (CHFV) is a bunyavirus, genus Nairovirus, with three genomic RNA segments: the largest encodes a polymerase (with strong homology to the Lassa L RdRp), the middle segment encodes a bunyavirus-like envelope glycoprotein Gc,Gn, and the smallest segment encodes a nucleocapsid protein with strong homology to the Lassa NP [154]. It is possible that arenaviruses and bunyaviruses shared an ancestor. For example, a precursor of CCHFV could have adapted to propagate in both insects and mammals and could have fragmented a genome segment to evolve from 2 to 3 segments. One can also imagine that a virus like CCHFV initiated during a co-infection of ticks or mammalian hosts with a bi-segmented arenavirus and a tri-segmented bunyavirus. Co-infection could enable the bunyavirus polymerase to jump templates from a bunyaviral replicative form to an arenaviral mRNA (Figure 6). Mixing of these viruses is helped by the fact that between 4%–30% of bunyaviruses package two genome equivalents per virion [155,156,157].
Figure 6.
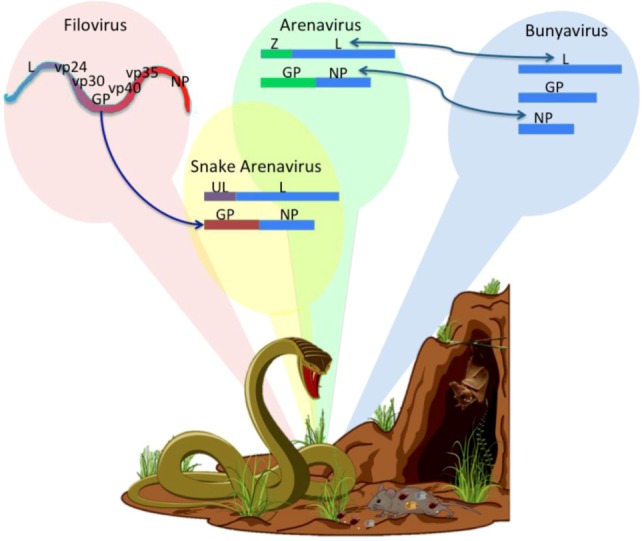
Arenavirus Evolution. The upper diagram shows the genomic structure and proteins encoded by filoviruses, arenaviruses, bunyaviruses, and the newly-discovered snake “arenavirus”. The blue arrows show the hypothetical changes that occurred in the arenaviral ancestors. The new snake virus has the L and NP genes of an arenavirus, but the GP of a filovirus and the Z gene similar to a cellular ubiquitin ligase (UL). The lower diagram shows how the reservoirs of arenaviruses, filoviruses, and bunyaviruses can interact with each other in the same ecological niche allowing co-infections and mixing of viral genomes to produce new viruses (Modify from [159]).
A new type of bi-segmented negative-strand RNA virus has recently been discovered in snakes. Three isolates of the new virus are characterized by ambisense coding and some sequence-homology with arenaviruses. The snake virus L and NP genes are homologous to those of arenaviruses, the GP sequences are homologous to filovirus envelope glycoproteins, and the Z gene sequences are homologous to host ubiquitin ligase [158]. Since the new virus was discovered in snakes suffering from Inclusion Body Disease (IBD) characterized by a build-up of virus particles in cellular cytoplasm, we speculate that the new virus carries a defective Z protein that is inefficient in packaging, exit, and transmissibility but better suited for chronic infection, a characteristic that would favor virus:host co-adaptation. Perhaps such viruses are only recently acquired by snakes since IBD is a lethal disease. Some snakes consume rodents and bats that carry arenaviruses and filoviruses; so a co-infected snake could conceivably harbor replication complexes in which the arenavirus polymerase jumps from an arenaviral replicative form to a filovirus GP mRNA or to a snake endogenous ubiquitin ligase (Figure 6). The snake viral Z protein could also be a primordial Z protein derived from a host ubiquitin ligase that functions perfectly well in budding. It would be highly interesting to substitute the new Z protein into functional-budding assays, or to monitor viral co-infection in snake cells to test the feasibility of recombination events.
Another possible source of arenavirus variation comes from co-infection with retroviruses or infection in the presence of a reverse transcriptase/retrotransposase. This could result in the integration of arenavirus sequences into the somatic genomic material of a host organism. The fact that this phenomenon takes place has resulted in speculation about its effect on low-level arenaviral gene expression leading to self-tolerance of arenaviral antigens [160,161].
Outside the laboratory, the real world is a very complicated microbiome, and the few examples of co-mingling of arenavirus sequences and host or other microbe sequences broadens the possibilities for arenavirus evolution.
3. Conclusion
In conclusion, viral genetic diversity plays an important role in the viral population fitness that is shaped by different selective pressures in the host, determining the host range of the viral offspring and the outcome of the infection. Therefore, it is important to study the heterogeneity and complexity of viral populations in the context of viral evolution and pathogenicity. Such observations will constitute a model for developing better viral classification systems, better vaccine candidates or vaccine screening techniques, and new drugs for viral treatments.
Supplementary Table 1.
List of all isolated arenavirus and their name, year of isolation, geographic distribution, reservoir hosts, and associated human diseases. The arenaviruses are divided into two serocomplexes: Old World and New World and based on the viral nucleocapsid protein gene sequences the NW viruses are divided into three phylogenetic groups
| Virus Species | Origen of name | Year | Distribution | Host | Associated Disease |
|---|---|---|---|---|---|
| Old World or LASV-LCMV serocomplex | |||||
| Lymphocytic choriomeningitis virus (LCMV) | Disease | 1933 [162] | Worldwide | House mouse (Mus musculus) and (Mus domesticus) and Syrian hamster (Mesocricetus auratus) | Flu like symptoms Meningitis |
| Encephalitis | |||||
| Congenital abnormalities/Abortion | |||||
| Multisystem organ failure in transplanted patients | |||||
| Lassa (LASV) | Town, Nigeria | 1969 [163] | West Africa | Multimammate mouse (Mastomys genus) | Hemorrhagic fever |
| Mopeia (MOPV) | Town, Mozambique | 1977 [164] | Southern Africa | Multimammate mouse (Mastomis natalensis) | Not associated with human disease |
| Merino Walk | Farm, South Africa | 1985 [55] | Eastern Cape | Karoo rat (Myotomys unisulcatus) | Unknown pathogenicity for humans |
| (MWV) | South Africa | ||||
| Mopeia/Lassa | M/L reassortant clone 29 | 1992 [72] | Russia | Laboratory virus. Passaged in Vero E6 and BHK cells | Vaccine candidate against LHF |
| reassortant | |||||
| (ML29) | |||||
| Morogoro | City, Tanzania | 2009 [165] | East Africa | Multimammate mouse (Mastomys natalensis) | Unknown pathogenicity for humans |
| Tanzania | |||||
| Mobala (MOBV) | Region, DR of Congo | 1983 [166] | Central African Republic | Soft-furred rat (Praomys sp.) | Not associated with human disease |
| IPPY (IPPYV) | Town, Central African Republic | 1985 [167] | Central African | Nile grass rat (Arvicanthis sp.) | Not associated with human disease |
| Lujo (LUJV) | Lusaka, Zambia Johannesburg, South Africa | 2009 [54] | Southern Africa | Unknown | Hemorrhagic fever |
| Luna (LUNV) | Lusaka-Namwala, Zambia | 2009 [168] | Southern Africa Zambia |
Multimammate mouse (Mastomys natalensis) | Unknown pathogenicity for humans |
| New World arenavirus or Tacaribe serocomplex South American group | |||||
| Group A | |||||
| Pichindé (PICV) | Valley, Colombia | 1965 [169] | South America | Tome’s rice rat (Oryzomys albigularis) | Not associated with human disease |
| Colombia | |||||
| Paraná (PARV) | River, Paraguay, Brazil, Argentina | 1965 [170] | South America | Paraguayan rice rat (Oryzomys buccinatus, Oryzomys angouya) | Not associated with human disease |
| Paraguay | |||||
| Flexal (FLEV) | Brazil | 1975 [171] | South America | Tome’s rice rat (Oryzomys albigularis, Nephelomys albigularis), Paraguayan rice rat (Oryzomys angouya, Oryzomys buccinatus), | Febril illness Associated with nonfatal laboratory-acquired infection |
| Brazil | |||||
| Pirital (PIRV) | Community, Venezuela | 1995 [172] | South America | Alston’s cotton rat (Sigmodon alstoni) | Not associated with human disease |
| Venezuela | |||||
| Allpaahuayo (ALLV) | National reserve, Peru | 1997 [173] | South America | Arboreal rice rats (Oecomys bicolor and Oecomys paricola) | Unknown pathogenicity for humans |
| Peru | |||||
| Group B | |||||
| Tacaribe (TCRV) | Beach, Trinidad | 1956 [174] | Caribbean Sea | fruit-eating bat (Artibeus sp.) | Associated only with single, nonfatal, laboratory-acquired infection. |
| Trinidad | |||||
| Junin (JUNV) | Town, Argentina | 1958 [175] | South America | Corn mouse, drylands vesper mouse (Calomys masculinus), grass field mouse (Akodon azarae), dark field mouse (Bolomys obscurus) | Hemorrhagic fever |
| Argentina | |||||
| Candid#1 | Argentina | 1985 [176] | South America | Passaged guinea pigs (GP2), mouse | Vaccine against Argentinian hemorrhagic fever |
| Argentina | (MB44), followed by clonal selection | ||||
| in fetal rhesus monkey lung cells (FRhL19). | |||||
| Machupo (MACV) | River, Bolivia | 1962 [28] | South America | large vesper mouse (Calomys callosus) | Hemorrhagic fever |
| Bolivia | |||||
| Amapari (AMAV) | Amapá region, Brazil | 1964 [177] | South America | rice rat (Oryzomys goeldii), bristly mouse (Neacomys guianae) | Not associated with human disease |
| Brazil | |||||
| Cupixi (CPXV) | Town, Brazil | 1970 [77] | South America | Large-headed Rice Rat (Oryzomys capito) | Not associated with human disease |
| Brazil | |||||
| Guanarito (GTOV) | Region, Venezuela | 1989 [178] | South America | Cane mouse (Zygodontomys brevicauda) | Hemorrhagic fever |
| Venezuela | |||||
| Sabiá (SABV) | Town, Brazil | 1990 [179] | South America | Unknown (Suspected rodent) | Hemorrhagic fever, Hemorrhagic fever associated with nonfatal laboratory-acquired infection |
| Brazil | |||||
| Chapare (CHPV) | Town, Bolivia | 2005 [180] | South America | Unknown | Hemorrhagic fever |
| Bolivia | |||||
| Group C | |||||
| Latino (LATV) | Bolivia | 1965 [31] | South America | Large vesper mouse (Calomys callosus) | Not associated with human disease |
| Bolivia, Brazil | |||||
| Oliveros (OLVV) | Town, Argentina | 1990 [181] | South America | Dark bolo mouse (Bolomys obscurus) | Not associated with human disease |
| Argentina | |||||
| Pampa virus (PAMV) | Region, Argentina | 1997 [182] | South America | Dark bolo mouse (Bolomys sp.) | Not associated with human disease |
| Argentina | |||||
| North American group | |||||
| Group A | |||||
| Tamiami (TAMV)* | Everglades, USA | 1964 [183] | North America Florida | Hispid cotton rat (Sigmodon hispidus) | Not associated with human disease |
| Whitewater Arroyo* (WWAV) | Whitewater Creek | 1993 [184] | North America New Mexico | White-throated wood rat (Neotoma albigula) | Febrile infection |
| Respiratory distress syndrome | |||||
| Catarina (CTNV) | Town, USA | 1999 [185] | North America Texas | Southern Plains Woodrat (Neotoma micropus) | Unknown Pathogenicity for humans |
| Skinner Tank virus | Reservoir, USA | 2002 [186] | North America Arizona | Mexican woodrat (Neotoma Mexicana) | Unknown pathogenicity for humans |
| (SKTV) | |||||
| Big Brushy Tank | USA | 2008 [187] | North America Arizona | White-throated woodrat (Neotoma albigula) | Unknown pathogenicity for humans |
| (BBTV) | |||||
| Tonto Creek | Creek, USA | 2008 [187] | North America Arizona | White-throated woodrat (Neotoma albigula) | Unknown pathogenicity for humans |
| (TTCV) | |||||
| Bear Canyon (BCNV)* | Trailhead, USA | 2002 [188] | North America California | California mouse (Peromyscus californico), Large-eared woodrat (Neotoma macrotis) | Unknown pathogenicity for humans |
*: represents recombinant viruses. [25]
Supplementary part 2 - Genetically detected arenaviruses
Old World
Gbagroube virus and Menekre virus: Mus setulosus, mice. Unknown pathogenicity for humans. Côte d’Ivoire, 2005 [56].
Kodoko virus: Guinea. Nannomys minutoides, Mus (Nannomys). Unknown pathogenicity for humans. West Africa, 2007 [189].
Dandenong (DANV): Town, Australia. Unknown host. Eastern Europe. Encephalitic illness in transplanted patients, 2008 [190].
New World
Black Mesa virus: Similar to WWAV. Black mesa state park. Oklahoma. USA. Neotoma albigula (Rat). Unknown pathogenicity for humans, USA, 1985 [191].
Oro Grande virus: Community California. USA. Neotoma micropus (Rat). Unknown pathogenicity for humans, 1998 [192].
Rio Cacarana virus (RCAV): Argentina, 2006 [193].
Real de Catorce (RDCV): Real de Catorce locality. San Luis Potosí. White-toothed woodrat (N. leucodon). Unknown pathogenicity for humans, Mexico, 2010 [194].
Pinhal virus: Close to LATV and OLVV. Calomys tener (delicate vesper mouse). Unknown pathogenicity for humans, Brazil, 2007 [195].
Ocozocoautla de Espinosa virus: Municipality, Chiapas, Mexico. Mexican deer mice (Peromyscus mexicanus). Closely related to South American Tacaribe complex. Unknown pathogenicity for humans, Mexico, 2012 [196]
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Auperin D.D., Romanowski V., Galinski M., Bishop D.H. Sequencing studies of pichinde arenavirus S RNA indicate a novel coding strategy, an ambisense viral S RNA. J. Virol. 1984;52:897–904. doi: 10.1128/jvi.52.3.897-904.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinschewer D.D., Perez M., de la Torre J.C. Role of the virus nucleoprotein in the regulation of lymphocytic choriomeningitis virus transcription and RNA replication. J. Virol. 2003;77:3882–3887. doi: 10.1128/JVI.77.6.3882-3887.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eichler R., Strecker T., Kolesnikova L., ter Meulen J., Weissenhorn W., Becker S., Klenk H.D., Garten W., Lenz O. Characterization of the lassa virus matrix protein Z: Electron microscopic study of virus-like particles and interaction with the nucleoprotein (NP) Virus Res. 2004;100:249–255. doi: 10.1016/j.virusres.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 4.Casabona J.C., Levingston Macleod J.M., Loureiro M.E., Gomez G.A., Lopez N. The ring domain and the l79 residue of Z protein are involved in both the rescue of nucleocapsids and the incorporation of glycoproteins into infectious chimeric arenavirus-like particles. J. Virol. 2009;83:7029–7039. doi: 10.1128/JVI.00329-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shtanko O., Imai M., Goto H., Lukashevich I.S., Neumann G., Watanabe T., Kawaoka Y. A role for the C terminus of mopeia virus nucleoprotein in its incorporation into Z protein-induced virus-like particles. J. Virol. 2010;84:5415–5422. doi: 10.1128/JVI.02417-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Sobrido L., Zuniga E.I., Rosario D., Garcia-Sastre A., de la Torre J.C. Inhibition of the type i interferon response by the nucleoprotein of the prototypic arenavirus lymphocytic choriomeningitis virus. J. Virol. 2006;80:9192–9199. doi: 10.1128/JVI.00555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Sobrido L., Emonet S., Giannakas P., Cubitt B., Garcia-Sastre A., de la Torre J.C. Identification of amino acid residues critical for the anti-interferon activity of the nucleoprotein of the prototypic arenavirus lymphocytic choriomeningitis virus. J. Virol. 2009;83:11330–11340. doi: 10.1128/JVI.00763-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hastie K.M., Kimberlin C.R., Zandonatti M.A., MacRae I.J., Saphire E.O. Structure of the lassa virus nucleoprotein reveals a dsRNA-specific 3' to 5' exonuclease activity essential for immune suppression. P. Natl. Acad. Sci. U.S.A. 2011;108:2396–2401. doi: 10.1073/pnas.1016404108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi X., Lan S., Wang W., Schelde L.M., Dong H., Wallat G.D., Ly H., Liang Y., Dong C. Cap binding and immune evasion revealed by lassa nucleoprotein structure. Nature. 2010;468:779–783. doi: 10.1038/nature09605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salvato M.S., Schweighofer K.J., Burns J., Shimomaye E.M. Biochemical and immunological evidence that the 11 kda zinc-binding protein of lymphocytic choriomeningitis virus is a structural component of the virus. Virus Res. 1992;22:185–198. doi: 10.1016/0168-1702(92)90050-J. [DOI] [PubMed] [Google Scholar]
- 11.Borden K.L., Campbelldwyer E.J., Carlile G.W., Djavani M., Salvato M.S. Two ring finger proteins, the oncoprotein PML and the arenavirus Z protein, colocalize with the nuclear fraction of the ribosomal P proteins. J. Virol. 1998;72:3819–3826. doi: 10.1128/jvi.72.5.3819-3826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell Dwyer E.J., Lai H., MacDonald R.C., Salvato M.S., Borden K.L. The lymphocytic choriomeningitis virus ring protein Z associates with eukaryotic initiation factor 4e and selectively represses translation in a ring-dependent manner. J. Virol. 2000;74:3293–3300. doi: 10.1128/JVI.74.7.3293-3300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kentsis A., Dwyer E.C., Perez J.M., Sharma M., Chen A., Pan Z.Q., Borden K.L. The ring domains of the promyelocytic leukemia protein PML and the arenaviral protein Z repress translation by directly inhibiting translation initiation factor eIf4e. J. Mol. Biol. 2001;312:609–623. doi: 10.1006/jmbi.2001.5003. [DOI] [PubMed] [Google Scholar]
- 14.Lukashevich I.S., Salvato M.S. Lassa virus genome. Curr. Genomics. 2006;7:351–379. doi: 10.2174/138920206778948673. [DOI] [Google Scholar]
- 15.Lee K.J., Novella I.S., Teng M.N., Oldstone M.B., de La Torre J.C. Np and l proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic rna analogs. J. Virol. 2000;74:3470–3477. doi: 10.1128/JVI.74.8.3470-3477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez N., Jacamo R., Franze-Fernandez M.T. Transcription and rna replication of tacaribe virus genome and antigenome analogs require N and L proteins: Z protein is an inhibitor of these processes. J. Virol. 2001;75:12241–12251. doi: 10.1128/JVI.75.24.12241-12251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvato M., Shimomaye E., Oldstone M.B. The primary structure of the lymphocytic choriomeningitis virus L gene encodes a putative rna polymerase. Virology. 1989;169:377–384. doi: 10.1016/0042-6822(89)90163-3. [DOI] [PubMed] [Google Scholar]
- 18.Salvato M.S., Shimomaye E.M. The completed sequence of lymphocytic choriomeningitis virus reveals a unique RNA structure and a gene for a zinc finger protein. Virology. 1989;173:1–10. doi: 10.1016/0042-6822(89)90216-X. [DOI] [PubMed] [Google Scholar]
- 19.Lenz O., ter Meulen J., Feldmann H., Klenk H.D., Garten W. Identification of a novel consensus sequence at the cleavage site of the Lassa virus glycoprotein. J. Virol. 2000;74:11418–11421. doi: 10.1128/JVI.74.23.11418-11421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.York J., Nunberg J.H. Intersubunit interactions modulate ph-induced activation of membrane fusion by the junin virus envelope glycoprotein gpc. J. Virol. 2009;83:4121–4126. doi: 10.1128/JVI.02410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfau C.J. Biochemical and biophysical properties of the arenaviruses. Prog. Med. Virol. 1974;18:64–80. [PubMed] [Google Scholar]
- 22.Salvato M.S., Clegg J.C.S., Buchmeier M.J., Charrel R.N., Gonzalez J.P., Lukashevich I.S., Peters C.J., Romanowski V. Arenaviridae. In: King A.M.Q., Adams M.J., Carstens E.B., Lefkowitz E.J., editors. Virus Taxonomy, Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier; San Diego, CA, USA: 2012. pp. 715–723. [Google Scholar]
- 23.Weissenbacher M.C., Coto C.E., Calello M.A., Rondinone S.N., Damonte E.B., Frigerio M.J. Cross-protection in nonhuman primates against argentine hemorrhagic fever. Infect. Immun. 1982;35:425–430. doi: 10.1128/iai.35.2.425-430.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez J.D., Duplantier J.M. The Arenaviruses and Rodent Co-Evolution Process : A Global View of a Theory. In: Saluzzo J.F., Dodet B., editors. Factors in the Emergence and Control of Rodent-Borne Viral Diseases (Hantaviral and Arenaviral Diseases) Elsevier; Paris: 1999. pp. 39–42. [Google Scholar]
- 25.Charrel R.N., Lemasson J.J., Garbutt M., Khelifa R., De Micco P., Feldmann H., de Lamballerie X. New insights into the evolutionary relationships between arenaviruses provided by comparative analysis of small and large segment sequences. Virology. 2003;317:191–196. doi: 10.1016/j.virol.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Jones M.S., 2nd, Harrach B., Ganac R.D., Gozum M.M., Dela Cruz W.P., Riedel B., Pan C., Delwart E.L., Schnurr D.P. New adenovirus species found in a patient presenting with gastroenteritis. J. Virol. 2007;81:5978–5984. doi: 10.1128/JVI.02650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackenzie R.B., Webb P.A., Johnson K.M. Detection of complement-fixing antibody after bolivian hemorrhagic fever, employing machupo, junin and tacaribe virus antigens. Am. J. Trop. Med. Hyge. 1965;14:1079–1084. doi: 10.4269/ajtmh.1965.14.1079. [DOI] [PubMed] [Google Scholar]
- 28.Johnson K.M., Wiebenga N.H., Mackenzie R.B., Kuns M.L., Tauraso N.M., Shelokov A., Webb P.A., Justines G., Beye H.K. Virus isolations from human cases of hemorrhagic fever in Bolivia. P. Soc. Exp. Biol. Med. 1965;118:113–118. doi: 10.3181/00379727-118-29772. [DOI] [PubMed] [Google Scholar]
- 29.Webb P.A., Johnson K.M., Mackenzie R.B. The measurement of specific antibodies in bolivian hemorrhagic fever by neutralization of virus plaques. P. Soc. Exp. Biol. Med. 1969;130:1013–1019. doi: 10.3181/00379727-130-33711. [DOI] [PubMed] [Google Scholar]
- 30.Murphy F.A., Webb P.A., Johnson K.M., Whitfield S.G. Morphological comparison of machupo with lymphocytic choriomeningitis virus: Basis for a new taxonomic group. J. Virol. 1969;4:535–541. doi: 10.1128/jvi.4.4.535-541.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowe W.P., Murphy F.A., Bergold G.H., Casals J., Hotchin J., Johnson K.M., Lehmann-Grube F., Mims C.A., Traub E., Webb P.A. Arenoviruses: Proposed name for a newly defined virus group. J. Virol. 1970;5:651–652. doi: 10.1128/jvi.5.5.651-652.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casals J., Buckley S.M., Cedeno R. Antigenic properties of the arenaviruses. B. World Health Organ. 1975;52:421–427. [PMC free article] [PubMed] [Google Scholar]
- 33.Buchmeier M.J., Parekh B.S. Protein structure and expression among arenaviruses. Curr. Top. Microbiol. Immunol. 1987;133:41–57. doi: 10.1007/978-3-642-71683-6_4. [DOI] [PubMed] [Google Scholar]
- 34.Ruo S.L., Mitchell S.W., Kiley M.P., Roumillat L.F., Fisher-Hoch S.P., McCormick J.B. Antigenic relatedness between arenaviruses defined at the epitope level by monoclonal antibodies. J. Gen. Virol. 1991;72:549–555. doi: 10.1099/0022-1317-72-3-549. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez A., Pifat D.Y., Kenyon R.H., Peters C.J., McCormick J.B., Kiley M.P. Junin virus monoclonal antibodies: Characterization and cross-reactivity with other arenaviruses. J. Gen. Virol. 1989;70:1125–1132. doi: 10.1099/0022-1317-70-5-1125. [DOI] [PubMed] [Google Scholar]
- 36.Howard C. Antigenic Diversity among the Arenaviruses. In: Salvato M, editor. The Arenaviridae. Plenum Press; New York, New York, USA: 1993. pp. 37–49. [Google Scholar]
- 37.Weissenbacher M.C., de Guerrero L.B., Boxaca M.C. Experimental biology and pathogenesis of junin virus infection in animals and man. B. World Health Organ. 1975;52:507–515. [PMC free article] [PubMed] [Google Scholar]
- 38.Jahrling P.B., Trotter R.W., Barrera Oro J.G., et al. Cross-Protection against Machupo Virus with Candid #1 Junin Virus Vaccine: Iii, Post-Challenge Clinical Findings. In: Kurstak E., editor. Proceedings of the Second International Conference on the Impact of Viral Diseases on the Development of Latin American Countries and the Caribbean Region; Mar del Plata, Argentina: 1988. [Google Scholar]
- 39.Lukashevich I.S., Carrion R., Jr., Salvato M.S., Mansfield K., Brasky K., Zapata J., Cairo C., Goicochea M., Hoosien G.E., Ticer A., et al. Safety, immunogenicity, and efficacy of the ML29 reassortant vaccine for lassa fever in small non-human primates. Vaccine. 2008;26:5246–5254. doi: 10.1016/j.vaccine.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCormick J.B. Epidemiology and control of lassa fever. Curr. Top. Microbiol. Immunol. 1987;134:69–78. doi: 10.1007/978-3-642-71726-0_3. [DOI] [PubMed] [Google Scholar]
- 41.Justines G., Johnson K.M. Use of oral swabs for detection of machupo-virus infection in rodents. Am. J. Trop. Med. Hyg. 1968;17:788–790. doi: 10.4269/ajtmh.1968.17.788. [DOI] [PubMed] [Google Scholar]
- 42.Skinner H.H., Knight E.H., Grove R. Murine lymphocytic choriomeningitis: The history of a natural cross-infection from wild to laboratory mice. Lab. Anim. 1977;11:219–222. doi: 10.1258/002367777780936422. [DOI] [PubMed] [Google Scholar]
- 43.Peralta L.A., Laguens R.P., Cossio P.M., Sabattini M.S., Maiztegui J.I., Arana R.M. Presence of viral particles in the salivary gland of calomys musculinus infected with junin virus by a natural route. Intervirology. 1979;11:111–116. doi: 10.1159/000149021. [DOI] [PubMed] [Google Scholar]
- 44.Vitullo A.D., Hodara V.L., Merani M.S. Effect of persistent infection with junin virus on growth and reproduction of its natural reservoir, calomys musculinus. Am. J. Trop. Med. Hyg. 1987;37:663–669. doi: 10.4269/ajtmh.1987.37.663. [DOI] [PubMed] [Google Scholar]
- 45.Vitullo A.D., Merani M.S. Is vertical transmission sufficient to maintain junin virus in nature? J. Gen. Virol. 1988;69:1437–1440. doi: 10.1099/0022-1317-69-6-1437. [DOI] [PubMed] [Google Scholar]
- 46.Borremans B., Leirs H., Gryseels S., Gunther S., Makundi R., de Bellocq J.G. Presence of mopeia virus, an African arenavirus, related to biotope and individual rodent host characteristics: Implications for virus transmission. Vector Borne and Zoonotic Dis. 2011;11:1125–1131. doi: 10.1089/vbz.2010.0010. [DOI] [PubMed] [Google Scholar]
- 47.Webb P.A., Justines G., Johnson K.M. Infection of wild and laboratory animals with machupo and latino viruses. B. World Health Organ. 1975;52:493–499. [PMC free article] [PubMed] [Google Scholar]
- 48.Vitullo A.D., Merani M.S. Vertical transmission of junin virus in experimentally infected adult calomys musculinus. Intervirology. 1990;31:339–344. doi: 10.1159/000150170. [DOI] [PubMed] [Google Scholar]
- 49.Mills J.N., Ellis B.A., Childs J.E., McKee K.T., Jr., Maiztegui J.I., Peters C.J., Ksiazek T.G., Jahrling P.B. Prevalence of infection with junin virus in rodent populations in the epidemic area of argentine hemorrhagic fever. Am. J. Trop. Med. Hyg. 1994;51:554–562. [PubMed] [Google Scholar]
- 50.Calisher C.H., Nabity S., Root J.J., Fulhorst C.F., Beaty B.J. Transmission of an arenavirus in white-throated woodrats (neotoma albigula), southeastern Colorado, 1995-1999. Emerg. Infect. Dis. 2001;7:397–402. doi: 10.3201/eid0703.010305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ostfeld S.R., Mills J.N. Social Behavior, Demography, and Rodent-Borne Pathogens. In: Wolf J.O., Sherman P.W., editors. Rodent Societies: An Ecological and Evolutionary Perspective. University of Chigago, Press; Chicago, Illinois, USA: 2007. pp. 478–486. [Google Scholar]
- 52.Jay M.T., Glaser C., Fulhorst C.F. The arenaviruses. J. Am. Vet. Med. Assoc. 2005;227:904–915. doi: 10.2460/javma.2005.227.904. [DOI] [PubMed] [Google Scholar]
- 53.Gonzalez J.P., Barbazan P., Baillon F., Capelle J., Chevallier D., Cornet J.P., Fournet F., Herbreteau V., Hugot J.P., Le Gouilh M., et al. Fundamentals, domains, and diffusion of disease emergence: Tools and strategies for a new paradigm. In encyclopedia of infectious diseases: Modern methodologies. In: Tibayrenc M., editor. Wiley & Sons, Inc.; Hoboken, New Jersey, USA: 2007. pp. 525–568. [Google Scholar]
- 54.Paweska J.T., Sewlall N.H., Ksiazek T.G., Blumberg L.H., Hale M.J., Lipkin W.I., Weyer J., Nichol S.T., Rollin P.E., McMullan L.K., et al. Nosocomial outbreak of novel arenavirus infection, southern Africa. Emerg. Infect. Dis. 2009;15:1598–1602. doi: 10.3201/eid1510.090211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palacios G., Savji N., Hui J., Travassos da Rosa A., Popov V., Briese T., Tesh R., Lipkin W.I. Genomic and phylogenetic characterization of merino walk virus, a novel arenavirus isolated in south Africa. J. Gen. Virol. 2010;91:1315–1324. doi: 10.1099/vir.0.017798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coulibaly-N'Golo D., Allali B., Kouassi S.K., Fichet-Calvet E., Becker-Ziaja B., Rieger T., Olschlager S., Dosso H., Denys C., Ter Meulen J., et al. Novel arenavirus sequences in hylomyscus sp. And mus (nannomys) setulosus from cote d'ivoire: Implications for evolution of arenaviruses in Africa. PLoS One. 2011;6:e20893. doi: 10.1371/journal.pone.0020893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bowen M.D., Peters C.J., Nichol S.T. Phylogenetic analysis of the arenaviridae: Patterns of virus evolution and evidence for cospeciation between arenaviruses and their rodent hosts. Mol. Phylogenet. Evol. 1997;8:301–316. doi: 10.1006/mpev.1997.0436. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez J.P., Emonet S., de Lamballerie X., Charrel R. Arenaviruses. Cur. Top. Microbiol. Iimmun. 2007;315:253–288. doi: 10.1007/978-3-540-70962-6_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Irwin N.R., Bayerlova M., Missa O., Martinkova N. Complex patterns of host switching in new world arenaviruses. Mol. Ecol. 2012;21:4137–4150. doi: 10.1111/j.1365-294X.2012.05663.x. [DOI] [PubMed] [Google Scholar]
- 60.Sabeti P.C., Varilly P., Fry B., Lohmueller J., Hostetter E., Cotsapas C., Xie X., Byrne E.H., McCarroll S.A., Gaudet R., et al. Genome-wide detection and characterization of positive selection in human populations. Nature. 2007;449:913–918. doi: 10.1038/nature06250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andersen K.G., Shylakhter I., Tabrizi S., Grossman S.R., Happi C.T., Sabeti P.C. Genome-wide scans provide evidence for positive selection of genes implicated in lassa fever. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2012;367:868–877. doi: 10.1098/rstb.2011.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao W., Henry M.D., Borrow P., Yamada H., Elder J.H., Ravkov E.V., Nichol S.T., Compans R.W., Campbell K.P., Oldstone M.B. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 63.Radoshitzky S.R., Abraham J., Spiropoulou C.F., Kuhn J.H., Nguyen D., Li W., Nagel J., Schmidt P.J., Nunberg J.H., Andrews N.C., et al. Transferrin receptor 1 is a cellular receptor for new world haemorrhagic fever arenaviruses. Nature. 2007;446:92–96. doi: 10.1038/nature05539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spiropoulou C.F., Kunz S., Rollin P.E., Campbell K.P., Oldstone M.B. New world arenavirus clade C, but not clade A and B viruses, utilizes alpha-dystroglycan as its major receptor. J. Virol. 2002;76:5140–5146. doi: 10.1128/JVI.76.10.5140-5146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kunz S. Receptor binding and cell entry of old world arenaviruses reveal novel aspects of virus-host interaction. Virology. 2009;387:245–249. doi: 10.1016/j.virol.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 66.Pasqual G., Rojek J.M., Masin M., Chatton J.Y., Kunz S. Old world arenaviruses enter the host cell via the multivesicular body and depend on the endosomal sorting complex required for transport. PLoS pathogens. 2011;7:e1002232. doi: 10.1371/journal.ppat.1002232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Botten J., Sidney J., Mothe B.R., Peters B., Sette A., Kotturi M.F. Coverage of related pathogenic species by multivalent and cross-protective vaccine design: Arenaviruses as a model system. Microbiol. Mol. Biol. Rev. 2010;74:157–170. doi: 10.1128/MMBR.00045-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salvato M.S., Lukashevich I.S. Vaccines Against Lassa Fever. Marcel Dekker, Inc; New York, New York, USA: 2009. p. 9. [Google Scholar]
- 69.Albarino C.G., Posik D.M., Ghiringhelli P.D., Lozano M.E., Romanowski V. Arenavirus phylogeny: A new insight. Virus Genes. 1998;16:39–46. doi: 10.1023/A:1007993525052. [DOI] [PubMed] [Google Scholar]
- 70.Archer A.M., Rico-Hesse R. High genetic divergence and recombination in arenaviruses from the Americas. Virology. 2002;304:274–281. doi: 10.1006/viro.2002.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riviere Y., Ahmed R., Southern P., Oldstone M.B. Perturbation of differentiated functions during viral infection in vivo. II. Viral reassortants map growth hormone defect to the S RNA of the lymphocytic choriomeningitis virus genome. Virology. 1985;142:175–182. doi: 10.1016/0042-6822(85)90431-3. [DOI] [PubMed] [Google Scholar]
- 72.Lukashevich I.S. Generation of reassortants between African arenaviruses. Virology. 1992;188:600–605. doi: 10.1016/0042-6822(92)90514-P. [DOI] [PubMed] [Google Scholar]
- 73.Teng M.N., Borrow P., Oldstone M.B., de la Torre J.C. A single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with the ability to cause growth hormone deficiency syndrome. J. Virol. 1996;70:8438–8443. doi: 10.1128/jvi.70.12.8438-8443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen M., Lan S., Ou R., Price G.E., Jiang H., de la Torre J.C., Moskophidis D. Genomic and biological characterization of aggressive and docile strains of lymphocytic choriomeningitis virus rescued from a plasmid-based reverse-genetics system. J. Gen. Virol. 2008;89:1421–1433. doi: 10.1099/vir.0.83464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ortiz-Riano E., Cheng B.Y., de la Torre J.C., Martinez-Sobrido L. The C-terminal region of lymphocytic choriomeningitis virus nucleoprotein contains distinct and segregable functional domains involved in NP-Z interaction and counteraction of the type I interferon response. J. Virol. 2011;85:13038–13048. doi: 10.1128/JVI.05834-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kerber R., Rieger T., Busch C., Flatz L., Pinschewer D.D., Kummerer B.M., Gunther S. Cross-species analysis of the replication complex of old world arenaviruses reveals two nucleoprotein sites involved in l protein function. J. Virol. 2011;85:12518–12528. doi: 10.1128/JVI.05091-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Charrel R.N., Feldmann H., Fulhorst C.F., Khelifa R., de Chesse R., de Lamballerie X. Phylogeny of new world arenaviruses based on the complete coding sequences of the small genomic segment identified an evolutionary lineage produced by intrasegmental recombination. Biochem. Bioph. Res. Co. 2002;296:1118–1124. doi: 10.1016/S0006-291X(02)02053-3. [DOI] [PubMed] [Google Scholar]
- 78.Emonet S., Lemasson J.J., Gonzalez J.P., de Lamballerie X., Charrel R.N. Phylogeny and evolution of old world arenaviruses. Virology. 2006;350:251–257. doi: 10.1016/j.virol.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 79.Holland J.J., De La Torre J.C., Steinhauer D.A. RNA virus populations as quasispecies. Curr. Top. Microbiol. Immunol. 1992;176:1–20. doi: 10.1007/978-3-642-77011-1_1. [DOI] [PubMed] [Google Scholar]
- 80.Drake J.W., Holland J.J. Mutation rates among RNA viruses. P. Natl. Acad. Sci. U.S.A. 1999;96:13910–13913. doi: 10.1073/pnas.96.24.13910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grande-Perez A., Sierra S., Castro M.G., Domingo E., Lowenstein P.R. Molecular indetermination in the transition to error catastrophe: Systematic elimination of lymphocytic choriomeningitis virus through mutagenesis does not correlate linearly with large increases in mutant spectrum complexity. P. Natl. Acad. Sci. U.S.A. 2002;99:12938–12943. doi: 10.1073/pnas.182426999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ruiz-Jarabo C.M., Ly C., Domingo E., de la Torre J.C. Lethal mutagenesis of the prototypic arenavirus lymphocytic choriomeningitis virus (LCMV) Virology. 2003;308:37–47. doi: 10.1016/S0042-6822(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 83.Sevilla N., de la Torre J.C. Arenavirus diversity and evolution: Quasispecies in vivo. Curr. Top. Microbiol. Immunol. 2006;299:315–335. doi: 10.1007/3-540-26397-7_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bui H.H., Botten J., Fusseder N., Pasquetto V., Mothe B., Buchmeier M.J., Sette A. Protein sequence database for pathogenic arenaviruses. Immunome. Res. 2007;3:1. doi: 10.1186/1745-7580-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fulhorst C.F., Charrel R.N., Weaver S.C., Ksiazek T.G., Bradley R.D., Milazzo M.L., Tesh R.B., Bowen M.D. Geographic distribution and genetic diversity of whitewater arroyo virus in the southwestern united states. Emerg. Infect. Dis. 2001;7:403–407. doi: 10.3201/eid0703.010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blasdell K.R., Becker S.D., Hurst J., Begon M., Bennett M. Host range and genetic diversity of arenaviruses in rodents, United Kingdom. Emerg. Infect. Dis. 2008;14:1455–1458. doi: 10.3201/eid1409.080209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bowen M.D., Rollin P.E., Ksiazek T.G., Hustad H.L., Bausch D.G., Demby A.H., Bajani M.D., Peters C.J., Nichol S.T. Genetic diversity among lassa virus strains. J. Virol. 2000;74:6992–7004. doi: 10.1128/jvi.74.15.6992-7004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weaver S.C., Salas R.A., de Manzione N., Fulhorst C.F., Travasos da Rosa A.P., Duno G., Utrera A., Mills J.N., Ksiazek T.G., Tovar D., et al. Extreme genetic diversity among pirital virus (arenaviridae) isolates from western Venezuela. Virology. 2001;285:110–118. doi: 10.1006/viro.2001.0954. [DOI] [PubMed] [Google Scholar]
- 89.Domingo E., Martin V., Perales C., Grande-Perez A., Garcia-Arriaza J., Arias A. Viruses as quasispecies: Biological implications. Curr. Top. Microbiol. Immunol. 2006;299:51–82. doi: 10.1007/3-540-26397-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mas A., Lopez-Galindez C., Cacho I., Gomez J., Martinez M.A. Unfinished stories on viral quasispecies and darwinian views of evolution. J. Mol. Biol. 2010;397:865–877. doi: 10.1016/j.jmb.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 91.de la Torre J.C., Holland J.J. RNA virus quasispecies populations can suppress vastly superior mutant progeny. J. Virol. 1990;64:6278–6281. doi: 10.1128/jvi.64.12.6278-6281.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buesa-Gomez J., Teng M.N., Oldstone C.E., Oldstone M.B., de la Torre J.C. Variants able to cause growth hormone deficiency syndrome are present within the disease-nil WE strain of lymphocytic choriomeningitis virus. J. Virol. 1996;70:8988–8992. doi: 10.1128/jvi.70.12.8988-8992.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stocker C., Martinez Peralta L., Kratzberg T., Lohmann F., Bruns M. Characterization of a virus variant produced by l cells persistently infected with lymphocytic choriomeningitis virus. J. Gen. Virol. 1994;75:3431–3439. doi: 10.1099/0022-1317-75-12-3431. [DOI] [PubMed] [Google Scholar]
- 94.Teng M.N., Oldstone M.B., de la Torre J.C. Suppression of lymphocytic choriomeningitis virus--induced growth hormone deficiency syndrome by disease-negative virus variants. Virology. 1996;223:113–119. doi: 10.1006/viro.1996.0460. [DOI] [PubMed] [Google Scholar]
- 95.Perales C., Mateo R., Mateu M.G., Domingo E. Insights into rna virus mutant spectrum and lethal mutagenesis events: Replicative interference and complementation by multiple point mutants. J. Mol. Biol. 2007;369:985–1000. doi: 10.1016/j.jmb.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 96.Romanowski V., Bishop D.H. The formation of arenaviruses that are genetically diploid. Virology. 1983;126:87–95. doi: 10.1016/0042-6822(83)90463-4. [DOI] [PubMed] [Google Scholar]
- 97.Pulkkinen A.J., Pfau C.J. Plaque size heterogeneity: A genetic trait of lymphocytic choriomeningitis virus. Appl. Microbiol. 1970;20:123–128. doi: 10.1128/am.20.1.123-128.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hotchin J., Sikora E. Low-pathogenicity variant of lymphocytic choriomeningitis virus. Infect. Immun. 1973;7:825–826. doi: 10.1128/iai.7.5.825-826.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Loeb L.A., Essigmann J.M., Kazazi F., Zhang J., Rose K.D., Mullins J.I. Lethal mutagenesis of hiv with mutagenic nucleoside analogs. P. Natl. Acad. Sci. U.S.A. 1999;96:1492–1497. doi: 10.1073/pnas.96.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moreno H., Gallego I., Sevilla N., de la Torre J.C., Domingo E., Martin V. Ribavirin can be mutagenic for arenaviruses. J. Virol. 2011;85:7246–7255. doi: 10.1128/JVI.00614-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grande-Perez A., Gomez-Mariano G., Lowenstein P.R., Domingo E. Mutagenesis-induced, large fitness variations with an invariant arenavirus consensus genomic nucleotide sequence. J. Virol. 2005;79:10451–10459. doi: 10.1128/JVI.79.16.10451-10459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grande-Perez A., Lazaro E., Lowenstein P., Domingo E., Manrubia S.C. Suppression of viral infectivity through lethal defection. P. Natl. Acad. Sci. U.S.A. 2005;102:4448–4452. doi: 10.1073/pnas.0408871102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pfeiffer J.K., Kirkegaard K. Increased fidelity reduces poliovirus fitness and virulence under selective pressure in mice. PLoS pathogens. 2005;1:e11. doi: 10.1371/journal.ppat.0010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vignuzzi M., Stone J.K., Arnold J.J., Cameron C.E., Andino R. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature. 2006;439:344–348. doi: 10.1038/nature04388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lukashevich I.S., Patterson J., Carrion R., Moshkoff D., Ticer A., Zapata J., Brasky K., Geiger R., Hubbard G.B., Bryant J., et al. A live attenuated vaccine for lassa fever made by reassortment of lassa and mopeia viruses. J. Virol. 2005;79:13934–13942. doi: 10.1128/JVI.79.22.13934-13942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moshkoff D.A., Salvato M.S., Lukashevich I.S. Molecular characterization of a reassortant virus derived from lassa and mopeia viruses. Virus Genes. 2007;34:169–176. doi: 10.1007/s11262-006-0050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zapata J., Goicochea M., Bryan J., Davis H., Pauza C., Sadzewicz L., Tallon L., Mahurkar A., Myers G., Fraser-Liggett C., et al. Genetic Stability of a Lassa Vaccine Candidate (ml29) in Vaccinated Animals. XV International Congress of Virology; Sopporo, Japan. 11–16 September 2011; VI-PO5–61. [Google Scholar]
- 108.Graci J.D., Cameron C.E. Therapeutically targeting RNA viruses via lethal mutagenesis. Future Virol. 2008;3:553–566. doi: 10.2217/17460794.3.6.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ahmed R., Oldstone M.B. Organ-specific selection of viral variants during chronic infection. J. Exp. Med. 1988;167:1719–1724. doi: 10.1084/jem.167.5.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Evans C.F., Borrow P., de la Torre J.C., Oldstone M.B. Virus-induced immunosuppression: Kinetic analysis of the selection of a mutation associated with viral persistence. J. Virol. 1994;68:7367–7373. doi: 10.1128/jvi.68.11.7367-7373.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cheng-Mayer C., Weiss C., Seto D., Levy J.A. Isolates of human immunodeficiency virus type 1 from the brain may constitute a special group of the AIDS virus. P. Natl. Acad. Sci. U.S.A. 1989;86:8575–8579. doi: 10.1073/pnas.86.21.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Trivedi P., Meyer K.K., Streblow D.N., Preuninger B.L., Schultz K.T., Pauza C.D. Selective amplification of simian immunodeficiency virus genotypes after intrarectal inoculation of rhesus monkeys. J. Virol. 1994;68:7649–7653. doi: 10.1128/jvi.68.11.7649-7653.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hall J.S., French R., Hein G.L., Morris T.J., Stenger D.C. Three distinct mechanisms facilitate genetic isolation of sympatric wheat streak mosaic virus lineages. Virology. 2001;282:230–236. doi: 10.1006/viro.2001.0841. [DOI] [PubMed] [Google Scholar]
- 114.Cabot B., Martell M., Esteban J.I., Sauleda S., Otero T., Esteban R., Guardia J., Gomez J. Nucleotide and amino acid complexity of hepatitis C virus quasispecies in serum and liver. J. Virol. 2000;74:805–811. doi: 10.1128/JVI.74.2.805-811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sanjuan R., Codoner F.M., Moya A., Elena S.F. Natural selection and the organ-specific differentiation of HIV-1 v3 hypervariable region. Evolution. 2004;58:1185–1194. doi: 10.1111/j.0014-3820.2004.tb01699.x. [DOI] [PubMed] [Google Scholar]
- 116.Deforges S., Evlashev A., Perret M., Sodoyer M., Pouzol S., Scoazec J.Y., Bonnaud B., Diaz O., Paranhos-Baccala G., Lotteau V., et al. Expression of hepatitis C virus proteins in epithelial intestinal cells in vivo. J. Gen. Virol. 2004;85:2515–2523. doi: 10.1099/vir.0.80071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jelcic I., Hotz-Wagenblatt A., Hunziker A., Zur Hausen H., de Villiers E.M. Isolation of multiple TT virus genotypes from spleen biopsy tissue from a hodgkin's disease patient: Genome reorganization and diversity in the hypervariable region. J. Virol. 2004;78:7498–7507. doi: 10.1128/JVI.78.14.7498-7507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jridi C., Martin J.F., Marie-Jeanne V., Labonne G., Blanc S. Distinct viral populations differentiate and evolve independently in a single perennial host plant. J. Virol. 2006;80:2349–2357. doi: 10.1128/JVI.80.5.2349-2357.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Brown R.J., Peters P.J., Caron C., Gonzalez-Perez M.P., Stones L., Ankghuambom C., Pondei K., McClure C.P., Alemnji G., Taylor S., et al. Intercompartmental recombination of HIV-1 contributes to env intrahost diversity and modulates viral tropism and sensitivity to entry inhibitors. J. Virol. 2011;85:6024–6037. doi: 10.1128/JVI.00131-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wright C.F., Morelli M.J., Thebaud G., Knowles N.J., Herzyk P., Paton D.J., Haydon D.T., King D.P. Beyond the consensus: Dissecting within-host viral population diversity of foot-and-mouth disease virus by using next-generation genome sequencing. J. Virol. 2011;85:2266–2275. doi: 10.1128/JVI.01396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rodas J.D., Lukashevich I.S., Zapata J.C., Cairo C., Tikhonov I., Djavani M., Pauza C.D., Salvato M.S. Mucosal arenavirus infection of primates can protect them from lethal hemorrhagic fever. J. Med. Virol. 2004;72:424–435. doi: 10.1002/jmv.20000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carrion R., Jr., Brasky K., Mansfield K., Johnson C., Gonzales M., Ticer A., Lukashevich I., Tardif S., Patterson J. Lassa virus infection in experimentally infected marmosets: Liver pathology and immunophenotypic alterations in target tissues. J. Virol. 2007;81:6482–6490. doi: 10.1128/JVI.02876-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baize S., Marianneau P., Loth P., Reynard S., Journeaux A., Chevallier M., Tordo N., Deubel V., Contamin H. Early and strong immune responses are associated with control of viral replication and recovery in lassa virus-infected cynomolgus monkeys. J. Virol. 2009;83:5890–5903. doi: 10.1128/JVI.01948-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aebischer T., Moskophidis D., Rohrer U.H., Zinkernagel R.M., Hengartner H. In vitro selection of lymphocytic choriomeningitis virus escape mutants by cytotoxic T lymphocytes. P. Natl. Acad. Sci. U.S.A. 1991;88:11047–11051. doi: 10.1073/pnas.88.24.11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ciurea A., Hunziker L., Martinic M.M., Oxenius A., Hengartner H., Zinkernagel R.M. CD4+ T-cell-epitope escape mutant virus selected in vivo. Nature Medicine. 2001;7:795–800. doi: 10.1038/89915. [DOI] [PubMed] [Google Scholar]
- 126.Ciurea A., Hunziker L., Zinkernagel R.M., Hengartner H. Viral escape from the neutralizing antibody response: The lymphocytic choriomeningitis virus model. Immunogenetics. 2001;53:185–189. doi: 10.1007/s002510100314. [DOI] [PubMed] [Google Scholar]
- 127.Wu-Hsieh B., Howard D.H., Ahmed R. Virus-induced immunosuppression: A murine model of susceptibility to opportunistic infection. J. Infect. Dis. 1988;158:232–235. doi: 10.1093/infdis/158.1.232. [DOI] [PubMed] [Google Scholar]
- 128.Ahmed R., Hahn C.S., Somasundaram T., Villarete L., Matloubian M., Strauss J.H. Molecular basis of organ-specific selection of viral variants during chronic infection. J. Virol. 1991;65:4242–4247. doi: 10.1128/jvi.65.8.4242-4247.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Salvato M., Shimomaye E., Southern P., Oldstone M.B. Virus-lymphocyte interactions. Iv. Molecular characterization of lcmv armstrong (CTL+) small genomic segment and that of its variant, clone 13 (CTL-) Virology. 1988;164:517–522. doi: 10.1016/0042-6822(88)90566-1. [DOI] [PubMed] [Google Scholar]
- 130.Salvato M., Borrow P., Shimomaye E., Oldstone M.B. Molecular basis of viral persistence: A single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with suppression of the antiviral cytotoxic T-lymphocyte response and establishment of persistence. J. Virol. 1991;65:1863–1869. doi: 10.1128/jvi.65.4.1863-1869.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bergthaler A., Flatz L., Hegazy A.N., Johnson S., Horvath E., Lohning M., Pinschewer D.D. Viral replicative capacity is the primary determinant of lymphocytic choriomeningitis virus persistence and immunosuppression. P. Natl. Acad. Sci. U.S.A. 2010;107:21641–21646. doi: 10.1073/pnas.1011998107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sevilla N., Kunz S., Holz A., Lewicki H., Homann D., Yamada H., Campbell K.P., de La Torre J.C., Oldstone M.B. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J. Exp. Med. 2000;192:1249–1260. doi: 10.1084/jem.192.9.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sullivan B.M., Emonet S.F., Welch M.J., Lee A.M., Campbell K.P., de la Torre J.C., Oldstone M.B. Point mutation in the glycoprotein of lymphocytic choriomeningitis virus is necessary for receptor binding, dendritic cell infection, and long-term persistence. P. Natl. Acad. Sci. U.S.A. 2011;108:2969–2974. doi: 10.1073/pnas.1019304108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Flatz L., Bergthaler A., de la Torre J.C., Pinschewer D.D. Recovery of an arenavirus entirely from RNA polymerase i/ii-driven cDNA. P. Natl. Acad. Sci. U.S.A. 2006;103:4663–4668. doi: 10.1073/pnas.0600652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zapata J., Salvato M.S. Areanvirus variation due to host-specifc adaptation. Viruses. 2013 doi: 10.3390/v5010241. Reported data on this manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kunz S., Sevilla N., McGavern D.B., Campbell K.P., Oldstone M.B. Molecular analysis of the interaction of LCMV with its cellular receptor [alpha]-dystroglycan. J. Cell Biol. 2001;155:301–310. doi: 10.1083/jcb.200104103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Traub E. An epidemic in a mouse colony due to the virus of acute lymphocytic choriomeningitis. J. Exp. Med. 1936;63:533–546. doi: 10.1084/jem.63.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dalldorf G. The simultaneous occurrence of the viruses of canine distemper and lymphocytic choriomeningitis : A correction of “canine distemper in the rhesus monkey”. J. Exp. Med. 1939;70:19–27. doi: 10.1084/jem.70.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smadel J.E., Wall M.J. A soluble antigen of lymphocytic choriomeningitis: II. Independence of anti-soluble substance antibodies and neutralizing antibodies, and the role of soluble antigen and inactive virus in immunity to infection. J. Exp. Med. 1940;72:389–405. doi: 10.1084/jem.72.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bowen G.S., Calisher C.H., Winkler W.G., Kraus A.L., Fowler E.H., Garman R.H., Fraser D.W., Hinman A.R. Laboratory studies of a lymphocytic choriomeningitis virus outbreak in man and laboratory animals. Am. J. Epidemiol. 1975;102:233–240. doi: 10.1093/oxfordjournals.aje.a112152. [DOI] [PubMed] [Google Scholar]
- 141.Biggar R.J., Schmidt T.J., Woodall J.P. Lymphocytic choriomeningitis in laboratory personnel exposed to hamsters inadvertently infected with LCM virus. J. Am. Vet. Med. Assoc. 1977;171:829–832. [PubMed] [Google Scholar]
- 142.Montali R.J., Scanga C.A., Pernikoff D., Wessner D.R., Ward R., Holmes K.V. A common-source outbreak of callitrichid hepatitis in captive tamarins and marmosets. J. Infect. Dis. 1993;167:946–950. doi: 10.1093/infdis/167.4.946. [DOI] [PubMed] [Google Scholar]
- 143.Greenwood A.G., Sanchez S. Serological evidence of murine pathogens in wild grey squirrels (sciurus carolinensis) in north wales. Vet. Rec. 2002;150:543–546. doi: 10.1136/vr.150.17.543. [DOI] [PubMed] [Google Scholar]
- 144.Zapata J.C., Pauza C.D., Djavani M.M., Rodas J.D., Moshkoff D., Bryant J., Ateh E., Garcia C., Lukashevich I.S., Salvato M.S. Lymphocytic choriomeningitis virus (LCMV) infection of macaques: A model for lassa fever. Antivir. Res. 2011;92:125–138. doi: 10.1016/j.antiviral.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Oldstone M.B., Ahmed R., Buchmeier M.J., Blount P., Tishon A. Perturbation of differentiated functions during viral infection in vivo. I. Relationship of lymphocytic choriomeningitis virus and host strains to growth hormone deficiency. Virology. 1985;142:158–174. doi: 10.1016/0042-6822(85)90430-1. [DOI] [PubMed] [Google Scholar]
- 146.Weaver S.C., Salas R.A., de Manzione N., Fulhorst C.F., Duno G., Utrera A., Mills J.N., Ksiazek T.G., Tovar D., Tesh R.B. Guanarito virus (arenaviridae) isolates from endemic and outlying localities in venezuela: Sequence comparisons among and within strains isolated from venezuelan hemorrhagic fever patients and rodents. Virology. 2000;266:189–195. doi: 10.1006/viro.1999.0067. [DOI] [PubMed] [Google Scholar]
- 147.Matloubian M., Kolhekar S.R., Somasundaram T., Ahmed R. Molecular determinants of macrophage tropism and viral persistence: Importance of single amino acid changes in the polymerase and glycoprotein of lymphocytic choriomeningitis virus. J. Virol. 1993;67:7340–7349. doi: 10.1128/jvi.67.12.7340-7349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Barresi R., Campbell K.P. Dystroglycan: From biosynthesis to pathogenesis of human disease. J. Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 149.Kunz S., Sevilla N., Rojek J.M., Oldstone M.B. Use of alternative receptors different than alpha-dystroglycan by selected isolates of lymphocytic choriomeningitis virus. Virology. 2004;325:432–445. doi: 10.1016/j.virol.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 150.Pasquato A., Rochat C., Burri D.J., Pasqual G., de la Torre J.C., Kunz S. Evaluation of the anti-arenaviral activity of the subtilisin kexin isozyme-1/site-1 protease inhibitor pf-429242. Virology. 2012;423:14–22. doi: 10.1016/j.virol.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Plotkin J.B., Robins H., Levine A.J. Tissue-specific codon usage and the expression of human genes. P. Natl. Acad. Sci. U.S.A. 2004;101:12588–12591. doi: 10.1073/pnas.0404957101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pandit A., Sinha S. Differential trends in the codon usage patterns in HIV-1 genes. PLoS One. 2011;6:e28889. doi: 10.1371/journal.pone.0028889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gallaher W.R., DiSimone C., Buchmeier M.J. The viral transmembrane superfamily: Possible divergence of arenavirus and filovirus glycoproteins from a common RNA virus ancestor. B.M.C. Microbiol. 2001;1:1. doi: 10.1186/1471-2180-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Carter S.D., Surtees R., Walter C.T., Ariza A., Bergeron E., Nichol S.T., Hiscox J.A., Edwards T.A., Barr J.N. Structure, function, and evolution of the crimean-congo hemorrhagic fever virus nucleocapsid protein. J. Virol. 2012;86:10914–10923. doi: 10.1128/JVI.01555-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Urquidi V., Bishop D.H. Non-random reassortment between the tripartite RNA genomes of la crosse and snowshoe hare viruses. J. Gen. Virol. 1992;73:2255–2265. doi: 10.1099/0022-1317-73-9-2255. [DOI] [PubMed] [Google Scholar]
- 156.Rodriguez L.L., Owens J.H., Peters C.J., Nichol S.T. Genetic reassortment among viruses causing hantavirus pulmonary syndrome. Virology. 1998;242:99–106. doi: 10.1006/viro.1997.8990. [DOI] [PubMed] [Google Scholar]
- 157.Borucki M.K., Chandler L.J., Parker B.M., Blair C.D., Beaty B.J. Bunyavirus superinfection and segment reassortment in transovarially infected mosquitoes. J. Gen. Virol. 1999;80:3173–3179. doi: 10.1099/0022-1317-80-12-3173. [DOI] [PubMed] [Google Scholar]
- 158.Stenglein M.D., Sanders C., Kistler A.L., Ruby J.G., Franco J.Y., Reavill D.R., Dunker F., Derisi J.L. Identification, characterization, and in vitro culture of highly divergent arenaviruses from boa constrictors and annulated tree boas: Candidate etiological agents for snake inclusion body disease. mBio. 2012;3:e00180–00112. doi: 10.1128/mBio.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Foto, C.s. [(accessed on 12 November 2012)]. Available online: http://ec.Comps.Canstockphoto.Com/can-stock-photo_csp7080858.jpg.
- 160.Klenerman P., Hengartner H., Zinkernagel R.M. A non-retroviral RNA virus persists in DNA form. Nature. 1997;390:298–301. doi: 10.1038/36876. [DOI] [PubMed] [Google Scholar]
- 161.Geuking M.B., Weber J., Dewannieux M., Gorelik E., Heidmann T., Hengartner H., Zinkernagel R.M., Hangartner L. Recombination of retrotransposon and exogenous RNA virus results in nonretroviral cdna integration. Science. 2009;323:393–396. doi: 10.1126/science.1167375. [DOI] [PubMed] [Google Scholar]
- 162.Armstrong C., Lillie R.D. Experimental lymphocytic choriomeningitis of monkeys and mice produced by a virus encountered in studies of the 1933 St. Louis encephalitis epidemic. Pub. Health Rep. 1934;49:1019–1027. doi: 10.2307/4581290. [DOI] [Google Scholar]
- 163.Frame J.D., Baldwin J.M., Jr., Gocke D.J., Troup J.M. Lassa fever, a new virus disease of man from west Africa. I. Clinical description and pathological findings. Am. J. Trop. Med. Hyg. 1970;19:670–676. doi: 10.4269/ajtmh.1970.19.670. [DOI] [PubMed] [Google Scholar]
- 164.Wulff H., McIntosh B.M., Hamner D.B., Johnson K.M. Isolation of an arenavirus closely related to lassa virus from mastomys natalensis in south-east Africa. B. World Health Organ. 1977;55:441–444. [PMC free article] [PubMed] [Google Scholar]
- 165.Gunther S., Hoofd G., Charrel R., Roser C., Becker-Ziaja B., Lloyd G., Sabuni C., Verhagen R., van der Groen G., Kennis J., et al. Mopeia virus-related arenavirus in natal multimammate mice, Morogoro, Tanzania. Emerg. Infect. Dis. 2009;15:2008–2012. doi: 10.3201/eid1512.090864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gonzalez J.P., McCormick J.B., Saluzzo J.F., Herve J.P., Georges A.J., Johnson K.M. An arenavirus isolated from wild-caught rodents (pramys species) in the central African Republic. Intervirology. 1983;19:105–112. doi: 10.1159/000149344. [DOI] [PubMed] [Google Scholar]
- 167.Swanepoel R., Leman P.A., Shepherd A.J., Shepherd S.P., Kiley M.P., McCormick J.B. Identification of ippy as a lassa-fever-related virus. Lancet. 1985;1:639. doi: 10.1016/s0140-6736(85)92175-0. [DOI] [PubMed] [Google Scholar]
- 168.Ishii A., Thomas Y., Moonga L., Nakamura I., Ohnuma A., Hang'ombe B., Takada A., Mweene A., Sawa H. Novel arenavirus, Zambia. Emerg. Infect. Dis. 2011;17:1921–1924. doi: 10.3201/eid1710.10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Trapido H., Sanmartin C. Pichinde virus, a new virus of the tacaribe group from Colombia. Am. J. Trop. Med. Hyg. 1971;20:631–641. [PubMed] [Google Scholar]
- 170.Webb P.A., Johnson K.M., Hibbs J.B., Kuns M.L. Parana, a new tacaribe complex virus from Paraguay. Arch. Gesamte Virusforsch. 1970;32:379–388. doi: 10.1007/BF01250066. [DOI] [PubMed] [Google Scholar]
- 171.Carlton M.L., Gillespie R.A., Garver J., Draguljic D., Vela E.M. The syrian golden hamster as a model to study flexal virus pathogenesis. Arch. Clin. Microbiol. 2012;3:1–9. [Google Scholar]
- 172.Fulhorst C.E., Bowen M.D., Salas R.A., de Manzione N.M., Duno G., Utrera A., Ksiazek T.G., Peters C.J., Nichol S.T., De Miller E., et al. Isolation and characterization of pirital virus, a newly discovered south American arenavirus. Am. J. Trop. Med. Hyg. 1997;56:548–553. doi: 10.4269/ajtmh.1997.56.548. [DOI] [PubMed] [Google Scholar]
- 173.Moncayo A.C., Hice C.L., Watts D.M., Travassos de Rosa A.P., Guzman H., Russell K.L., Calampa C., Gozalo A., Popov V.L., Weaver S.C., et al. Allpahuayo virus: A newly recognized arenavirus (arenaviridae) from arboreal rice rats (oecomys bicolor and oecomys paricola) in northeastern Peru. Virology. 2001;284:277–286. doi: 10.1006/viro.2000.0803. [DOI] [PubMed] [Google Scholar]
- 174.Downs W.G., Anderson C.R., Spence L., Aitken T.H., Greenhall A.H. Tacaribe virus, a new agent isolated from artibeus bats and mosquitoes in Trinidad, west Indies. Am. J. Trop. Med. Hyg. 1963;12:640–646. doi: 10.4269/ajtmh.1963.12.640. [DOI] [PubMed] [Google Scholar]
- 175.Parodi A.S., Rugiero H.R., Greenway D.J., Mettler N., Martinez A., Boxaca M., De La Barrera J.M. Isolation of the junin virus (epidemic hemorrhagic fever) from the mites of the epidemic area (echinolaelaps echidninus, barlese) Prensa Med. Argent. 1959;46:2242–2244. [PubMed] [Google Scholar]
- 176.Barrera Oro J.G., McKee K.T., Jr. Toward a vaccine against argentine hemorrhagic fever. Bull. Pan. Am. Health Organ. 1991;25:118–126. [PubMed] [Google Scholar]
- 177.Pinheiro F., Shope R., Paes de Andrade A., Bensabath H., Cacios G., Casals J. Amapari, a new virus of the tacaribe group from rodents and mites of amapa territory, Brazil. Proc. Soc. Exp. Biol. Med. 1966;122:531–535. [Google Scholar]
- 178.Salas R., de Manzione N., Tesh R.B., Rico-Hesse R., Shope R.E., Betancourt A., Godoy O., Bruzual R., Pacheco M.E., Ramos B., et al. Venezuelan haemorrhagic fever. Lancet. 1991;338:1033–1036. doi: 10.1016/0140-6736(91)91899-6. [DOI] [PubMed] [Google Scholar]
- 179.Lisieux T., Coimbra M., Nassar E.S., Burattini M.N., de Souza L.T., Ferreira I., Rocco I.M., da Rosa A.P., Vasconcelos P.F., Pinheiro F.P., et al. New arenavirus isolated in Brazil. Lancet. 1994;343:391–392. doi: 10.1016/s0140-6736(94)91226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Delgado S., Erickson B.R., Agudo R., Blair P.J., Vallejo E., Albarino C.G., Vargas J., Comer J.A., Rollin P.E., Ksiazek T.G., et al. Chapare virus, a newly discovered arenavirus isolated from a fatal hemorrhagic fever case in Bolivia. PLoS pathogens. 2008;4:e1000047. doi: 10.1371/journal.ppat.1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bowen M.D., Peters C.J., Mills J.N., Nichol S.T. Oliveros virus: A novel arenavirus from Argentina. Virology. 1996;217:362–366. doi: 10.1006/viro.1996.0124. [DOI] [PubMed] [Google Scholar]
- 182.Lozano M.E., Posik D.M., Albarino C.G., Schujman G., Ghiringhelli P.D., Calderon G., Sabattini M., Romanowski V. Characterization of arenaviruses using a family-specific primer set for RT-PCR amplification and RFLP analysis. Its potential use for detection of uncharacterized arenaviruses. Virus Res. 1997;49:79–89. doi: 10.1016/S0168-1702(97)01458-5. [DOI] [PubMed] [Google Scholar]
- 183.Calisher C.H., Tzianabos T., Lord R.D., Coleman P.H. Tamiami virus, a new member of the tacaribe group. Am. J. Trop. Med. Hyg. 1970;19:520–526. doi: 10.4269/ajtmh.1970.19.520. [DOI] [PubMed] [Google Scholar]
- 184.Fulhorst C.F., Bowen M.D., Ksiazek T.G., Rollin P.E., Nichol S.T., Kosoy M.Y., Peters C.J. Isolation and characterization of whitewater arroyo virus, a novel north American arenavirus. Virology. 1996;224:114–120. doi: 10.1006/viro.1996.0512. [DOI] [PubMed] [Google Scholar]
- 185.Cajimat M.N., Milazzo M.L., Bradley R.D., Fulhorst C.F. Catarina virus, an arenaviral species principally associated with neotoma micropus (southern plains woodrat) in Texas. Am. J. Trop. Med. Hyg. 2007;77:732–736. [PubMed] [Google Scholar]
- 186.Cajimat M.N., Milazzo M.L., Borchert J.N., Abbott K.D., Bradley R.D., Fulhorst C.F. Diversity among tacaribe serocomplex viruses (family arenaviridae) naturally associated with the Mexican woodrat (neotoma mexicana) Virus Res. 2008;133:211–217. doi: 10.1016/j.virusres.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Milazzo M.L., Cajimat M.N., Haynie M.L., Abbott K.D., Bradley R.D., Fulhorst C.F. Diversity among tacaribe serocomplex viruses (family arenaviridae) naturally associated with the white-throated woodrat (neotoma albigula) in the southwestern United States. Vector Borne and Zoonotic Dis. 2008;8:523–540. doi: 10.1089/vbz.2007.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fulhorst C.F., Bennett S.G., Milazzo M.L., Murray H.L., Jr., Webb J.P., Jr., Cajimat M.N., Bradley R.D. Bear canyon virus: An arenavirus naturally associated with the california mouse (peromyscus californicus) Emerg. Infect. Dis. 2002;8:717–721. doi: 10.3201/eid0807.010281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lecompte E., ter Meulen J., Emonet S., Daffis S., Charrel R.N. Genetic identification of kodoko virus, a novel arenavirus of the African pigmy mouse (mus nannomys minutoides) in west africa. Virology. 2007;364:178–183. doi: 10.1016/j.virol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 190.Palacios G., Druce J., Du L., Tran T., Birch C., Briese T., Conlan S., Quan P.L., Hui J., Marshall J., et al. A new arenavirus in a cluster of fatal transplant-associated diseases. The New England J. Med. 2008;358:991–998. doi: 10.1056/NEJMoa073785. [DOI] [PubMed] [Google Scholar]
- 191.Ayers S., Bradley R. Genetic diversity in the transferrin receptor 1 (tfr1) among natural hosts of the north american arenaviruses. Mus. Texas Tech. 2011;304:1–15. [Google Scholar]
- 192.Virus Pathogens and Database and Analysis Resource (ViPR)-Arenaviridae. [(accessed on 22 May 2012)]. Available online: http://www.viprbrc.org/brc/viprDetails.do?ncbiAccession=EU938669&context=1332876702165.
- 193.Salvato M.S., Clegg J.C.S., Buchmeier M.J., Charrel R.N., Gonzalez J.P., Lukashevich I.S., Peters C.J., Rico-Hesse R., Romanowski V. Index of viruses - arenaviridae (2006) In: Büchen-Osmond C., editor. Ictvdb— the Universal Virus Database, Version 4. Columbia University; New York, New York, USA: 2006. [Google Scholar]
- 194.Inizan C.C., Cajimat M.N., Milazzo M.L., Barragan-Gomez A., Bradley R.D., Fulhorst C.F. Genetic evidence for a tacaribe serocomplex virus, Mexico. Emerg. Infect. Dis. 2010;16:1007–1010. doi: 10.3201/eid1606.091648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Charrel R.N., de Lamballerie X. Zoonotic aspects of arenavirus infections. Vet. Microbiol. 2010;140:213–220. doi: 10.1016/j.vetmic.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 196.Cajimat M.N., Milazzo M.L., Bradley R.D., Fulhorst C.F. Ocozocoautla de espinosa virus and hemorrhagic fever, Mexico. Emerg. Infect. Dis. 2012;18:401–405. doi: 10.3201/eid1803.111602. [DOI] [PMC free article] [PubMed] [Google Scholar]