Abstract
A new spatane diterpenoid, leptoclalin A (1), along with two previously reported known norcembranoid diterpenes (2 and 3), were isolated from a cultured soft coral Sinularia leptoclados. The structures were determined by extensive spectroscopic analyses and by comparison with the spectral data of related known compounds. Metabolite 1 is rarely found in spatane skeletons reported from soft corals. In addition, compound 1 exhibited weak cytotoxicity towards human tumor cell lines T-47 D and K-562.
Keywords: spatane diterpenoid, soft coral, Sinularia leptoclados
1. Introduction
In previous studies, soft corals have emerged as one of the most prolific sources of novel secondary metabolites [1]. Some of these have exhibited various biological activities, such as cytotoxic [2,3], anti-inflammatory [4,5,6], antiviral [6] and antifouling [7] activities. However, the bioactive secondary metabolites contents of wild soft coral are small, and extraction of large amounts of bioactive metabolites from wild coral is not feasible. Therefore, scientists are searching for new ways to obtain large sources of bioactive metabolites. In recent years, the farming techniques of soft corals have improved considerably. Therefore, researchers have been able to obtain greater amounts of soft corals, and thus larger amounts of bioactive metabolites, and have been engaged in examining the various pharmacological activities. During the course of our group’s (National Museum of Marine Biology & Aquarium, Taiwan) search for bioactive metabolites from cultured soft corals, several diterpenoids have been isolated from the soft corals Klyxum simplex [8,9,10,11], Sinularia flexibilis [12], Sarcophyton trocheliophorum [13], and Lobophytum crassum [14]. In continuation of our search for biologically active secondary metabolites from the cultured soft coral Sinularia leptoclados (Figure 1), we have isolated one new spatane diterpenoid, leptoclalin A (1), along with two known norcembranoid diterpenes, 5-episinuleptolide (2) and sinuleptolide (3) [15] (Chart 1). The structure of 1 was established by detailed spectroscopic analysis, including extensive examination of 2D NMR (1H–1H COSY, HMQC and HMBC) correlations. The cytotoxicity of compounds 1–3 against four cancer cells, DLD-1 (human colon adenocarcinoma), HCT 116 (human colorectal carcinoma), T-47D (hormone-dependent breast cancer) and K-562 (human chronic myelogenous leukemia) was studied.
Figure 1.
Soft coral Sinularia leptoclados.
Chart 1.
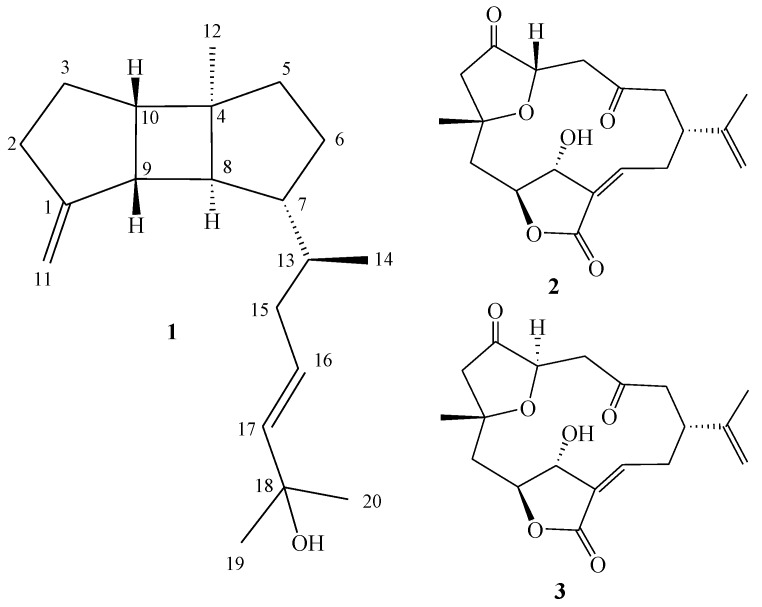
Structures of metabolites 1–3.
2. Results and Discussion
The EtOAc extract of the freeze-dried specimen was fractionated by silica gel column chromatography and the eluted fractions were further separated utilizing normal phase HPLC to yield metabolites 1–3. The new compound was given the trivial name leptoclalin A (1). The known compounds were identified as 5-episinuleptolide (2) and sinuleptolide (3).
Leptoclalin A (1) was isolated as a colorless oil. Its molecular formula was determined to be C20H32O on the basis of HR-ESI-MS (m/z 311.2353 [M + Na]+), implying five degrees of unsaturation. The IR spectrum of 1 revealed the presence of a hydroxy functionality (νmax = 3365 cm−1). The 13C-NMR and DEPT spectroscopic data (Table 1) of 1 indicated the presence of four methyls, six methylenes, seven methines, and three quaternary carbons, implying, from the required degrees of unsaturation, a tricyclic diterpene framework. The NMR signal at δC 70.7 (C) showed the presence of a hydroxy group. A 1,1-disubstituted and a 1,2-disubstituted double bond were also identified from NMR signals appearing at δC 157.5 (C), 103.7 (CH2), and δH 4.74 (1H, s) and 4.70 (1H, s), and at δC 139.2 (CH), 125.8 (CH), and δH 5.58 (2H, m), respectively. Moreover, the 1H NMR spectroscopic data of 1 revealed evidence of four methyl groups at δ 0.83 (3H, d, J = 6.5 Hz), 1.00 (3H, s) and 1.31 (6H, s).
Table 1.
1H and 13C NMR data for 1.
| δH (J in Hz) a | δC (mult.) b | δH (J in Hz) c | δC (mult.) d | |
|---|---|---|---|---|
| 1 | 157.5 (C) | 157.8 (C) | ||
| 2 | 2.54 m; 2.28 m | 33.7 (CH2) | 2.55 m; 2.27 m | 34.4 (CH2) |
| 3 | 1.81 m; 1.59 m | 27.2 (CH2) | 1.74 m; 1.52 m | 28.0 (CH2) |
| 4 | 43.1 (C) | 43.8 (C) | ||
| 5 | 1.50 m | 42.0 (CH2) | 1.48 m | 42.7 (CH2) |
| 6 | 1.89 m; 1.55 m | 29.7 (CH2) | 1.84 m; 1.54 m | 30.6 (CH2) |
| 7 | 1.62 m | 54.5 (CH) | 1.62 m | 55.3 (CH) |
| 8 | 1.62 m | 55.0 (CH) | 1.67 m | 55.8 (CH) |
| 9 | 2.35 m | 47.8 (CH) | 2.46 m | 48.6 (CH) |
| 10 | 2.32 m | 45.7 (CH) | 2.24 m | 46.4 (CH) |
| 11 | 4.74 s; 4.70 s | 103.7 (CH2) | 4.89 s; 4.87 s | 104.8 (CH2) |
| 12 | 1.00 s | 21.6 (CH3) | 0.94 s | 22.0 (CH3) |
| 13 | 1.20 m | 36.3 (CH) | 1.20 m | 37.4 (CH) |
| 14 | 0.83 d (6.5) | 17.8 (CH3) | 0.88 d (6.5) | 18.4 (CH3) |
| 15 | 2.15 ddd (13.5, 3.5, 3.5); 1.74 m | 38.2 (CH2) | 2.27 m; 1.85 m | 39.1 (CH2) |
| 16 | 5.58 m | 125.8 (CH) | 5.90 m | 125.1 (CH) |
| 17 | 5.58 m | 139.2 (CH) | 5.90 m | 142.0 (CH) |
| 18 | 70.7 (C) | 70.1 (C) | ||
| 19 | 1.31 s | 29.9 (CH3) | 1.53 s | 31.3 (CH3) |
| 20 | 1.31 s | 29.8 (CH3) | 1.53 s | 31.2 (CH3) |
a 500 MHz in CDCl3; b 125 MHz in CDCl3; c 500 MHz in pyridine-d5; d 125 MHz in pyridine-d5.
The planar structure and all of the 1H and 13C chemical shifts of 1 were elucidated by 2D NMR experiments, in particular 1H–1H COSY and HMBC experiments (Figure 2). From the 1H–1H COSY spectrum (CDCl3), it was possible to establish the proton sequences from H2-2 to H-10 through H2-3; H2-5 to H2-6; H-8 to H-10 through H-9; and H-13 to H3-14. The overlapping of proton singals of H-7 and H-8 at δH 1.62 ppm, measured in CDCl3, was clearly resolved by measuring the 1H NMR spectrum in pyridine-d5 (see Table 1) into two separate protons at δH1.62 and 1.67, respectively. 1H–1H COSY correlations (pyridine-d5) were observed between H-6 to H-13 through H-7. Therefore, the 1H–1H COSY experiment allowed the building of the two partial structures of the consecutive proton spin systems indicated in bold in Figure 2. These data, together with the HMBC correlations (Figure 2) from H2-2 to C-1, H2-3 to C-1 and C-9, H2-5 to C-4 and C-10, H2-6 to C-4 and C-8, H-9 to C-7 and H-10 to C-8 established the connectivity within the 5-membered, 4-membered and 5-membered rings. Moreover, the correlations of H2-11 to C-2 and C-9 indicated the attachment of an sp2 methylene at C-1, and the methyl was attached at C-4 on the basis of the correlations of H3-12 to C-4, C-5, C-8 and C-10. The planar structure of the side chain was elucidated mainly from the key HMBC correlations from H3-14 to C-7, C-13, and C-15, H2-15 to C-16 and C-17, and both methyls H3-19 and H3-20 to C-18 and C-17, and thus the connectivity from C-13 to C-20 was fully established. The above findings suggested that 1 has a tricyclic carbon skeleton.
Figure 2.
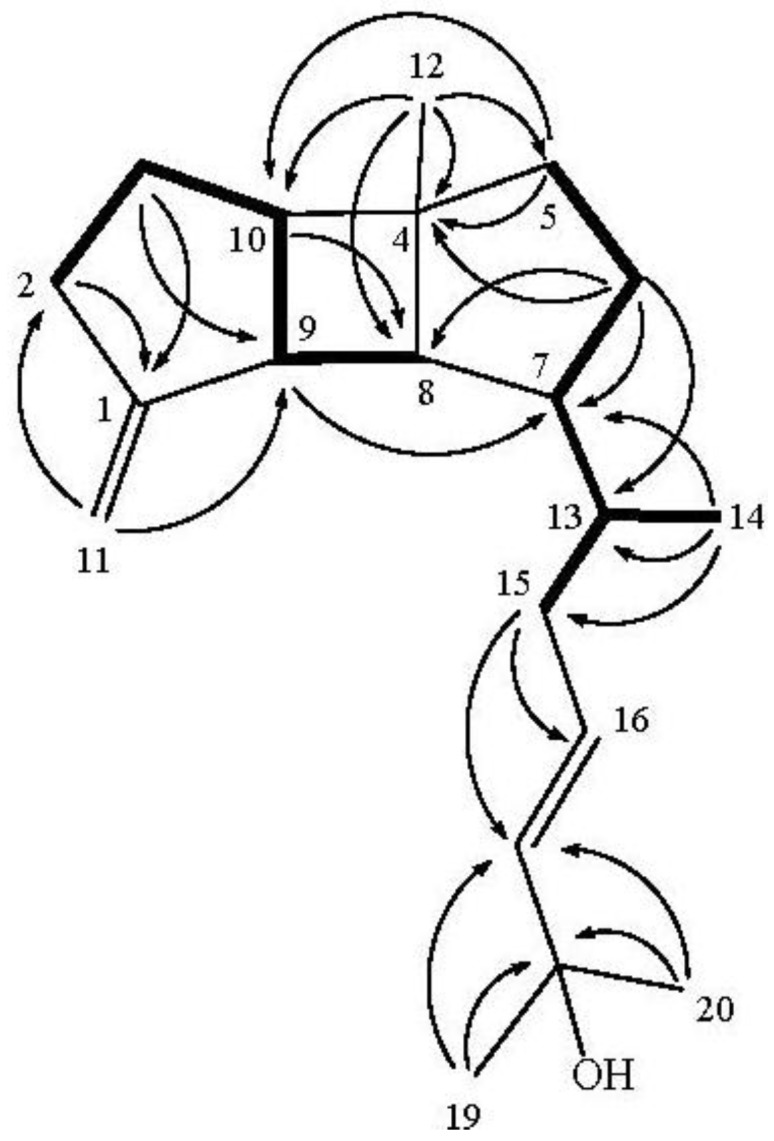
Selected 1H−1H COSY (▬) and HMBC (→) correlations of 1.
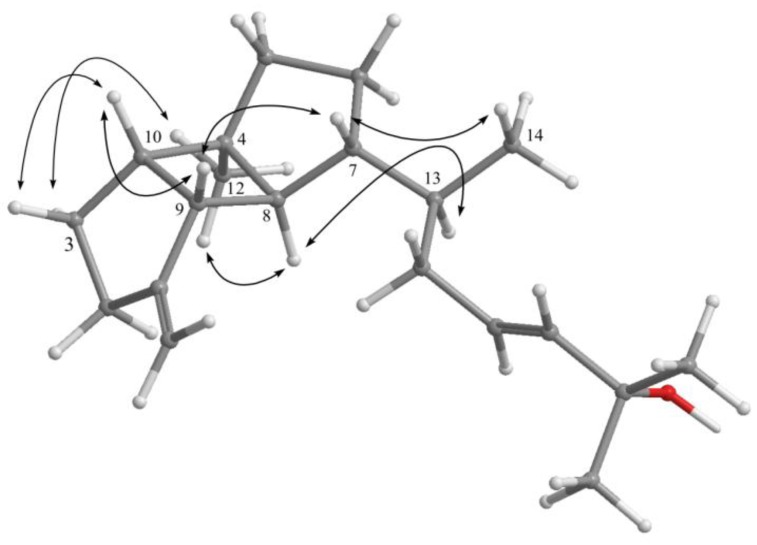
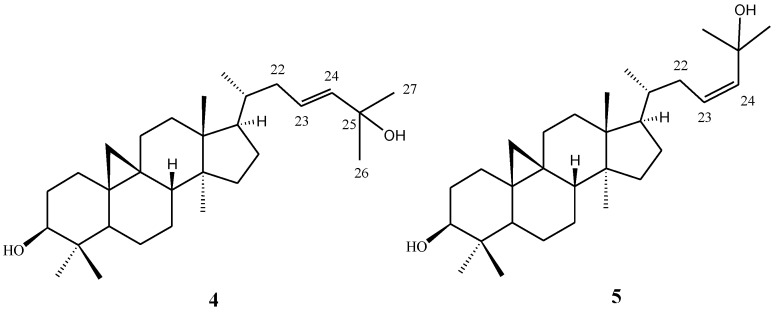
A computer-modeled 3D structure of 1 (Figure 3) was generated using the molecular modeling program Chem3D Ultra version 9.0 and MM2 force-field calculations for energy minimization. The relative structure of 1, assigned by the analysis of NOE correlations, was compatible with that of 1 offered by computer modeling, in which the close contacts of the atoms in space were consistent with the NOE correlations. By the NOESY spectrum (pyridine-d5), it was found that H-9 (δ 2.46) showed NOE interactions with H-7 (δ 1.62) and H-10 (δ 2.24); therefore, assuming the β-orientation of H-9, H-7 and H-10 should also be positioned on the β face. One of the methylene protons at C-3 (δ 1.52) exhibited NOE correlations with H-10 and was characterized as H-3β, while the other (δ 1.74) was designated H-3α. NOE correlations observed between H-3α and H3-12 (δ 0.94) and H3-12 and H-8 (δ 1.67) reflected the α-orientations of H3-12 and H-8. Moreover, NOE correlations were observed between H-8 with H-13 but not with H3-14, and between H-7 with H3-14, indicating that H3-14 has a β-orientation. Furthermore, the configuration of the double bond at C-16/C-17 was determined by comparison of the NMR data of 1 in CDCl3 with those of two related synthetic compounds, (23E)-cycloart-23-ene-3β,25-diol (4) and (23E)-cycloart-23-ene-3β,25-diol (5) (Figure 4), also measured in CDCl3 (Table 2) [16]. Comparison of the NMR data of 1 and 4 confirmed that both compounds have the same partial structure from C-15 to C-20 of 1 and from C-22 to C-27 of 4. Furthermore, the geometry at C-16 double bond is deducible with the 13C chemical shift of C-15 methylene (the downfield chemical shift at 38.2 ppm clearly indicates the E geometry). Thus, it was suggested that the geometry of 1 at C-16/C-17 is E. On the basis of the above analysis, the structure of 1 was established.
Figure 3.
Computer-generated model of 1 using MM2 force field calculations and selected NOE correlations of 1.
Figure 4.
Structures of 4 and 5.
Table 2.
Selective 1H and 13C NMR data for 4 and 5 a.
| 4 b | 5 b | |||
|---|---|---|---|---|
| position | δH (J in Hz) | δC (mult.) | δH (J in Hz) | δC (mult.) |
| 22a | 1.74 m | 39.0 (CH2) | 2.13 dddd (14.8, 9.9, 8.8, 1.7) | 34.6 (CH2) |
| 22b | 2.17 ddd (14.0, 3.3, 3.3) | 2.38 dddd (14.8, 6.1, 3.8, 1.7) | ||
| 23 | 5.60 m | 125.6 (CH) | 5.31 ddd (12.1, 8.8, 6.1) | 130.2 (CH) |
| 24 | 5.60 m | 139.4 (CH) | 5.53 ddd (12.1, 1.7, 1.7) | 137.4 (CH) |
| 25 | 70.7 (C) | 71.6 (C) | ||
| 26 | 1.31 s | 30.0 (CH3) | 1.36 s | 31.3 (CH3) |
| 27 | 1.32 s | 29.9 (CH3) | 1.37 s | 31.1 (CH3) |
a Reference 10; b NMR data in CDCl3.
Finally, we used the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay to examine the cytotoxic activities of compounds 1–3 against four cancer cells, DLD-1 (human colon adenocarcinoma), HCT 116 (human colorectal carcinoma), T-47D (hormone-dependent breast cancer), and K-562 (human chronic myelogenous leukemia). Cells were treated with different concentrations of 1–3 for 72 h. The results show that compound 1 was found to show weak cytotoxicity towards the growth of T-47D and K-562 tumor cells (the IC50 values were 15.4 and 12.8 μg/mL for T-47D and K-562, respectively) (Table 3). The other tested compounds were not cytotoxic (IC50 > 20 μg/mL) towards the above four cancer cell lines (Table 3).
Table 3.
Cytotoxicity (IC50μg/mL) of compounds 1–3.
| Cell Lines | ||||
|---|---|---|---|---|
| Compound | DLD-1 | HCT-116 | T-47D | K-562 |
| 1 | NA b | NA b | 15.4 | 12.8 |
| 2 | NA b | NA b | NA b | NA b |
| 3 | NA b | NA b | NA b | NA b |
| Doxorubicin a | 0.42 | 0.89 | 0.28 | 0.14 |
a Clinical anticancer drug used as a positive control; b NA, not active at 20 μg/mL.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotation values were measured with a Jasco P-1010 digital polarimeter. IR spectra were recorded on a Varian Digilab FTS 1000 Fourier transform infrared spectrophotometer. The NMR spectra were recorded on a Varian Mercury Plus 400 FT-NMR (or Varian Unity INOVA 500 FT-NMR) instrument at 400 MHz (or 500 MHz) for 1H-NMR and 100 MHz (or 125 MHz) for 13C-NMR, respectively, in CDCl3 and pyridine-d5. ESI-MS-spectra were obtained with a Bruker APEX II mass spectrometer. Gravity column chromatography was performed on silica gel (230–400 mesh, Merck, Darmstadt, Germany). TLC was carried out on precoated Kieselgel 60 F254 (0.2 mm, Merck, Darmstadt, Germany) and spots were visualized by spraying with 10% H2SO4 solution followed by heating. High-performance liquid chromatography (HPLC) was performed using a system comprised of a Hitachi L-7100 pump and a Rheodyne 7725 injection port. A preparative normal phase column (Hibar 250 × 21.2 mm, Supelco, silica gel 60, 5 μm) was used for HPLC.
3.2. Animal Material
Specimens of the soft coral Sinularia leptoclados were collected off the coast of Pingtung, southern Taiwan, and transplanted to a 120-ton cultivating tank equipped with a flow-through sea water system in June 2005. The cultured soft coral was harvested in December 2010. A voucher specimen (specimen No. 2010CSC-2) was deposited in the National Museum of Marine Biology and Aquarium, Pingtung, Taiwan.
3.3. Extraction and Separation
The soft coral Sinularia leptoclados (12.5 kg fresh wt) was frozen for storage and then freeze dried. The freeze-dried material (3.0 kg) was minced and extracted exhaustively with EtOAc (5 × 5 L). The EtOAc extract was evaporated to yield a residue (100.5 g), which was subjected to open column chromatography on silica gel, eluting with n-hexane (H)–EtOAc (E) gradient and EtOAc (E)–acetone (A) gradient to give 12 fractions: Fr-1 (eluted by H–E 100:1), Fr-2 (eluted by H–E 50:1), Fr-3 (eluted by H–E 30:1), Fr-4 (eluted by H–E 20:1), Fr-5 (eluted by H–E 10:1), Fr-6 (eluted by H–E 8:1), Fr-7 (eluted by H–E 5:1), Fr-8 (eluted by H–E 3:1), Fr-9 (eluted by H–E 1:1), Fr-10 (eluted by EtOAc), Fr-11 (eluted by E–A 1:1) and Fr-12 (eluted by acetone). Fraction 5 (2.2 g) was separated by silica gel column chromatography with gradient elution (n-hexane–EtOAc, 10:1 to 5:1) to yield five subfractions (5A–5E). Subfraction 5B (108 mg) was subjected to normal-phase HPLC with n-hexane–EtOAc (10:1) elution to afford 1 (3.4 mg). Fraction 8 (6.5 g), was further separated by silica gel open column chromatography with gradient elution (n-hexane–EtOAc, 1:2 to 2:1) to yield seven subfractions (8A–8G). Subfraction 8C (4.5 g) was further chromatographed over silica gel using n-hexane–acetone (5:1) to afford 3 (750 mg) and a mixture, which was further purified by normal phase HPLC using n-hexane–EtOAc (3:2) to afford 3 (350 mg) and 2 (1.2 g), respectively.
Leptoclalin A (1): colorless oil; [α]24D +24 (c 0.14, CHCl3); IR (neat) νmax 3365, 2927, 2865, 1456, and 1374 cm−1; 13C and 1H NMR data, see Table 1; ESIMS m/z 311 [M + Na]+; HRESIMS m/z 311.2353 [M + Na]+ (calcd for C20H32ONa, 311.2351).
3.4. Cytotoxicity Testing
Cell lines were purchased from the American Type Culture Collection (ATCC). Cytotoxicity assays of compounds 1–3 were performed using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] colorimetric method [17,18].
4. Conclusions
Spatane diterpenoids were first reported in 1980 as having been obtained from the brown alga Spatoglossum schmittii from the Galapagos Islands [19]. Brown algae are now known to be a rich source of novel spatane diterpenoids [20,21,22,23,24,25,26,27]. In contrast, they are generally the minor components of marine soft coral [28]. Our investigation of the chemical constituents of the soft coral Sinularia leptoclados led to the obtainment of one new spatane diterpenoid (1). Moreover, to the best of our knowledge, metabolite 1has a spatane skeleton rarely found in soft corals.
Acknowledgements
This study was supported in part by grants from National Science Council of Taiwan (NSC 98-2313-B-276-001-MY3 and NSC 101-2313-B-276-002) and Antai Medical Care Cooperation Antai Tian-Sheng Memorial Hospital Research Fund (Project No. 101-FI-DBS-IAC-R-002).
Supplementary Files
Supporting Information (PDF, 782 KB)
Footnotes
Samples Availability: Available from the authors.
References
- 1.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2012;29:144–222. doi: 10.1039/c2np00090c. [DOI] [PubMed] [Google Scholar]
- 2.Wright A.D., Nielson J.L., Tapiolas D.M., Liptrot C.H., Motti C.A. A great barrier reef Sinularia sp. yields two new cytotoxic diterpenes. Mar. Drugs. 2012;10:1619–1630. doi: 10.3390/md10081619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su T.-R., Tsai F.-J., Lin J.-J., Huang H.H., Chiu C.-C., Su J.-H., Yang Y.-T., Chen J.-F., Wong B.-S., Wu Y.-J. Induction of apoptosis by 11-dehydrosinulariolide via mitochondrial dysregulation and ER stress pathways in human melanoma Cells. Mar. Drugs. 2012;10:1883–1898. doi: 10.3390/md10081883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su J.-H., Wen Z.-H. Bioactive cembrane-based diterpenoids from the soft coral Sinularia triangula. Mar. Drugs. 2011;9:944–951. doi: 10.3390/md9060944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao C.-H., Chou K.-J., Huang C.-Y., Wen Z.-H., Hsu C.-H., Wu Y.-C., Dai C.-F., Sheu J.-H. Bioactive cembranoids from the soft coral Sinularia crassa. Mar. Drugs. 2011;9:1955–1968. doi: 10.3390/md9101955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng S.-Y., Huang K.-J., Wang S.-K., Wen Z.-H., Chen P.-W., Duh C.-Y. Antiviral and anti-inflammatory metabolites from the soft coral Sinularia capillosa. J. Nat. Prod. 2010;73:771–775. doi: 10.1021/np9008078. [DOI] [PubMed] [Google Scholar]
- 7.Shi H., Yu S., Liu D., van Ofwegen L., Proksch P., Lin W. Sinularones A–I, new cyclopentenone and butenolide derivatives from a marine soft coral Sinularia sp. and their antifouling activity. Mar. Drugs. 2012;10:1331–1344. doi: 10.3390/md10061331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen B.-W., Wu Y.-C., Chiang M.Y., Su J.-H., Wang W.-H., Fang T.-Y., Sheu J.-H. Eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Tetrahedron. 2009;65:7016–7022. [Google Scholar]
- 9.Chen B.-W., Chao C.-H., Su J.-H., Wen Z.-H., Sung P.-J., Sheu J.-H. Anti-inflammatory eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 2010;8:2363–2366. doi: 10.1039/b926353e. [DOI] [PubMed] [Google Scholar]
- 10.Chen B.-W., Chao C.-H., Su J.-H., Tsai C.-W., Wang W.-H., Wen Z.-H., Hsieh C.-H., Sung P.-J., Wu Y.-C., Sheu J.-H. Klysimplexins I-T, eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Org. Biomol. Chem. 2011;9:834–844. doi: 10.1039/c0ob00351d. [DOI] [PubMed] [Google Scholar]
- 11.Chen B.-W., Huang C.-Y., Wen Z.-H., Su J.-H., Wang W.-H., Sung P.-J., Wu Y.-C., Sheu J.-H. Klysimplexins U-X, eunicellin-based diterpenoids from the cultured soft coral Klyxum simplex. Bull. Chem. Soc. Jpn. 2011;84:1237–1242. doi: 10.1246/bcsj.20110156. [DOI] [PubMed] [Google Scholar]
- 12.Su J.-H., Lin Y.-F., Lu Y., Huang C.-Y., Wang W.-H., Fang T.-Y., Sheu J.-H. Oxygenated cembranoids from the cultured and wild-type soft corals Sinularia flexibilis. Chem. Pharm. Bull. 2009;57:1189–1192. doi: 10.1248/cpb.57.1189. [DOI] [PubMed] [Google Scholar]
- 13.Su J.-H., Lu Y., Lin W.-Y., Wang W.-H., Sung P.-J., Sheu J.-H. A cembranoid, trocheliophorol, from the cultured soft coral Sarcophyton trocheliophoru. Chem. Lett. 2010;39:172–173. doi: 10.1246/cl.2010.172. [DOI] [Google Scholar]
- 14.Lee N.-L., Su J.-H. Tetrahydrofuran cembranoids from the cultured soft coral Lobophytum crassum. Mar. Drugs. 2011;9:2526–2536. doi: 10.3390/md9122526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y., Pattenden G. Novel macrocyclic and polycyclic norcembranoid diterpenes from Sinularia species of soft coral: Structural relationships and biosynthetic speculations. Nat. Prod. Rep. 2011;28:429–440. doi: 10.1039/c0np00029a. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi S., Satoh H., Hongo Y., Koshino H. Structural revision of terpenoids with a (3Z)-2-methyl-3-penten-2-ol moiety by the synthesis of (23E)- and (23Z)-cycloart-23-ene-3β,25-diols. J. Org. Chem. 2007;72:4578–4581. doi: 10.1021/jo070478m. [DOI] [PubMed] [Google Scholar]
- 17.Alley M.C., Scudiero D.A., Monks A., Hursey M.L., Czerwinski M.J., Fine D.L., Abbott B.J., Mayo J.G., Shoemaker R.H., Boyd M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 18.Scudiero D.A., Shoemaker R.H., Paull K.D., Monks A., Tierney S., Nofziger T.H., Currens M.J., Seniff D., Boyd M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 19.Gerwick W.H., Fenical W., Engen D.V., Clardy J. Isolation and structure of spatol, a potent inhibitor of cell replication from the brown seaweed Spatoglossum schmittii. J. Am. Chem. Soc. 1980;102:7991–7993. doi: 10.1021/ja00547a055. [DOI] [Google Scholar]
- 20.De Rosa S., Iodice C., Khalaghdoust M., Oryan S., Rustaiyan A. Spatane diterpenoids from the brown alga Stoechospermum marginatum (Dictyotaceae) Phytochemistry. 1999;51:1009–1012. [Google Scholar]
- 21.Yamase H., Umemoto K., Ooi T., Kusumi T. Structures and absolute stereochemistry of five new secospatanes and a spatane isolated from the brown alga Dilophus okamurai DAWSON. Chem. Pharm. Bull. 1999;47:813–818. doi: 10.1248/cpb.47.813. [DOI] [Google Scholar]
- 22.Venkateswarlu Y., Biabani M. A spatane diterpene from the brown alga Stoechospermum marginatum. Phytochemistry. 1995;40:331–333. doi: 10.1016/0031-9422(95)00231-U. [DOI] [Google Scholar]
- 23.Vanaltena I.A., Mcgivern J. New spatane-derived diterpenoids from the Australian brown alga Dictyota fenestrata. Aust. J. Chem. 1992;45:541–551. doi: 10.1071/CH9920541. [DOI] [Google Scholar]
- 24.Kurata K., Suzuki M., Shiraishi K., Taniguchi K. Spatane-type diterpenes with biological activity from the brown alga Dilophus okamurai. Phytochemistry. 1988;27:1321–1324. [Google Scholar]
- 25.Rao C.B., Pullaiah K.C., Surapaneni R.K., Suryaprabha R., Raju V.S.N. A new spatane diterpenoid from Stoechospermum marginatum C. Agardh. Indian J. Chem. Sect. B. 1987;26:79–80. [Google Scholar]
- 26.Gerwick W.H., Fenical W. Spatane diterpenoids from the tropical marine algae Spatoglossum schmittii and Spatoglossum howleii (Dictyotaceae) J. Org. Chem. 1983;48:3325–3329. doi: 10.1021/jo00167a033. [DOI] [Google Scholar]
- 27.Gerwick W.H., Fenical W., Sultanbawa M.U.S. Spatane diterpenoids from the tropical marine alga Stoechospermum marginatum (Dictyotaceae) J. Org. Chem. 1981;46:2233–2241. [Google Scholar]
- 28.Li L., Sheng L., Wang C.-Y., Zhou Y.-B., Huang H., Li X.-B., Li J., Mollo E., Gavagnin M., Guo Y.-W. Diterpenes from the Hainan soft coral Lobophytum cristatum Tixier-Durivault. J. Nat. Prod. 2011;74:2089–2094. doi: 10.1021/np2003325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information (PDF, 782 KB)