Abstract
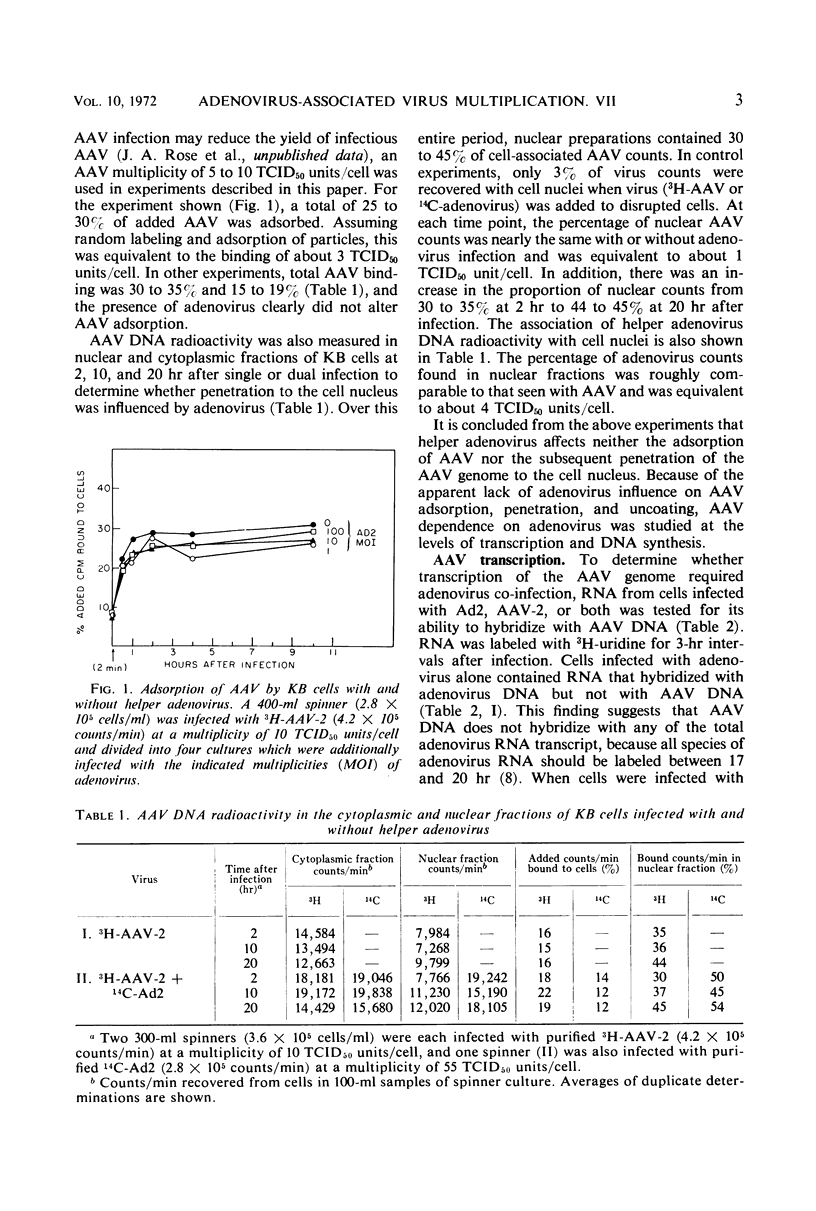
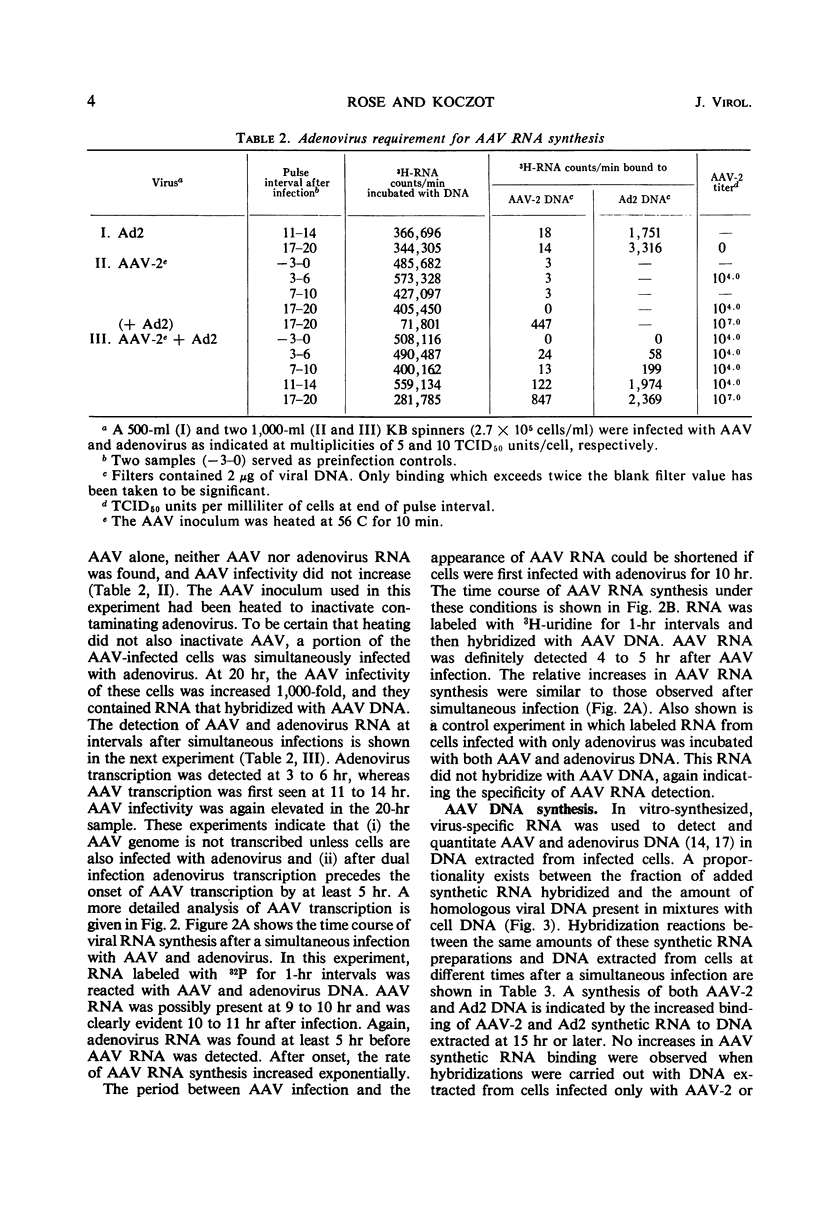
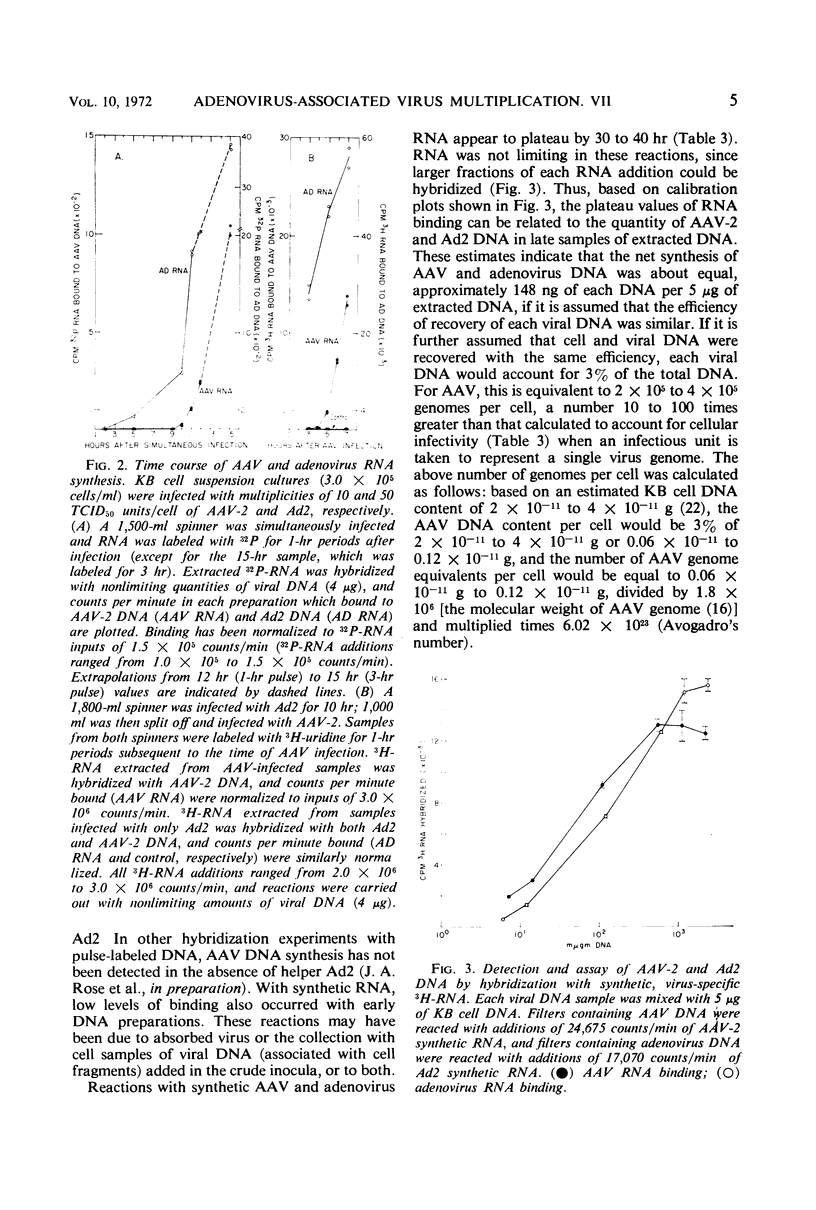
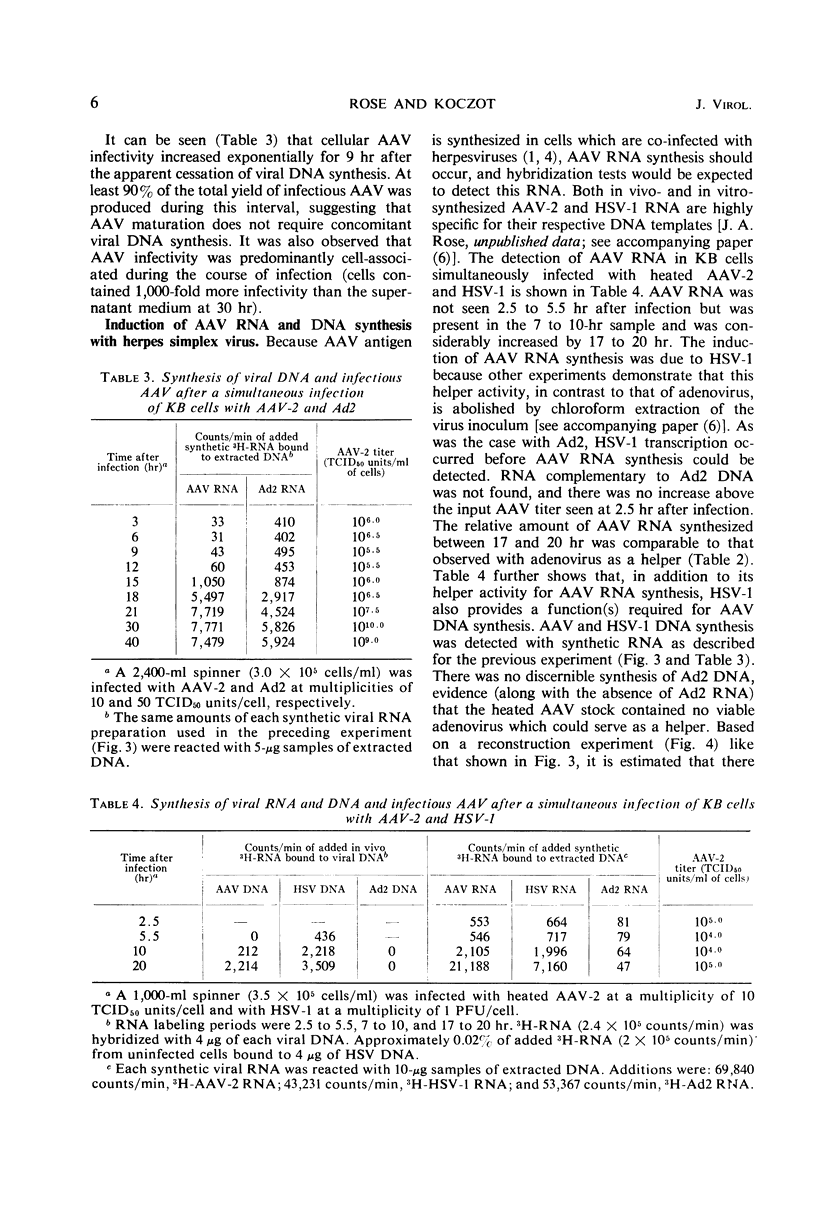
The adsorption of adenovirus-associated virus (AAV) type 2 by KB cells and the subsequent penetration of the AAV genome to the cell nucleus was measured with and without helper adenovirus type 2 (Ad2). It was found that the helper virus did not enhance either process. On the other hand, a synthesis of AAV deoxyribonucleic acid (DNA) or ribonucleic acid (RNA) was not detected by nucleic acid hybridization after KB cells were infected with AAV type 2 alone, whereas both AAV DNA and RNA synthesis were readily detected when cells were additionally infected with Ad2 or herpes simplex virus type 1, a partial helper of AAV replication. AAV RNA synthesis was initially observed 10 to 11 hr after simultaneous infection with Ad2, but the interval between AAV infection and AAV transcription could be reduced to 4 to 5 hr when cells were first infected with Ad2 for 10 hr. It was estimated that AAV DNA synthesis accounted for 3% of the total DNA in cells after a simultaneous infection with Ad2. These findings, together with the previous observation that adenovirus provides a helper function(s) after AAV uncoating, suggest that AAV are defective only subsequent to the uncoating process, and that helper viruses may provide a factor(s) needed for initiating synthesis of AAV DNA, RNA, or both.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATCHISON R. W., CASTO B. C., HAMMON W. M. ADENOVIRUS-ASSOCIATED DEFECTIVE VIRUS PARTICLES. Science. 1965 Aug 13;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Atchison R. W. The role of herpesviruses in adenovirus-associated virus replication in vitro. Virology. 1970 Sep;42(1):155–162. doi: 10.1016/0042-6822(70)90248-5. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Rose J. A. Evidence for a single-stranded adenovirus-associated virus genome: isolation and separation of complementary single strands. J Virol. 1970 Jun;5(6):693–699. doi: 10.1128/jvi.5.6.693-699.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklow N. R., Hoggan M. D., McClanahan M. S. Adenovirus-associated viruses: enhancement by human herpesviruses. Proc Soc Exp Biol Med. 1970 Sep;134(4):952–954. doi: 10.3181/00379727-134-34919. [DOI] [PubMed] [Google Scholar]
- Blacklow N. R., Hoggan M. D., Rowe W. P. Immunofluorescent studies of the potentiation of an adenovirus-associated virus by adenovirus 7. J Exp Med. 1967 May 1;125(5):755–765. doi: 10.1084/jem.125.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAWFORD L. V., CRAWFORD E. M. A COMPARATIVE STUDY OF POLYOMA AND PAPILLOMA VIRUSES. Virology. 1963 Oct;21:258–263. doi: 10.1016/0042-6822(63)90265-4. [DOI] [PubMed] [Google Scholar]
- Carter B. J., Rose J. A. Adenovirus-associated virus multiplication. 8. Analysis of in vivo transcription induced by complete or partial helper viruses. J Virol. 1972 Jul;10(1):9–16. doi: 10.1128/jvi.10.1.9-16.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Mak S., Green M. A method for determining the fraction of the viral genome transcribed during infection and its application to adenovirus-infected cells. Proc Natl Acad Sci U S A. 1968 Jul;60(3):959–966. doi: 10.1073/pnas.60.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggan M. D., Blacklow N. R., Rowe W. P. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggan M. D., Shatkin A. J., Blacklow N. R., Koczot F., Rose J. A. Helper-dependent infectious deoxyribonucleic acid from adenovirus-associated virus. J Virol. 1968 Aug;2(8):850–851. doi: 10.1128/jvi.2.8.850-851.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Melnick J. L., Mayor H. D. An immunofluorescence assay for studying replication of adeno-satellite virus. J Gen Virol. 1967 Apr;1(2):199–209. doi: 10.1099/0022-1317-1-2-199. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- RUSSELL W. C., CRAWFORD L. V. SOME CHARACTERISTICS OF THE DEOXYRIBONUCLEIC ACID FROM HERPES SIMPLEX VIRUS. Virology. 1963 Nov;21:353–361. doi: 10.1016/0042-6822(63)90196-x. [DOI] [PubMed] [Google Scholar]
- Rabson A. S., Tyrrell S. A., Legallais F. Y. Growth of ultraviolet-damaged herpesvirus in xeroderma pigemntosum. Proc Soc Exp Biol Med. 1969 Nov;132(2):802–806. [PubMed] [Google Scholar]
- Reich P. R., Baum S. G., Rose J. A., Rowe W. P., Weissman S. M. Nucleic acid homology studies of adenovirus type 7-SV40 interactions. Proc Natl Acad Sci U S A. 1966 Feb;55(2):336–341. doi: 10.1073/pnas.55.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roizman B., Spear P. G. Preparation of herpes simplex virus of high titer. J Virol. 1968 Jan;2(1):83–84. doi: 10.1128/jvi.2.1.83-84.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Berns K. I., Hoggan M. D., Koczot F. J. Evidence for a single-stranded adenovirus-associated virus genome: formation of a DNA density hybrid on release of viral DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):863–869. doi: 10.1073/pnas.64.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Hoggan M. D., Koczot F., Shatkin A. J. Genetic relatedness studies with adenovirus-associated viruses. J Virol. 1968 Oct;2(10):999–1005. doi: 10.1128/jvi.2.10.999-1005.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Hoggan M. D., Shatkin A. J. Nucleic acid from an adeno-associated virus: chemical and physical studies. Proc Natl Acad Sci U S A. 1966 Jul;56(1):86–92. doi: 10.1073/pnas.56.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Koczot F. Adenovirus-associated virus multiplication. VI. Base compostion of the deoxyribonucleic acid strand species and strand-specific in vivo transcription. J Virol. 1971 Nov;8(5):771–777. doi: 10.1128/jvi.8.5.771-777.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALZMAN N. P. Systematic fluctuations in the cellular protein, RNA and DNA during growth of mammalian cell cultures. Biochim Biophys Acta. 1959 Jan;31(1):158–163. doi: 10.1016/0006-3002(59)90451-2. [DOI] [PubMed] [Google Scholar]



