Abstract
Spreading depression of Leão is an intense spreading depolarization (SD) wave associated with massive transmembrane ionic, water, and neurotransmitter shifts. Spreading depolarization underlies migraine aura, and occurs in brain injury, making it a potential therapeutic target. While susceptibility to SD can be modulated pharmacologically, much less is known about modulation by systemic physiological factors, such as the glycemic state. In this study, we systematically examined modulation of SD susceptibility by blood glucose in anesthetized rats under full physiological monitoring. Hyperglycemia and hypoglycemia were induced by insulin or dextrose infusion (blood glucose ∼40 and 400 mg/dL, respectively). Spreading depolarizations were evoked by direct cortical electrical stimulation to determine the intensity threshold, or by continuous topical KCl application to determine SD frequency. Hyperglycemia elevated the electrical SD threshold and reduced the frequency of KCl-induced SDs, without significantly affecting other SD properties. In contrast, hypoglycemia significantly prolonged individual and cumulative SD durations, but did not alter the electrical SD threshold, or SD frequency, amplitude or propagation speed. These data show that increased cerebral glucose availability makes the tissue resistant to SD.
Keywords: animal models, electrophysiology, glucose, migraine, spreading depression
Introduction
Spreading depression of Leão is an intense spreading depolarization (SD) wave associated with massive ionic, water, and neurotransmitter fluxes typically lasting less than a minute in normal brain. Spreading depolarization is believed to be the electrophysiological substrate of migraine aura and a trigger for headache. Moreover, SDs occur in ischemic, hemorrhagic, or traumatic brain injury, and worsen tissue outcome by increasing the metabolic burden and diminishing the perfusion.1 Therefore, SD is a potential therapeutic target in migraine and brain injury.2
Genetic and pharmacological modulation of SD susceptibility is well recognized;3, 4, 5, 6 however, modulation by systemic physiological factors is poorly understood. Glycemic status in particular is a clinically relevant and modifiable factor in the management of brain injury and migraine. Spreading depolarization rapidly stimulates glucose consumption, leading to a lasting depletion of tissue glucose, and hypoglycemia delays SD recovery, suggesting that glucose is an important immediate source of energy required to restore the transmembrane ionic gradients after SD.7, 8, 9 Indeed, hyperglycemia suppresses peri-infarct SDs.10, 11, 12 However, it is not clear whether this is because of improved energy status in ischemic penumbra stabilizing the polarization state, or because tissue glucose availability directly modulates SD susceptibility. In support of the latter, hypoglycemia appears to lower topical KCl concentrations required to trigger a SD,13 and hyperglycemia has been reported to markedly reduce the amplitude of KCl-induced SDs.11 However, there has never been a systematic analysis of the impact of blood and cerebrospinal fluid (CSF) glucose levels on SD susceptibility. Therefore, using electrical and KCl stimulation as two independent but complementary methods for SD induction, we tested the hypothesis that blood glucose is inversely related to SD susceptibility.
Materials and methods
National and institutional guidelines for animal care and use for research purposes were strictly followed, and study protocol was approved by the institutional review board.
Surgical Preparation
A total of 28 rats (Sprague–Dawley, male, 275 to 400 g) were fasted overnight, anesthetized with urethane (1.3 to 1.5 g/kg, intraperitoneal), and intubated for mechanical ventilation (70% N2O/30% O2; SAR-830, CWE, Ardmore, PA, USA). Femoral vein and artery were cannulated for drug infusions, continuous mean arterial blood pressure recording (PowerLab, ADInstruments, Colorado Springs, CO, USA), and arterial blood gas and pH measurements every 15 to 30 minutes to maintain arterial pCO2 around 35 mm Hg (Rapidlab 248 blood gas/pH analyzer, Siemens HealthCare, Eschborn, Germany). Arterial blood pressure, pH, pCO2, and pO2 were comparable among different glycemic states (Table 1). Rectal temperature was kept at 37°C using a thermostatically controlled heating pad (Harvard Apparatus, Holliston, MA, USA). Rats were placed in a stereotaxic frame (Stoelting, Wood Dale, IL, USA) and three burr holes were drilled under saline cooling on each hemisphere at the following coordinates (mm from bregma): (1) posterior 7, lateral 2 (parieto-occipital cortex) for direct electrical stimulation (2 mm diameter), or topical KCl application (1 mm diameter); (2) posterior 5, lateral 2 (fronto-parietal cortex) for proximal recording site; (3) posterior 1, lateral 2 mm (frontal cortex) for distal recording site. Dura was gently removed at the KCl site, and care was taken to avoid bleeding. Following surgical preparation, the cortex was allowed to rest for 10 minutes under saline irrigation and dura was covered with mineral oil to prevent drying.
Table 1. Systemic physiological parameters.
| Group | N | BP (mm Hg) | pH | pCO2 (mm Hg) | pO2 (mm Hg) | Blood glucose (mg/dL) | CSF glucose (mg/dL) |
|---|---|---|---|---|---|---|---|
| Hypoglycemia | 6 | 104±4 | 7.38±0.01 | 36±1 | 181±9 | 39±1 | 21±3 |
| Normoglycemia | 12 | 100±4 | 7.41±0.01 | 35±1 | 185±9 | 128±7 | 98±10 |
| Hyperglycemia | 10 | 104±3 | 7.41±0.01 | 37±1 | 177±10 | 425±16 | 228±12 |
BP, blood pressure; CSF, cerebrospinal fluid.
Electrophysiology
The extracellular steady (DC) potential and electrocorticogram were recorded with glass micropipettes (150 mmol/L NaCl), 300 μm below the dural surface (EX1 differential amplifiers, Dagan Corporation, Minneapolis, MN, USA). Ag/AgCl reference electrode was placed subcutaneously in the neck.
Spreading Depolarization Susceptibility
Spreading depolarization susceptibility was determined using two distinct but complementary methods. On one hemisphere, electrical threshold for SD induction was determined by direct cortical stimulation using a constant current unit (WPI, Sarasota, FL, USA), a bipolar stimulation electrode placed on the cortical pial surface (400 μm tip diameter, 1 mm tip separation; Frederick Haer Company, Bowdoin, ME, USA), and a Ag/AgCl ground electrode placed subcutaneously in the neck, as described previously.4 Cathodal pulses of increasing intensity (100 to 4,000 μC) were applied at 4-minute intervals by adjusting the stimulus current and duration until an SD was observed. At 1 mA current, pulses of 100, 200, 300, and 400 milliseconds were applied followed by 2 mA current of 300, 400, and 500 milliseconds. If SD was not evoked, additional stimuli of 3 mA, 400 milliseconds, and 4 mA, 400, 500, 1,000 milliseconds then were applied. After the completion of threshold determination, the other hemisphere was surgically prepared in the same manner. A cotton ball (2 mm diameter) soaked with 1 M KCl was placed on the pial surface and replaced every 30 minutes. The number of KCl-induced SDs was counted for 1 hour. Small amplitude shifts in extracellular DC potential (<5 mV) were not included in the SD count.
Spreading Depolarization Attributes
In addition to the electrical SD threshold and KCl-induced SD frequency, the amplitude of DC shift, and its duration at half maximal amplitude were measured. The duration of first SD upon KCl application, as well as the average and cumulative duration of all SDs during continuous KCl application were calculated. Spreading depolarization propagation speed was measured based on its latency and the distance between the proximal and distal recording electrodes. In addition, propagation block between the two recording sites (%) was calculated as: 100 × (1−(number of SDs detected at the distal site/proximal site)).
Experimental Protocol
Three cohorts of rats were studied in alternating order. Hypoglycemia was induced by combined insulin and glucose (10%) infusion. After an initial bolus of 3 mU/g, insulin infusion was maintained at 1.5 mU per kg per minute. Blood glucose was initially monitored every 5 to 10 min, and then every 30 min to adjust the infusion rate for a target blood glucose of 40 mg/dL. Hyperglycemia was induced by intravenous 20% dextrose infusion for 1 hour; an additional dose (1 to 2 ml) of 40% dextrose was given intraperitoneally to achieve target blood glucose (∼400 mg/dL). Saline infusion served as time control in normoglycemic group. Once target blood glucose was achieved, glycemic condition was maintained for 1 hour before SD susceptibility assessment and continued throughout the experiment. At the end of each experimental protocol, cerebrospinal fluid (CSF) was sampled via cisternal puncture and glucose concentrations were measured. Cerebrospinal fluid glucose levels showed excellent correlation with blood glucose in each cohort (R2=0.83; Table 1).
Data Analysis
Data are expressed as mean±s.d. One-way analysis of variance or Kruskal–Wallis tests were used to determine statistically significant differences in parametric and nonparametric datasets, respectively (GraphPad Prism 5, La Jolla, CA, USA).
Results
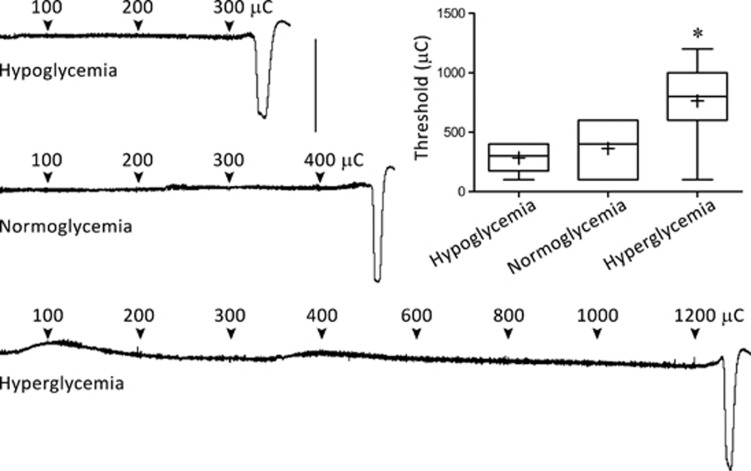
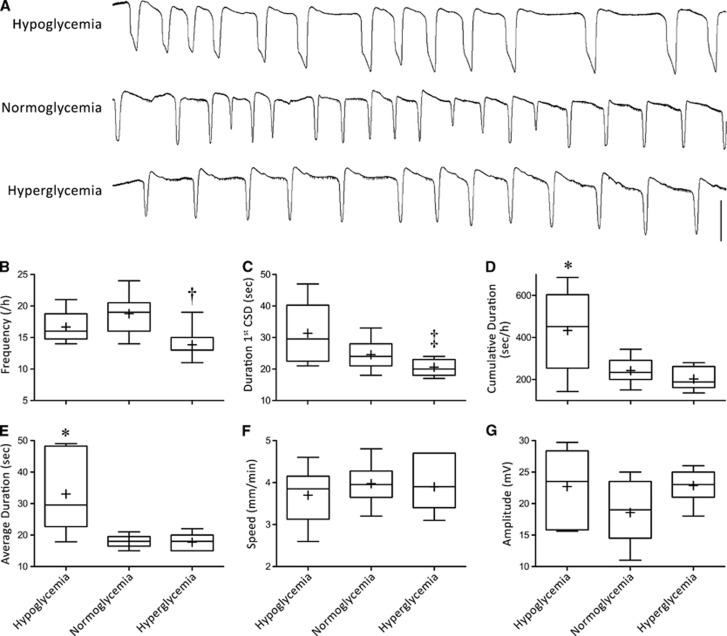
Hyperglycemia elevated the electrical threshold for SD induction (Figure 1), and suppressed KCl-induced SD frequency (Figures 2A and 2B). The duration of first SD triggered upon initial KCl application also tended to be shorter compared with normoglycemic rats (Figure 2C). However, in normoglycemic rats, SD durations quickly decreased after the first SD (subsequent SDs 32% shorter lasting compared with first SD, P<0.01). Therefore, the average and total cumulative duration of all SDs triggered during 1 hour KCl application did not significantly differ between normoglycemic and hyperglycemic groups (Figures 2D and 2E).
Figure 1.
Glycemic state and electrical stimulation threshold for spreading depolarization (SD) induction. Representative DC potential recordings show the timing and intensity of cathodal stimulation in the three glycemic groups, and the threshold intensity that triggered an SD. The graph shows the median (horizontal line), mean (+), 25% to 75% range (box), and 10% to 90% range (whisker) in each group. *P<0.01 versus both hypoglycemic and normoglycemic groups. Vertical calibration bar=25 mV. μC, microCoulomb.
Figure 2.
Glycemic state and susceptibility to KCl-induced spreading depolarization (SD). (A) Representative DC potential recordings show repetitive SDs during continuous topical KCl (1 M) application in the three glycemic groups. Box and whisker plots show SD frequency (B), duration of first SD upon initial topical KCl application (C), total cumulative and average SD durations during 1 hour continuous topical KCl application (D, E), propagation speed of first SD upon initial topical KCl application (F), and SD amplitude (G). Hypoglycemia prolonged the SDs, so that although SD frequency was not increased, total cumulative depolarization duration was significantly longer. In contrast, hyperglycemia reduced SD frequency, and the duration of first SD. †P<0.05 versus normoglycemia; ‡P<0.05 versus hypoglycemia; *P<0.01 versus both hyperglycemia and normoglycemia. Vertical calibration bar=25 mV.
In contrast to hyperglycemia, hypoglycemia did not alter SD threshold (Figure 1) or frequency (Figures 2A and 2B). As expected, SD durations were significantly prolonged (Figure 2C). Indeed, the average and total cumulative depolarization durations during 1 hour topical KCl application were more than doubled in hypoglycemic rats (Figures 2D and 2E).
Glycemic state did not impact SD propagation speed or amplitude (Figures 2F and 2G), or the incidence of propagation block between the two recording sites (23%±13%, 23%±18%, and 9%±27% in hypoglycemic, normoglycemic, and hyperglycemic groups, respectively; P>0.05).
Discussion
Our data demonstrate that glycemic state and cerebral glucose availability are important modulators of SD susceptibility and duration in otherwise normal rat brain. Hyperglycemia rendered the cortex more resistant to SD initiation and hastened SD recovery, whereas hypoglycemia had the opposite effect on SD durations. More severe hyperglycemia was reported to greatly diminish the occurrence and duration of peri-infarct SDs and decrease the amplitude of KCl-induced SDs, although SD frequency and duration did not appear to differ from normoglycemic controls.11 In another study, hyperglycemia delayed and hypoglycemia hastened the onset of anoxic depolarization.14 This was later confirmed using diffusion-weighted MRI, which also showed that hyperglycemia shortened the duration of peri-infarct SDs in ischemic penumbra, but interestingly not in nonischemic cortex.15 The latter may reflect better preservation of the tissue glucose pool, and therefore, membrane stability. Data from isolated retina preparations also suggest that glucose availability is an important physiological regulator of SD occurrence and properties.16
The mechanism of hyperglycemic suppression of SD susceptibility is not known. In both experimental animals and in humans, SD is associated with a rapid surge in glucose utilization that leads to a marked decrease in tissue glucose levels within minutes,7, 9, 17, 18, 19, 20, 21, 22 which can be stepwise cumulative upon repeated exposure.23 Hyperglycemia does increase cerebral glucose availability and diminish the drop in tissue glucose during SD.19, 24 It is therefore possible that hyperglycemic increase in glucose availability provides an immediate source of substrate for rapidly stimulated glycolysis to help the ATP-dependent pumps quell the stimulus-induced rises in extracellular [K+], preventing them from reaching the SD threshold. Hyperglycemia may also augment lactate production and lower the tissue pH, thereby suppressing the membrane excitability.9, 25 It is unlikely that changes in plasma or tissue osmolarity play a role since mannitol was reportedly ineffective.26 A vascular mechanism is also unlikely because hyperglycemia does not alter the resting blood flow or the hemodynamic response to SD.27
In general, hypoglycemia has been reported to have the opposite effect. For example, insulin-induced hypoglycemia reportedly reduced the KCl concentration threshold for SD by half in rats.13 However, glucose levels were not reported, and the increase in SD susceptibility developed a couple of hours after a single dose of insulin, and thus did not appear to temporally correspond to the transient hypoglycemia induced by this method. In another study, hypoglycemia (<75 mg/dL) was associated with higher frequency of peri-infarct SDs in cats.10 Although in our study hypoglycemia did not appear to enhance SD susceptibility in nonischemic brain, it is possible that depleted glucose pool makes ischemic penumbra more sensitive to hypoglycemia to facilitate peri-infarct SD occurrence. We did not observe spontaneous SD events analogous to anoxic depolarization in any of the hypoglycemic animals,28 but hypoglycemia did delay SD recovery as blood glucose levels were generally below 50 mg/dL.12 Therefore, the absence of an increase in KCl-induced SD frequency in hypoglycemic rats should be interpreted with caution, because prolonged SDs are expected to limit the SD repetition rate presumably by extending the absolute refractory period during which a subsequent SD could not be triggered. Indeed, the cumulative depolarization duration was significantly prolonged in hypoglycemic rats, suggesting that hypoglycemia can be detrimental in brain injury.
In summary, the inverse relationship between plasma glucose and peri-infarct SD occurrence10, 11 appears to be at least in part due to a general suppression of SD susceptibility by higher plasma glucose levels, which is observed even in nonischemic brain. Our data suggest that the optimal normoglycemic range in the management of brain injury may be higher than previously targeted. However, hyperglycemia can also be detrimental for injury outcome via unrelated mechanisms,29 and thus cannot be endorsed solely based on these data. Lastly, it is interesting to note that fasting is a commonly quoted trigger for migraine, and diabetes has been reported to abolish or reduce the frequency and severity of migraine attacks.30, 31, 32 The mechanism of these clinical associations may be plasma glucose modulation of SD susceptibility.
Acknowledgments
The authors thank Dr Brian Healy of the MGH Biostatistics Center for his critical insights during data analysis.
The authors declare no conflict of interest.
Footnotes
This study was supported by the National Institutes of Health (NS055104; NS061505).
References
- Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17:439–447. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2010;31:17–35. doi: 10.1038/jcbfm.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayata C. Spreading depression: from serendipity to targeted therapy in migraine prophylaxis. Cephalalgia. 2009;29:1095–1114. doi: 10.1111/j.1468-2982.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol. 2006;59:652–661. doi: 10.1002/ana.20778. [DOI] [PubMed] [Google Scholar]
- Eikermann-Haerter K, Dilekoz E, Kudo C, Savitz SI, Waeber C, Baum MJ, et al. Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. J Clin Invest. 2009;119:99–109. doi: 10.1172/JCI36059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikermann-Haerter K, Hyun Lee J, Yuzawa I, Liu CH, Zhou Z, Kyoung Shin H, et al. Migraine mutations increase stroke vulnerability by facilitating ischemic depolarizations. Circulation. 2012;125:335–345. doi: 10.1161/CIRCULATIONAHA.111.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Dollinger B, Brown G, Rapoport S, Sokoloff L. Cerebral glucose utilization: local changes during and after recovery from spreading cortical depression. Science. 1979;203:188–190. doi: 10.1126/science.758688. [DOI] [PubMed] [Google Scholar]
- Csiba L, Paschen W, Mies G. Regional changes in tissue pH and glucose content during cortical spreading depression in rat brain. Brain Res. 1985;336:167–170. doi: 10.1016/0006-8993(85)90430-5. [DOI] [PubMed] [Google Scholar]
- Hashemi P, Bhatia R, Nakamura H, Dreier JP, Graf R, Strong AJ, et al. Persisting depletion of brain glucose following cortical spreading depression, despite apparent hyperaemia: evidence for risk of an adverse effect of Leao's spreading depression. J Cereb Blood Flow Metab. 2009;29:166–175. doi: 10.1038/jcbfm.2008.108. [DOI] [PubMed] [Google Scholar]
- Strong AJ, Smith SE, Whittington DJ, Meldrum BS, Parsons AA, Krupinski J, et al. Factors influencing the frequency of fluorescence transients as markers of peri-infarct depolarizations in focal cerebral ischemia. Stroke. 2000;31:214–222. doi: 10.1161/01.str.31.1.214. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Astrup J. Infarct rim: effect of hyperglycemia on direct current potential and [14C]2-deoxyglucose phosphorylation. J Cereb Blood Flow Metab. 1986;6:607–615. doi: 10.1038/jcbfm.1986.108. [DOI] [PubMed] [Google Scholar]
- Gido G, Katsura K, Kristian T, Siesjo BK. Influence of plasma glucose concentration on rat brain extracellular calcium transients during spreading depression. J Cereb Blood Flow Metab. 1993;13:179–182. doi: 10.1038/jcbfm.1993.21. [DOI] [PubMed] [Google Scholar]
- Bures J, Buresova O. Activation of latent foci of spreading cortical depression in rats. J Neurophysiol. 1960;23:225–236. doi: 10.1152/jn.1960.23.3.225. [DOI] [PubMed] [Google Scholar]
- Hansen AJ. The extracellular potassium concentration in brain cortex following ischemia in hypo- and hyperglycemic rats. Acta Physiol Scand. 1978;102:324–329. doi: 10.1111/j.1748-1716.1978.tb06079.x. [DOI] [PubMed] [Google Scholar]
- Els T, Rother J, Beaulieu C, de Crespigny A, Moseley M. Hyperglycemia delays terminal depolarization and enhances repolarization after peri-infarct spreading depression as measured by serial diffusion MR mapping. J Cereb Blood Flow Metab. 1997;17:591–595. doi: 10.1097/00004647-199705000-00015. [DOI] [PubMed] [Google Scholar]
- Vercesi A, Martins-Ferreira H. Oxygen and glucose requirements in chick retinal spreading depression. An Acad Bras Cienc. 1983;55:309–316. [PubMed] [Google Scholar]
- Mies G, Paschen W. Regional changes of blood flow, glucose, and ATP content determined on brain sections during a single passage of spreading depression in rat brain cortex. Exp Neurol. 1984;84:249–258. doi: 10.1016/0014-4886(84)90222-x. [DOI] [PubMed] [Google Scholar]
- Krivanek J. Some metabolic changes accompanying Leao's spreading cortical depression in the rat. J Neurochem. 1961;6:183–189. doi: 10.1111/j.1471-4159.1961.tb13463.x. [DOI] [PubMed] [Google Scholar]
- Silver IA, Erecinska M. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. J Neurosci. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood SE, Parkin MC, Bezzina EL, Boutelle MG, Strong AJ. Transient changes in cortical glucose and lactate levels associated with peri-infarct depolarisations, studied with rapid-sampling microdialysis. J Cereb Blood Flow Metab. 2005;25:391–401. doi: 10.1038/sj.jcbfm.9600050. [DOI] [PubMed] [Google Scholar]
- Parkin M, Hopwood S, Jones DA, Hashemi P, Landolt H, Fabricius M, et al. Dynamic changes in brain glucose and lactate in pericontusional areas of the human cerebral cortex, monitored with rapid sampling on-line microdialysis: relationship with depolarisation-like events. J Cereb Blood Flow Metab. 2005;25:402–413. doi: 10.1038/sj.jcbfm.9600051. [DOI] [PubMed] [Google Scholar]
- Krivanek J. Concerning the dynamics of the metabolic changes accompanying cortical spreading depression. Physiol Bohemoslov. 1962;11:383–391. [PubMed] [Google Scholar]
- Feuerstein D, Manning A, Hashemi P, Bhatia R, Fabricius M, Tolias C, et al. Dynamic metabolic response to multiple spreading depolarizations in patients with acute brain injury: an online microdialysis study. J Cereb Blood Flow Metab. 2010;30:1343–1355. doi: 10.1038/jcbfm.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjedde A, Hansen AJ, Quistorff B. Blood-brain glucose transfer in spreading depression. J Neurochem. 1981;37:807–812. doi: 10.1111/j.1471-4159.1981.tb04465.x. [DOI] [PubMed] [Google Scholar]
- Mutch WA, Hansen AJ. Extracellular pH changes during spreading depression and cerebral ischemia: mechanisms of brain pH regulation. J Cereb Blood Flow Metab. 1984;4:17–27. doi: 10.1038/jcbfm.1984.3. [DOI] [PubMed] [Google Scholar]
- Ximenes-da-Silva A, Guedes RC. Differential effect of changes in blood glucose levels on the velocity of propagation of cortical spreading depression in normal and malnourished rats. Braz J Med Biol Res. 1991;24:1277–1281. [PubMed] [Google Scholar]
- Wolf T, Lindauer U, Villringer A, Dirnagl U. Excessive oxygen or glucose supply does not alter the blood flow response to somatosensory stimulation or spreading depression in rats. Brain Res. 1997;761:290–299. doi: 10.1016/s0006-8993(97)00354-5. [DOI] [PubMed] [Google Scholar]
- Astrup J, Norberg K. Potassium activity in cerebral cortex in rats during progressive severe hypoglycemia. Brain Res. 1976;103:418–423. doi: 10.1016/0006-8993(76)90817-9. [DOI] [PubMed] [Google Scholar]
- de Courten-Myers GM, Kleinholz M, Wagner KR, Myers RE. Normoglycemia (not hypoglycemia) optimizes outcome from middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1994;14:227–236. doi: 10.1038/jcbfm.1994.29. [DOI] [PubMed] [Google Scholar]
- Blau JN, Pyke DA. Effect of diabetes on migraine. Lancet. 1970;2:241–243. doi: 10.1016/s0140-6736(70)92588-2. [DOI] [PubMed] [Google Scholar]
- Martins I, Blau JN. Headaches in insulin-dependent diabetic patients. Headache. 1989;29:660–663. doi: 10.1111/j.1526-4610.1989.hed2910660.x. [DOI] [PubMed] [Google Scholar]
- Latsko M, Silberstein S, Rosen N. Frovatriptan as preemptive treatment for fasting-induced migraine. Headache. 2011;51:369–374. doi: 10.1111/j.1526-4610.2010.01827.x. [DOI] [PubMed] [Google Scholar]