Abstract
A recently discovered protein phosphatase PHLPP (PH domain Leucine-rich repeat Protein Phosphatase) has been shown to dephosphorylate Akt on its hydrophobic motif (Ser473) thereby decreasing Akt kinase activity. We generated PHLPP1 knockout (KO) mice and used them to explore the ability of enhanced in vivo Akt signaling to protect the brain against ischemic insult. Brains from KO mice subjected to middle cerebral artery occlusion (MCAO) for 2 hours showed significantly greater increases in Akt activity and less neurovascular damage after reperfusion than wild-type (WT) mice. Remarkably, infarct volume in the PHLPP1 KO was significantly reduced compared with WT (12.7±2.7% versus 22.9±3.1%) and this was prevented by Akt inhibition. Astrocytes from KO mice and neurons in which PHLPP1 was downregulated showed enhanced Akt activation and diminished cell death in response to oxygen-glucose deprivation. Thus, deletion of PHLPP1 can enhance Akt activation in neurons and astrocytes, and can significantly increase cell survival and diminish infarct size after MCAO. Inhibition of PHLPP could be a therapeutic approach to minimize damage after focal ischemia.
Keywords: Akt, ischemia, middle cerebral artery occlusion (MCAO), PH domain leucine-rich repeat protein phosphatase (PHLPP), stroke
Introduction
Stroke is the second leading cause of death worldwide according to the World Health Organization.1 The extent of cerebral damage after stroke is determined by the balance between apoptosis, necrosis, and survival in the various cells forming the neurovascular unit, which maintains blood–brain barrier integrity and adequate blood supply to the brain.2, 3, 4 The serine/threonine kinase Akt has a well-recognized role in cell survival, including neuronal protection against cerebral ischemia.5, 6, 7 However, interpretation of the studies published to date concerning involvement of Akt in stroke is complicated. Neuronal-specific transgenic mice overexpressing an activated form of Akt after injury showed reduced infarct area after middle cerebral artery occlusion (MCAO),6 but it is not clear whether these levels of expression and activity of Akt are physiologic. Indeed mice deficient in Akt1, a major contributor to neuronal survival, do not show differences in stroke outcome8 but the observation that Akt1-deficient mice and control mice have equivalent levels of phosphorylated Akt suggests that alternate isoforms of Akt compensate for germline loss of Akt1.8 Recent studies revealed that increasing Akt activity by removal of its endogenous inhibitor, carboxyl terminal modulator protein (CTMP), protects neurons from ischemia-induced death.9 This provides encouraging evidence that increasing endogenous activation of all Akt isoforms are protective, although how CTMP affects Akt activity has not been clearly defined.
The activation of Akt by growth factors or cytokines is initiated by stimulation of phosphoinositide-3-kinase and generation of PIP3, which recruits Akt to the plasma membrane. Akt is phosphorylated at threonine (Thr)308 by its upstream kinase, phosphoinositide-dependent kinase 1, and at the hydrophobic motif on serine (Ser)473 by TORC2.10, 11, 12 The duration and amplitude of Akt phosphorylation is important for determining its physiologic effects, thus termination of Akt activation by phosphatases could significantly affect its functional activity. Several phosphatases have been found to negatively regulate Akt activity. The tumor suppressor, PTEN (phosphatase and tensin homolog deleted on chromosome 10), antagonizes Akt signaling by dephosphorylating PIP3 to PIP2, thereby limiting Akt activation.13 Based on its site of action, many additional PIP3-regulated processes are also affected by PTEN. Akt can be dephosphorylated at Thr308 and Ser473 by protein phosphatase 2A (PP2A), however, it has limited substrate selectivity based on its pleiotropic effects on numerous targets.14, 15 Recently, a novel serine/threonine protein phosphatase, PH domain Leucine-rich repeat Protein Phosphatase (PHLPP) was reported to specifically dephosphorylate Akt on its hydrophobic motif, thereby attenuating Akt activity.16
The PHLPP family of phosphatases is comprised of three members PHLPP (α and β splice variants) and PHLPP2. The PHLPP1α and PHLPP1β are splice variants of the same gene. PHLPP1 and 2 possess an identical domain structure composed of a PH domain followed by a region of leucine-rich repeats, a PP2C phosphatase domain, and a C-terminal PDZ ligand domain.16, 17, 18 Cellular localization studies reveal that PHLPP1 and PHLPP2 are present throughout the cell16, 17, 18 and are expressed in both neurons19 and astrocytes.20 In addition to its effects on Akt, PHLPP has been shown to dephosphorylate the hydrophobic motif of conventional and novel PKC isoforms in vitro, affecting their stability and thus the level of expression in cancer cells.18
The role of PHLPP in regulating Akt activity in the brain and its effect on cerebral ischemia has not been examined. The studies reported here show that deletion of PHLPP1 from the brain leads to transient but accentuated Akt activation and protects the brain from ischemic damage after MCAO. The salutary effect of PHLPP1 removal on ischemic damage is also observed in isolated astrocytes and neurons and suggests that PHLPP1 could represent an important therapeutic target for protection against damage induced by stroke.
Materials and methods
Animals
All procedures were performed in accordance with NIH Guide for the Care and use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the University of California San Diego. Generation of homozygous Black Swiss/129Sv PHLPP1 knockout (KO) mice has been described previously.21
Model of Cerebral Ischemia
Middle cerebral artery occlusion (MCAO) was performed using the intraluminal filament technique as previously described.22, 23 Wild-type (WT) and PHLPP1 KO littermates having an average weight of 30 g and ∼10 to 12 weeks of age were used for surgical experiments. Mice were anesthetized with 2% isoflurane mixed with 0.8% oxygen. For FITC-dextran experiments, mice received tail vein injections (40 μL) of FITC conjugated to high molecular weight dextran (2 MDa; Sigma, St Louis, MO, USA) dissolved in 5% (wt/vol) solution in sterile phosphate-buffered saline ∼10 minutes before occlusion of the middle cerebral artery.24 A midline neck incision was made to expose the left common carotid artery. The external and left common carotid were excised and ligated with 7-0 silk (Ethicon, Somerville, NJ, USA). A small incision was made in the common carotid artery close to the bifurcation point of the external and internal carotid arteries. To occlude the middle cerebral artery, silicon-coated (Heraeus, South Bend, IN, USA) 6-0 nylon suture (Ethicon) was advanced 9 to 10 mm through the internal carotid artery to the anterior cerebral artery. To ensure induction of focal brain ischemia, filament diameter was measured using microscopy. Image analysis software and the suture size used were proportional to animal weight.25 Regional cerebral blood flow was measured by a flexible Laser-Doppler Flowmetry (LDF) probe (Moor Instruments, Wilmington, DE, USA). After occlusion and before reperfusion, the mice were allowed to awaken from anesthesia and neurologic examinations were performed 1 hour after MCAO using a published rodent neurologic grading system.26, 27 Mice were reanesthetized and the suture removed after 2 hours of ischemia and reperfusion was performed for various times. For histologic examination of the brain, the animal was transcardially perfused with saline followed by 4% (wt/vol) paraformaldehyde. After euthanasia via an overdose of penbarbitol, brains were removed and sectioned and subsequently stained with 2% (wt/vol) 2,3,5-triphenyltetrazolium chloride (TTC) (Sigma) and later area of infarct subsequently quantitated using light microscopy and ImageJ software (version 1.40 g, NIH, Bethesda, MD, USA).
Akt Inhibitor Studies
Inhibitor studies were performed as previously described using a modified protocol.8, 28 Briefly, MCAO was followed by the administration of a pan-Akt inhibitor triciribine (2 μL, 1 mmol/L, dissolved in 5% (wt/vol) DMSO (Sigma) and injected intracerebroventricularly (coordinates 1.0 mm lateral, 0.2 mm posterior, 3.1 mm deep) to male WT and KO mice. The compound was injected: 1 hour before MCAO, again 1 hour after stroke onset, and 1 hour after reperfusion. After 24 hours of reperfusion, mice were euthanized via an overdose of penbarbitol, brains were removed and sectioned and subsequently stained with 2% (wt/vol) TTC (Sigma). Area of infarct was quantitated using light microscopy and ImageJ software (NIH).
Immunohistochemistry
Fixed brains were paraffin-embedded and sectioned (15 μm). Vascular disruption was represented by FITC-dextran labeling in the brain sections. For each brain, three sections spanning Bregma level −3 mm to 2 mm were mounted and imaged by epifluorescence microscopy at low power using a CCD camera (Apogee Instruments, Auburn, CA, USA). The area of FITC-dextran was quantified in Image-Pro Plus (Media Cybernetics Inc., Bethesda, MD, USA) as previously described.24 In brief, an operator blinded to treatment set the brightness and contrast levels to optimize the appearance of the fluorescence. Using thresholding and segmentation, the operator measured the total area of fluorescence. Cellular death associated with focal ischemia was detected using a TRITC deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) protocol (Roche, San Francisco, CA, USA) and costained with DAPI for nuclear visualization.29
Cell Culture
Cultured astrocytes were prepared from postnatal day P1 to P3 WT and PHLPP KO mice as previously described.30 Briefly, cerebral cortices were isolated from adherent meninges, and the tissue dissociated and trypsinized. Cells were maintained in high-glucose DMEM supplemented with 10% (w/vol) FBS/2 mmol/L glutamine/100 units/mL penicillin/100 μg/mL streptomycin (Gibco, Carlsbad, CA, USA). After 11 days in culture, cells were shaken overnight to remove oligodendrocytes and plated at one-third their confluency and maintained in an incubator at 37°C with 5% CO2. Experiments involving WT and KO astrocytes were plated at equal cell number and experiments performed at the second passage. HT22 mouse hippocampal cells (gift from Dr David Shubert) were maintained in high-glucose DMEM supplemented with 10% (w/vol) FBS/2 mmol/L glutamine/100 units/mL penicillin/100 μg/mL streptomycin (Gibco) in an incubator at 37°C with 10% CO2. Experiments involving HT22 cells were plated at 5 × 104 cells per 35 mm dish. Primary mouse striatal neurons (including glia) were purchased from Lonza (Walkersville, MD, USA) and maintained in primary neuron basal medium supplemented with PNGM SingleQuots (2 mmol/L glutamine, 50 μg/mL Gentamicin/37 ng/mL Amphotericin, and 2% (wt/vol) Neural Survival Factor (NSF)-1) (Lonza) in an incubator at 37°C with 5% CO2. After 7 days in culture, medium was changed and the cells were transfected with siRNA. Astrocytes from WT and KO mice as well as HT22 cells were serum starved for 24 hours before stimulation with 1 nmol/L IGF-1 (Austral Biologicals, San Ramon, CA, USA). Protein extracts from the primary astrocytes and HT22 cells were examined by western blotting for activation of Akt and multiple downstream targets.31, 32
Immunoblotting
The left cortex was obtained from control mice and those subjected to 2 hours MCAO with various times of reperfusion. Astrocytes and HT22 cells treated with agonist or vehicle were used for cell culture experiments. Tissue or cells were homogenized and extracts made in lysis buffer31 and protein concentrations determined (Bradford Assay; BioRad, Hercules, CA, USA). Individual samples were subjected to SDS-polyacrylamide gel electrophoresis (Invitrogen, Carlsbad, CA, USA) and transferred onto PVDF membranes (Millipore, Billerica, MA, USA). Membranes were blocked in 5% (wt/vol) milk TBS-Tween for 1 hour and probed overnight at 4°C using various antibodies (PHLPP1 and PHLPP2 antibodies were from Bethyl Laboratories (Montgomery, TX, USA) and total and phosphorylated Akt Ser473, Thr308, Akt1, Akt2, Akt3, PKCα, cleaved caspase 3, pERK1/2, and gapdh were from Cell Signaling Technology (Boston, MA, USA)). Fold changes in cleaved caspase 3 and phosphorylation of Akt at Ser473 were determined by densitometry and normalized to accompanying GAPDH blots, changes were expressed as relative values compared with WT samples.
Akt Kinase Assay
Akt catalytic activity was assessed using a nonradioactive Akt kinase assay kit (Cell Signaling Technology). Extracts (150 μg) from the injured hemisphere, astrocytes, or HT22 cells were incubated with an antibody to total Akt overnight. Immunocomplexes were spun down and washed twice with Kinase Buffer from the kit. For the kinase reaction, immunocomplexes were resuspended in 50 μL Kinase Buffer with 200 μmol/L ATP and 1 μg GST-GSK3 at 30°C for 30 minutes. Reactions were terminated with the addition of 10 μL 5 × SDS and the samples were subjected to immunoblotting with the phosphorylated GSK-3 antibody and total Akt. The change in catalytic activity of Akt was determined by densitometry of phosphorylated GSK-3 substrate and normalized to total immunoprecipitated Akt. The activity of Akt was expressed as fold change relative to WT or control samples.
Transfection with siRNA
Predesigned mouse PHLPP1 and PHLPP2 ON-TARGET plus and control siRNA were purchased from Thermo Scientific (Waltham, MA, USA). siRNA (2 μmol/L) was transfected with DharmaFECT-3 (astrocytes and primary neurons) and DharmaFECT-4 reagent (HT22 cells) (Thermo Scientific) (in a 1:3 ratio, respectively) as previously described.32 After overnight incubation, cells were washed and cultured for another 24 hours in complete media.
Oxygen-Glucose Deprivation
For simulated ischemia experiments, cells were transfected with various siRNAs for 48 hours before oxygen-glucose deprivation (OGD). Control cells were placed in normoxic buffer (140 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 10 mmol/L glucose, 10 mmol/L HEPES and 2 mmol/L CaCl2 at pH 7.4) at 37°C with 5 or 10% CO2 depending on the cell type under investigation. For OGD, cells containing hypoxic buffer (140 mmol/L NaCl, 12 mmol/L KCl, 1 mmol/L MgCl2, and 2 mmol/L CaCl2 at pH 6.5) without glucose were placed in a humidified chamber filled with 95% N2 and 5% CO2 at 37°C for 18 hours (astrocytes) or subjected to 90 minutes OGD and 18 hours reperfusion in normoxic buffer (neurons).
Cell Death ELISA
DNA fragmentation indicative of apoptosis was assayed using the cell death detection ELISAPLUS (Roche Applied Science, Indianapolis, IN, USA) as previously described.32 Briefly, supernatants from astrocytes and HT22 cells (20 μL) exposed to OGD or normoxic conditions were incubated with anti-histone-biotin antibody and anti-DNA-peroxidase antibody in a streptavidin-coated 96-well plate on an orbital shaker (60 r.p.m.) at room temperature for 3 hours. Subsequently, wells were washed with incubation buffer (200 μL per well) three times, 2, 2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic) acid substrate (100 μL per well) was added and absorbance was measured at 405 nm using plate reader. All readings were normalized to protein concentration.
Cell Death Propidium Iodide Assay
Viability of striatal neurons after OGD was determined by simultaneous fluorescent labeling of live and dead cells. After 18 hours of reperfusion, medium was removed and 200 μL of Hank's Balanced Salt Solution (Gibco) containing 2.5 μm/L propidium iodine (PI) (Invitrogen), 3 μm/L calcein-AM (Invitrogen), and 5 μg/ml Hoechst33258 (Invitrogen) was added to each well and the plate incubated at 37°C for 30 minutes. Absorbance was measured on a plate reader. The calcein-AM reading was normalized to Hoechst and the calcein/PI ratio determined. For normalization of the experiments, the calcein/PI ratio for the siControl neurons subjected to OGD was set at 100% and the relative percent cell death for neurons with PHLPP1 knockdown was calculated. After the spectrophotometer reading, cell death was visualized by fluorescence microscopy.
Lactate Dehydrogenase Assay
Cell death or cytotoxicity was assayed by measuring lactate dehydrogenase (LDH) release into the cell culture medium by the LDH Cytotoxicity Assay Kit II (MBL International Corporation, Woburn, MA, USA) according to manufacturer's protocol. Samples were run in duplicate and allowed to incubate for 4 hours at room temperature. The LDH activity was detected on a plate reader at OD450 nm. The relative percent LDH release between the groups was determined by normalizing the siControl cells subjected to ischemia/reperfusion to 100%.
Data Analysis
Researchers were blinded to the treatment group during analyses. Significance (P<0.05) was determined by Student's t-test or one-way ANOVA with post hoc Tukey analysis using GraphPad Prism software (GraphPad, La Jolla, CA, USA).
Results
Deletion of PHLPP1 Does Not Affect PHLPP2 or Akt Isoform Expression in the Brain
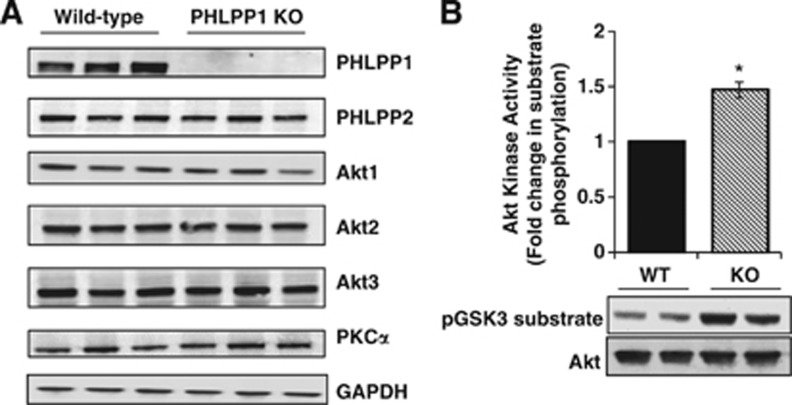
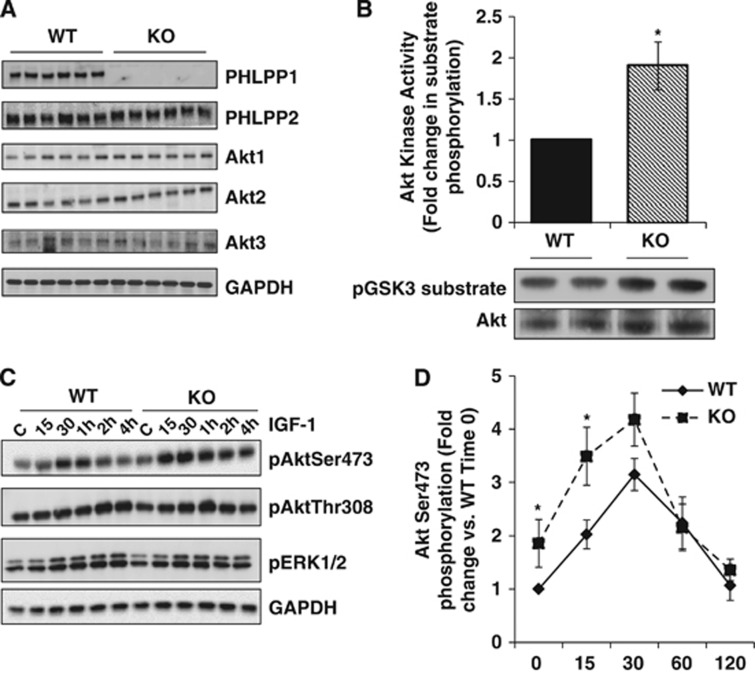
The generation and characterization of PHLPP1 gene-targeted mice has been previously described21, 32 but the effects of deleting PHLPP on Akt activation and protection in the brain have not been investigated. Conventional PHLPP1 KO mice are viable and show no overt changes in brain size or development (data not shown). Extracts from whole brain of WT and PHLPP1 KO mice were analyzed for PHLPP expression. The PHLPP1 protein was undetectable in null mice and there was no compensatory change in PHLPP2 (Figure 1A). Removal of PHLPP1 also had no effect on the level of expression of any of the three Akt isoforms (Figure 1A). The catalytic activity of Akt was, however, significantly elevated in KO compared with WT brains at baseline (Figure 1B), consistent with a role for PHLPP in regulating basal Akt activity. PHLPP1 has been shown to regulate a phosphorylation site on conventional and novel PKCs that alters its stability in some systems;18 however, PKCα, a conventional PKC isoform in the brain, did not show altered expression in the absence of PHLPP1 (Figure 1A) nor did the novel PKC, PKCδ (data not shown).
Figure 1.
Deletion of PHLPP1 increases Akt activity without affecting PHLPP2 or Akt isoform expression. (A) Cerebral extracts (25 μg) from wild-type (WT) and PHLPP1 knockout (KO) mice were analyzed for PHLPP, Akt, and PKCα expression. n=3 samples/genotype. (B) Fold change of Akt catalytic activity in WT and KO cerebral extracts (150 μg). A representative blot of GSK3 phosphorylation and total immunoprecipitated Akt is shown. (The graph represents n=6/independent-samples t-test *P⩽0.05 compared with WT.) PHLPP, PH domain Leucine-rich repeat Protein Phosphatase.
PHLPP1 Knockout Mice Have Reduced Neurovascular Damage and Infarct Volume After Focal Cerebral Ischemia
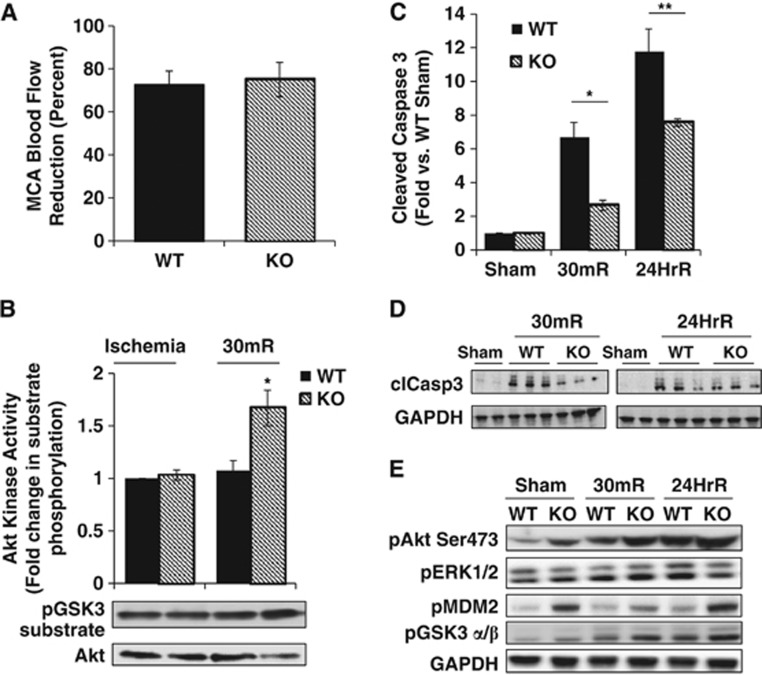
Having established that removal of PHLPP1 has an effect on brain Akt activity, we went on to determine if the ability of PHLPP deletion to accentuate physiologic levels of Akt activation would protect the brain from in vivo stroke damage. The WT and PHLPP1 KO mice were subjected to 2 hours of focal cerebral ischemia followed by reperfusion for various times. The reduction in cerebral blood flow after MCAO was equivalent in WT and PHLPP1 KO mice (∼75% reduction versus baseline value; Figure 2A). Mice were awakened within 5 minutes after MCAO surgery (ischemia) and behavioral changes were scored 1 hour after ischemia onset to assess the severity of neurologic impairment. There was no significant difference in the neurologic function score obtained in WT and KO mice immediately after MCAO (data not shown). During the 2 hours ischemia imposed by MCAO, Akt catalytic activity was increased to equivalent levels in WT and KO mice. Notably however, after 30 minutes reperfusion, Akt activity in the KO was ∼70% higher than that of WT mouse brain (Figure 2B). The known protective effect of Akt suggested that increased Akt activation would be accompanied by decreased cell death. To test this, we examined caspase 3 cleavage in WT and KO mouse brains at both 30 minutes and 24 hours of reperfusion (Figures 2C and 2D). Caspase 3 cleavage was markedly increased at both times in WT mouse brains and the response was significantly attenuated at both 30 minutes and 24 hours in the PHLPP KO. The attenuated caspase activation after reperfusion was a predicted consequence of the increase in Akt phosphorylation and activity resulting from PHLPP1 deletion, and was not associated with changes in phosphorylation of another potentially protective kinase, ERK1/2 (Figure 2E).
Figure 2.
Increased Akt catalytic activity and decreased apoptosis in the PHLPP1 knockout (KO) mice after middle cerebral artery occlusion (MCAO). Wild-type (WT) and KO mice were subjected to 2 hours of cerebral ischemia by MCAO and Akt activity and downstream signaling were measured after 30 minutes or 24 hours of reperfusion. (A) Laser-Doppler Flowmetry (LDF) determined no significant difference between WT (n=9) and KO (n=11) in the reduction of blood flow in the middle cerebral artery (MCA) during ischemia. (B) The catalytic activity of Akt was determined in WT and KO brain extracts (150 μg) during ischemia and after 30 minutes reperfusion (n=3; independent-sample t-test *P⩽0.05). (C, D) Quantification and western blot of cleaved caspase 3 levels in the brain after MCAO (n=3; independent-sample t-test *P⩽0.05 compared with WT 30mR;**P⩽0.05 compared with WT 24hR). Levels of cleaved caspase 3 were normalized to GAPDH levels. Representative western blot (25 μg) of cleaved caspase 3 in Sham operated (1 WT and 1 KO) and in mice after 30 minutes (30mR) and 24 hours reperfusion (24HrR) (n=3 WT/KO independent samples). (E) Phosphorylation of Akt and its downstream targets are increased in cerebral extracts (25 μg) from KO mice compared with WT at baseline (Sham operated) and after 2 hours of ischemia and 30 minutes (30mR) or 24 hours reperfusion (24HrR). There was no significant difference in ERK 1/2 phosphorylation between WT and KO mice.
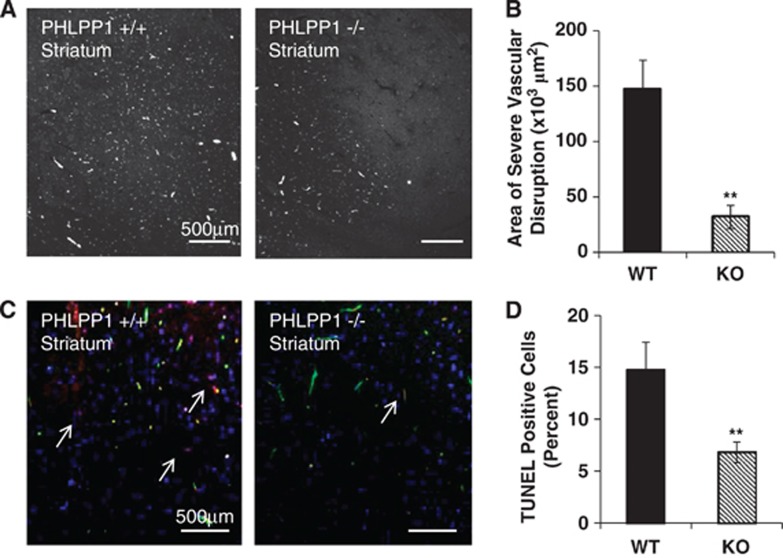
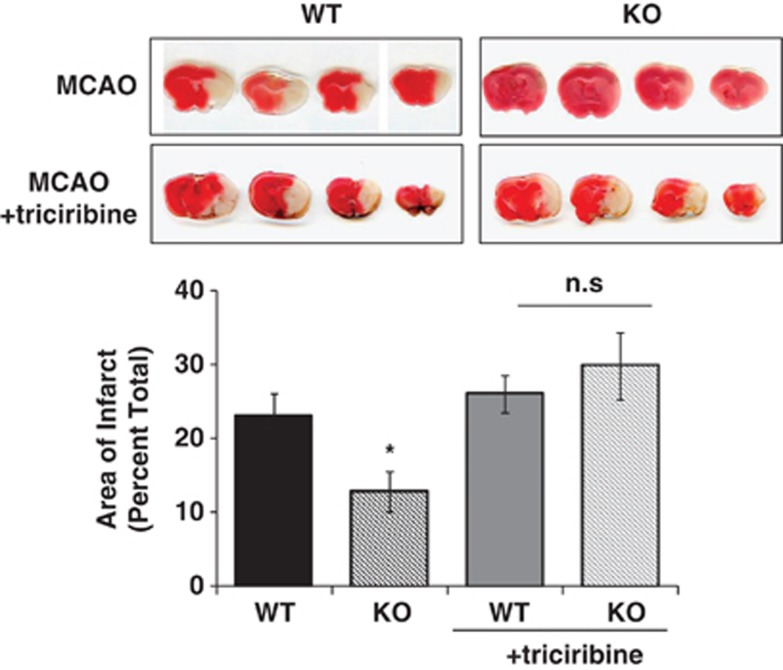
The effect of ischemic damage on vascular integrity after 2 hours MCAO and 30 minutes reperfusion was assessed by FITC-dextran fluorescence (Figures 3A and 3B). The area of vascular disruption was decreased by >80% in PHLPP1 KO compared with WT mice. There was also a significant decrease in TUNEL-positive cells in the striatum, further indicating diminished cell death in KO versus WT mouse brain (Figures 3C and 3D). Finally, the protective effect of PHLPP1 removal on infarct size after 2 hours MCAO and 24 hours reperfusion was investigated using TTC staining. The lesion volume in the brains of KO mice was significantly smaller (12.7±2.7%) than that in brains of WT mice (22.9±3.1%) (Figure 4). To prove that the reduction in infarct volume after MCAO in the PHLPP1 KO mice was because of the enhanced Akt activity, we examined the effect of the Akt inhibitor, triciribine. Treatment with triciribine fully prevented the protection (12.7±2.7% in KO versus 29.7±4.5% in KO with inhibitor) conferred by PHLPP deletion.
Figure 3.
PHLPP1 knockout (KO) mice have reduced neurovascular damage after middle cerebral artery occlusion (MCAO). Wild-type (WT) and KO mice were subjected to 2 hours of cerebral ischemia and 30 minutes reperfusion. (A) The striatum region of the brain was visualized for severe vascular disruption using FITC-dextran. (B) The amount of vascular damage was significantly reduced in the PHLPP1 KO mice (n=6; independent-sample t-test **P⩽0.001 compared with WT). (C) Cell death was reported by TUNEL staining (Red-TUNEL, Green-FITC-dextran, and Blue-DAPI). (D) PHLPP1 KO mice had significantly less TUNEL-positive cells in the striatum of the brain after injury. The graph represents three brains and six areas analyzed (independent-sample t-test **P⩽0.01 compared with WT). PHLPP, PH domain Leucine-rich repeat Protein Phosphatase; TUNEL, TRITC deoxynucleotidyltransferase-mediated dUTP nick end labeling.
Figure 4.
PHLPP1 knockout (KO) mice specifically alter Akt activity and protect the brain from middle cerebral artery occlusion (MCAO) injury. Wild-type (WT) and KO mice (12 to 14 weeks old) were subjected to 2 hours of cerebral ischemia and 24 hours reperfusion. For Akt inhibitor studies, triciribine (1 mmol/L in 5% DMSO) was injected introcerebroventricularly three times, 1 hour before surgery, 1 hour after ischemia, and 1 hour after reperfusion. After 24 hours reperfusion, brains were removed and stained with 2,3,5-triphenyltetrazolium chloride (TTC) and area of infarct was quantitated. The PHLPP1 KO mice have a significant reduction in infarct size after MCAO that is reversed on treatment with the Akt inhibitor triciribine (n=20 WT/13 KO; independent-sample t-test *P⩽0.02 compared with WT; n=7 WT/4 KO in inhibitor study; n.s., no significant difference between WT and KO).
PHLPP1 Knockout Astrocytes Have Accentuated Akt Activity and Protection After Ischemia
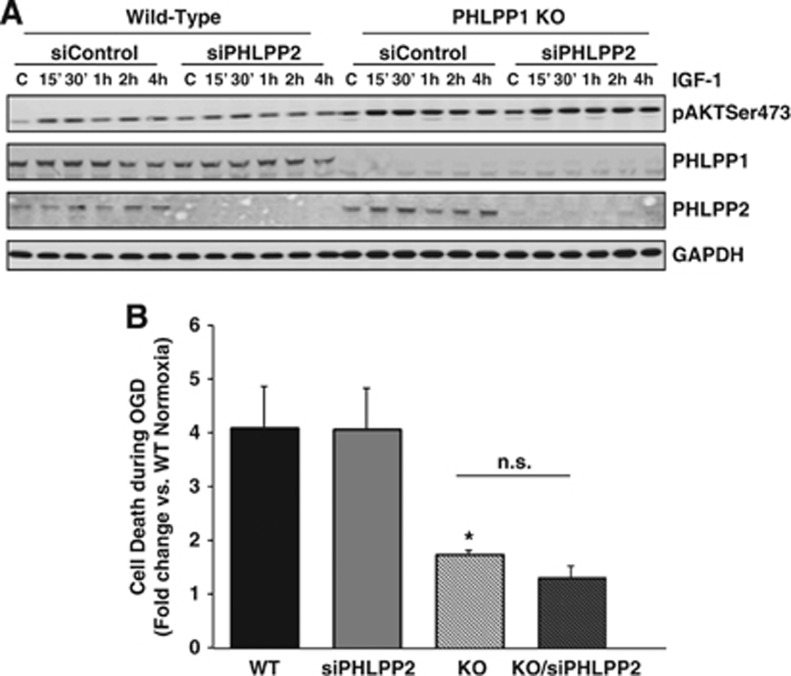
Since removal of PHLPP1 attenuates damage to the neurovascular unit after stroke, the effect of PHLPP1 deletion on Akt activation in the brain was examined at the cellular level. Cortical astrocytes were isolated from WT and KO mice. As observed in whole brain, astrocytes from KO mice showed complete loss of PHLPP1 protein, and no change in Akt isoform or PHLPP2 expression (Figure 5A). Akt activity was significantly increased in these cells at baseline (Figure 5B) as observed in whole brain. We used this isolated cell preparation to examine responses to agonists that could activate Akt in the brain. The IGF-1 (1 nmol/L) increased phosphorylation of Akt at Ser473 and this was significantly enhanced in PHLPP1 KO astrocytes (Figures 5C and 5D) with no significant difference at Thr308 (Figure 5C). Recent studies from the Newton laboratory have determined that PHLPP deletion increases MAP (ERK1/2) kinase activation through altering receptor tyrosine kinase signaling (personal communication). However, we observed no significant difference in IGF-1 stimulated ERK activation in KO versus WT astrocytes (Figure 5C). To determine whether removal of the other PHLPP isoform, PHLPP2, would further potentiate Akt activation, we examined the effect of siRNA-mediated PHLPP2 knockdown in WT and PHLPP1 KO astrocytes. There was no further increase in Akt activation in response to IGF-1 in WT or PHLPP1 null cells after siRNA knockdown of PHLPP2, thus PHLPP2 and PHLPP1 do not appear to serve redundant functions in this system (Figure 6A). These data indicate a selective ability of PHLPP1 gene deletion to accentuate Akt activation in cortical astrocytes.
Figure 5.
PHLPP knockout (KO) astrocytes have increased Akt activity. (A) Wild-type (WT) and KO astrocytes were immunoblotted for changes in PHLPP and Akt isoform expression at baseline. (B) Catalytic activity of Akt was determined in WT and KO astrocytes (150 μg) at baseline (n=7; independent-samples t-test *P⩽0.05 compared with WT). Representative blots of GSK3 substrate phosphorylation and immunoprecipitated Akt from WT and KO astrocytes are shown. (C) Phosphorylation of Akt at Ser473, Thr308, and ERK1/2 after IGF-1 (1 nmol/L) stimulation for various times (up to 4 hours) in WT and KO astrocytes (25 μg). (D) Quantification of Akt phosphorylation at Ser473 in WT and KO astrocytes stimulated with IGF-1 and normalized to GAPDH (n=6 independent experiments/ANOVA *P⩽0.05 compared with WT (Time 0)).
Figure 6.
Removal of PHLPP1 not PHLPP2 accentuates Akt activation and protects astrocytes from oxygen-glucose deprivation (OGD). Wild-type (WT) and knockout (KO) astrocytes were transfected with control or PHLPP2 siRNA (2 μmol/L) for 48 hours. Cells were serum starved for 24 hours and (A) stimulated with IGF-1 (1 nmol/L) for various times and blotted for phosphorylation of Akt at Ser473 and PHLPP isoform expression or (B) exposed to either normoxic conditions or 18 hours OGD. Apoptosis of the astrocytes was quantified by DNA fragmentation using an ELISA. The graph represents fold OGD induced apoptosis of each group compared with WT cells under normoxic conditions (n=7 independent experiments *P⩽0.05 compared with WT OGD).
Finally, to directly show that removal of PHLPP1 can protect central nervous system cells from ischemic insult, WT and PHLPP1 KO cells were exposed to either normoxic conditions or OGD for 18 hours. Apoptosis was quantified using an ELISA to measure DNA fragmentation. Simulated ischemia induced by OGD lead to ∼4-fold increase in apoptosis in WT astrocytes or PHLPP2 knockdown cells; apoptosis was greatly attenuated in the PHLPP1 KO astrocytes (Figure 6B). No further protection was achieved when PHLPP2 was downregulated using siRNA, consistent with the aforementioned observation that PHLPP2 does not regulate Akt activation in these cells.
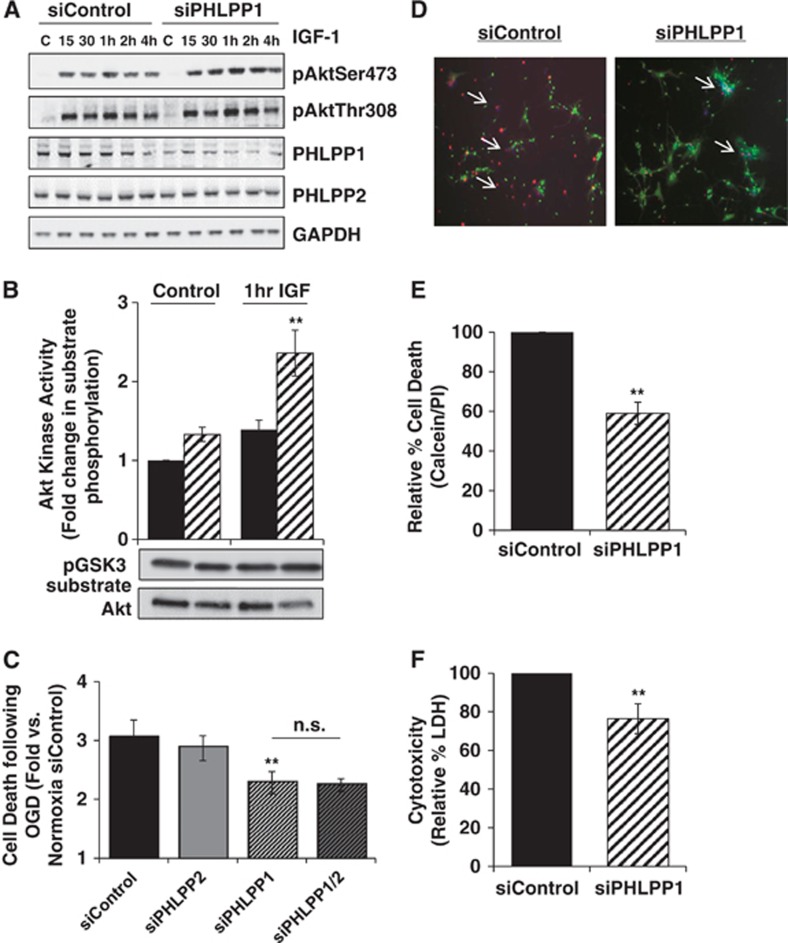
Knockdown of PHLPP1 in Neurons Increases Agonist-Induced Activation and Protects them from Ischemia/Reperfusion Injury
Since removal of PHLPP1 increased Akt activity and provided protection to astrocytes from ischemic damage in vitro, we determined whether neurons lacking PHLPP1 would also be protected. Hippocampal neurons (HT22 cells) were transfected with control or PHLPP1 siRNA for 48 hours. Knockdown of PHLPP1 accentuated phosphorylation of Akt at Ser473 after treatment with IGF-1 (1 nmol/L), knockdown of PHLPP1 accentuated phosphorylation of Akt at Ser473 compared with siControl without significantly increasing Thr308 phosphorylation (Figure 7A). Also, the catalytic activity of Akt was also significantly greater after 1 hour of IGF-1 stimulation in neurons with PHLPP1 knockdown compared with control (Figure 7B). To determine whether removal of PHLPP1 could confer protection to neurons, HT22 cells were subjected to either normoxia or OGD for 90 minutes and 18 hours reperfusion. Apoptosis was quantified using an ELISA to measure DNA fragmentation. The OGD induced an ∼3-fold increase in apoptosis (DNA fragmentation as assessed by ELISA) in control or PHLPP2 knockdown neurons and this response was modest but significantly attenuated in the PHLPP1 knockdown cells (Figure 7C).
Figure 7.
Downregulation of PHLPP1 accentuates Akt activation and protects neurons from oxygen-glucose deprivation (OGD). HT22 cells were transfected with control, PHLPP1, PHLPP2 or both PHLPP1 and PHLPP2 siRNA (2 μmol/L) for 48 hours. Cells were serum starved for 24 hours followed by stimulation with IGF-1 (1 nmol/L) and (A) lysed and immunoblotted for PHLPP and phosphorylation of Akt at Ser473 and Thr308 at various times or (B) assayed for Akt catalytic activity after 1 hour IGF-1 addition (n=3 independent experiments; t-test **P⩽0.03 compared with baseline siControl cells). In (C–F), HT22 cells (C) or primary striatal neurons (D–F) were treated with siRNAs as described above and then subjected to 90 minutes of OGD and 18 hours reperfusion under normoxic conditions. For HT22 cells (C) apoptosis was quantified by DNA fragmentation using an ELISA. The graph represents fold increase in OGD induced apoptosis for each group compared with siControl cells under normoxic conditions (n=6 independent experiments; t-test **P⩽0.03 compared with siControl OGD). For primary neurons (D–F) cell death after OGD was determined by propidium iodine (PI) staining (Red-PI, Green-calcein-AM, and Blue-Hoechst) and lactate dehydrogenase (LDH) release into the medium (n=3 independent experiments performed in triplicate; t-test **P⩽0.03 compared with siControl OGD).
Finally, to show that removal of PHLPP1 protects primary mouse striatal neurons from ischemia/reperfusion injury, PHLPP1 was downregulated by transfection of PHLPP1 siRNA. Downregulation of PHLPP1 mRNA to ∼86% control levels was achieved, as measured by quantitative PCR, and neurons were subjected to OGD for 90 minutes followed by 18 hours reperfusion. Knockdown of PHLPP1 significantly reduced neuronal cytotoxicity as determined by both decreases in the percent PI-positive cells and LDH release (Figures 7D to F). Thus, removal of PHLPP1 increases Akt activation and protects both astrocytes and neurons from ischemic damage.
Discussion
Transient focal brain ischemia can occur as a consequence of thrombosis or embolism. The sequelae of ischemia are well established but the signaling pathways that contribute to or protect the brain from cerebral damage are less well understood. Akt is a serine/threonine kinase that regulates downstream targets involved in many cellular processes and is well known to have a role in cell survival. The dysregulation of Akt has been implicated in many disorders, including cardiovascular and neurologic diseases and cancer.7 The importance of the recently discovered protein phosphatase PHLPP1 in regulating pathophysiological responses in the brain has not been previously examined. Here, we use PHLPP1 gene deletion to show for the first time that loss of PHLPP1 enhances Akt activity and diminishes cell death and vascular disruption after transient focal brain ischemia.
Akt is activated through its phosphorylation on two residues, Thr308 and Ser473. Alessi et al33 showed that phosphorylation on both residues is critical for full activation of Akt. Consistent with this, overexpression of PHLPP1 in vitro dramatically reduces Akt catalytic activity by dephosphorylating Akt at Ser473 without affecting Thr308.16 Conversely, low levels of PHLPP expression are associated with increased levels of Ser473 phosphorylated Akt and concomitant activation of downstream Akt targets.16, 17, 32 Dephosphorylation of the hydrophobic motif on PKC can also be catalyzed by PHLPP, however, this regulates PKC stability rather than its activation state.18 Our previous work showed that PHLPP1 deletion does not affect either conventional or novel PKC levels in the heart or isolated cardiac myocytes.32 The data presented here further show that loss of PHLPP1 does not change PKCα levels in the brain. Earlier studies reported that the protein termed as SCOP (aka PHLPP1), acting through its Ras association domain and LRR region, inhibited ERK signaling and impaired long-term memory formation.34, 35 However, removal of PHLPP1 did not alter ERK signaling at the cellular level (Figure 5C) or in the brain at baseline or after ischemic injury (Figure 2E). Accordingly, we suggest that the observed effects of PHLPP1 deletion on infarct size and vascular leakage result from changes in Akt activation and not alterations in signaling through protective kinase signaling pathways using PKC or ERK. This hypothesis is also supported by our studies showing that pharmacological inhibition of Akt fully prevents the protection conferred by PHLPP1 deletion.
Numerous studies have documented changes in phosphorylation of Akt after brain reperfusion and increases in phosphorylation of Akt have been linked to protection.12, 36, 37 Transient upregulation of Akt phosphorylation was observed after permanent occlusion of the middle cerebral artery in mice,37 while blocking the increased Akt activation after ischemia with LY294002, a phosphoinositide-3-kinase inhibitor, potentiated neuronal cell death.38 To increase neuroprotection from ischemic insult in vivo, many studies have overexpressed the activated kinase6 or focused on neuroprotective factors that non-selectively activate Akt.39, 40 Our data suggest an alternative approach, i.e., to prolong Akt signaling during reperfusion after transient ischemia by inhibiting its phosphatase PHLPP.
The findings presented here show that deletion of the Akt phosphatase PHLPP1 enhances the extent of Akt activity transiently and provides significant protection of the neurovascular unit in response to reperfusion after MCAO. Brains from PHLPP1 KO mice showed decreases in vascular leakage, caspase 3 cleavage, and cell death after ischemia and 30 minutes reperfusion. Notably, the area of infarct after 24 hours reperfusion is decreased by >55% in the PHLPP1 KO mice compared with WT and this can be completely prevented by treatment with an Akt inhibitor. Interestingly, WT mice treated with the Akt inhibitor did not show significantly greater infarct volume than WT after MCAO as shown by others.8 This finding shows that activation of Akt during reperfusion is not sufficient to limit the extent of MCAO damage unless its phosphatase is inactivated.
To provide cellular evidence that the protective effect of PHLPP deletion in the brain results from diminished Akt dephosphorylation and enhanced Akt activation, we isolated astrocytes from WT and KO mouse brain and downregulated PHLPP in neuronal cultures. Activation of Akt by IGF-1 in KO astrocytes was potentiated for up to 30 minutes; subsequent recovery of Akt activity to basal levels occurred at similar rates in WT and KO cells, suggesting that PHLPP1 deletion in astrocytes provides an accentuated response to agonist without disrupting the normal transient kinetic profile of kinase activation. The time course for Akt activation by IGF-1 was more sustained in HT22 cells; IGF-1 increased Akt phosphorylation for up to 4 hours and this was accentuated throughout by PHLPP deletion. Most importantly, PHLPP1 KO astrocytes, HT22, and primary striatal neurons with downregulated PHLPP1 are all protected from cell death in response to simulated ischemia induced by OGD in vitro compared with WT or control cells.
In summary, our studies in vivo and in primary astrocytes and neurons in which PHLPP1 is genetically deleted or downregulated confirm that PHLPP1 is an important regulator of Akt activity and survival in response to ischemic stress in the central nervous system. Since our in vivo studies use mice with global KO, of PHLPP1, the highly striking protection against stroke injury may reflect effects of increased Akt activation in neurons, astrocytes, and other cells in the neurovascular unit to cerebral protection. Determining which cell type within the neurovascular unit is most critical will require generation of tissue-specific PHLPP1 KO. Regardless of the answer, the evidence presented here shows for the first time that PHLPP is an important regulator of Akt activity in the brain and suggests that pharmacological inhibition of PHLPP could have a therapeutic impact on ischemic damage caused by stroke.
Acknowledgments
The authors thank Dr Alexandra C Newton for her collaborative efforts in generating the PHLPP1 gene targeted mice and Melissa Barlow for her technical assistance.
The authors declare no conflict of interest.
Footnotes
This work was supported by the Tobacco-Related Disease Research Program of the University of California 20KT-0048 (NHP), NIH grant HL08557 and GM036927 (JHB), National Institutes of Neurological Disorders and Stroke grants R01NS043300 and R01NS075930 (PDL), and American Heart Association Predoctoral Fellowship (BC).
References
- WHO . World Health Statistics. Geneva: WHO; 2012. [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–414. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Lo EH, Rosenberg GA. The neurovascular unit in health and disease: introduction. Stroke. 2009;40:S2–S3. doi: 10.1161/STROKEAHA.108.534404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ. The neurovascular unit in the setting of stroke. J Intern Med. 2010;267:156–171. doi: 10.1111/j.1365-2796.2009.02199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Activating Akt and the brain's resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005;20:299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba N, Kiryu-Seo S, Maeda M, Muraoka M, Ishii M, Kiyama H. Transgenic mouse overexpressing the Akt reduced the volume of infarct area after middle cerebral artery occlusion. Neurosci Lett. 2004;359:159–162. doi: 10.1016/j.neulet.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Yang ZZ, Tschopp O, Baudry A, Dummler B, Hynx D, Hemmings BA. Physiological functions of protein kinase B/Akt. Biochem Soc Trans. 2004;32:350–354. doi: 10.1042/bst0320350. [DOI] [PubMed] [Google Scholar]
- Li J, Lang J, Zeng Z, McCullough LD. Akt1 gene deletion and stroke. J Neurol Sci. 2008;269:105–112. doi: 10.1016/j.jns.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T, Ofengeim D, Noh KM, Latuszek-Barrantes A, Hemmings BA, Follenzi A, et al. The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death. Nat Neurosci. 2009;12:618–626. doi: 10.1038/nn.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfel NA, Newton AC. Pleckstrin homology domain leucine-rich repeat protein phosphatase (PHLPP): a new player in cell signaling. J Biol Chem. 2012;287:3610–3616. doi: 10.1074/jbc.R111.318675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duronio V. The life of a cell: apoptosis regulation by the PI3K/PKB pathway. Biochem J. 2008;415:333–344. doi: 10.1042/BJ20081056. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Kawano T. Akt is a molecular target for signal transduction therapy in brain ischemic insult. J Pharmacol Sci. 2003;92:317–327. doi: 10.1254/jphs.92.317. [DOI] [PubMed] [Google Scholar]
- Liao Y, Hung MC. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010;2:19–42. [PMC free article] [PubMed] [Google Scholar]
- Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY, Chiang CW. Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J Biol Chem. 2008;283:1882–1892. doi: 10.1074/jbc.M709585200. [DOI] [PubMed] [Google Scholar]
- Zwaenepoel K, Goris J, Erneux C, Parker PJ, Janssens V. Protein phosphatase 2A PR130/B''alpha1 subunit binds to the SH2 domain-containing inositol polyphosphate 5-phosphatase 2 and prevents epidermal growth factor (EGF)-induced EGF receptor degradation sustaining EGF-mediated signaling. FASEB J. 2010;24:538–547. doi: 10.1096/fj.09-140228. [DOI] [PubMed] [Google Scholar]
- Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18:13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Brognard J, Newton AC. PHLiPPing the switch on Akt and protein kinase C signaling. Trends Endocrinol Metab. 2008;19:223–230. doi: 10.1016/j.tem.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson TC, Verrier JD, Semple-Rowland S, Kumar A, Foster TC. PHLPP1 splice variants differentially regulate AKT and PKCalpha signaling in hippocampal neurons: characterization of PHLPP proteins in the adult hippocampus. J Neurochem. 2010;115:941–955. doi: 10.1111/j.1471-4159.2010.06984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfel NA, Niederst M, Stevens MW, Brennan PM, Frame MC, Newton AC. Mislocalization of the E3 ligase, beta-transducin repeat-containing protein 1 (beta-TrCP1), in glioblastoma uncouples negative feedback between the pleckstrin homology domain leucine-rich repeat protein phosphatase 1 (PHLPP1) and Akt. J Biol Chem. 2011;286:19777–19788. doi: 10.1074/jbc.M111.237081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masubuchi S, Gao T, O'Neill A, Eckel-Mahan K, Newton AC, Sassone-Corsi P. Protein phosphatase PHLPP1 controls the light-induced resetting of the circadian clock. Proc Natl Acad Sci USA. 2010;107:1642–1647. doi: 10.1073/pnas.0910292107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST. Rodent models of focal stroke: size, mechanism, and purpose. NeuroRx. 2005;2:396–409. doi: 10.1602/neurorx.2.3.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ZA, Namiranian K, Klaus J, Kibler K, Dore S. Use of an optimized transient occlusion of the middle cerebral artery protocol for the mouse stroke model. J Stroke Cerebrovasc Dis. 2006;15:133–138. doi: 10.1016/j.jstrokecerebrovasdis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Chen B, Friedman B, Cheng Q, Tsai P, Schim E, Kleinfeld D, et al. Severe blood-brain barrier disruption and surrounding tissue injury. Stroke. 2009;40:e666–e674. doi: 10.1161/STROKEAHA.109.551341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, McCullough LD. Middle cerebral artery occlusion model in rodents: methods and potential pitfalls. J Biomed Biotechnol. 2011;2011:464701. doi: 10.1155/2011/464701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Hara H, Huang PL, Panahian N, Fishman MC, Moskowitz MA. Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. J Cereb Blood Flow Metab. 1996;16:605–611. doi: 10.1097/00004647-199607000-00010. [DOI] [PubMed] [Google Scholar]
- Song YS, Narasimhan P, Kim GS, Jung JE, Park E-H, Chan PH. The role of Akt signaling in oxidative stress mediates NF-[kappa]B activation in mild transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:1917–1926. doi: 10.1038/jcbfm.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Cheng Q, Yang K, Lyden PD. Thrombin mediates severe neurovascular injury during ischemia. Stroke. 2010;41:2348–2352. doi: 10.1161/STROKEAHA.110.584920. [DOI] [PubMed] [Google Scholar]
- Citro S, Malik S, Oestreich EA, Radeff-Huang J, Kelley GG, Smrcka AV, et al. Phospholipase Cepsilon is a nexus for Rho and Rap-mediated G protein-coupled receptor-induced astrocyte proliferation. Proc Natl Acad Sci USA. 2007;104:15543–15548. doi: 10.1073/pnas.0702943104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell NH, Wilkins BJ, York A, Saba-El-Leil MK, Meloche S, Robbins J, et al. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci USA. 2007;104:14074–14079. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Purcell NH, Smith JM, Gao T, Whittaker R, Huang K, et al. PHLPP-1 negatively regulates Akt activity and survival in the heart. Circ Res. 2010;107:476–484. doi: 10.1161/CIRCRESAHA.109.215020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Phan T, Mansuy IM, Storm DR. Proteolytic degradation of SCOP in the hippocampus contributes to activation of MAP kinase and memory. Cell. 2007;128:1219–1229. doi: 10.1016/j.cell.2006.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K, Okada M, Takano A, Nagai K. SCOP, a novel gene product expressed in a circadian manner in rat suprachiasmatic nucleus. FEBS Lett. 1999;458:363–369. doi: 10.1016/s0014-5793(99)01190-4. [DOI] [PubMed] [Google Scholar]
- Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006;34:249–270. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamawaki T, Sasaki T, Hattori H, Hamada J, Fukuuchi Y, et al. Upregulation of Akt phosphorylation at the early stage of middle cerebral artery occlusion in mice. Brain Res. 2002;942:1–10. doi: 10.1016/s0006-8993(02)02474-5. [DOI] [PubMed] [Google Scholar]
- Noshita N, Lewen A, Sugawara T, Chan PH. Evidence of phosphorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2001;21:1442–1450. doi: 10.1097/00004647-200112000-00009. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kilic E, Kilic U, Weber B, Bassetti CL, Marti HH, et al. VEGF overexpression induces post-ischaemic neuroprotection, but facilitates haemodynamic steal phenomena. Brain. 2005;128:52–63. doi: 10.1093/brain/awh325. [DOI] [PubMed] [Google Scholar]
- Kilic E, Kilic U, Wang Y, Bassetti CL, Marti HH, Hermann DM. The phosphatidylinositol-3 kinase/Akt pathway mediates VEGF's neuroprotective activity and induces blood brain barrier permeability after focal cerebral ischemia. FASEB J. 2006;20:1185–1187. doi: 10.1096/fj.05-4829fje. [DOI] [PubMed] [Google Scholar]