Abstract
Therapeutic hypothermia is of relevance to treatment of increased body temperature and brain injury, but drugs inducing selective, rapid, and safe cooling in humans are not available. Here, we show that injections of adenosine 5′-monophosphate (AMP), an endogenous nucleotide, promptly triggers hypothermia in mice by directly activating adenosine A1 receptors (A1R) within the preoptic area (POA) of the hypothalamus. Inhibition of constitutive degradation of brain extracellular AMP by targeting ecto 5′-nucleotidase, also suffices to prompt hypothermia in rodents. Accordingly, sensitivity of mice and rats to the hypothermic effect of AMP is inversely related to their hypothalamic 5′-nucleotidase activity. Single-cell electrophysiological recording indicates that AMP reduces spontaneous firing activity of temperature-insensitive neurons of the mouse POA, thereby retuning the hypothalamic thermoregulatory set point towards lower temperatures. Adenosine 5′-monophosphate also suppresses prostaglandin E2-induced fever in mice, having no effects on peripheral hyperthermia triggered by dioxymetamphetamine (ecstasy) overdose. Together, data disclose the role of AMP, 5′-nucleotidase, and A1R in hypothalamic thermoregulation, as well and their therapeutic relevance to treatment of febrile illness.
Keywords: AMP, A1R, fever, hypothalamus, hypothermia
Introduction
Hypothermia is one of the most effective neuroprotective strategies, and mechanisms underlying prevention of neuronal loss by brain cooling are being understood at the molecular levels.1 In humans, small decreases of brain temperature (3°C to 4°C) are well tolerated, with shivering and infections being the main drawbacks of body cooling. On the clinical side, trials of therapeutic hypothermia in patients suffering from stroke, neonatal hypoxia, or cardiac arrest proved promising.1, 2, 3 Yet, current cooling devices suffer from excessive degree of invasiveness and/or considerable delay in inducing the hypothermic effects.4, 5 Because of that, drugs triggering rapid and safe hypothermia could be of remarkable relevance to treatment of different neurological disorders. These tools could also be of therapeutic significance in conditions of altered thermoregulation such as febrile illness, drug-induced hyperthermia, or heat stroke.6 Despite their therapeutic significance and intense investigation at the preclinical and clinical level, hypothermic drugs are still an unmet need.2, 7 A major problem in pharmacological induction of hypothermia is the availability of tools able to prompt transient cooling without excessive reduction of body temperature (Tb) and/or suspended animation.8 Of course, development of safe hypothermic drugs able to selectively trigger Tb cooling not affecting cardiovascular and respiratory functions necessitates deeper understanding of the neurochemistry underpinning hypothalamic thermoregulation. Indeed, the hypothalamic circuitry integrating signals originating from both peripheral thermoreceptors and different brain regions of the brainstem and diencephalon is currently only in part understood. Also, the precise nature of the neurotransmitters and receptors adopted by the different hypothalamic neuronal populations to bring about heat retention or loss responses is unsolved.
Reportedly, circadian oscillations of serum levels of adenosine 5′-monophosphate (AMP) regulate expression of key metabolic enzymes and correlate to torpor in mice. Of note, the nucleotide induces profound and long-lasting hypothermia when injected at high doses in rodents.9, 10 Despite the apparent potential relevance of this naturally occurring compound to therapeutic hypothermia, the site of action of AMP as well as the molecular mechanisms underlying its hypothermic effect are still unknown. In the present study, we sought to identify such mechanisms as well as the effects of AMP-dependent cooling to altered thermogenesis. We originally report that, although adenosine (Ado) is the prototypical specific ligand of Ado A1 receptors (A1R), both exogenous and endogenous AMP prompt hypothermia by directly (i.e., without necessitating conversion into Ado) activating A1R onto spontaneously firing hypothalamic neurons dictating the Tb set point. Importantly, the hypothermic effect prompted by AMP readily counteracted the pyretic response in mice, having no effects in a model of peripheral hyperthermia triggered by acute intoxication by 3,4-methylenedioxymetamphetamine (MDMA or ecstasy).
Materials and methods
Animals, Drug Treatment, ICV Injection, and Tb Measurement
C57/Bl6 male mice of 20 to 25 g were used (Harlan Nossan, UK). Adenosine 5′-monophosphate, AMPCP, Ado, 8-phenyl theopylline and MDMA were dissolved in saline, all the other compounds were dissolved in dimethyl sulfoxide (DMSO). Since rectal and temporalis muscle temperature similarly decreased upon drug treatment, a rectal probe (Harvard Apparatus, Holliston, MA, USA) was used to measure Tb in mice.11 In experiments designed to study the heat loss response, animals were exposed to a 40°C RT (room temperature) by placing cages in a box equipped with thermostat and air recycling. As for i.c.v. injection, 3 μL (1 μ/min) were injected i.c.v. (P+1, L+1 and V-3 mm from bregma) in anesthetized mice (2% isoflurane, 70% nitrous oxide, and 30% oxygen). All animal procedures were conducted according to the European Community Guidelines for Animal Care, DL 116/92, and to the ARRIVE guidelines.
PCR
In all, 1 μg of total RNA isolated from mouse or rat hypothalamus was retro-transcribed using iScript kit (Bio-Rad, Hercules, CA, USA) and amplified as described.12 The following primers were used: Mouse 5′-NT: Forward 5′-GGCTCTTTACCAAGGTGCAGC-3′ and Reverse 5′-ATCAATCAGTCCTTCCACACCG-3′ Rat 5′-NT: Forward 5′-TTCACCAAGGTGCAGCAGATC-3′ and Reverse 5′-ATCAATCAGTCCTTCCACACCG-3′ Mouse/Rat 18S ribosomal RNA: Forward 5′-GGGAGGTAGTGACGAAAAATAACAAT-3′ and Reverse 5′-TTGCCCTCCAATGGATCCT-3′. Real-time PCR assays were performed by Rotor-Gene SYBR Green PCR Kit (Qiagen, Milan, Germany) and analyzed using the Rotor-Gene 3000 cycler system (Corbett Research, Mortlake, Australia).
5′-NT Activity Analysis
Mouse or rat hypothalami were dissected from brain (total of four hypothalami per species) and rapidly homogenized in a 50 mmol/L Tris buffer pH 8 containing KCl 50 mmol/L, DTT 1 mmol/L, Protease Inhibitor Cocktail 10 μL/mL, MgCl2 2 mmol/L. Homogenates were spun at 16,000 g and the pellet discarded. The supernatants were spun at 100,000 g for 40 minutes. The resulting pellet containing the membrane fraction was resuspended in 500 μL of the same buffer containing 2% v/v Triton X-100 and kept at 0°C for 30 minutes. The extract was soon utilized for enzyme assay. The 5′-NT assay was run in tubes containing 50 μL of brain extract and 50 μL of homogenization buffer containing AMP 100 μmol/L in the presence or absence of different concentrations of AMP (0.01 to 1 mmol/L). The reaction was incubated at 37°C and stopped after 5 minutes with an equal volume of HClO4 0.4 N. The mixture was centrifuged at 10,000 g for 10 minutes. Adenosine formation was measured after standard derivatization procedure by means of high-performance liquid chromatography (HPLC) and fluorimeter detection.13
Hippocampal Field Excitatory Postsynaptic Potential Recording
Experiments were carried out on acute hippocampal slices.14 Briefly, male Wistar rats or C57/Bl6 male mice (Harlan, Udine, Italy) were killed under isoflurane-induced anesthesia and their hippocampi were rapidly removed and placed in ice-cold oxygenated (95% O2 to 5% CO2) artificial cerebrospinal fluid (ACSF). Rats brains and slices were maintained in ACSF containing (in mmol/L): NaCl 125, KCl 3, NaH2PO4 1.25, MgSO4 1, CaCl2 2, NaHCO3 25, and 𝒟-glucose 10. Mouse brains and slices were maintained in ACSF containing (in mmol/L): NaCl 124, KCl 2.75, NaH2PO4 1.25, MgSO4 1.3, CaCl2 2, NaHCO3 26, and 𝒟-glucose 10. Parasagittal rat or mouse hippocampal slices (400 μm thick) were prepared using a McIlwain tissue chopper (The Mickle Lab. Engineering, Gomshall, UK) and kept in oxygenated ACSF for at least 1 hour at RT. A single slice was then placed on a nylon mesh in a small chamber (0.8 mL), completely submerged and superfused with oxygenated ACSF (31°C to 32°C) at a constant flow rate of 1.5 to 1.8 mL/min. All drugs were stored at −20°C in stock solutions at 1,000 to 10,000 times the desired final concentration, dissolved in ACSF and applied to the slice by superfusion. Changes in superfusing solutions reached the preparation in 60 seconds and this delay was taken into account in our calculations. Test pulses (100 microseconds, 0.066 Hz) were delivered through a bipolar nichrome electrode positioned in the CA1 stratum radiatum to stimulate Schaffer collateral/commissural fibers. Evoked extracellular field excitatory postsynaptic potentials (fEPSPs) were recorded with glass microelectrodes (2 to 10 MΩ, Harvard Apparatus, Edenbridge, UK) filled with 150 mmol/L NaCl and placed in the stratum radiatum of the CA1 hippocampal region. Responses were amplified (BM 622, Mangoni, Pisa, Italy), digitized (sample rate, 33.33 kHz), and stored for later analysis with LTP (version 2.30D) program (University of Bristol, Bristol, UK). After the responses were stabilized, a baseline input–output curve was constructed. Stimulation strength was set to elicit a fEPSP of half-maximal slope. Field excitatory postsynaptic potential slope was routinely measured and expressed as the percentage of the average slope of responses measured during the 5 minutes preceding any treatment.
Single Unit Recordings of Spontaneous Activity from Preoptic Area Neurons
Coronal sections of 300 μmol/L thickness were obtained from the preoptic area (POA) of adult CD1 mice. Mice were anesthetized with isoflurane and decapitated. The brains were sliced in ice-cold, oxygenated (95% O2+5% CO2) artificial cerebral spinal solution (aCSF) containing (in mmol/L) 130 NaCl, 3.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 10 glucose, 2 CaCl2, and 2 MgCl2. The anterior commissure was used as an anatomical marker of the region of interest. Slices were allowed to recover in the same solution kept at 37°C with constant oxygenation. Slices were then transferred to a recording chamber mounted on an upright microscope (Nikon Eclipse E600 FN, Tokyo, Japan) equipped with an infrared camera (Hamamatsu) for visually guided experiments. Flow rate of oxygenated aCSF in the recording chamber was 1 mL/min. Bath temperature was controlled within the desired range (32°C to 39°C) with a TC344B temperature controller (Warner, Hamden, CT, USA). Pipettes were filled with aCSF and had a resistance of 3 to 4 MΩ. Recordings were made in cell-attached configuration (RSEAL below 100 MΩ) in voltage clamp mode (VHOLD=0). Signals were sampled at 20 KHz and low-pass filtered at 10 KHz, acquired with an Axon Multiclamp 700B and digitized with a Digidata 1440A and Clampex 10 (Axon, Molecular Devices, Sunnyvale, CA, USA). Trace and statistical analysis were made with Clampfit 10 (Axon) and Origin 8.1 (Microcal, Northampton, MA, USA). All drugs were bath applied and diluted × 1,000.
Oxygen Consumption Rate
Mice treated or not with AMP or AMPCP were immediately placed in a closed respirometer (Columbus Instruments, Columbus, OH, USA) and oxygen consumption measured for 20 minutes.11
Data Analysis
Data were analyzed using WinLTP 1.11 reanalysis program and the software package Gℛ𝒜𝒫ℋP𝒜𝒟 Pℛℐ𝒮ℳ (version 4.0; GraphPad Software, San Diego, CA, USA). All numerical data are expressed as mean±s.e.m. Statistical significance was evaluated using paired two-tailed Student's t-test or one-way analysis of variance plus Tukey's post hoc test. Differences were considered significant at P<0.05.
Results
Pharmacological Modulation of Adenosine 5′-Monophosphate-Dependent Hypothermia
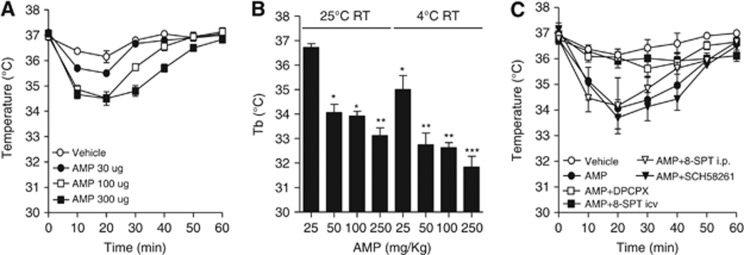
We first attempted to understand whether AMP-dependent hypothermia is due to a central or peripheral action of the nucleotide. To this aim, AMP was injected i.c.v. (30 to 300 μg) and Tb was reduced transiently and dose dependently (Figure 1A). Higher doses of AMP (25 to 250 mg/kg) were necessary to trigger hypothermia when the nucleotide was injected intraperitoneally, and the extent of the hypothermic effect was higher when RT decreased from 25°C to 4°C (Figure 1B). Blood parameters were also not affected by AMP, except for mild hyperglycemia (Table 1). At doses comprised between 50 and 250 mg/kg, no impairment of locomotor activity and only mild sedation occurred (not shown). Because extracellular AMP is rapidly converted into Ado,15 we next investigated whether purinergic receptor activation underlined AMP-dependent hypothermia. We found that cooling was almost completely prevented by the A1R antagonist 8-Cyclopentyl-t,3-dipropylxanthine (DPCPX) given intraperitoneally. The blood–brain barrier-impermeant A1R antagonist 8-sulfophenyl theophylline also prevented hypothermia but only when injected i.c.v. Conversely, the A2AR blocker SCH-58261 had no effects (Figure 1C). The A1/P2Y1 receptor agonist 2-methylthio-ADP had no cooling effects, ruling out an involvement of the dimeric receptor16 (not shown). A role of the A3R was also excluded because the receptor is insensitive to DPCPX antagonism.17 However, even though these findings indicated an exclusive involvement of brain A1R, to our surprise we found that Ado injected intraperitoneally or i.c.v. was less hypothermic than AMP (Figures 2A and 2B). This might be due to the prompt uptake of Ado by equilibrative nucleoside transporters (ENTs).18 In keeping with this interpretation, both dipiridamole (nonspecific ENT blocker) and nitrobenzylthioinosine (specific ENT1 blocker) increased the hypothermic effect of Ado (Figure 2C).
Figure 1.
Effects of adenosine 5′-monophosphate (AMP) and purine receptor-modulating drugs on body temperature (Tb) in mice. (A) Effects of i.c.v. injections of AMP on Tb (single i.c.v. injection at T=0, at least eight mice per group were injected). (B) Effects of room temperature (RT) on maximal Tb loss (T=20 minutes) induced by intraperitoneal injections of AMP. (C) Effects of the A1R antagonists 8-Cyclopentyl-t,3-dipropylxanthine (DPCPX) (0.1 mg/kg intraperitoneally) and 8-sulfophenyl theophylline (50 μg i.c.v., or 10 mg/kg intraperitoneally) as well as the A2AR blocker SCH-58261 (50 μg i.c.v.) on Tb loss induced by AMP (50 mg/kg intraperitoneally). Antagonists were injected 10 minutes before AMP. Drugs were injected at T=0. For all graphs, each point/column represents the mean±s.e.m., at least eight animals per group were used. (B) *P<0.05, **P<0.01, ***P<0.001 versus control Tb, analysis of variance (ANOVA) plus Tukey's post hoc test.
Table 1. Effects of AMP (50 mg/kg i.p.) on different mouse blood parameters.
| Control | AMP | |
|---|---|---|
| Blood pH | 7.21±0.02 | 7.15±0.07 |
| Blood pCO2 (mm Hg) | 43.24±3.56 | 52.17±7.3 |
| Blood pO2 (mm Hg) | 136.26±8.2 | 131.32±5.1 |
| Blood glucose (mg/dL) | 194.04±7.5 | 233.17±12.7* |
| Blood electrolytes (meq/L) | ||
| K+ | 4.44±0.07 | 5.08±0.76 |
| Na+ | 142.4±0.75 | 142.17±1.19 |
| Ca2+ | 5.16±0.06 | 5.23±0.1 |
| Cl− | 121±1.22 | 122.83±1.19 |
Abbreviations: AMP, adenosine 5′-monophosphate; i.p., intraperitoneally.
Values represent the mean±s.e.m. of blood samples taken from six different mice 20 minutes after AMP injection. *P<0.05 versus Control, Student's t-test.
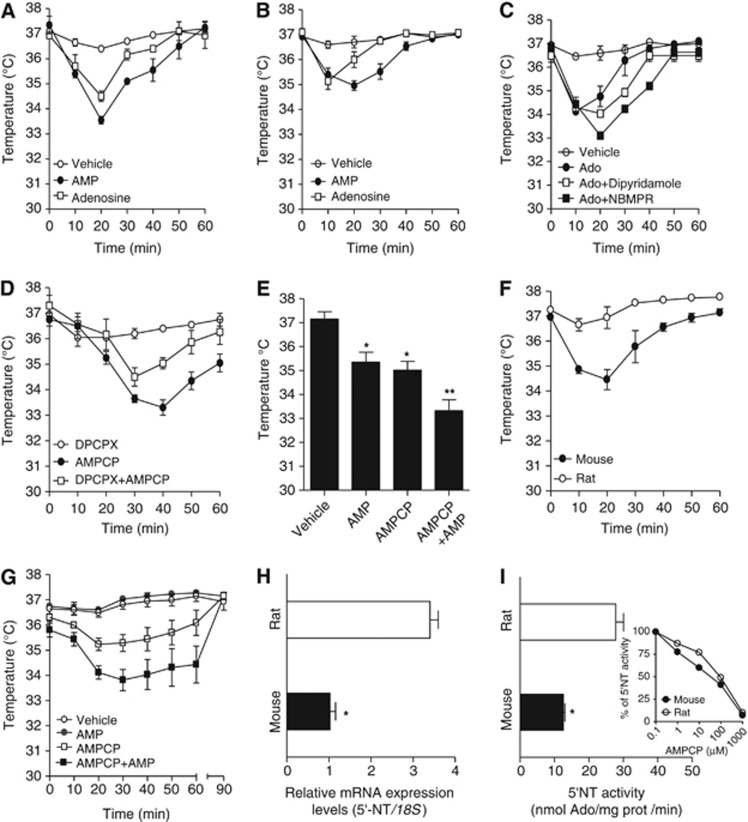
Figure 2.
Effects of adenosine 5′-monophosphate (AMP) and Ado on body temperature (Tb) in mice and rats. Comparison of the hypothermic effects of adenosine and AMP injected at 50 mg/kg intraperitoneally. (A) or 30 μg i.c.v. (B) in mice. (C) Effect of specific (nitrobenzylthioinosine, NBMPR, 50 μg i.c.v.) and nonspecific (dipiridamole 10 mg/kg intraperitoneally) nucleoside transporter inhibitor on hypothermia induced by Ado 50 μg i.c.v. (D) The A1R antagonists 8-Cyclopentyl-t,3-dipropylxanthine (DPCPX) (0.1 mg/kg intraperitoneally) counteracts the effects of the 5′-NT inhibitor AMPCP (50 μg i.c.v.) on Tb in mice. (E) Summation effect of AMPCP (50 μg i.c.v.) and AMP (50 mg/kg intraperitoneally) on Tb loss in mice. (F) Comparison of the hypothermic effect of AMP (50 mg/kg intraperitoneally) in mice and rats. (G) Effects of AMPCP (50 μg i.c.v.), AMP (50 mg/kg intraperitoneally) or a combination of both on Tb in rats. (H) Transcript levels of 5′-NT within the rat and mouse hypothalamus revealed by reverse transcriptase polymerase chain reaction (RT–PCR). (I) 5′-NT activity in the rat or mouse hypothalamus. The activity is fully inhibited by addition of AMPCP to the enzymatic assay (I inset). (A–G) Each point/column represents the mean±s.e.m., at least eight animals per group were used. (H, I) Each column represents the mean±s.e.m. of three experiments conducted in duplicate. *P<0.05, **P<0.01 versus vehicle (E) or rat (H, I). (E) analysis of variance (ANOVA) plus Tukey's post hoc test (H, I) Student's t-test.
We next tested the effect of methylene-ADP (AMPCP), a classic inhibitor of 5′-NT (the plasma membrane enzyme responsible for extracellular conversion of AMP to Ado).15, 19 Interestingly, we found that AMPCP i.c.v. reduced Tb in mice, and intraperitoneal injection of DPCPX counteracted cooling by AMPCP i.c.v. (Figure 2D). We found that the slight reduction of Tb induced by DPCPX (Figure 2D) was due to DMSO used to dissolve the drug (not shown). When AMPCP and AMP were concomitantly injected, a summation effect was found (Figure 2E). Taken together, these findings suggest that both exogenous and endogenous AMP prompt hypothermia through direct A1R activation within the mouse brain. Interestingly, we noticed that rats were less sensitive to the hypothermic effect of AMP (Figure 2F). AMPCP, however, reduced Tb in rats, and rendered them sensitive to the cooling effects of AMP (Figure 2G). This suggested that the reduced sensitivity of rats to AMP was due to their ability to readily degrade AMP to Ado. We therefore compared the expression level of 5′-NT in the mouse and rat hypothalamus. In keeping with low sensitivity of rats to AMP, both transcript levels and enzymatic activity of 5′-NT were higher in rat than mouse hypothalamus (Figures 2H and I). As a whole, these findings suggest that h5′-NT has a key role in central thermoregulation by modulating extracellular [AMP]/[Ado] within the hypothalamus. Data also indicate that hypothalamic 5′-NT activity dictates the sensitivity of a given species to the hypothermic effect of AMP, and give explanation of why high doses of the nucleotide were necessary to induce hypothermia in rats.10
Adenosine 5′-Monophosphate Decreases Excitatory Synaptic Activity in a A1R-Dependent Manner
To corroborate the unexpected findings that exogenous AMP or AMP accumulation due to 5′-NT inhibition directly activate A1R within the rodent brain, we next analyzed the nucleotide's effects in the hippocampal CA1 region, a well-characterized circuitry in which A1R reduce excitatory synaptic activity.20 We found that AMP reduced fEPSP slope in both mouse and rat hippocampal slices in a DPCPX-sensitive manner (Figures 3A–C). Consistent with data obtained in vivo, AMPCP potentiated AMP-dependent inhibition of neurotransmission in slices from mice (Figures 3D and 3F) and rats (Figures 3G and 3I), indicating active degradation of exogenous AMP by 5′-NT. Of note, AMPCP alone sufficed to reduce fEPSP in rat slices (Figures 3G and 3H), having no effects in those of mice (Figures 3D and 3E). This finding, along with evidence that 5-NT transcript levels were almost twofold higher in rat than mouse hippocampus (Figure 3J) further corroborate the hypothesis that extracellular conversion of AMP to Ado by 5′-NT is more active in rat than mouse brain. Data suggest that increased concentrations of AMP within the brain extracellular space leads to a degree of A1R activation higher than that induced by a similar increase of Ado. This is consistent with the notion that AMP, at variance with Ado, is not uptaken by nucleoside transporters.18, 21
Figure 3.
Effect of adenosine 5′-monophosphate (AMP) on hippocampal neuronal activity. (A) Reprentative plot of the effect of AMP alone or in combination with 8-Cyclopentyl-t,3-dipropylxanthine (DPCPX) on field excitatory postsynaptic potential (fEPSP) slope recorded in the CA1 region of mouse hippocampal slices. The effect of DPCPX alone on fEPSP reveals a tonic A1R-dependent inhibitory tone in the hippocampal slice. Representative fEPSP traces taken under the different treatments are shown above the plot. Scale bars: 10 milliseconds, 0.5 mV. Quantitation of the effect of 25 μmol/L AMP alone or in the presence of 100 nmol/L DPCPX on fEPSP in mouse (B) or rat (C) CA1 region of hippocampal slices. (D) Reduction of fEPSP slope in mouse CA1 hippocampal slices induced by 25 μmol/L AMP is potentiated by 25 μmol/L AMPCP and inhibited by 100 μmol/L DPCPX. Quantitation of the effect of 25 μmol/L AMPCP alone (E), or in the presence of 25 μmol/L AMP (F) on fEPSP in mouse hippocampal slices. (G) Reduction of fEPSP slope in rat CA1 hippocampal slices induced by 25 μmol/L AMP is potentiated by 25 μmol/L AMPCP and inhibited by 100 μmol/L DPCPX. Quantitation of the effect of 25 μmol/L AMPCP alone (H) or in combination with 25 μmol/L AMP (I) on fEPSP in rat hippocampal slices. Note that AMPCP alone suffices to reduce fEPSP in rat (H) but not in mouse (E) slices. (J) Reverse transcriptase polymerase chain reaction (RT–PCR) evaluation of transcript levels for 5′-NT in the hippocampus of mice and rats. fEPSP plots representative of 5 (A, D) or 4 (G) independent experiments. Each column represents the mean±s.e.m. of recordings from 5 (B, C, E, F) or 4 (H, I) hippocampal slices. *P<0.05, **P<0.01, versus first AMP application (B, C, F, I), control (H), or rat (J). Student's t-test was adopted for the statistical analysis.
Adenosine 5′-Monophosphate Reduces Activity of Spontaneously Firing Hypothalamic Neurons
We next investigated whether AMP directly affects hypothalamic neurons involved in thermoregulation. Several lines of evidence suggest that within the hypothalamic POA two spontaneously firing neuronal populations, namely warm-sensitive and temperature-insensitive neurons (WSN and TIN, respectively), are key effectors of hypothalamic thermoregulation.22, 23 In the rat, TIN account for ∼70% of POA neurons and behave like a Tb pace-maker, dictating the thermoregulatory set point. Conversely, WSN are less represented (∼20% of POA neurons) and are thought to be involved in the heat loss response.22, 23 By means of single-cell electrophysiological recording from the POA of acute hypothalamic mouse slices, we confirmed the existence of spontaneously active neurons. To identify the possible presence of TIN and WSN, we evaluated whether firing activity changed when bath temperature raised from 32°C to 37°C. We found that firing rate displayed low sensitivity to temperature increase in 86% of cells (TIN), whereas a population of cells corresponding to 14% showed high intrinsic warm sensitivity (WSN) (Figures 4A and 4B). Evidence that at 37°C firing activity of WSN was twofold than that of TIN (consistent with data obtained in rats)22 further indicated the distinct nature of these two neuronal populations. To our knowledge, this is the first evidence that WSN and TIN exist in the mouse hypothalamus. Remarkably, AMP added to the bath solution promptly reduced spontaneous firing rate of both TIN and WSN (Figures 4C–F). This effect was blocked by DPCPX (Figure 4G), indicating the involvement of A1R. These findings, along those obtained in vivo, suggest that AMP prompts hypothermia by directly acting within the POA. We also reason that suppression of TIN firing activity by AMP can well be the mechanisms underlying the cooling effect of the nucleotide. Indeed, TIN activity dictates the hypothalamic thermoregulatory set point,22, 23 and reduction of their firing frequency leads to a resetting to lower temperatures of their reference signal. Upon retuning the hypothalamic set point to lower temperatures, a series of autonomic responses are set into motion, including a reduction of basal metabolism and oxygen consumption. In keeping with this, and with the ability of AMP to reset hypothalamic thermogenesis, we found that both AMP and AMPCP reduced oxygen consumption in mice (Figure 4H).
Figure 4.
Effect of adenosine 5′-monophosphate (AMP) on hypothalamic neuronal activity, as well as oxygen consumption and body temperature (Tb) loss response in mice. Effect of bath temperature on spontaneous firing frequency of warm-sensitive and temperature-insensitive neurons (WSN and TIN) of hypothalamic mouse slices. (A) Representative traces of the spontaneous activity at 32°C and 37°C are shown. (B) Thermal sensitivity (expressed as Hz/°C) of WSN and TIN when bath temperature increases from 32°C to 37°C. (C) Representative traces of the spontaneous firing activity of a TIN or WSN kept at 37°C under control conditions or in the presence of AMP 50 μmol/L. Representative frequency plots showing the effect of AMP 50 μmol/L on spontaneous firing activity of a TIN (D) or WSN (E) kept at 37°C. (F) Effect of AMP 50 μmol/L on spontaneous firing frequency of TIN and WSN of the mouse hypothalamic preoptic area (POA). *P<0.05 versus control. (G) Representative frequency plot showing the effect of AMP 50 μmol/L or AMP 50 μmol/L+8-Cyclopentyl-t,3-dipropylxanthine (DPCPX) 1 μmol/L on spontaneous firing activity of WSN kept at 37°C. (H) Time course of the effects of AMP (50 mg/kg intraperitoneally) or AMPCP (50 μg i.c.v.) on oxygen consumption in mice. Total amount of oxygen consumed in 20 minutes by the three animal groups is shown in the inset. (I) Effect of AMP (50 mg/kg intraperitoneally) on the heat loss response of mice exposed at T=0 to a RT (room temperature) of 40°C. (A, C) Representative traces of eight cells per group. (B) Each point represents the mean±s.e.m. of five different cells. ***P<0.001 (paired t-test). (D, E) Representative plots of five cells per group. (F) Each column represents the mean±s.e.m. of recordings from eight cells per group. (H) Each point or column (inset) represents the mean±s.e.m. of four animals per group. (I) Each point represents the mean±s.e.m. of six mice per group. (F) Student's t-test or (H, inset) analysis of variance (ANOVA) plus Tukey's post hoc test.
The negative impact of AMP on firing activity of WSN, however, is at odds with the nucleotide's hypothermic effect. Indeed, according to a proposed model of hypothalamic thermoregulation,22 reduction of WSN firing activity should promote heat retention and contrast heat loss. Thus, to better understand whether WSN inhibition by AMP in vitro had a functional correlate in vivo, we next investigated if the WSN-dependent heat loss response was impaired in AMP-challenged mice. To this end, we exposed mice to RT of 40°C and analyzed their thermoregulatory response. In principle, blockade of the WSN-mediated heat loss response by AMP should impair the animals' ability to avoid hyperthermia. When exposed to 40°C, control mice started panting and showed perioral saliva spreading for evaporative cooling (not shown), thereby limiting Tb increase to 38°C±0.7°C. Of note, as established by two blind evaluators, comparable panting and perioral saliva spreading occurred in AMP-injected mice (not shown). Accordingly, mice injected with AMP underwent Tb increase similar to those receiving vehicle (Figure 4I). Findings therefore suggested that AMP-dependent inhibition of WSN does not compromise the heat loss response in mice, in keeping with the hypothermic effects of the nucleotide.
Hypothermia Induced by Adenosine 5′-Monophosphate Reduces the Febrile Response but not MDMA-Dependent Hyperthermia
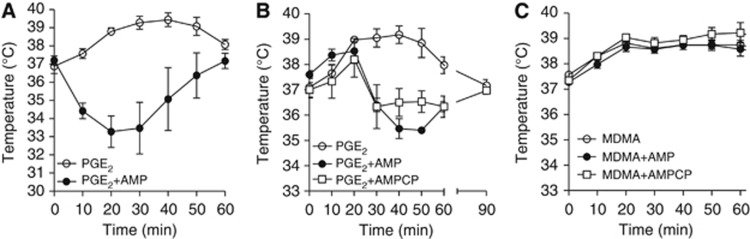
To investigate whether the impact of AMP on hypothalamic thermoregulation could be harnessed for therapeutic purposes, we next tested the nucleotide's effect in conditions of increased Tb such as fever (as a model of central hyperthermia) and dioxymetamphetamine (MDMA) overdose (a form of peripheral hyperthermia). We found that AMP both prevented (Figure 5A) and aborted (Figure 5B) prostaglandin E2 (PGE2)-induced fever in mice. Adenosine 5′-monophosphate, however, had no effect on hyperthermia prompted by MDMA (Figure 5C). Given that MDMA causes hyperthermia through mechanisms unrelated to hypothalamic resetting,24 the latter finding further supports the hypothesis that A1R activation within the hypothalamus is the sole mechanisms responsible for the cooling effects of AMP.
Figure 5.
Effect of adenosine 5′-monophosphate (AMP) on fever and 3,4-methylenedioxymetamphetamine (MDMA)-induced hyperthermia in mice. (A) Effects of AMP on PGE2-induced fever in mice. Animals received AMP (50 mg/kg intraperitoneally) and 3 minutes later an i.c.v. injection of PGE2 (10 nmol). (B) Effects of AMP (50 mg/kg intraperitoneally) or AMPCP (50 μg i.c.v.) on fever induced in mice by PGE2 (10 nmol i.c.v.). AMP and AMPCP were injected 20 minutes after PGE2. (C) Effects of AMP (50 mg/kg intraperitoneally) or AMPCP (50 μg i.c.v.) on hyperthermia induced by MDMA (10 mg/kg intraperitoneally). Each point represents the mean±s.e.m. of eight animals per group.
Discussion
The present study provides the first evidence that the endogenous nucleotide AMP prompts Tb cooling by activating A1R within the hypothalamus. This is in keeping with expression of A1R by hypothalamic neurons.25, 26 Findings also indicate that 5′-NT is a key determinant of A1R-dependent thermoregulation. 5′-NT catalyzes a key step in extracellular Ado formation from ATP, thereby playing a central role in P1 purinergic receptor activation.15 In this perspective, it has been proposed that genetic or pharmacological manipulation of 5′-NT activity is a suitable strategy to modulate Ado neurotransmission.27 Very recent findings are partially at odds with this scenario by showing that exit of intracellular Ado through ENTs of neurons, and not extracellular conversion of AMP into Ado, is the mechanism of extracellular Ado accumulation in conditions of excessive firing.19 However, our results demonstrate that under physiological conditions inhibition of brain 5′-NT potentiates A1R signaling, thereby indicating that the enzyme actively participates to homeostatic modulation of purinergic neurotransmission. We reason that potentiation of A1R activation by 5′-NT inhibition is due to accumulation of extraneuronal AMP which, by itself, is able to activate A1R but is not uptaken by ENTs, a feature that prolongs A1R activation. Conversely, Ado, which shows A1R activating efficacy similar to AMP,28 is rapidly uptaken by ENTs,29 thereby triggering reduced activation of A1R compared with the nucleotide. In keeping with this, we found that hypothermia prompted by i.c.v. injections of AMP lasts longer than that induced by an equal dose of Ado injected through the same routes, and, strikingly, was potentiated by 5′-NT inhibition. Also, the fact that ENT inhibitors potentiate the cooling effects of Ado, is in keeping with the hypothesis that the nucleoside exerts weaker hypothermic effects because of rapid intracellular uptake. Taken together, our findings indicate that within specific brain regions 5′-NT is a negative, rather than positive,27 regulator of A1R-dependent neurotransmission. On this basis, extracellular AMP should be considered a bona fide purinergic neuromodulator selectively acting on A1R, rather than a mere metabolic precursor of Ado. Very recent data obtained in transfected tumor cells indicate that AMP is a specific ligand of A1R.28 Remarkably, 5′-NT appears the only enzyme responsible for extracellular degradation of AMP into Ado,19 and its expression levels are quite low in most areas of the brain.30 These findings suggest that the enzyme is constitutively limiting rate of Ado formation from AMP, a condition that may favor extraneuronal accumulation of the nucleotide, and ensuing A1R activation within specific brain regions.
Data on the effects of AMP on hypothalamic neurotransmission add important new information to mechanisms of central thermogenesis. For the first time we describe the key role of A1R in reducing firing activity of TIN, thereby furthering our understanding of the hypothalamic neurochemistry regulating Tb. Evidence that inhibition of firing activity of WSN in vitro correlates with cooling in vivo, is also of importance to reevaluate the role of this neuronal population in the heat loss response. In this regard, our findings allow to identify a functional, hierarchical order between TIN and WSN. Data suggest that suppression of TIN activity suffices to prompt heat loss, irrespective of whether firing of WSN is concomitantly reduced (a condition that should lead to heat retention). This can be ascribed, at least in part, to the fact that TIN considerably outnumber WSN (86% versus 14%, respectively, see above). Consequently, entity of the heat loss response due to reduction of TIN activity can mask heat retention signals originating from inhibition of WSN.
The antipyretic effects of AMP are also in keeping with a model in which TIN play a dominant role in hypothalamic thermoregulation. Indeed, AMP efficiently counteracted fever notwithstanding its negative impact on WSN that, by itself, would have facilitated PGE2-dependent WSN inhibition and the ensuing pyretic response. Notably, all the available antipyretics, such as nonsteroidal antiinflammatory drugs or corticosteroids, are thought to relieve fever by counteracting PGE2-dependent WSN inhibition.22, 23 In this light, the antipyretic effect of AMP provides the first pharmacological demonstration that resetting the reference signal originating from TIN is an alternative, efficient strategy to counteract the pyretic response. This notion points to the A1R of TIN as a new, druggable target for febrile disorders, and has potential implications in developing new strategies for treating nonsteroidal antiinflammatory drug-insensitive stubborn fevers. Also, data showing that AMP significantly reduces excitatory hippocampal neurotransmission widens the neurochemical properties of the nucleotide. Endogenous formation of AMP in the synaptic cleft might therefore play unexpected roles in purinergic neurotransmission and contribute to A1R-dependent regulation of neuronal (dys)functions such as memory and pain, as well as seizures and neurodegeneration. We also reason that deregulation of AMP-dependent A1R activation might concur to neurological impairment associated with inborn errors of purine metabolism.31
In conclusion, the present study discloses the role of AMP and 5′-NT in central thermoregulation via hypothalamic A1R activation. It also indicates that AMP-dependent hypothermia can be harnessed to treatment of patients with febrile illness. It might be worth investigating human responses to intravenous AMP injections.
Acknowledgments
The authors thank Professor R Corradetti for providing with MDMA.
The authors declare no conflict of interest.
Footnotes
This work was supported by European Program in Stroke Research and AstraZeneca.
References
- Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13:267–278. doi: 10.1038/nrn3174. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Hemmen TM. Therapeutic hypothermia for brain ischemia: where have we come and where do we go. Stroke. 2010;41:S72–S74. doi: 10.1161/STROKEAHA.110.595371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PS. Hypothermia: a systematic review and meta-analysis of clinical trials. Semin Fetal Neonatal Med. 2010;15:238–246. doi: 10.1016/j.siny.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Diller KR, Zhu L. Hypothermia therapy for brain injury. Annu Rev Biomed Eng. 2009;11:135–162. doi: 10.1146/annurev-bioeng-061008-124908. [DOI] [PubMed] [Google Scholar]
- Testori C, Sterz F, Behringer W, Spiel A, Firbas C, Jilma B. Surface cooling for induction of mild hypothermia in conscious healthy volunteers - a feasibility trial. Crit Care. 2011;15:R248. doi: 10.1186/cc10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346:1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Macleod MR, Kollmar R. Therapeutic hypothermia for acute ischemic stroke: ready to start large randomized trials. J Cereb Blood Flow Metab. 2010;30:1079–1093. doi: 10.1038/jcbfm.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC. Is human hibernation possible. Annu Rev Med. 2008;59:177–186. doi: 10.1146/annurev.med.59.061506.110403. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kaasik K, Blackburn MR, Lee CC. Constant darkness is a circadian metabolic signal in mammals. Nature. 2006;439:340–343. doi: 10.1038/nature04368. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang S, Luo Y, Ji X, Nemoto EM, Chen J. When hypothermia meets hypotension and hyperglycemia: the diverse effects of adenosine 5′-monophosphate on cerebral ischemia in rats. J Cereb Blood Flow Metab. 2009;29:1022–1034. doi: 10.1038/jcbfm.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzi M, Felici R, Cavone L, Gerace E, Minassi A, Appendino G, et al. Ischemic neuroprotection by TRPV1 receptor-induced hypothermia. J Cereb Blood Flow Metab. 2012;32:978–982. doi: 10.1038/jcbfm.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco G, Pittelli M, Cavone L, Fossati S, Porcu M, Mascagni P, et al. Histone deacetylase (HDAC) inhibitors reduce the glial inflammatory response in vitro and in vivo. Neurobiol Dis. 2009;36:269–279. doi: 10.1016/j.nbd.2009.07.019. [DOI] [PubMed] [Google Scholar]
- Latini S, Bordoni F, Pedata F, Corradetti R. Extracellular adenosine concentrations during in vitro ischaemia in rat hippocampal slices. Br J Pharmacol. 1999;127:729–739. doi: 10.1038/sj.bjp.0702591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppi E, Pugliese AM, Stephan H, Muller CE, Pedata F. Role of P2 purinergic receptors in synaptic transmission under normoxic and ischaemic conditions in the CA1 region of rat hippocampal slices. Purinergic Signal. 2007;3:203–219. doi: 10.1007/s11302-006-9049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73) Purinergic Signal. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata H, Yoshioka K, Kamiya T, Tsuga H, Oyanagi K. Functions of heteromeric association between adenosine and P2Y receptors. J Mol Neurosci. 2005;26:233–238. doi: 10.1385/JMN:26:2-3:233. [DOI] [PubMed] [Google Scholar]
- Fishman P, Bar-Yehuda S, Liang BT, Jacobson KA. Pharmacological and therapeutic effects of A(3) adenosine receptor agonists. Drug Discov Today. 2012;17:359–366. doi: 10.1016/j.drudis.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber-Ruano I, Pastor-Anglada M. Transport of nucleoside analogs across the plasma membrane: a clue to understanding drug-induced cytotoxicity. Curr Drug Metab. 2009;10:347–358. doi: 10.2174/138920009788499030. [DOI] [PubMed] [Google Scholar]
- Lovatt D, Xu Q, Liu W, Takano T, Smith NA, Schnermann J, et al. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc Natl Acad Sci USA. 2012;109:6265–6270. doi: 10.1073/pnas.1120997109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherington JP, Lambert NA. Differential desensitization of responses mediated by presynaptic and postsynaptic A1 adenosine receptors. J Neurosci. 2002;22:1248–1255. doi: 10.1523/JNEUROSCI.22-04-01248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgorska M, Kocbuch K, Pawelczyk T. Recent advances in studies on biochemical and structural properties of equilibrative and concentrative nucleoside transporters. Acta Biochim Pol. 2005;52:749–758. [PubMed] [Google Scholar]
- Boulant JA. Neuronal basis of Hammel's model for set-point thermoregulation. J Appl Physiol. 2006;100:1347–1354. doi: 10.1152/japplphysiol.01064.2005. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrott AC. MDMA and temperature: A review of the thermal effects of 'Ecstasy' in humans. Drug Alcohol Depend. 2012;121:1–9. doi: 10.1016/j.drugalcdep.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Sagara M, Sekino Y, Shirao T, Honda K, Yoshioka T, et al. The sulphydryl reagent, N-ethylmaleimide, disrupts sleep and blocks A1 adenosine receptor-mediated inhibition of intracellular calcium signaling in the in vitro ventromedial preoptic nucleus. Neuroscience. 2001;106:733–743. doi: 10.1016/s0306-4522(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Winston S, McCarley RW. Orexin neurons of the hypothalamus express adenosine A1 receptors. Brain Res. 2002;944:190–194. doi: 10.1016/s0006-8993(02)02873-1. [DOI] [PubMed] [Google Scholar]
- Zylka MJ. Pain-relieving prospects for adenosine receptors and ectonucleotidases. Trends Mol Med. 2011;17:188–196. doi: 10.1016/j.molmed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittiner JE, Korboukh I, Hull-Ryde EA, Jin J, Janzen WP, Frye SV, et al. AMP is an adenosine A1 receptor agonist. J Biol Chem. 2012;287:5301–5309. doi: 10.1074/jbc.M111.291666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Xiong W, Albensi BC, Parkinson FE. Expression of human equilibrative nucleoside transporter 1 in mouse neurons regulates adenosine levels in physiological and hypoxic-ischemic conditions. J Neurochem. 2011;118:4–11. doi: 10.1111/j.1471-4159.2011.07242.x. [DOI] [PubMed] [Google Scholar]
- Langer D, Hammer K, Koszalka P, Schrader J, Robson S, Zimmermann H. Distribution of ectonucleotidases in the rodent brain revisited. Cell Tissue Res. 2008;334:199–217. doi: 10.1007/s00441-008-0681-x. [DOI] [PubMed] [Google Scholar]
- Camici M, Micheli V, Ipata PL, Tozzi MG. Pediatric neurological syndromes and inborn errors of purine metabolism. Neurochem Int. 2010;56:367–378. doi: 10.1016/j.neuint.2009.12.003. [DOI] [PubMed] [Google Scholar]