Abstract
Coated-platelets are procoagulant platelets observed upon dual-agonist stimulation with collagen and thrombin. Coated-platelet levels are elevated in patients with nonlacunar (large-vessel) ischemic stroke and decreased in patients with spontaneous intracerebral hemorrhage as compared with controls. The purpose of this study was to investigate a possible relationship between coated-platelet levels and stroke recurrence in patients with nonlacunar ischemic stroke. We assayed coated-platelet levels in 190 consecutive patients with nonlacunar stroke who were followed for up to 12 months; 20 subjects experienced recurrent stroke. Subjects were categorized into tertiles of coated-platelet levels. The distributions of time-to-recurrent stroke were estimated for each tertile using cumulative incidence curves and compared statistically using a log-rank test. The cumulative incidence of recurrent stroke at 12 months differed among the coated-platelet tertiles: 2% for the first tertile (lowest coated-platelet levels), 18% for the second tertile, and 17% for the third tertile (overall log-rank test, P=0.019). These data suggest that higher levels of coated-platelets, measured shortly after a nonlacunar stroke, are associated with an increased incidence of stroke recurrence. This observation offers an additional tool for identifying patients at highest risk for stroke recurrence following a nonlacunar (large-vessel) infarct.
Keywords: platelets, recurrence, stroke, thrombosis
Introduction
Coated-platelets are a subpopulation of platelets observed upon dual-agonist stimulation with collagen and thrombin.1, 2 First described in 2000, this subset of activated platelets expresses surface phosphatidylserine, supports a robust prothrombinase activity, and retains high levels of several procoagulant proteins on the cell surface, including factor V, fibrinogen, and von Willebrand factor.1, 2, 3, 4, 5 Because thrombin generation is the central event in coagulation, coated-platelets are considered to be prothrombotic.1 This conclusion is supported by a genetically determined absence of coated-platelets in German shepherd dogs that results in a bleeding diathesis,6 as well as by several observations detailed below concerning coated-platelets in stroke.
In healthy controls without a history of stroke, the coated-platelet subpopulation represents on average 30% of all platelets, although the range observed is quite wide.1, 7 Studies in humans and experimental animals have identified several modifiers of coated-platelet levels, including inflammation and some antiplatelet medications.7, 8, 9
Previous studies in ischemic stroke indicated that mean coated-platelet levels are elevated in patients with nonlacunar (large-vessel) ischemic stroke compared with either controls without a history of stroke or patients with lacunar (small-vessel) ischemic stroke.10 Furthermore, the presence of early hemorrhagic transformation in patients with nonlacunar ischemic stroke was associated with lower coated-platelet levels.11 In contrast to the findings in ischemic stroke, we noted that patients with spontaneous intracerebral hemorrhage had significantly lower coated-platelet levels shortly after the hemorrhagic event compared with normal controls and that these levels inversely correlated with the size of the bleed.12, 13
Because of the wide range of coated-platelet levels observed within the nonlacunar stroke population,10, 11 we decided to investigate a possible relationship between coated-platelet levels and stroke recurrence in patients with nonlacunar ischemic stroke. We hypothesized that higher levels of coated-platelets measured at the time of the initial nonlacunar ischemic stroke would be associated with stroke recurrence.
Materials and methods
Subjects
The study was approved by the Institutional Review Board of the University of Oklahoma Health Sciences Center (OUHSC). Individual informed consent was obtained for all study subjects in accordance with the OUHSC and the Oklahoma City Veterans Administration Medical Center (VAMC) rules and regulations. The study was carried out in accordance with the Helsinki Declaration of 1975 (and as revised in 1983).
One hundred and ninety consecutive, eligible patients with a diagnosis of nonlacunar ischemic stroke based on TOAST criteria14 were recruited from the Neurology service at the OUHSC and the VAMC between 15 October 2007 and 15 January 2011. During this time interval, a total of 374 patients were admitted with diagnosis of nonlacunar ischemic stroke based on TOAST criteria. Of these patients, 92 declined to participate. Of the remaining 282 patients, 190 (67%) were recruited for our study. The main factors precluding recruitment were >96 hours between the actual onset of the symptoms and enrollment and administration of thrombolytic therapy or anticoagulation for the current stroke before collection of blood for determining coated-platelet levels.
Exclusion criteria included administration of anticoagulants or thrombolytics for the current stroke before enrollment, prolonged coagulation tests (prothrombin time, partial thromboplastin time and international normalized ratio) on admission, >96 hours between the onset of the symptoms and enrollment, imaging evidence of small-vessel (lacunar) stroke as defined by TOAST criteria,14 prior dementia or evidence of primary intracerebral hemorrhage or tumor. These exclusion criteria were prompted by the potentially confounding effects of heparin and thrombolytics on coated-platelet measurements, the observation that changes in coated-platelet production in animals after physiological manipulation require a minimum of 4 days to manifest,1 and previously published coated-platelet abnormalities in Alzheimer disease and intracerebral hemorrhage.12, 13, 15, 16
All patients were diagnosed with nonlacunar ischemic stroke by a board certified neurologist and underwent initial brain CT (computed tomography) scan followed by brain MRI (magnetic resonance imaging) studies, or repeat brain CT (if MRI was contraindicated) within 12 to 24 hours to confirm the presence of cerebral ischemia.17 The diagnosis of nonlacunar ischemic stroke was based on clinical and imaging data and published criteria.14 Additional investigations included carotid ultrasound studies and echocardiogram/ECG, along with routine laboratory studies including coagulation studies and complete blood count, serum chemistry, troponin, and creatine kinase levels. The neuroradiologist reading the imaging studies and the neurologist establishing the diagnosis were not aware of the coated-platelet measurements.
Smoking status,7 gender, race, age, National Institutes of Health Stroke Scale score, history of atrial fibrillation, hypertension, hypercholesterolemia, and large artery disease, and use of medications that may influence coated-platelet levels, such as SSRI (selective serotonin reuptake inhibitors), HMG-CoA reductase inhibitors (statins) or antiplatelet medications,1, 7, 8 were recorded at the time of enrollment for each patient and reflected prehospitalization status and medication use. The use of medications that may influence coated-platelet levels (listed above), and additional treatments, such as anticoagulation or surgical/endovascular treatment for stroke prevention were recorded at the time of discharge. Additional information recorded included a history of diabetes or end stage renal disease as previous research has noted abnormal coated-platelet levels in these conditions,9, 18 and cardioembolism as the source of stroke due to potential differences in recurrence rates between cardioembolic and noncardioembolic strokes.19, 20, 21
Stroke recurrence was defined as a new neurologic deficit with sudden onset occurring after study enrollment and >24 hours after the initial diagnosis of stroke with symptoms lasting >24 hours and not due to edema, mass effect, brain shift syndrome, hemorrhagic transformation, or secondary to a procedure.19, 20, 22, 23 The diagnosis of recurrent ischemic stroke was established by a neurologist. Repeat brain MRI and CT studies were performed and this information was recorded in the medical chart. All stroke recurrence data were obtained through review of medical records at the time of discharge, progress notes from the outpatient stroke clinic visits scheduled at ∼1, 3, 6, 9, and 12 months following the initial infarct, notes from the primary care provider visits and medical records from outside facilities, if the patient was admitted to another medical facility for recurrent medical problems. In addition, telephone contact was maintained with patients and/or caregivers at 3, 6, 9, and 12 months as an additional source of information regarding stroke recurrence and to minimize loss to follow-up.
Coated-Platelet Assay
After obtaining informed consent, 5 mL of blood was drawn into a plastic syringe containing 0.5 mL of acid citrate dextrose, and PRP (platelet-rich plasma) was prepared as previously described.10 Coated-platelets were assayed10 with 1 μL of PRP in a 100-μL assay with the following reagents (final concentrations): 1.0 μg/mL biotin-fibrinogen, 0.4 mmol/L gly-pro-arg-pro-amide, 500 ng/mL convulxin, 0.5 U/mL bovine thrombin, 2 mmol/L CaCl2, 1 mmol/L MgCl2, 150 mmol/L NaCl, and 10 mmol/L N-(2-hydroxyethyl)-piperazine-N′-(4-butanesulfonic acid) (HEPES), pH 7.5. After 5 minutes at 37°, 0.8 μg of phycoerythrin-streptavidin and 0.5 μg of FITC-abciximab were added. After an additional 5 minutes at 37°, the reaction was stopped with 0.2 mL of 1.5% (w/v) formalin in 150 mmol/L NaCl, 10 mmol/L HEPES, pH 7.5. The percentage of abciximab-positive events (platelets) with bound biotin-fibrinogen was quantitated by flow cytometry. Results are reported as percent of cells converted to coated-platelets. Individuals performing the coated-platelet assay were not aware of the clinical diagnosis corresponding to the blood sample analyzed.
Statistical Analyses
Data were analyzed using SAS (SAS System for Windows, ver. 9.1, SAS Institute, Cary, NC, USA) and SPSS (SPSS for Windows, rel. 15.0, SPSS, Chicago, IL, USA). Descriptive statistics were used to summarize the distribution of baseline patient and clinical characteristics. Subjects were grouped into tertiles of the observed coated-platelet level distribution. The categorization approach was defined a priori to allow for nonlinear associations between coated-platelet level and the risk of stroke recurrence. The distributions of baseline characteristics were compared among the three coated-platelet groups using analysis of variance for continuous measures or a χ2 test (or Fisher's exact test for low expected cell counts) for categorical measures. A Pearson correlation coefficient was calculated to quantify the strength of the linear association between coated-platelet levels and the time elapsed from the onset of symptoms to the time of the blood draw.
Subjects were recruited over a 39-month period (15 October 2007 to 15 January 2011) and analyses were based on follow-up data gathered as of 1 May 2011. Maximum follow-up for a given subject was 12 months. The median length of follow-up was compared among groups using a Kruskal–Wallis test. Distributions of time to recurrent stroke were estimated for subgroups of subjects using cumulative incidence curves, which were calculated using the Kaplan–Meier method, and compared using a log-rank test, where the size of the asymptotic test is expected to be correct given the equal sizes (n=60) in each coated-platelet tertile.24 Stroke recurrence and death were considered as separate events. The time to recurrence was censored at the 12-month follow-up visit or the end of study follow-up, whichever came first, for subjects who did not experience a stroke recurrence during follow-up. In addition, the time to recurrence was censored at the time of death for subjects who died before experiencing a stroke recurrence. A two-sided, Bonferroni-adjusted α level of 0.0167 was used when making all pairwise comparisons among the coated-platelet tertile groups, but was otherwise set at a two-sided 0.05 level. Univariate and multivariate regression models adjusting for confounding factors were not fit given the small number of observed events (recurrent stroke) in the lowest tertile.25
A total sample size of 180 subjects with a maximum follow-up of 12 months was targeted to achieve >80% power to detect a difference in the time-to-recurrence distributions between pairs of groups defined by tertiles of coated-platelet levels, where the lowest and highest risk groups have a 12-month recurrence rate of 2% and 25%, respectively, using a log-rank test and a two-sided 0.0167 α level. The event rate estimates were based on our previously published research data on coated-platelet levels in ischemic stroke.10, 11 Sample size calculations were performed using PASS software (NCSS, LLC, Kaysville, UT, USA).26
Results
Of the initial 190 subjects, follow-up data are available for 180 (95%) and subsequent analyses are based only on subjects with follow-up data. Table 1 lists demographic variables, relevant comorbidities, and pertinent medications for all nonlacunar ischemic stroke patients with follow-up (n=180). The mean coated-platelet level for the entire group was 40.3% (s.d. 13.3%), with a minimum of 9.6% and maximum of 69.7%, consistent with our previous data regarding the distribution of coated-platelet levels in patients with nonlacunar ischemic stroke.10, 11 Stroke patients included 46 women and 134 men (Table 1). Of these patients, 104 were veterans of the United States armed forces resulting in an over-representation of men, a consequence of the military composition during the time these veterans served.
Table 1. Summary of baseline demographic variables and relevant comorbidities and medications for all patients with follow-up.
| Baseline valuesa |
All subjects (n=180) |
Stroke recurrence (n=20) |
No recurrence (n=160) |
P valueb | |||||
|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | s.d. | Mean | s.d. | Mean | s.d. | ||
| Age (years) | 34 | 95 | 65.8 | 11.6 | 67.9 | 13.8 | 65.5 | 11.3 | 0.69 |
| NIHSS | 2 | 38 | 12.7 | 8.4 | 9.7 | 6.4 | 13.1 | 8.6 | 0.20 |
| Count (%) |
Count (%) |
Count (%) |
|||||||
| Male | 134 (74%) | 13 (65%) | 121 (76%) | 0.21 | |||||
| Race/ethnicity | 0.83 | ||||||||
| Caucasian | 127 (71%) | 14 (70%) | 113 (71%) | ||||||
| African American | 50 (28%) | 6 (30%) | 44 (28%) | ||||||
| Hispanic | 1 (<1%) | 0 | 1 (<1%) | ||||||
| American Indian | 1 (<1%) | 0 | 1 (<1%) | ||||||
| Other | 1 (<1%) | 0 | 1 (<1%) | ||||||
| Smokers | 85 (47%) | 8 (40%) | 77 (48%) | 0.39 | |||||
| Antiplatelet use | 103 (57%) | 13 (65%) | 90 (56%) | 0.60 | |||||
| SSRI use | 14 (8%) | 0 | 14 (9%) | 0.20 | |||||
| Statin use | 70 (39%) | 10 (50%) | 60 (38%) | 0.33 | |||||
| Diabetes | 59 (33%) | 6 (30%) | 53 (33%) | 0.81 | |||||
| End stage renal disease | 7 (4%) | 0 | 7 (4%) | 0.41 | |||||
| Atrial fibrillation | 30 (17%) | 3 (15%) | 27 (17%) | 0.86 | |||||
| Hypertension | 135 (75%) | 15 (75%) | 120 (75%) | 0.99 | |||||
| Hypercholesterolemia | 85 (47%) | 10 (50%) | 75 (47%) | 0.90 | |||||
| Large artery disease | 55 (31%) | 10 (50%) | 45 (28%) | 0.040 | |||||
NIHSS, National Institutes of Health Stroke Scale; SSRI, selective serotonin reuptake inhibitors.
Data are summarized using the minimum (Min), maximum (Max), mean and s.d., or count (%).
P value comparing the time-to-recurrence distribution between groups defined by the indicated characteristic at baseline using the log-rank test. Age comparison is between groups above and below the median (64 years) and race/ethnicity comparison is between Caucasians and all others.
Follow-up among subjects who did not develop a recurrent stroke and did not die ranged from 3 months, driven by the time lag between recruitment of the last subject and the study closure date, to 12 months, the maximum patient follow-up period, with a median follow-up of 12 months. Among the 180 subjects with follow-up information, 26 (14%) died without a prior recurrence, 19 (11%) experienced a recurrent stroke and remained alive during the follow-up period, 1 (<1%) experienced a recurrent stroke and later died during the follow-up period, and 134 (74%) did not experience a recurrence and did not die over the entire follow-up period. Among the 20 subjects who experienced a recurrent stroke, 12 were either still admitted (n=4) or were readmitted to our medical center (n=8) and 8 were admitted to other medical facilities. Baseline demographic, medication use, and medical history characteristics were not significantly associated with the time-to-recurrence distributions, with the exception of a history of large artery disease, which was associated with a higher risk of recurrence (P=0.040) (Table 1).
Subjects were categorized into tertiles based on the observed coated-platelet distribution (9.6% to 34%, 34.1% to 46%, and 46.1% to 69.7%). Median follow-up duration among subjects who did not develop a recurrence and remained alive was not significantly different among the coated-platelet level groups (P=0.15).
Table 2 summarizes the distribution of patient characteristics, including use of medications that may influence coated-platelet levels (SSRIs, statins, or antiplatelet medications),1, 7, 8 at baseline according to coated-platelet tertile. There were no significant differences among the groups in terms of baseline characteristics.
Table 2. Summary of baseline demographic variables and relevant comorbidities and medications for all patients according to coated-platelet tertiles.
| Baseline valuesa |
Coated-platelet level (%) |
P value | ||
|---|---|---|---|---|
| Lowest tertile (n=60) | Middle tertile (n=60) | Upper tertile (n=60) | ||
| Age (years) | 65.8 (12.0) | 68.3 (11.1) | 63.2 (11.1) | 0.053 |
| NIHSS | 14.4 (9.3) | 11.0 (7.0) | 12.6 (8.6) | 0.086 |
| Male sex | 46 (77%) | 40 (67%) | 48 (80%) | 0.22 |
| Race/ethnicity (white versus nonwhite) | 0.18 | |||
| White | 37 (62%) | 45 (75%) | 45 (75%) | |
| African American | 22 (36%) | 14 (23%) | 14 (23%) | |
| Hispanic | 0 | 0 | 1 (2%) | |
| Native American | 1 (2%) | 0 | 0 | |
| Other | 0 | 1 (2%) | 0 | |
| Current smoker | 31 (52%) | 23 (38%) | 31 (52%) | 0.24 |
| Statin use | 25 (42%) | 22 (37%) | 23 (38%) | 0.85 |
| Antiplatelet use | 39 (65%) | 33 (55%) | 31 (52%) | 0.31 |
| SSRI use | 3 (5%) | 5 (8%) | 6 (10%) | 0.69 |
| Prior TIA/stroke | 8 (13%) | 12 (20%) | 7 (12%) | 0.40 |
| Prior CAD | 21 (35%) | 16 (27%) | 14 (23%) | 0.34 |
| Diabetes | 23 (38%) | 16 (27%) | 20 (33%) | 0.39 |
| End stage renal disease | 3 (5%) | 2 (3%) | 2 (3%) | 0.80 |
| Cardioembolism | 18 (30%) | 18 (30%) | 14 (23%) | 0.64 |
| Atrial fibrillation | 11 (18%) | 10 (17%) | 9 (15%) | 0.89 |
| Hypertension | 39 (65%) | 48 (80%) | 48 (80%) | 0.091 |
| Hypercholesterolemia | 29 (48%) | 28 (47%) | 28 (47%) | 0.98 |
| Large artery disease | 16 (27%) | 23 (38%) | 16 (27%) | 0.28 |
CAD, coronary artery disease; NIHSS, National Institutes of Health Stroke Scale; SSRI, selective serotonin reuptake inhibitors; TIA, transient ischemic attack.
Data are summarized using the mean and s.d. or count (%).
Table 3 summarizes the use of medications that may influence coated-platelet levels (listed above), and additional treatments, such as anticoagulation or surgical/endovascular treatment for stroke prevention, all recorded at the time of discharge according to coated-platelet tertile. There were no significant differences among the groups in terms of these parameters at discharge. No significant linear correlation was found in stroke patients between coated-platelet levels and the time elapsed from the onset of symptoms of the initial stroke to the time of the blood draw (r=0.06, P=0.4).
Table 3. Summary of medications pertinent to coated-platelet levels, use of anticoagulation, antiplatelet therapy, and surgical/endovascular treatments at the time of discharge for all patients according to coated-platelet tertiles.
| Treatment at the time of discharge |
Coated-platelet level (%) |
P value | ||
|---|---|---|---|---|
| Lowest tertile (n=60) | Middle tertile (n=60) | Upper tertile (n=60) | ||
| Statin use | 45 (75%) | 47 (78%) | 43 (72%) | 0.70 |
| Antiplatelet use | 54 (90%) | 55 (92%) | 54 (90%) | 0.94 |
| Aspirin | 25 (42%) | 26 (43%) | 25 (42%) | 0.98 |
| Clopidogrel | 20 (33%) | 21 (35%) | 22 (37%) | 0.93 |
| Combination aspirin/extended-release dipyridamole | 9 (15%) | 8 (13%) | 7 (12%) | 0.87 |
| Anticoagulation use | 12 (20%) | 13 (22%) | 13 (22%) | 0.97 |
| Concomitant antiplatelet and anticoagulation use | 7 (12%) | 9 (15%) | 8 (13%) | 0.87 |
| SSRI use | 4 (7%) | 4 (7%) | 5 (8%) | 0.90 |
| Surgical/endovascular procedures | 7 (12%) | 5 (8%) | 9 (15%) | 0.52 |
SSRI, selective serotonin reuptake inhibitors.
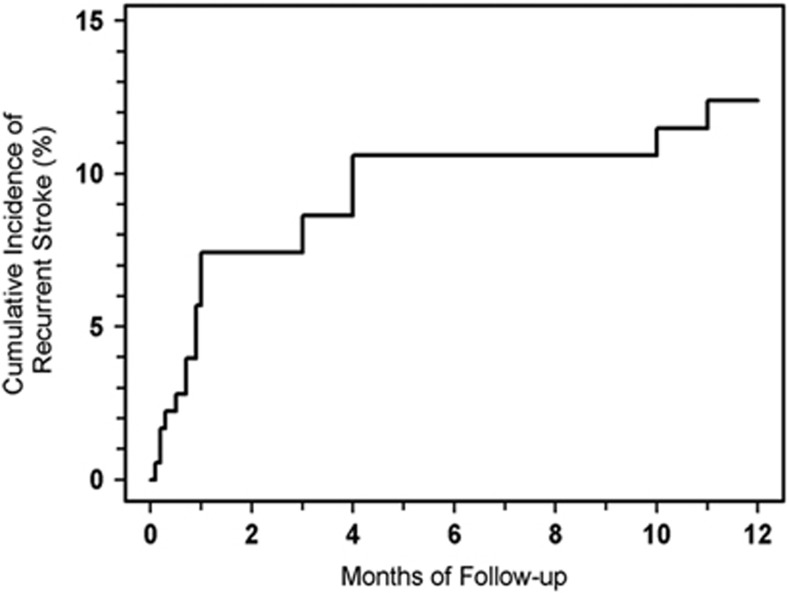
The cumulative incidence of recurrent stroke for all patients (n=180) increased from 9% (95% confidence interval (CI): 5% to 14%, n=15 recurrences) at 3 months to 11% (95% CI: 7% to 16%, n=18) at 6 and 9 months, and to 12% (95% CI: 8% to 19%, n=20) at 12 months (Figure 1).
Figure 1.
Cumulative incidence of stroke recurrence. Patients with nonlacunar stroke (n=180) were followed for up to 12 months. The cumulative incidence of stroke recurrence (%) is plotted as a function of follow-up time.
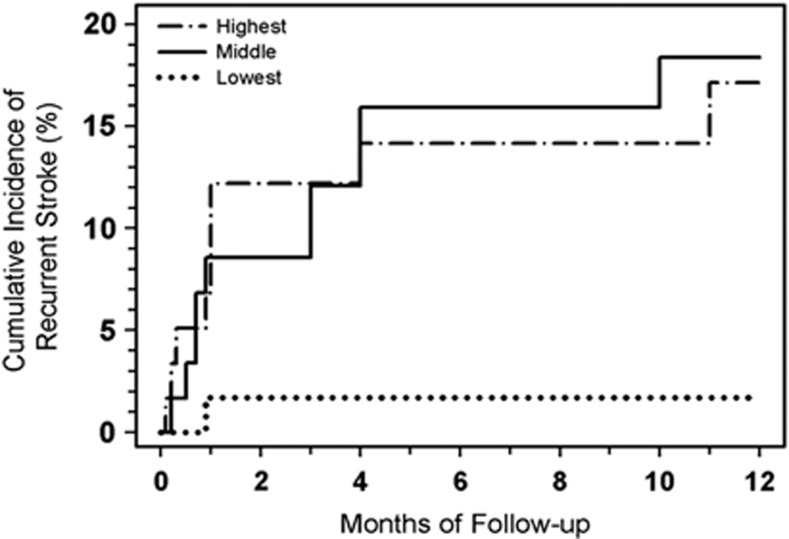
The estimated cumulative incidence of recurrent stroke according to coated-platelet tertile is presented in Figure 2. A total of 9 subjects in the highest (n=60) and 10 subjects in the middle (n=60) coated-platelet tertile groups experienced a recurrent stroke during the follow-up period, resulting in a cumulative incidence of recurrent stroke at 12 months of 17% (95% CI: 9% to 31%) and 18% (95% CI: 10% to 32%), respectively (Table 4). In contrast, only one subject with a coated-platelet level in the lowest tertile (n=60) experienced a recurrent stroke, resulting in a cumulative incidence of recurrent stroke at 12 months of 2% (95% CI: <1% to 11%) (Table 4). The time-to-recurrent stroke differed significantly among the coated-platelet level tertiles (overall log-rank test, P=0.019). Pairwise comparisons, using an adjusted α level of 0.0167, suggest that the risk of recurrent stroke is higher among subjects in the highest or middle coated-platelet tertile compared with those in the lowest tertile (P=0.0075 and P=0.0052, respectively) and that the risk does not differ significantly between subjects in the middle and highest tertile groups (P=0.88).
Figure 2.
Cumulative incidence of stroke recurrence analyzed by coated-platelet tertiles. Nonlacunar stroke patients (n=180) from Figure 1 were subdivided according to coated-platelet levels at time of initial stroke. Recurrence incidence (%) is plotted as a function of follow-up time for lowest coated-platelet tertile group (··········· coated-platelet levels from 9.6% to 34.0%); middle tertile group (———— 34.1% to 46.0%); and highest tertile group (· - · - · - · - · 46.1% to 69.7%). Each tertile represents 60 patients.
Table 4. Cumulative incidence of recurrent stroke among groups of patients defined by tertiles of coated-platelet levels.
| Time point |
Lowest tertilea (n=60) |
Middle tertilea (n=60) |
Highest tertilea (n=60) |
|||
|---|---|---|---|---|---|---|
| Cumulative incidence of recurrent stroke | 95% CI | Cumulative incidence of recurrent stroke | 95% CI | Cumulative incidence of recurrent stroke | 95% CI | |
| 3 months | 2% (n=1) | <1–11% | 12% (n=7) | 6–24% | 12% (n=7) | 6–24% |
| 6 months | 2% (n=1) | <1–11% | 16% (n=9) | 9–28% | 14% (n=8) | 7–26% |
| 9 months | 2% (n=1) | <1–11% | 16% (n=9) | 9–28% | 14% (n=8) | 7–26% |
| 12 months | 2% (n=1) | <1–11% | 18% (n=10) | 10–32% | 17% (n=9) | 9–31% |
CI, confidence interval.
Lowest coated-platelet tertile group (coated-platelets from 9.6% to 34.0%), middle tertile group (34.1% to 46.0%), and highest tertile group (46.1% to 69.7%)
The cumulative incidence of death due to all causes at 12 months in all patients (n=180) was estimated to be 16% (95% CI: 11% to 22%, n=27 deaths). Because different mortality rates across tertiles could explain differences in stroke risk (competing causes), the cumulative incidence of stroke recurrence was estimated and compared among coated-platelet groups with death due to all causes considered as a competing event for stroke recurrence. Estimated differences in the cumulative incidence of recurrent stroke among the coated-platelet level groups were similar between the scenarios when death was considered as a competing event and when death was not considered a competing event.
Discussion
The current study indicates that higher levels of coated-platelets are associated with stroke recurrence in patients with an initial nonlacunar ischemic stroke. The risk of recurrent stroke was higher in those patients with coated-platelet levels in the highest and middle tertiles compared with those in the lowest tertile, even though the risk of recurrence for the group as a whole was similar to that reported in the literature.23 These differences are not likely due to confounding variables given the balance in demographic and clinical factors among the coated-platelet groups, although confounding could not be formally addressed given the small number of recurrences observed in the lowest tertile.
The association between increased coated-platelet production and stroke recurrence in nonlacunar stroke patients is consistent with the prothrombotic potential of these activated platelets.1, 10 The phenotypic differences between coated- and noncoated-platelets center on the presence of bound procoagulant proteins on the coated-platelet surface.2 The presence of these procoagulant proteins is responsible for the prothrombinase activity associated with coated-platelets but not with noncoated-platelets. Furthermore, the activated fibrinogen receptors on coated-platelets are occupied2 and not available for ligand-dependent binding/aggregation with noncoated-platelets.2 The biochemical distinctions responsible for these phenotypic differences include mitochondrial depolarization4 and serotonin derivatization of α-granule proteins,2 two events not observed with noncoated-platelets.
When the current observations are coupled with the previous findings of low coated-platelet levels in spontaneous intracerebral hemorrhage and early hemorrhagic transformation,11, 12, 13 an attractive hypothesis emerges: coated-platelet levels may help identify ischemic stroke patients with a higher risk for recurrence and a lower risk for hemorrhagic complications. These patients may prove to benefit from more aggressive secondary prevention strategies.27, 28 Conversely, coated-platelet levels may also identify those patients at greater risk for hemorrhagic complications as a result of aggressive antiplatelet treatment and thereby avert untoward consequences noted in previous trials assessing the efficacy of combination antiplatelet treatment in stroke prevention.28, 29, 30, 31, 32
Limitations of this study include a relatively modest number of recurrent strokes, a larger percentage of men than women, crosssectional measures of coated-platelet levels and the exclusion of patients who received thrombolysis or were treated with anticoagulants for the current stroke before enrollment. Nevertheless, these results demonstrate an association between coated-platelet levels and stroke recurrence in nonlacunar stroke patients, setting the stage for additional studies examining possible uses for coated-platelet determinations in the treatment of nonlacunar stroke patients.
Acknowledgments
The authors thank Robert Cox and Paul Friese for assistance.
The authors declare no conflict of interest.
Footnotes
This study was supported by grants from the Department of Veterans Affairs Clinical Science Research and Development Service (Award Number 1I01CX000340) to Dr Prodan, and the American Heart Association (10GRNT4010005) to Dr Dale.
References
- Dale GL. Coated-platelets: an emerging component of the procoagulant response. J Thromb Haemostasis. 2005;3:2185–2192. doi: 10.1111/j.1538-7836.2005.01274.x. [DOI] [PubMed] [Google Scholar]
- Dale GL, Friese P, Batar P, Hamilton SF, Reed GL, Jackson KW, et al. Stimulated platelets use serotonin to enhance their retention of procoagulant proteins on the cell surface. Nature. 2002;415:175–179. doi: 10.1038/415175a. [DOI] [PubMed] [Google Scholar]
- Dale GL, Remenyi G, Friese P. Quantitation of microparticles released from coated-platelets. J Thromb Haemostasis. 2005;3:2081–2088. doi: 10.1111/j.1538-7836.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- Remenyi G, Szasz R, Friese P, Dale GL. Role of mitochondrial permeability transition pore in coated-platelet formation. Arterioscler Thromb Vasc Biol. 2005;25:467–471. doi: 10.1161/01.ATV.0000152726.49229.bf. [DOI] [PubMed] [Google Scholar]
- Alberio L, Safa O, Clemetson KJ, Esmon CT, Dale GL. Surface expression and functional characterization of alpha-granule factor V in human platelets: effects of ionophore A23187, thrombin, collagen and convulxin. Blood. 2000;95:1694–1702. [PubMed] [Google Scholar]
- Brooks MB, Catalfamo JL, Friese P, Dale GL. Scott syndrome dogs have impaired coated-platelet formation and calcein release but normal mitochondrial depolarization. J Thromb Haemostasis. 2007;5:1972–1974. doi: 10.1111/j.1538-7836.2007.02683.x. [DOI] [PubMed] [Google Scholar]
- Prodan CI, Joseph PM, Vincent AS, Dale GL. Coated-platelet levels are influenced by smoking, aspirin and selective serotonin reuptake inhibitors. J Thromb Haemostasis. 2007;5:2149–2151. doi: 10.1111/j.1538-7836.2007.02691.x. [DOI] [PubMed] [Google Scholar]
- Norgard NB, Saya S, Hann CL, Hennebry TA, Schechter E, Dale GL. Clopidogrel attenuates coated-platelet production in patients undergoing elective coronary catheterization. J Cardiovasc Pharmacol. 2008;52:536–539. doi: 10.1097/FJC.0b013e3181907390. [DOI] [PubMed] [Google Scholar]
- Valaydon ZS, Lee P, Dale GL, Januszewski AS, Rowley KG, Nandurkar H, et al. Increased coated-platelet levels in chronic haemodialysis patients. Nephrology (Carlton ) 2009;14:148–154. doi: 10.1111/j.1440-1797.2008.01026.x. [DOI] [PubMed] [Google Scholar]
- Prodan CI, Joseph PM, Vincent AS, Dale GL. Coated-platelets in ischemic stroke: differences between lacunar and cortical stroke. J Thromb Haemostasis. 2008;6:609–614. doi: 10.1111/j.1538-7836.2008.02890.x. [DOI] [PubMed] [Google Scholar]
- Prodan CI, Stoner JA, Cowan LD, Dale GL. Lower coated-platelet levels are associated with early hemorrhagic transformation in patients with non-lacunar brain infarction. J Thromb Haemostasis. 2010;8:1185–1190. doi: 10.1111/j.1538-7836.2010.03851.x. [DOI] [PubMed] [Google Scholar]
- Prodan CI, Vincent AS, Padmanabhan R, Dale GL. Coated-platelet levels are low in patients with spontaneous intracerebral hemorrhage. Stroke. 2009;40:2578–2580. doi: 10.1161/STROKEAHA.109.549014. [DOI] [PubMed] [Google Scholar]
- Prodan CI, Vincent AS, Dale GL. Coated platelet levels correlate with bleed volume in patients with spontaneous intracerebral hemorrhage. Stroke. 2010;41:1301–1303. doi: 10.1161/STROKEAHA.110.581447. [DOI] [PubMed] [Google Scholar]
- Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- Prodan CI, Szasz R, Vincent AS, Ross ED, Dale GL. Coated-platelets retain amyloid precursor protein on their surface. Platelets. 2006;17:56–60. doi: 10.1080/09537100500181913. [DOI] [PubMed] [Google Scholar]
- Prodan CI, Ross ED, Stoner JA, Cowan LD, Vincent AS, Dale GL. Coated-platelet levels and progression from mild cognitive impairment to Alzheimer disease. Neurology. 2011;76:247–252. doi: 10.1212/WNL.0b013e3182074bd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams H, Adams R, Del Zoppo G, Goldstein LB. Guidelines for the early management of patients with ischemic stroke: 2005 guidelines update a scientific statement from the Stroke Council of the American Heart Association/American Stroke Association. Stroke. 2005;36:916–923. doi: 10.1161/01.STR.0000163257.66207.2d. [DOI] [PubMed] [Google Scholar]
- Jenkins AJ, Gosmanova AK, Lyons TJ, May KD, Dashti A, Baker MZ, et al. Coated-platelet levels in patients with type 1 and with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2008;81:e8–10. doi: 10.1016/j.diabres.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Petty GW, Brown RD, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31:1062–1068. doi: 10.1161/01.str.31.5.1062. [DOI] [PubMed] [Google Scholar]
- Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62:569–573. doi: 10.1212/01.wnl.0000110311.09970.83. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- Coull AJ, Rothwell PM. Underestimation of the early risk of recurrent stroke: evidence of the need for a standard definition. Stroke. 2004;35:1925–1929. doi: 10.1161/01.STR.0000133129.58126.67. [DOI] [PubMed] [Google Scholar]
- Jackson C, Sudlow C. Comparing risks of death and recurrent vascular events between lacunar and non-lacunar infarction. Brain. 2005;128:2507–2517. doi: 10.1093/brain/awh636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellerer AM, Chmelevsky D. Small-sample properties of censored-data rank tests. Biometrics. 2012;39:675–682. [Google Scholar]
- Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–1510. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- Hintze J. PASS 2008. NCSS, LLC: Kaysville, Utah; 2008. [Google Scholar]
- Biller J. Antiplatelet therapy in ischemic stroke: variability in clinical trials and its impact on choosing the appropriate therapy. J Neurol Sci. 2009;284:1–9. doi: 10.1016/j.jns.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371:1612–1623. doi: 10.1016/S0140-6736(08)60694-7. [DOI] [PubMed] [Google Scholar]
- Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- Topol EJ, Easton D, Harrington RA, Amarenco P, Califf RM, Graffagnino C, et al. Randomized, double-blind, placebo-controlled, international trial of the oral IIb/IIIa antagonist lotrafiban in coronary and cerebrovascular disease. Circulation. 2003;108:399–406. doi: 10.1161/01.CIR.0000084501.48570.F6. [DOI] [PubMed] [Google Scholar]
- Rothwell PM, Algra A, Amarenco P. Medical treatment in acute and long-term secondary prevention after transient ischaemic attack and ischaemic stroke. Lancet. 2011;377:1681–1692. doi: 10.1016/S0140-6736(11)60516-3. [DOI] [PubMed] [Google Scholar]