Abstract
Myogenic tone is a fundamental aspect of vascular behavior in resistance arteries. This contractile response to changes in intravascular pressure is critically involved in blood flow autoregulation in tissues such as the brain, kidneys, and heart. Myogenic tone also helps regulate precapillary pressure and provides a level of background tone upon which vasodilator stimuli act to increase tissue perfusion when appropriate. Despite the importance of these processes in the brain, little is known about the mechanisms involved in control of myogenic tone in the cerebral microcirculation. Here, we report that pharmacological inhibition of P2Y4 and P2Y6 pyrimidine receptors nearly abolished myogenic tone in cerebral parenchymal arterioles (PAs). Molecular suppression of either P2Y4 or P2Y6 receptors using antisense oligodeoxynucleotides reduced myogenic tone by 44%±8% and 45%±7%, respectively. These results indicate that both receptor isoforms are activated by increased intravascular pressure, which enhances the activity of voltage-dependent calcium channels and increases myogenic tone in PAs. Enhancement or inhibition of ectonucleotidase activity had no effect on parenchymal arteriolar myogenic tone, indicating that this response is not mediated by local release of nucleotides, but rather may involve direct mechanical activation of P2Y receptors in the smooth muscle cells.
Keywords: arterioles, cerebral, myogenic, P2Y receptors, vasoconstriction
Introduction
While much is known about the mechanisms of myogenic regulation in pial arteries on the surface of the brain,1, 2, 3, 4 much less is known about this fundamental vascular response in arteries within the brain, the so-called parenchymal arterioles (PAs). The myogenic response is an important element of the brain's ability to autoregulate blood flow over a substantial range of intravascular pressures. Myogenic tone in the brain microcirculation also provides a background constricted state that is appropriate to control local perfusion pressure and protect capillaries from the potentially damaging effects of high intravascular pressure near the capillaries. Further, tonic constriction of cerebral arterioles contributes to the tightly regulated process of neurovascular coupling, whereby inhibition of such tone can lead to an appropriate increase in local blood flow when local neuronal activity increases. Nevertheless, the fundamental processes involved in myogenic response in the brain microcirculation have not been clarified. Given the specific environment in which the PAs reside and their unique interacting partners, that is, neruons, astrocytes, pericytes, etc., as well as other distinct functional aspects involving calcium signaling,5, 6 ion channel function,7 and tone development8 in PAs versus the larger diameter pial arteries, it is reasonable to propose that the mechanisms of myogenic regulation in PAs may be unique as well.
A recent study involving heterologous expression of G-protein-coupled receptors (GPCRs) and TRPC6 channels as well as examination of myogenic mechanisms in brain pial and systemic arteries, lead to the interesting proposal that mechanical activation of Angiotensin II receptors in vascular smooth muscle cells is linked to myogenic tone development.9 Although the mechanisms by which Angiotensin II receptors are actually mechanically activated were not defined in this study, linkage to activation of a depolarizing mechanism via PLC and DAG signaling was apparent. In light of these interesting observations, but given the unique behaviors of PAs versus pial arteries presented above, a major goal of the current study was to determine the contributions of GPCRs to the regulation of myogenic tone in the PAs. Our results indicate that, in cerebral parenchymal arterioles, GPCRs indeed mediate pressure-induced tone. However, we have found that P2Y4 and P2Y6 pyrimidine receptors, rather than Ang II receptors, are involved in this response. This behavior is likely produced by mechanoactivation of the pyrimidine GPCRs and does not appear to involve release of endogenous pyrimidine nucleotides or autocrine/paracrine activity.
Materials and methods
Animals and Tissues
All animal use was conducted according to the guidelines approved by the University of Vermont IACUC. Male, Sprague Dawley rats, 15 to 20 weeks old, were euthanized by an overdose of sodium pentobarbital (120 mg/kg, intraperitoneally) followed by exsanguination. The brain was rapidly removed and place in ice-cold, MOPS buffered PSS (physiological salt solution) of the following composition (in mmol/L): 3 MOPS, 145 NaCl, 5 KCl, 1 MgSO4, 2.5 CaCl2, 1 KH2PO4, 2 pyruvate, 5 glucose, 1% BSA, pH 7.3. Middle cerebral arteries and attached parenchymal arterioles were isolated from the brain as previously described10 and transferred to a small volume of MOPS buffered PSS for further dissection before mounting in a myograph for diameter measurements.
Diameter Recordings
Endothelial cell-denuded arterial segments were mounted on glass pipettes in an arteriograph chamber (Living Systems, Burlington, VT, USA) containing a bicarbonate-buffered artificial cerebral spinal fluid (aCSF) of the following composition (in mmol/L): 136 NaCl, 3 KCl, 15 NaHCO3, 1.25 NaH2PO4, 1 MgCl2, 4 glucose, 2 CaCl2 (temperature 37.5°C and pH 7.3). The endothelium was removed by introducing an air bubble into the arterial lumen for 7.5 minutes followed by flushing with aCSF. Arteries were then pressurized to 10 mm Hg (with no flow), and superfused with warmed (37°C), gassed (20% O2/5% CO2/balance N2) aCSF. In experiments using Ca2+-free aCSF, CaCl2 was omitted, and 5 mmol/L EGTA was added. As an initial check of tissue viability and for verification of endothelial cell removal, arteries were constricted with the thromboxane receptor activator U46619 (100 nmol/L) and then exposed to 1 μmol/L NS 309, an activator of SKCa and IKCa channels which mediate endothelium-dependent vasodilator responses in these arteries.11 Arteries were rejected for study if constrictions in response to U46619 were less than a 60% decrease in diameter. Absence of a dilation to NS 309 indicated successful endothelial cell removal. In experiments where steady myogenic tone was desired, pial arteries were pressurized to 80 mm Hg and PAs were pressurized to 40 mm Hg and studied after tone developed. In experiments where no myogenic tone was desired, for example, in studies of agonist-induced vasoconstriction, pial arteries, and PAs were pressurized to 10 and 5 mm Hg, respectively.
Oligonucleotides and Reversible Permeabilization
Antisense and sense oligodeoxynucleotides (ODNs) were designed based on published sequences for rat P2Y2 (RGD:62088), P2Y4 (RGD:61798), and P2Y6 (RGD:620269) receptors; the ODN sequences are shown in Supplementary Table 1. The last five bases on the 5′ and 3′ ends were phosphorothioated to limit ODN degradation. All ODNs were synthesized by Integrated DNA Technologies Inc. (San Diego, CA, USA) Sense and antisense ODNs (2 μmol/L) were introduced into the arterial smooth muscle cells via a reversible permeabilization procedure.3 The arterial segments were then organ cultured for 3 days in minimal essential medium (DMEM-F-12) with ℒ-glutamine (2 mmol/L), penicillin (50 U/mL), and streptomycin (50 μg/mL) before use. Similar to studies on freshly isolated arteries, organ cultured arteries were rejected for further analysis if U46619 induced less than a 60% decrease in diameter.
RT–PCR for P2Y Receptor Message
RNA was prepared from isolated arterioles with endothelium present, as previously described for assay of ion channel message.3 First-strand complementary DNA was prepared from 720 ng total RNA using the Qiagen (Valencia, CA, USA) Sensiscript Reverse Transcriptase kit. PCR was performed using HotStarTaq DNA Polymerase (Qiagen). PCR reactions were hot started at 94°C for 10 minutes and exposed to 38 cycles of 94°C for 60 seconds, 60°C for 90 seconds, and 72°C for 60 seconds, then followed by 72°C final extension for 10 minutes. All reaction products were resolved on 1.8% agarose gels. Primer sequences are shown in Supplementary Table 2.
Chemicals and Reagents
Buffer reagents, UTP and UDP were purchased from Sigma-Aldrich (St Louis, MO, USA). Nimodipine was dissolved in ethyl alcohol to a final solvent concentration of 0.1%. All other compounds were dissolved in the appropriate salt solution.
Statistical Analysis
Values are mean values±s.e., and n indicates the number of animals. Arterial tone under different conditions is typically normalized as percentage Tone, and calculated as follows:
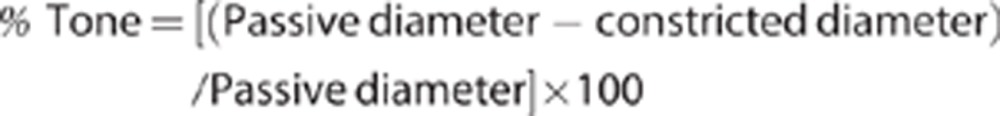 |
where ‘Passive diameter' equals the diameter in Ca2+-free aCSF.
For P2Y receptor agonist concentration/response relationships, responses are expressed as percent of the maximum contraction induced by the thromboxane receptor activator U46619. For P2Y receptor antagonist studies, data are expressed as percent inhibition of active tone.
Student's t-test was used to compare experimental groups. Mean values were considered significantly different at P≤0.05.
Results
Angiotensin Receptor Inhibition Reduces Myogenic Tone in Pial Arteries, but not in Parenchymal Arterioles
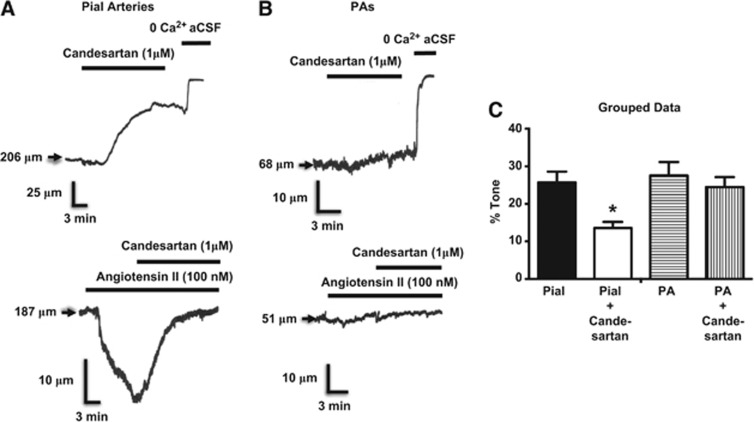
The initial objective of the present study was to determine the possible contributions of Angiotensin II receptors to the mechanisms of myogenic tone in parenchymal arterioles. However, although we were able to confirm the involvement of these GPCRs in the myogenic behavior of pial arteries9 (Figure 1A), we found no evidence of a similar role for angiotensin II receptors in isolated PAs (Figure 1B). Specifically, candesartan, an angiotensin II receptor blocker, had no effect on parenchymal arteriolar myogenic tone, a finding that was consistent with the absence of vasoconstrictor responses to angiotensin II itself in these arterioles (Figure 1B).
Figure 1.
Effects of the AT1 receptor inverse agonist candesartan on myogenic tone in cerebral arteries. (A) Candesartan inhibits myogenic tone and Ang II constricts pial arteries, but these Ang II receptor modulators have no significant effects on parenchymal arterioles (PAs) (B). (C) Grouped data show significant effect of cadesartan on pial artery myogenic tone and the absence of effect on myogenic tone in PAs. *P<0.05 versus response without candesartan. N=4 for pial and parenchymal artery groups. Pial arteries and PAs were pressurized to 80 and 40 mm Hg, respectively, to induce myogenic tone, and to 10 and 5 mm Hg, respectively, where myogenic tone is very low or absent, for assessment of Ang II-induced vasoconstrictions.
Inhibition of P2Y Receptors Inhibits Pyrimidine Nucleotide-Mediated Constrictions and Myogenic Tone in Isolated Parenchymal Arterioles
In view of the negligible angiotensin II receptor-mediated vasoconstriction of parenchymal arterioles, we next explored the possible contributions of other GPCRs in the myogenic behavior of these blood vessels. Pyrimidine nucleotides, in particular UTP and UDP, are known to cause substantial and sustained contraction of cerebrovascular smooth muscle12, 13, 14 and previous evidence in mesenteric resistance arteries indicated that pyrimidine receptors may contribute to myogenic reactivity of systemic arteries.15 Thus, we next considered the possible role of these receptors in parenchymal arteriolar myogenic behavior.
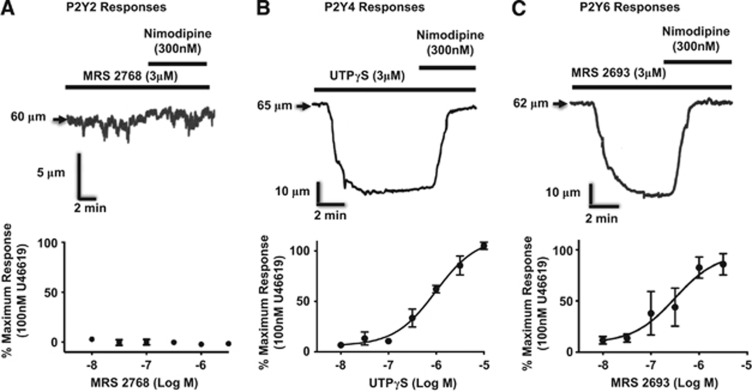
We first determined that message for the predominant vascular P2Y receptors (P2Y2, P2Y4, P2Y6) is present in parenchymal artery extracts. In agreement with previous studies examining localization of P2Y receptors in coronary16 and pial13 arteries, message for each of these receptors is present in PAs (Supplementary Figure 1). We subsequently explored the vasomotor activity of parenchymal arterioles in response to activation of P2Y receptors. Although a P2Y2-selective agonist (MRS 276817) had no effect on vascular tone in PAs (Figure 2A), pyrimidine receptor ligands with relative affinities for P2Y2 and P2Y4 (UTPγS18) or P2Y6 (MRS 2693=5-iodo-UDP19) receptors induced robust, dihydropyridine-sensitive constrictions of isolated parenchymal arterioles without endothelium (Figures 2B and 2C). These studies suggested possible roles for P2Y4 or P2Y6 receptors in the constriction of PAs in response to extrinsic or intrinsic excitatory stimuli, mediated by calcium entry through voltage-dependent calcium channels (CaV), which is a hallmark of myogenic responses in most arteries.
Figure 2.
Responses of parenchymal arterioles (PAs) to P2Y receptor agonists. (A) Although a P2Y2 ligand (n=3) does not constrict PAs, (B) P2Y4 (n=3), and (C) P2Y6 (n=4) ligands cause robust, dihydropyridine-sensitive constrictions of isolated PAs without endothelium. PAs were pressurized to 5 mm Hg, where myogenic tone is very low or absent.
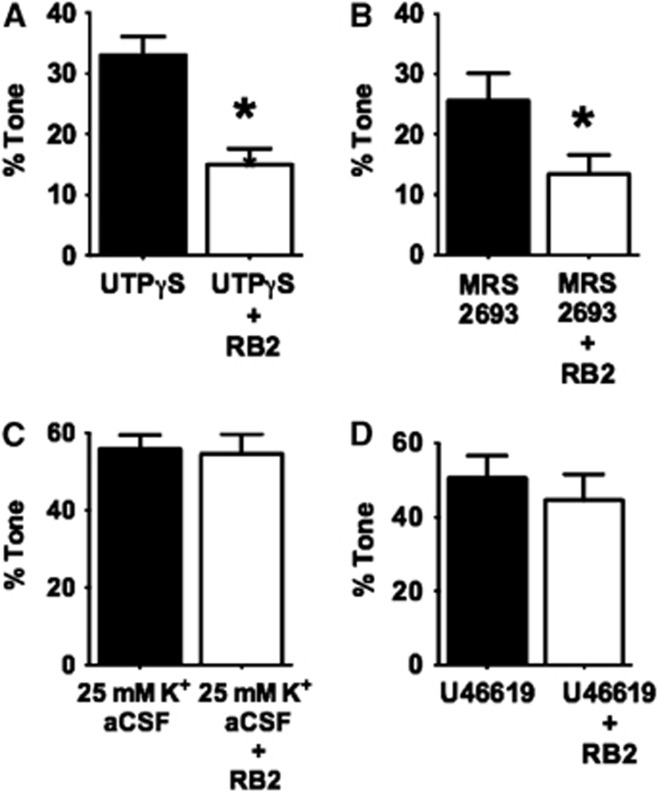
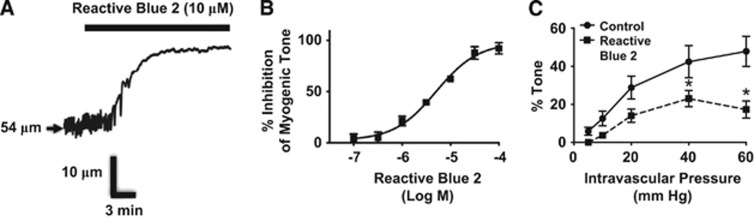
To establish a possible link between P2Y receptor activity and myogenic tone in PAs, we next tested the effects of P2Y-receptor inhibition on the vasoconstrictor responses to P2Y4 and P2Y6 receptor ligands and on myogenic tone in PAs. Reactive Blue 2 (RB2, 10 μmol/L), which inhibits multiple P2 purinergic receptors, including P2Y4 and P2Y6 receptors (P2Y4 IC50: 19 μmol/L;20 P2Y6 IC50: 1 μmol/L21), substantially reduced constrictor responses to UTPγS or MRS 2693 (Figures 3A and 3B). Control experiments revealed no effect of RB2 on vasoconstriction induced by elevated bath K+ (Figure 3C) or by the thromboxane receptor activator U46619 (Figure 3D), indicating that the effects of RB2 are relatively selective for P2Y receptors. Interestingly, RB2 also inhibited pressure-induced myogenic tone in PAs (Figure 4), with an IC50 of 5.1 μmol/L (95% CI: 3.2 to 7.9 μmol/L). Supportive of a possible general role of P2Y receptors in regulation of cerebral myogenic tone, RB2 abolished the residual, candesartan-resistant myogenic tone in pial arteries (data not shown, n=3).
Figure 3.
Reactive Blue 2 (RB2, 10 μmol/L) inhibits (A) P2Y4 (n=4) and (B) P2Y6 (n=4) receptor-induced constrictions, but not (C) depolarization (K+)-mediated (n=8) or (D) U46619-induced (n=5) responses in parenchymal arterioles (PAs) pressurized to 5 mm Hg. *P<0.05 versus response without RB2.
Figure 4.
Reactive Blue 2 inhibits myogenic tone. (A) RB2 (10 μmol/L) substantially reduces myogenic tone in a parenchymal arteriole (PA) held at 40 mm Hg intravascular pressure. (B) RB2 concentration/response relationship in PAs with tone (P=40 mm Hg) (n=3). (C) Effects of RB2 (10 μmol/L) on myogenic tone over the full pressure/diameter relationship in PAs. *P<0.05 versus Control (n=5).
A substantial role for P2Y6 receptors in myogenic tone was further implied in studies using the relatively selective P2Y6 receptor blocker MRS 2578, which potently inhibited myogenic tone in PAs by a maximum of about 80% (Supplementary Figure 2). Selective pharmacological inhibitors of P2Y4 receptors are not available. Therefore, to corroborate and extend the previous observations, we next tested the effects of suppression of P2Y receptor expression on myogenic and pyrimidine nucleotide-induced tone.
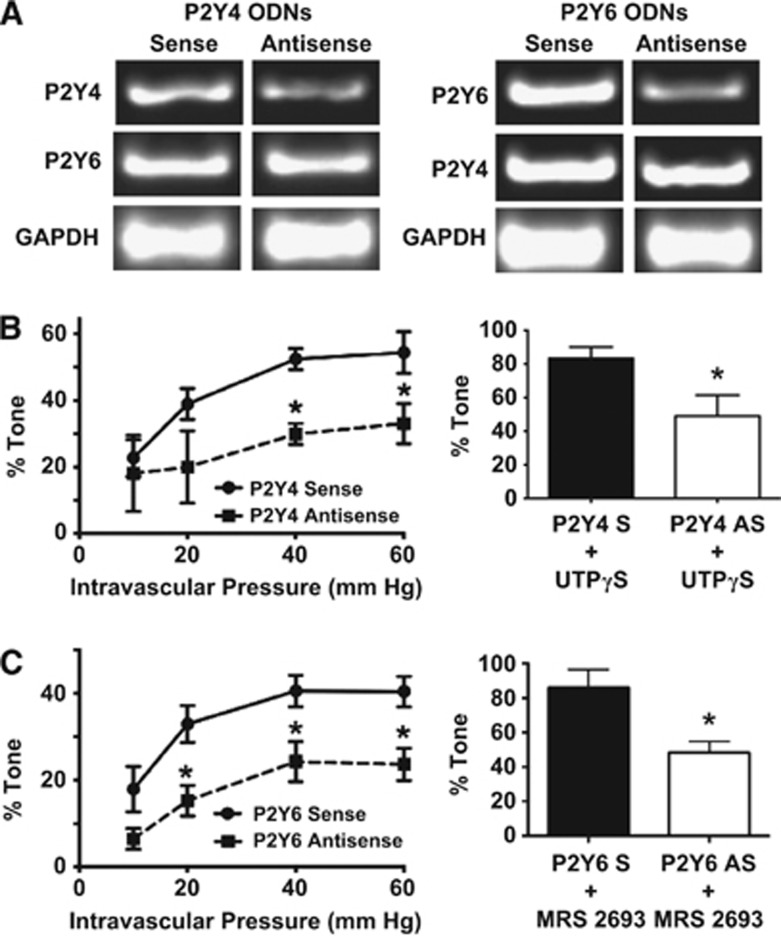
Expression of P2Y4 and P2Y6 receptors was substantially reduced by antisense ODNs, as indicated by semiquantitative PCR (Figure 5A). Parallel functional studies revealed that myogenic tone in PAs treated with antisense ODNs targeting P2Y4 or P2Y6 receptors was reduced by ∼50% compared with control (sense-treated) arterioles, as were responses to the respective P2Y4 or P2Y6 receptor ligands (Figures 5B and 5C). As predicted by the absence of P2Y2-mediated vasoconstrictor responses (Figure 2A), P2Y2 receptor antisense treatment did not affect myogenic responses in PAs (data not shown).
Figure 5.
Effects of antisense oligonucleotides on P2Y receptor message levels, myogenic tone, and responses to P2Y4- and P2Y6-specific ligands. (A) Semiquantitative PCR indicates a specific and substantial decrease in P2Y4- or P2Y6- message levels in arteries exposed to antisense versus sense oligodeoxynucleotides (ODNs) (sense or antisense ODN-treated parenchymal arterioles (PAs) from three animals were pooled in each case to assess relative message abundance). Myogenic tone and constrictions to receptor-specific ligands were significantly reduced in arterial samples treated with (B) P2Y4 (n=4 to 5) and (C) P2Y6 (n=8) receptor antisense ODNs. *P<0.05 versus sense-treated.
Potentiation or Inhibition of Ectonucleotidase Activity has no Effect on Myogenic Tone in Parenchymal Arterioles
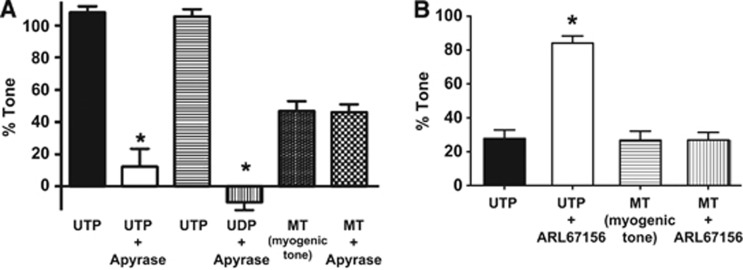
We next addressed the question whether the P2Y receptor-mediated activation underlying myogenic tone in PAs is due to the actions of endogenous pyrimidine nucleotides. If so, we predicted that enhanced ectonucleotidase activity by addition of exogenous ectonucleotidase enzyme (apyrase) should reduce myogenic tone and, conversely, inhibition of endogenous ectonucleotidase activity should increase myogenic tone. However, to the contrary, we found that exogenous ectonucleotidase activity sufficient to nearly abolish maximum UTP- or UDP-induced vasoconstrictions (Figure 6A), had no effect on parenchymal arteriolar myogenic tone. Similarly, inhibition of endogenous ectonucleotidase activity using ARL67156, such that constrictions to exogenous UTP were nearly tripled, did not alter myogenic tone in parenchymal arterioles (Figure 6B).
Figure 6.
Effects of exogenous ectonucleotidase (apyrase) and an ectonucleotidase inhibitor (ARL67156) on pyrimidine nucleotide-induced constrictions and myogenic tone in isolated parenchymal arterioles (PAs). (A) Apyrase inhibits responses to UTP and UDP but has no effect on myogenic tone (n=4). (B) The ectonucleotidase inhibitor ARL67156 enhances UTP-induced constrictions, but not myogenic tone (n=4). For UTP and UDP-induced responses, arteries were held at 5 mm Hg to minimize myogenic tone; myogenic tone was induced by increasing intravascular pressure to 60 mm Hg. *P<0.05 versus responses without apyrase or ARL67156.
Discussion
P2Y Pyrimidine Receptor Presence and Function in Parenchymal Arterioles
The present study provides the first evidence that P2Y pyrimidine receptors are critical regulators of intrinsic (myogenic) tone in cerebral parenchymal arterioles. There is considerable evidence for the presence of functional, pyrimidine nucleotide-sensitive receptors in the cerebral circulation. These receptors are well-represented in endothelial and vascular smooth muscle cells in the brain. Activation of cerebral endothelial P2Y receptors is uniformly associated with endothelium-dependent vasodilation;22, 23, 24 this response is thought to be mediated primarily by P2Y1 and P2Y2 receptors located on the endothelial cells.25, 26
The P2Y receptors that contribute to myogenic tone as shown in the present study are located on the cerebral vascular smooth muscle cells. P2Y6 receptors appear to be the predominant pyrimidine nucleotide receptors in cerebrovascular smooth muscle.13, 27 However, P2Y4 receptors are also present in the vascular smooth muscle of cerebral arteries and these receptors can be selectively activated by pyrimidine nucleotide derivatives27 to cause vasoconstriction. P2Y4 receptors play a predominant role in the constrictor responses of rat intraparenchymal cerebral arterioles to UTP.24 In the present study, we found that activation of P2Y4 or P2Y6, but not P2Y2 receptors, induced robust vasoconstriction that was abolished by block of voltage-dependent Ca2+ channels in PAs. In arteries outside the brain circulation, pyrimidine receptor-mediated vasoconstriction is mediated primarily by calcium release from stores.28 In cerebral arteries, calcium entry via CaV channels is the major mechanism of P2Y receptor-induced constriction.29, 30 This is consistent with the prevalence of CaV-dependent constrictor responses in cerebral arteries in general. The responses of PAs to excitatory stimuli, including responses to other receptor ligands31 as well as to increased intravascular pressure,10, 31, 32 are particularly sensitive to CaV channel inhibition.
P2Y4 and P2Y6 Receptors Mediate Myogenic Tone in Parenchymal Arterioles
A major finding in the present study is the central involvement of P2Y4 and P2Y6 pyrimidine nucleotide receptors in the mechanisms of myogenic tone in brain parenchymal arterioles. An initial goal of our study was to test the hypothesis that, similar to recent observations for pial arteries on the surface of the brain,9 pressure-induced vascular tone of intraparenchymal arterioles is mediated by mechanoactivation of GPCRs. In the aforementioned study, pharmacological block of Angiotensin II receptors inhibited the myogenic responses of rat pial arteries by about 50%. This effect was proposed to be due to direct, physical activation of angiotensin II receptors, since inhibition of the formation of endogenous Angiotensin II by pretreatment of isolated pial arteries with an ACE inhibitor had no effect on myogenic tone development or the inhibitory effect of Angiotensin II receptor blockade. In the present study, we confirmed the potential contributions of angiotensin II receptors to the myogenic responses of isolated pial arteries. In PAs, however, we found no evidence for a role of angiotensin II receptors as mediators of myogenic tone. Our findings are in fact consistent with a role for GPCRs as mediators of myogenic tone, but in the case of the intraparenchymal arterioles P2Y4 and P2Y6 receptors are the key players. It is not clear why the particular receptors involved in the myogenic behavior of PAs should be different from those involved in the responses of pial arteries to increased intravascular pressure. This may be related in part to unique aspects of the environment and cellular interactions experienced by the PAs, specifically the associations among blood vessels, astrocytes, and neurons forming the so-called neurovascular unit.
There is abundant evidence in support of a role for signaling mechanisms downstream of GPCR activation in regulation of myogenic tone, in particular involving Gq/11 type receptors that signal through phospholipase C and reaction products derived from phosphatidylinositol. For instance, myogenic activation of canine renal arteries is correlated with elevated levels of IP3 and diacylglycerol.33 More recent reports emphasize the significant involvement of IP3 receptors in myogenic tone.34, 35 In addition, protein kinase C is clearly involved in the mechanisms underlying myogenic tone.4, 36, 37 Although the contributions of the above PLC-related signaling pathways may vary among vascular beds, a common denominator of these signaling mechanisms is their ability to induce depolarization and activation of calcium entry through voltage-dependent calcium channels,33, 36, 38 which as emphasized above, is a prime characteristic of myogenic tone in general. Pyrimidine receptor signaling is associated with activation of Gq/11 G-protein signaling39 and therefore is a logical candidate mediator of the unique signaling mechanisms linking mechanical stimulation with vascular smooth muscle depolarization and contraction in cerebral parenchymal arterioles. The depolarization and constriction following activation of P2Y receptors in PA myocytes most likely involves several distinct mechanisms, including activation of transient receptor potential channels,3 inhibition of K+ channels,16 or through direct activation of CaV channels via mechanisms that involve PKC activity.40 However, the relative contributions of these various mechanisms in PAs have not yet been resolved.
P2Y Receptors in Parenchymal Arterioles Are Activated by Mechanical Stimulation and not by Autocrine/Paracrine Mechanisms
The present results are consistent with the proposal that activation of P2Y receptors in the parenchymal arterioles is due to direct receptor mechanoactivation rather than via release of endogenous pyrimidine nucleotides from local sources. This proposal is based on the absence of effects on myogenic tone in the PAs when ectonucleotidase activity is either inhibited or potentiated. In contrast, myogenic tone is enhanced in mesenteric arteries isolated from ectonucleotidase null mice,15 suggesting that endogenous pyrimidine nucleotides may modulate or directly stimulate myogenic responses in peripheral arteries. Explanations for the apparent differential contribution of endogenous pyrimidines to myogenic tone in peripheral arteries versus PAs are not obvious, but perhaps are consistent with the diverse nature of vascular structure and physiological control mechanisms in central versus peripheral blood vessels, ranging from endothelial permeability differences to fundamental differences in neurovascular regulatory function. A possible alternative explanation for the absence of effect of ectonucleotidase inhibition or activation on myogenic tone in the PAs is insufficient penetration of compounds (apyrase, ARL67156) into sites of release and activity of endogenous pyrimidines. This possibility would seem unlikely for the ectonucleotidase inhibitor since it is a small molecule (MW:785 Da) that is capable of reaching sites of ectonucleotidase activity near pyrimidine receptors in sufficient amounts to greatly potentiate the action of exogenous pyrimidine nucleotides. Nevertheless, this issue would be most clearly resolved by direct measurement of endogenous pyrimidine nucleotide content and release during myogenic stimulation.
The physical interactions leading to mechanoactivation of P2Y receptors are not clear. Several models by which GPCRs could be mechanically activated have been proposed, including a tethered model whereby direct conformational changes of the receptor proteins within the cell membrane leads to receptor activation, as well as mechanisms involving receptor/cytoskeletal interactions that lead to receptor conformational changes (for review see ref. 41). There is evidence that both RB242 and the selective P2Y6 inhibitor MRS 2578 (ref. 43) can act as noncompetitive inhibitors of P2Y receptors. This suggests that through binding to sites on the receptor distinct from the natural ligand binding site, these inhibitors could have allosteric effects that inhibit receptor activity due to either the natural ligand binding or to the postulated mechanoactivation mechanisms associated with changes in intravascular pressure. Clarification of these issues will require further, detailed molecular and biophysical analysis.
Conclusions
The present results demonstrate that Gq/11-coupled P2Y4 and P2Y6 receptors contribute substantially to myogenic mechanisms of vasoconstriction in intraparenchymal cerebral arterioles. This response is mediated by mechanoactivation of the pyrimidine GPCRs and does not appear to involve release of endogenous pyrimidine nucleotides and autocrine/paracrine activity. A deeper appreciation of the specific signaling pathways and mechanisms involved in regulation of vascular tone within the brain may contribute to the development of novel approaches for treatment of brain microcirculatory disorders associated with stroke, hypertension, vasospasm, or other small vessel diseases.
Acknowledgments
The authors thank Fabrice Dabertrand and Rachael Baylie for their insightful comments about this manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by NIH Grant P01 HL095488 and the Totman Trust for Medical Research.
Supplementary Material
References
- Harder DR. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circ Res. 1984;55:197–202. doi: 10.1161/01.res.55.2.197. [DOI] [PubMed] [Google Scholar]
- Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res. 2004;95:922–929. doi: 10.1161/01.RES.0000147311.54833.03. [DOI] [PubMed] [Google Scholar]
- Osol G, Laher I, Cipolla M. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circ Res. 1991;68:359–367. doi: 10.1161/01.res.68.2.359. [DOI] [PubMed] [Google Scholar]
- Dabertrand F, Nelson MT, Brayden JE. Acidosis dilates brain parenchymal arterioles by conversion of calcium waves to sparks to activate BK channels. Circ Res. 2012;110:285–294. doi: 10.1161/CIRCRESAHA.111.258145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95:e73–e81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci USA. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. SKCa and IKCa Channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: effect of ischemia and reperfusion. Stroke. 2009;40:1451–1457. doi: 10.1161/STROKEAHA.108.535435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, et al. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystoriak MA, O'Connor KP, Sonkusare SK, Brayden JE, Nelson MT, Wellman GC. Fundamental increase in pressure-dependent constriction of brain parenchymal arterioles from subarachnoid hemorrhage model rats due to membrane depolarization. Am J Physiol Heart Circ Physiol. 2011;300:H803–H812. doi: 10.1152/ajpheart.00760.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial SK(Ca) and IK(Ca) channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab. 2011;31:1175–1186. doi: 10.1038/jcbfm.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasawa Y, White RP, Robertson JT. Mechanisms of the contractile effect induced by uridine 5-triphosphate in canine cerebral arteries. Stroke. 1983;14:347–355. doi: 10.1161/01.str.14.3.347. [DOI] [PubMed] [Google Scholar]
- Malmsjo M, Hou M, Pendergast W, Erlinge D, Edvinsson L. Potent P2Y6 receptor mediated contractions in human cerebral arteries. BMC Pharmacol. 2003;3:4. doi: 10.1186/1471-2210-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmsjo M, Adner M, Harden TK, Pendergast W, Edvinsson L, Erlinge D. The stable pyrimidines UDPbetaS and UTPgammaS discriminate between the P2 receptors that mediate vascular contraction and relaxation of the rat mesenteric artery. Br J Pharmacol. 2000;131:51–56. doi: 10.1038/sj.bjp.0703536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffenstein G, Drouin A, Thorin-Trescases N, Bachelard H, Robaye B, D'Orleans-Juste P, et al. NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc Res. 2010;85:204–213. doi: 10.1093/cvr/cvp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DG, Brayden JE. Mechanisms of coronary artery depolarization by uridine triphosphate. Am J Physiol Heart Circ Physiol. 2001;280:H2545–H2553. doi: 10.1152/ajpheart.2001.280.6.H2545. [DOI] [PubMed] [Google Scholar]
- Ko H, Carter RL, Cosyn L, Petrelli R, de Castro S, Besada P, et al. Synthesis and potency of novel uracil nucleotides and derivatives as P2Y2 and P2Y6 receptor agonists. Bioorg Med Chem. 2008;16:6319–6332. doi: 10.1016/j.bmc.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Watt WC, Stutts MJ, Brown HA, Boucher RC, Harden TK. Enzymatic synthesis of UTP gamma S, a potent hydrolysis resistant agonist of P2U-purinoceptors. Br J Pharmacol. 1996;117:203–209. doi: 10.1111/j.1476-5381.1996.tb15175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besada P, Shin DH, Costanzi S, Ko H, Mathe C, Gagneron J, et al. Structure-activity relationships of uridine 5′-diphosphate analogues at the human P2Y6 receptor. J Med Chem. 2006;49:5532–5543. doi: 10.1021/jm060485n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov YD, Wildman SS, Clements MP, King BF, Burnstock G. Molecular cloning and characterization of rat P2Y4 nucleotide receptor. Br J Pharmacol. 1998;124:428–430. doi: 10.1038/sj.bjp.0701880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaye B, Boeynaems JM, Communi D. Slow desensitization of the human P2Y6 receptor. Eur J Pharmacol. 1997;329:231–236. [PubMed] [Google Scholar]
- Marrelli SP, Khorovets A, Johnson TD, Childres WF, Bryan RM. P2 purinoceptor-mediated dilations in the rat middle cerebral artery after ischemia-reperfusion. Am J Physiol. 1999;276:H33–H41. doi: 10.1152/ajpheart.1999.276.1.H33. [DOI] [PubMed] [Google Scholar]
- Marrelli SP, O'Neil RG, Brown RC, Bryan RM. PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am J Physiol Heart Circ Physiol. 2007;292:H1390–H1397. doi: 10.1152/ajpheart.01006.2006. [DOI] [PubMed] [Google Scholar]
- Horiuchi T, Dietrich HH, Tsugane S, Dacey RG. Analysis of purine- and pyrimidine-induced vascular responses in the isolated rat cerebral arteriole. Am J Physiol Heart Circ Physiol. 2001;280:H767–H776. doi: 10.1152/ajpheart.2001.280.2.H767. [DOI] [PubMed] [Google Scholar]
- Pirotton S, Communi D, Motte S, Janssens R, Boeynaems JM. Endothelial P2-purinoceptors: subtypes and signal transduction. J Auton Pharmacol. 1996;16:353–356. doi: 10.1111/j.1474-8673.1996.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Marrelli SP. Mechanisms of endothelial P2Y(1)- and P2Y(2)-mediated vasodilatation involve differential [Ca2+]i responses. Am J Physiol Heart Circ Physiol. 2001;281:H1759–H1766. doi: 10.1152/ajpheart.2001.281.4.H1759. [DOI] [PubMed] [Google Scholar]
- Malmsjo M, Hou M, Pendergast W, Erlinge D, Edvinsson L. The stable pyrimidines UDPbetaS and UTPgammaS discriminate between contractile cerebrovascular P2 receptors. Eur J Pharmacol. 2003;458:305–311. doi: 10.1016/s0014-2999(02)02787-5. [DOI] [PubMed] [Google Scholar]
- Inscho EW, LeBlanc EA, Pham BT, White SM, Imig JD. Purinoceptor-mediated calcium signaling in preglomerular smooth muscle cells. Hypertension. 1999;33:195–200. doi: 10.1161/01.hyp.33.1.195. [DOI] [PubMed] [Google Scholar]
- Zhao G, Adebiyi A, Blaskova E, Xi Q, Jaggar JH. Type 1 inositol 1,4,5-trisphosphate receptors mediate UTP-induced cation currents, Ca2+ signals, and vasoconstriction in cerebral arteries. Am J Physiol Cell Physiol. 2008;295:C1376–C1384. doi: 10.1152/ajpcell.00362.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading SA, Earley S, Waldron BJ, Welsh DG, Brayden JE. TRPC3 mediates pyrimidine receptor-induced depolarization of cerebral arteries. Am J Physiol Heart Circ Physiol. 2005;288:H2055–H2061. doi: 10.1152/ajpheart.00861.2004. [DOI] [PubMed] [Google Scholar]
- Takayasu M, Dacey RG. Calcium dependence of intracerebral arteriolar vasomotor tone and constrictor responses in rats. Stroke. 1989;20:778–782. doi: 10.1161/01.str.20.6.778. [DOI] [PubMed] [Google Scholar]
- Takayasu M, Bassett JE, Dacey RG. Effects of calcium antagonists on intracerebral penetrating arterioles in rats. J Neurosurg. 1988;69:104–109. doi: 10.3171/jns.1988.69.1.0104. [DOI] [PubMed] [Google Scholar]
- Narayanan J, Imig M, Roman RJ, Harder DR. Pressurization of isolated renal arteries increases inositol trisphosphate and diacylglycerol. Am J Physiol. 1994;266:H1840–H1845. doi: 10.1152/ajpheart.1994.266.5.H1840. [DOI] [PubMed] [Google Scholar]
- Westcott EB, Jackson WF. Heterogeneous function of ryanodine receptors, but not IP3 receptors, in hamster cremaster muscle feed arteries and arterioles. Am J Physiol Heart Circ Physiol. 2011;300:H1616–H1630. doi: 10.1152/ajpheart.00728.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales AL, Amberg GC, Earley S. Ca2+ release from the sarcoplasmic reticulum is required for sustained TRPM4 activity in cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2010;299:C279–C288. doi: 10.1152/ajpcell.00550.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Straub SV, Brayden JE. Protein kinase C regulates vascular myogenic tone through activation of TRPM4. Am J Physiol Heart Circ Physiol. 2007;292:H2613–H2622. doi: 10.1152/ajpheart.01286.2006. [DOI] [PubMed] [Google Scholar]
- Crnich R, Amberg GC, Leo MD, Gonzales AL, Tamkun MM, Jaggar JH, et al. Vasoconstriction resulting from dynamic membrane trafficking of TRPM4 in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2010;299:C682–C694. doi: 10.1152/ajpcell.00101.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slish DF, Welsh DG, Brayden JE. Diacylglycerol and protein kinase C activate cation channels involved in myogenic tone. Am J Physiol Heart Circ Physiol. 2002;283:H2196–H2201. doi: 10.1152/ajpheart.00605.2002. [DOI] [PubMed] [Google Scholar]
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navedo MF, Amberg GC, Nieves M, Molkentin JD, Santana LF. Mechanisms underlying heterogeneous Ca2+ sparklet activity in arterial smooth muscle. J Gen Physiol. 2006;127:611–622. doi: 10.1085/jgp.200609519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch U, Mederos y Schnitzler M, Gudermann T. G protein-mediated stretch reception. Am J Physiol Heart Circ Physiol. 2012;302:H1241–H1249. doi: 10.1152/ajpheart.00818.2011. [DOI] [PubMed] [Google Scholar]
- Inoue K, Nakazawa K, Ohara-Imaizumi M, Obama T, Fujimori K, Takanaka A. Antagonism by reactive blue 2 but not by brilliant blue G of extracellular ATP-evoked responses in PC12 phaeochromocytoma cells. Br J Pharmacol. 1991;102:851–854. doi: 10.1111/j.1476-5381.1991.tb12265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamedova LK, Joshi BV, Gao ZG, von Kugelgen I, Jacobson KA. Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem Pharmacol. 2004;67:1763–1770. doi: 10.1016/j.bcp.2004.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.