Abstract
Utilizing a classic stroke model in rodents, middle cerebral artery occlusion (MCAo), we describe a novel neuroregenerative approach using the repeated intranasal administration of cocaine- and amphetamine-regulated transcript (CART) peptide starting from day 3 poststroke for enhancing the functional recovery of injured brain. Adult rats were separated into two groups with similar infarction sizes, measured by magnetic resonance imaging on day 2 after MCAo, and were treated with CART or vehicle. The CART treatment increased CART level in the brain, improved behavioral recovery, and reduced neurological scores. In the subventricular zone (SVZ), CART enhanced immunolabeling of bromodeoxyuridine, a neural progenitor cell marker Musashi-1, and the proliferating cell nuclear antigen, as well as upregulated brain-derived neurotrophic factor (BDNF) mRNA. AAV–GFP was locally applied to the SVZ to examine migration of SVZ cells. The CART enhanced migration of GFP(+) cells from SVZ toward the ischemic cortex. In SVZ culture, CART increased the size of neurospheres. The CART-mediated cell migration from SVZ explants was reduced by anti-BDNF blocking antibody. Using 1H-MRS (proton magnetic resonance spectroscopy), increases in N-acetylaspartate levels were found in the lesioned cortex after CART treatment in stroke brain. Cocaine- and amphetamine-regulated transcript increased the expression of GAP43 and fluoro-ruby fluorescence in the lesioned cortex. In conclusion, our data suggest that intranasal CART treatment facilitates neuroregeneration in stroke brain.
Keywords: BDNF, CART, MRI, neuroregeneration, stroke
Introduction
Current clinical treatment strategies for stroke primarily focus on reducing the size of ischemic damage and on rescuing dying cells early after occurrence. The therapy for stroke is limited by a narrow therapeutic time window after onset of stroke, lack of effective remedy days after stroke, and invasive treatment with cell transplantation. No pharmacological agent has shown effectiveness when therapy is initiated 3 days after stroke in patients.1
The cocaine- and amphetamine-regulated transcript (CART) is a peptide that was found in various brain regions, including striatum, hippocampus, and cortex.2 The expression of CART in the brain was initially considered to be regulated by exogenous amphetamine.2 It was later found that CART mRNA level expression was not regulated by amphetamine in the forebrain, hypothalamus,3 and cerebral cortex.4 Its expression in hypothalamus is influenced by glucocorticoids.5 The interaction of CART with amphetamine is thus still not clearly defined. Cocaine- and amphetamine-regulated transcript is involved in several important physiological responses in energy metabolism and neural injury. The expression of CART is upregulated in the brain after focal cerebral ischemia or electroconvulsive shock in rats.6 Oxygen–glucose deprivation increases CART expression in cultured cortical neurons.7 Treatment with CART before stroke reduces cerebral infarction in mice.7, 8 The protective effect of CART involves increasing ATP production,9 activation of MAPK/ERK,8 and upregulation of brain-derived neurotrophic factor (BDNF).10 These data suggest that CART is protective against ischemic brain injury when given before stroke. However, its regenerative action in poststroke brain has not been investigated.
Several pharmacological approaches have been used preclinically to activate endogenous repair processes after cerebral ischemia. For example, de novo neurogenesis was found in the subventricular zone (SVZ) of adult mammalian brain after stroke.11 Enhancing survival of the endogenous neural progenitor cells (NPCs) in SVZ by the p53 inhibitor pifithrin-α improved the functional recovery in stroke animals.12 Another pharmacological approach for neuroregeneration after cerebral ischemia is through reinnervation to the lesioned site. For example, posttreatment with low-dose amphetamine facilitates functional behavioral recovery by reinnervation in the lesioned side cortex of the stroke brain.4 Since activation of NPC proliferation in SVZ, or reinnervation to the lesioned sites occurs at a later stage,12, 13 compared with the cell death occurring in ischemic core or penumbra, pharmacological intervention to promote neuroregeneration may be effective late after the onset of stroke.
In this study, we demonstrated a neuroregenerative effect of CART, administered noninvasively via an intranasal route, by enhancing the proliferation of endogenous NPCs in SVZ and reinnervation to the lesioned cortex in adult rats days after onset of stroke.
Materials and methods
Surgery and Drug Administration
The use of animals and all experimental protocols were approved by the Animal Care and Use Committee of National Institute on Drug Abuse in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Adult male Sprague-Dawley rats, purchased from Charles River Laboratories (Frederick, MD, USA), were housed in an enriched environment by providing a toy (nylabone) or crinkle paper in their home cages with a 12-hour dark (6 pm to 6 am) and 12-hour light (6 am to 6 pm) cycle. Rats were anesthetized with chloral hydrate (0.4 g/kg, intraperitoneally). The right middle cerebral artery (MCA) was ligated with a 10-O suture and common carotids were clamped bilaterally by nontraumatic arterial clips to generate focal infarction in the cerebral cortex on day 0. The ligature and clips were then removed after 90 minutes of ischemia to allow reperfusion. Core body temperature was maintained at 37°C. The volume of infarction was measured at 2 days after MCA occlusion (MCAo) by T2-weighted imaging (see below). The size of lesion (infarction) was limited to the right cerebral cortex (Supplementary Figure 1A). Animals were separated into two groups with similar lesion sizes, and were treated with CART (lesioned volume=228.6±18.1 mm3) or saline (lesioned volume=226.5±27.6 mm3). Behavioral activity was examined in some animals from both groups on day 2 before treatment. Lesion volume (P=0.950, t test, Supplementary Figure 1B) and behavioral activity (see below) were not different between these two groups before treatment.
Cocaine- and amphetamine-regulated transcript or vehicle was given intranasally. Rats were anesthetized with isoflurane and were placed in a supine position. The CART55–102 (0.1 nmol/10 μL, Peptide Institute, Osaka, Japan) or saline was delivered into the nostrils of each rat at a dose of 40 μL on day 3 after MCAo and then 20 μL daily for another 6 to 9 days. For each daily injection, drugs were equally separated into two doses, given 5 minutes apart, to be delivered via the left and right nostrils. After drug delivery, animals were maintained with isoflurane in a supine position for additional 5 minutes to avoid loss of chemical fluid from the nose. Animals were sent back to their home cages after recovery from anesthesia. The CART treatment did not alter body weight (Supplementary Figure 1C). No animal died during surgery or during poststroke drug treatment.
BrdU Injection
After the 10th dose of CART or vehicle, animals were injected in the peritoneum with 5-bromo-2′-deoxyuridine (BrdU), 50 mg/kg, every 2 hours, four doses, Sigma-Aldrich (St Louis, MI, USA). Animals were killed. The brain tissues were collected for immunostaining 1 day later.
Intracortical Administration of Fluoro-Ruby
Fluoro-ruby (10% in saline, Fluorochrome, LLC, Denver, CO, USA) was administered to the right cortex (AP: −0.23 mm; Lateral 3.0 mm, DV: 1.5 mm from the brain surface) through a glass micropipette (AM Systems, Sequim, WA, USA) on day 14 after MCAo or sham surgery (2 days after 10 day intranasal CART or vehicle treatment). The volume (207±5 nL) of injection was controlled by a pneumatic pump. The brain samples were harvested on day 28 poststroke. The brain sections were mounted on slides, and cover slipped. Histological images were acquired using a Qimage Retiga Exi camera and Nikon 80i (Melville, NY, USA). A total of five images (each 1,114 μm × 637 μm) in the penumbra or corresponding cortex of each section were taken. Intensity of fluorescence was measured by the ImageJ (http://rsb.info.nih.gov/ij/).
Behavioral Measurements
(a) Body asymmetry was analyzed using an elevated body swing test.14 Rats were examined for lateral movements/turning when their bodies were suspended 20 cm above the testing table by lifting their tails. The frequency of initial turning of the head or upper body contralateral to the ischemic side was counted in 20 consecutive trials. The maximum impairment in body asymmetry in stroke animals is 20 contralateral turns/20 trials. In nonstroke rats, the average body asymmetry is 10 contralateral turns/20 trials (i.e., the animals turn in each direction with equal frequency).15
(b) Neurological deficits were also evaluated using Bederson's score.16 In a postural reflex test, rats were examined for the degree of abnormal posture when suspended 20 to 30 cm above the testing table. They were scored according to the following criteria.
0 Rats extend both forelimbs straight. No observable deficit.
1 Rats keep the one forelimb to the breast and extend the other forelimb straight.
2 Rats show decreased resistance to a lateral push in addition to the behavior in score 1 without circling.
3 Rats twist the upper half of their body in addition to behavior in score 2.
(c) Rotarod test: Animals received 3 days of training (twice daily) before MCAo. Each animal was placed in the respective lane, 13.75 inches above the testing platform. For training, the rod was rotated at 5 r.p.m. initially and then accelerated to 20 r.p.m. The test was performed again at 20 r.p.m. 2 days after MCAo as well as after 7 and 11 days of CART or saline treatment. Cutoff time for each test was 360 seconds. Each animal was tested five times per day. The two longest endurance times (ETime) on the rotating rod in five trials were averaged and used for analysis.
AAV Production and Unilateral AAV Injection into Subventricular Zone
The construction of the dsAAV–GFP packaging plasmid is based on AAV serotype 2 genome and has been described previously.17, 18 Viral stocks of AAV serotype 1 were prepared using the triple-transfection method.17 Plasmids used for packaging AAV were generously provided by Dr Xiao Xiao (UNC, Chapel Hill, NC, USA). AAV–GFP (2 μL, 5 × 109 viral genomes/μL) was stereotactically delivered at a speed of 0.5 μL/min into left SVZ (AP: 0.12 mm, ML: 2.1 mm, DV: 3.5 mm to bregma).
Magnetic Resonance Imaging4
Rats underwent MRI (magnetic resonance imaging) on days 2 and 10 after MCAo under isoflurane anesthesia (3% for induction and 1.5% for maintenance) in air/O2 (80:20). Magnetic resonance imaging experiments were performed on a Brucker Biospin 9.4 T animal MRI scanner (Bruker Medizintechnik, Karlsruhe, Germany) equipped with an actively shielded gradient coil. The inner diameter of the gradient coil was 0.12 m, and the maximum gradient strength was 400 mT/m. A birdcage coil driven in linear mode was used for radiofrequency excitation, and a single-turn circular surface coil (2 cm in diameter) was used for signal reception. The animals were secured on a custom made holder equipped with a nose cone for administration of anesthetic gases and ear bars to minimize head motion. Rectal temperature was maintained at 37.5°C±0.5°C with a feedback-controlled, water-circulated heating pad.
T2-Weighted Magnetic Resonance Imaging4
T2-weighted imaging was acquired using a RARE (rapid acquisition with relaxation enhancement) sequence with the following parameters: field of view=3.2 × 3.2 cm2, matrix size=192 × 192 (zero-filled to 256 × 256), TR (repetition time)=2,750 milliseconds, TE (echo time)=13.3 milliseconds, and 23 slices with 1-mm thickness. Ischemic injury size was determined from the hyperintensity region in the T2-weighted images. An area containing ischemic tissue and perilesioned boundaries was first manually traced and mirrored onto the contralateral side in each slice. Voxels in the lesion area with image intensities higher than the mean +2 s.d. of the intensity in the mirrored contralateral area were defined as the infarct zone. The volume of infarction in each animal was obtained from the product of the slice thickness (1 mm) and the sum of lesion areas in all slices.
Proton Magnetic Resonance Spectroscopy (1H-MRS)
Stimulated echo acquisition mode 1H-spectroscopy was used for spectral data acquisition. Single-voxel MR spectral data were obtained with TR/TE=3,000/2 milliseconds, and 512 averages, with a total acquisition time of 25 minutes. For each animal, the voxels for spectral data acquisition were placed on the infarcted and cerebellar (control) brain areas. The volume localized in the infarcted cortex that includes both the core and the penumbra regions was central to the infarcted region, ranging between 28 and 32 cm,3 conforming to the animal's lesion size. The dimensions of the voxel in the cerebellum were 3.8 mm (L/R), 3 mm (A/P), and 4 mm (S/I). In the A/P direction, the cerebellar voxel extended from approximately −7.36 to −9.36 mm, relative to Bregma. Positioning of voxels for all subjects was performed by the same operator to avoid the interoperator variability. The MRS (magnetic resonance spectroscopy) raw data were transferred to an off-line workstation and were processed using the linear combination model (LCModel) software (Oakville, Ontario, Canada). An unsuppressed water signal (TR=3,000 milliseconds, TE=2 milliseconds and number of average=16) was used as an internal reference, and the basis set provided by the vendor with the same scanning parameters was employed for absolute quantifications of metabolite concentrations. Metabolite concentrations are reported in units of mmol/L (mM). The quantification results were rejected if the Cramer-Rao lower bounds were >20%. Peaks of N-acetylaspartate (NAA) and creatine plus phosphocreatine (Cr+PCr) were included in the final analyses. To reduce variability among animals, the change of metabolite concentration in lesion cortex was normalized to the corresponding metabolite concentration in the cerebellum at each stage for each animal.
Enzyme-Linked Immunosorbent Assay, Western Blot Analysis, and Immunohistochemistry
The brain CART level was determined by enzyme-linked immunosorbent assay. Animals were treated with CART or vehicle for 7 days. Animals were killed at 2 hours after the last dose of intranasal delivery of CART or vehicle. For extraction of CART peptide from the brain tissue, cortex tissues were homogenized. Lysates were cleared by centrifugation. The supernatant was loaded on an equilibrated Strata C18-E column (Phoenix Pharmaceuticals, Waldorf, MD, USA). Equilibration was performed by washing with 1 mL of buffer B (60% acetonitril in 1% trifluoroacetic acid) and with 3 mL of buffer A (Phoenix Pharmaceuticals). The peptides were then eluted with 3 mL of buffer B (60% acetonitril in 1% trifluoroacetic acid) and collected in a 15-mL centrifuge tube, evaporated to dryness, and the residue was dissolved in 150 μL of EIA buffer (Phoenix Pharmaceuticals). The CART levels were measured by a sensitive EIA employing an antibody against CART (Phoenix Pharmaceuticals). For the EIA, 50 μL of each sample were used in each assay and duplicate samples were measured for each brain sample.
The GAP43 protein level was determined by Western analysis. The frozen cortical tissues were homogenized. Lysates were cleared by centrifugation and total protein concentration in each sample was determined. After preblocking in Odyssey blocking buffer (Li-Cor, Lincoln, NE, USA) overnight at 4°C, the membranes were incubated with primary antibody to anti-Actin (1:2,500, Sigma, St Louis, MO, USA) and anti-GAP43 (1:2,000, Santa Cruz, Santa Cruz, CA, USA) at room temperature for 2 hours, then incubated for 90 minutes in goat anti-rabbit IR-700 nm or goat-anti-mouse IR-800 nm secondary antibodies (1:2,500, Li-Cor). The membranes were scanned using an Odyssey Infrared Imager (Li-Cor). Immunoblots were quantified with ImageJ.
BrdU, Musashi-1, and proliferating cell nuclear antigen (PCNA) immunoreactivity was determined by immunohistochemistry. Animals were anesthetized with chloral hydrate (400 mg/kg, intraperitoneally) and perfused transcardially with saline followed by 4% paraformaldehyde in phosphate buffer (PB; 0.1 mol/L; pH 7.2). The brains were dissected, postfixed in paraformaldehyde for 18 to 20 hours, and transferred to 18% sucrose in 0.1 mol/L PB for at least 16 hours. Serial sections of the entire brain were cut at 25 μm thickness on a cryostat. BrdU and PCNA immunostaining were carried out as described.12 One series from every sixth section was stained for each antibody used. To control for staining variability, specimens from all experimental groups were included in every batch and reacted together in a net well tray under the same conditions. Sections were rinsed in PB and were blocked with 4% BSA with 0.3% Triton X-100 in 0.1 mol/L PB. Sections were then incubated with polyclonal anti-Musashi-1 (1:100, Sigma), polyclonal anti-BrdU (1:500, Millipore, Billerica, MA, USA), or anti-PCNA (1:400, Dako, Carpinteria, CA, USA) at 4°C for 24 hours. Sections were rinsed in 0.1 mol/L PB and incubated in Alexa Fluor 568 secondary antibody solution (1:500; Molecular Probes, Eugene, OR, USA). For PCNA or BrdU immunoreactivity, sections were rinsed in 0.1 mol/L PB and incubated in biotinylated IgG in PB (1:200; Vector Laboratories, Burlingame, Billerica, CA, USA) for 1 hour, followed by incubation for 1 hour with avidin–biotin–horseradish peroxidase complex. Staining was developed with 2,3′diaminobenzidine tetrahydrochloride (0.5 mg/mL in 50 mmol/L Tris–HCl buffer 7.4). Control sections were incubated without primary antibody. Sections were mounted on slides and cover slipped. Histological images were acquired using a Qimage Retiga Exi camera and Nikon 80i. Unbiased stereological analysis of the PCNA-positive cell population volume within the SVZ was performed using stereological principles and analyzed with StereoInvestigator software (Microbrightfield, Williston, VT, USA). The Cavalieri estimator was used and carried out on a Leica DM5000B microscope (Leica Microsystems, Bannockburn, IL, USA) equipped with a motorized stage and Lucivid attachment ( × 40 objective). Proliferating cells in the SVZ area were outlined on the basis of PCNA immunolabeling. Every sixth section was measured, and values from a total of 10 sections were calculated for each animal. For each tissue section analyzed, section thickness was assessed at each sampling site and a grid size of 25 μm was used. A point was clicked if it was within the outlined area and overlayed with a PCNA+ cell body. A total of eight animals (four control+four CART-treated animals) were counted. The actual points that were clicked ranged from 1,849 to 2,621/animal depending on treatment. Coefficients of error were all <0.10 (mean CE for CART-treated group equals 0.0145 and mean CE for control-treated group equals 0.0130).
Quantitative Reverse Transcription–Polymerase Chain Reaction
The SVZ was dissected out from stroke brains and processed for RNA isolation and DNAse I treatment using the RNAqueous-Micro Ambion kit (Applied Biosystems, Grand Island, NY). Total RNA was reverse transcribed into cDNA using the Superscript III reverse transcriptase kit. cDNA levels for HPRT, Hmbs, BDNF, GDNF (glial cell line-derived neurotrophic factor), and bone morphogenetic protein-7 (BMP7) (Table 1) were determined by specific universal probe Library primer probe sets (Roche, South San Francisco, CA) using quantitative reverse transcription–polymerase chanin reaction (RT–PCR).
Table 1. Oligonucleotide sequences used for quantitative RT–PCR.
| Forward primer | Reverse primer | Mouse/rat universal probe Library #, Roche | |
|---|---|---|---|
| HPRT1 | 5′-TGATAGATC CATTCC TATGACTGTAGA | 5′-AAGACATTCTTTCCAGTTAAAGTTGAG | 22 |
| Hmbs | 5′-TCC CTG AAG GAT GTG CCT AC | 5′-ACA AGG GTT TTC CCG TTT G | 79 |
| BDNF | 5′-CACTTTTGAGCACGTCATCG | 5′-TCCTTATGGTTTTCTTCGTTGG | 42 |
| GDNF | 5′-TAAGATGAAGTTATGGGATGTCG | 5′-CTTCGAGAAGCCTCTTACCG | 112 |
| BMP7 | 5′-CGAGACCTTCCAGATCACAGT | 5′-CAGCAAGAAGAGGTCCGACT | 1 |
Abbreviations: BDNF, brain-derived neurotrophic factor; BMP7, bone morphogenetic protein-7; GDNF, glial cell line-derived neurotrophic factor; RT–PCR, reverse transcription–polymerase chain reaction.
Subventricular Zone Neurosphere Cultures
Subventricular zone cells were collected from adult rat brains.12 Cells were plated in 24-well plates in 10% DMEM/F12 medium supplemented with 2% B27 supplement, 2 mmol/L ℒ-glutamine, 0.5% HEPES, 1% Pen/Strep, 0.02% heparin, epidermal growth factor (40 ng/mL), and basic Fibroblast growth factor (FGF) (10 ng/mL). Cocaine- and amphetamine-regulated transcript peptide, vehicle, or anti-human BDNF blocking antibody (1:1,000) was added from DIV1 to DIV7. The size of neurospheres was counted on DIV8.
Subventricular Zone Explant Culture
Subventricular zone explant cultures were prepared from adult male rats. Subventricular zone explants were cultured within Matrigel (BD Biosciences, San Jose, CA) in Neural basal-A medium containing 2% B27 supplement (Invitrogen, Grand Island, NY). The cultured SVZ explants were treated with CART (0, 100 nmol/L, 200 nmol/L) on days 1, 2, and 3 and some explants cultured with anti-human BDNF blocking antibody (G1641, Promega, 1:1,000, Madison, WI) on days 2 to 4. The distance of SVZ cell migration was examined from days 2 to 7 after culture.
Statistical Analysis
Values are represented as mean±s.e.m. Unpaired t-test and one- or two-way analysis of variance (ANOVA) with post-hoc Bonferroni tests were used for statistical analysis. A statistically significant difference was defined as P<0.05.
Results
Intranasal Administration of Cocaine- and Amphetamine-Regulated Transcript Increases Brain Cocaine- and Amphetamine-Regulated Transcript Levels
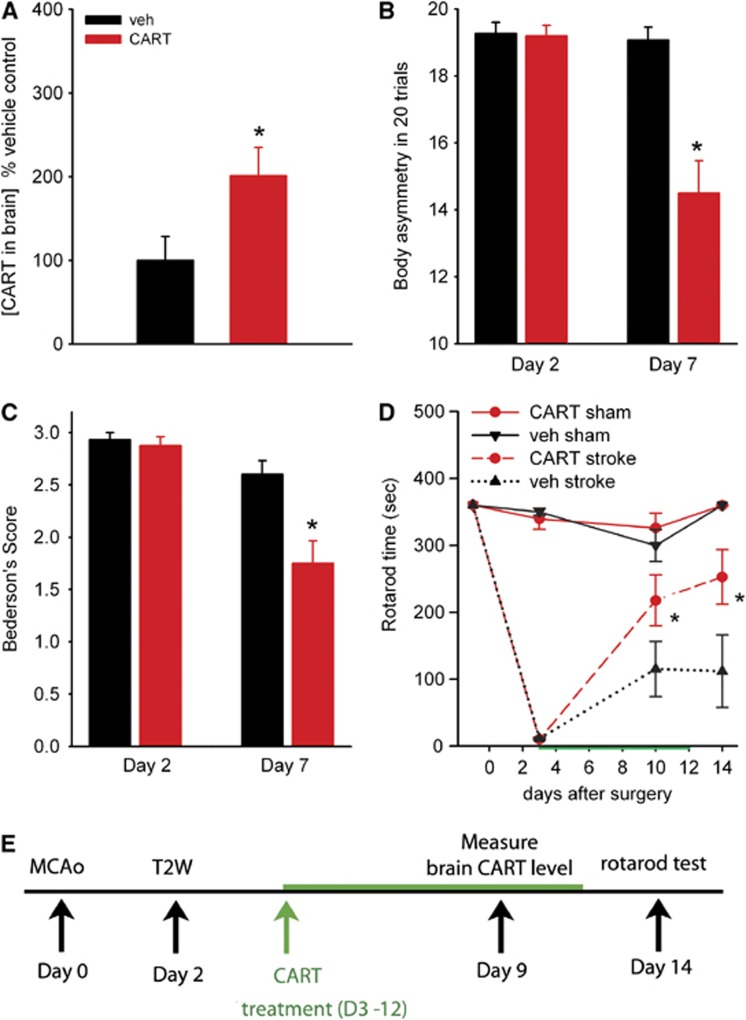
A total of 14 stroke animals were treated with CART (n=6) or vehicle (n=8) for 7 days starting from the 3rd day after MCAo (see timeline Figure 1E). Cortical tissue was harvested at 2 hours after the last dose of CART or vehicle. There was a significant elevation of CART levels in animals receiving CART treatment (P=0.039, Figure 1A). Our data thus demonstrate an efficient route for delivering exogenous CART to the stroke brain in that, at 2 hours after intranasal delivery, CART concentrations in the brain were doubled. More importantly, this approach may have a great potential for clinical use in stroke patients, especially when repeated administration is needed.
Figure 1.
Poststroke treatment with cocaine- and amphetamine-regulated transcript (CART) improves behavioral recovery. (A) Intranasal delivery of CART increased brain CART concentration. CART (n=6) or vehicle (n=8) was delivered into nostrils of each rat at dose of 40 μL on day 3 after middle cerebral artery occlusion (MCAo) and then 20 μL daily for another 6 days. Cerebral cortical tissue was harvested at 2 hours after the last dose of CART or vehicle. CART levels were measured by enzyme-linked immunosorbent assay (ELISA). All data were normalized to the mean CART level in the animals receiving vehicle. There is a significant elevation of brain CART protein level in animals receiving CART, compared with vehicle (P=0.04). (B) Poststroke treatment with CART improves behavioral recovery. Before intranasal treatment, all animals demonstrated close to 100% body asymmetry in 20 trials at 2 days after MCAo. CART treatment significantly reduced body asymmetry on day 7 post-MCAo (P<0.001). (C) Bederson's neurological tests were carried out on day 2 (before treatment) and on day 7 (after treatment). Intranasal CART treatment significantly reduced Bederson's score on day 7 post-MCAo (P<0.001). (D) Rotorod tests were taken before and days 2, 10, and 14 after MCAo or sham surgery. Treatment with CART significantly increased rotarod Etime in stroke animals (P=0.010, two-way analysis of variance (ANOVA), *P=0.031, post-hoc Bonferroni test). Error bars show mean values±s.e.m. (E) Timeline of treatment.
Behavioral Recovery Induced by Cocaine- and Amphetamine-Regulated Transcript Treatment
We next studied if poststroke intranasal treatment with CART would facilitate functional recovery using three behavioral tests on different days.
(i) Body asymmetry. Elevated body swing test14 was used to examine body asymmetry with 20 trials per each animal. Previous studies have shown that there is almost no body asymmetry (i.e., close to 10 in 20 trials) in nonstroke rats.15 Body asymmetry tests were thus examined only in stroke rats. A total of 31 stroke rats were examined on days 2 and 7 after MCAo. Before intranasal treatment, all animals demonstrated close to 100% body asymmetry at 2 days after MCAo. After CART treatment, there was a significant reduction in body asymmetry (P<0.001, two-way ANOVA). Post-hoc analysis indicated that CART treatment significantly reduced body asymmetry on day 7 post-MCAo (P<0.001, Figure 1B).
(ii) Bederson's neurological test was carried out on days 2 and 7 after MCAo (n=31, same animals used for body asymmetry test) or sham (no stroke) surgery (n=11). In all nonstroke rats, no neurological deficit was found and Bederson's score was zero. In stroke animals, no difference was found before CART treatment on day 2 (P=0.767, Figure 1C). Intranasal CART treatment significantly reduced Bederson's score on day 7 post-MCAo (P<0.001, Figure 1C).
(iii) Rotarod test. A total of 27 rats were used for the rotarod test 1 day before (or day −1), 3, 10, and 14 days after MCAo or sham surgery. These animals received 3 days of rotarod training before MCAo (n=16) or sham (n=11) surgery. The average endurance times (ETime) before surgery in all groups was 360 seconds (cutoff time, Figure 1D). At 3 days after MCAo (before treatment), ETime significantly dropped to <30 seconds in stroke animals, compared with control animals (P<0.001, F3,23=692.936, one-way ANOVA; P<0.001, post-hoc Bonferroni test). A significant increase in Etime was found in stroke animals receiving CART, comparing to vehicle, on day 10 (Figure 1D, P=0.023, two-way ANOVA + post-hoc Bonferroni test) and day 14 (P=0.004). No difference was found on day −1 (P=1.000 or day 3 (P=0.978) before treatment in these animals. Taken together, these data suggest that posttreatment with CART can induce behavioral improvement in stroke animals.
Cocaine- and Amphetamine-Regulated Transcript Induces Proliferation of Neural Progenitor Cells in Subventricular Zone in Stroke Rats
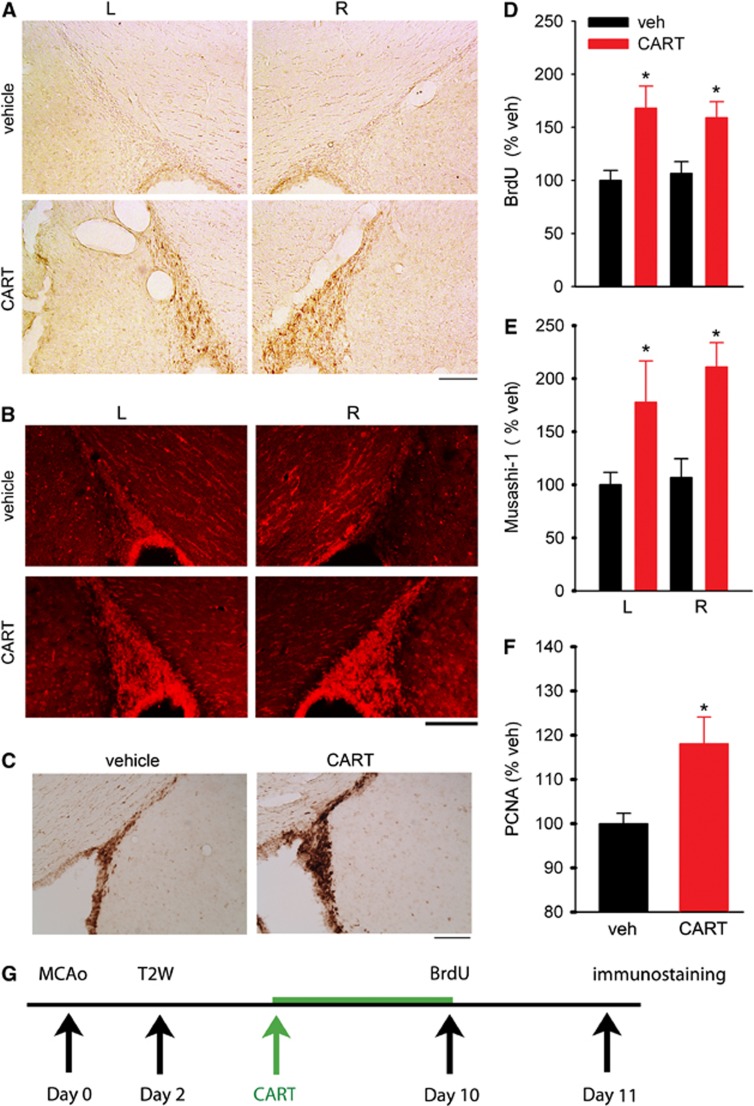
Stroke rats were treated with either CART (n=8) or vehicle (n=8) from days 3 to 10 after MCAo; BrdU was given on day 10. The brain tissues were collected on day 11 (see timeline Figure 2G). The CART treatment enhanced BrdU incorporation in SVZ, both ipsilateral and contralateral to the lesion (Figure 2A). We previously reported that anterior SVZ (AP: +0.2 mm to bregma) showed more BrdU incorporation than the posterior SVZ.12 The density of BrdU immunoreactivity in SVZ was averaged from three slices at the level of anterior commissure (AP: +0.2 mm from bregma) as described previously.12 Posttreatment with CART significantly enhanced BrdU immunoreactivity in SVZ in stroke rats (P<0.001, F1,28=16.701, two-way ANOVA, Figure 2D). Similar to previous reports,12 no difference was found between ipsilateral and contralateral SVZ (P=0.931, two-way ANOVA). The enhanced BrdU labeling in SVZ in CART-treated animals suggests an increase in cell proliferation in the SVZ after ischemic insults. Immunoreactivity for Musashi-1, an NPC marker,19 was examined in 15 stroke rats on day 11 poststroke. The CART treatment significantly enhanced Musashi-1 immunoreactivity in SVZ (P<0.001, F1,26=14.433, two-way ANOVA, Figures 2B and 2E). No difference was found between ipsilateral and contralateral SVZ (P=0.408, two-way ANOVA). The proliferation in SVZ was further confirmed by an increase in immunoreactivity of PCNA, an endogenous protein specifically expressed during the cell proliferation cycle, in the rostral subependymal zone of SVZ in another eight stroke rats. An example of the increase in PCNA immunoreactivity by CART in stroke animals is demonstrated in Figure 2C. Similar to BrdU and Musashi-1, the increase in PCNA was found in both hemispheres. In all animals studied, a significant increase in PCNA(+) cell density (P=0.033, Figure 2F) was found in the rostral SVZ (AP: +0.2 mm to bregma) in CART-treated rats (n=4), compared with vehicle-treated rats (n=4), on day 11 after MCAo.
Figure 2.
Treatment with cocaine- and amphetamine-regulated transcript (CART) increased cell proliferation in subventricular zone (SVZ) in stroke rats. (A) BrdU immunoreactivity was enhanced in SVZ bilaterally in a stroke animal receiving intranasal CART (lower panels), compared with vehicle (upper panels). (B) CART treatment increased Musashi-1 immunoreactivity bilaterally in SVZ in stroke animals (lower panels). (C) Photomicrographs demonstrate that CART treatment increased proliferating cell nuclear antigen (PCNA) immunostaining in the anterior subependymal zone of a stroke rat (right panel). (A–C) Calibration=100 μm. (D–F) In all animals studied, intranasal treatment with CART significantly enhanced (D) BrdU and (E) Musashi-1 immunoreactivity in SVZ. Data in (D, E) were normalized to the mean immunoreactivity in the left SVZ of stroke rats. (F) Density of PCNA-positive cells in the anterior subependymal zone was significantly increased by CART in stroke rats as determined by the Cavalieri method using unbiased stereological analysis. *P<0.05. (G) Timeline of treatment.
Cocaine- and Amphetamine-Regulated Transcript Induces Migration of Subventricular Zone Cells in Stroke Rats
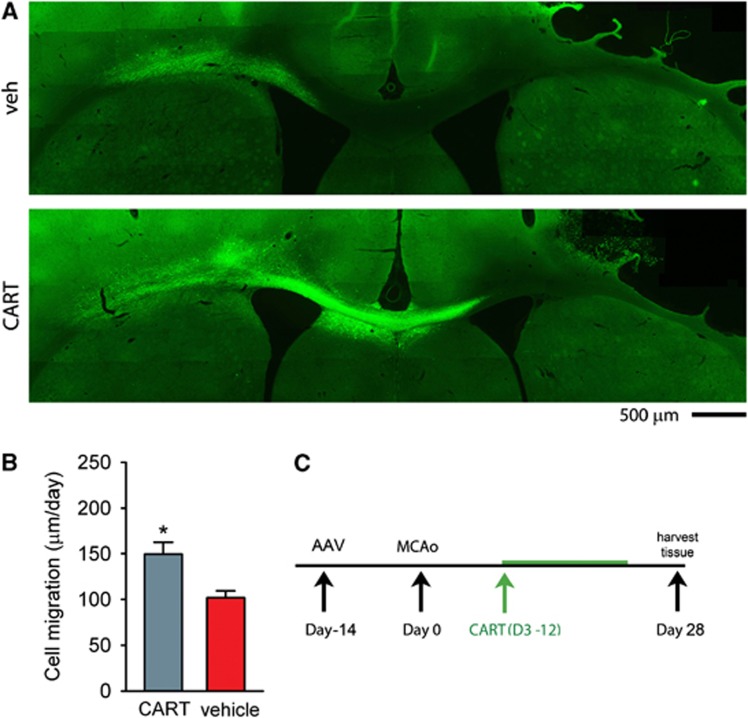
AAV–GFP was given to the contralateral (left) SVZ at 14 days before MCAo (n=13) or sham surgery (n=8). Animals were treated with CART or vehicle for 10 days starting from 3rd day after MCAo (Figure 3C). The brain tissues were harvested 28 days after MCAo. GFP(+) cells migrated bilaterally from the injection site, mainly toward to the lesioned hemisphere in stroke animals (Figure 3A). Limited cell migration along the corpus callosum was found in the nonstroke animals. Treatment with CART enhanced GFP(+) cell migration from the contralateral SVZ toward the ischemic cortex only in stroke animals (Figure 3A). The rate of cell migration (μm per day) was calculated by averaging the distance that GFP(+) cells traveled from the contralateral SVZ toward the ischemic hemisphere after CART treatment (i.e., 25 days). The CART treatment significantly increased the rate of cell migration in stroke animals (Figure 3B, P=0.011, t test).
Figure 3.
Cocaine- and amphetamine-regulated transcript (CART) enhances migration of cells from subventricular zone (SVZ) in stroke rats. (A) AAV–GFP was given into the contralateral (left) SVZ at 14 days before middle cerebral artery occlusion (MCAo). Animals were treated with vehicle (upper panel) or CART (lower panel) for 10 days after MCAo. CART increased migration of GFP(+) cells from SVZ, along the corpus callosum, toward lesioned side hemisphere (right). (B) In 13 stroke animals studied, the rate of cell migration (μm per day) was calculated by averaging the distance of GFP(+) cells traveling from the contralateral SVZ toward ischemic hemisphere after CART treatment (i.e., 25 days). CART treatment significantly increased the rate of cell migration in stroke animals (P=0.011). (C) Timeline.
Cocaine- and Amphetamine-Regulated Transcript Upregulates Brain-Derived Neurotrophic Factor mRNA Expression in Subventricular Zone of Stroke Rats
Previous studies have shown that trophic factors can induce neuroregeneration through their action in SVZ. The expression of selective trophic factors was examined in 13 stroke rats. Subventricular zone tissue was collected after 7-day CART (n=6) or vehicle (n=7) treatment in stroke rats. A significant increase in BDNF mRNA levels was found in rats receiving CART treatment (P=0.018, Figure 4A). Glial cell line-derived neurotrophic factor or BMP7 levels were not altered by CART treatment (GDNF, P=0.07; BMP7, P=0.803).
Figure 4.
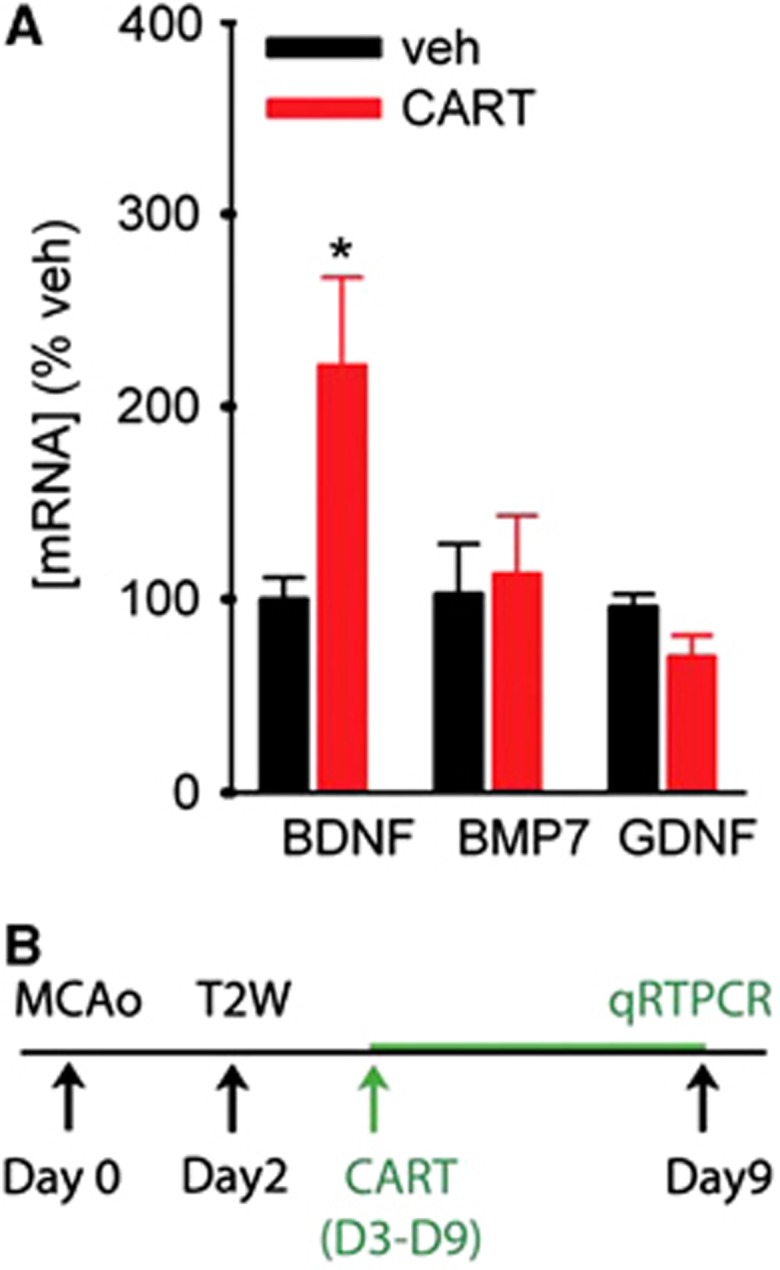
Cocaine- and amphetamine-regulated transcript (CART) treatment significantly increased brain-derived neurotrophic factor (BDNF) in subventricular zone (SVZ) of stroke rats. SVZ tissue was collected after 7-day CART or vehicle treatment from 13 stroke rats. (A) CART treatment significantly increased BDNF, but not bone morphogenetic protein-7 (BMP7) or glial cell line-derived neurotrophic factor (GDNF), mRNA expression in SVZ (*P=0.018). All the gene levels are normalized to the reference gene Hmbs. (B) Timeline.
Trophic Response to Cocaine- and Amphetamine-Regulated Transcript in Subventricular Zone Culture
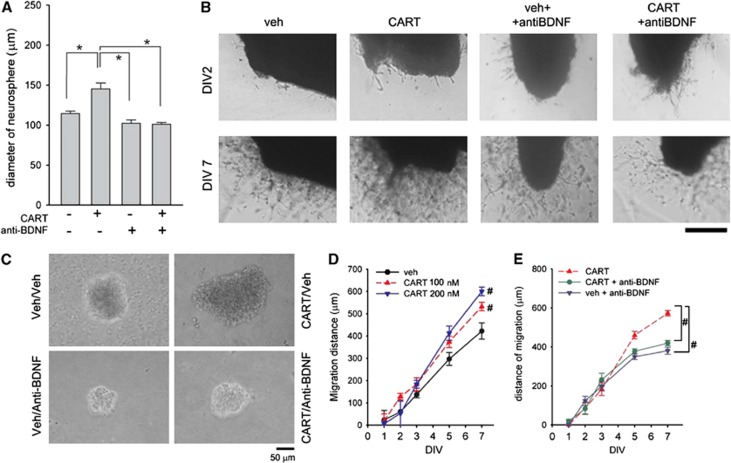
Subventricular zone cells were collected from adult rat brains for neurosphere cultures. The CART or vehicle was added to the SVZ progenitor cell cultures from DIV1 to DIV7. Representative images of the neurospheres treated with vehicle or CART (200 nmol/L) were taken on DIV8 (Figure 5C). Neurospheres with a diameter >90 μm were used to analyze the trophic response to CART. Administration of CART, as compared with vehicle controls, significantly increased the diameter of neurospheres (Figure 5A, P<0.001, F3,94=11.560, one-way ANOVA). This response was significantly antagonized by cotreatment with antihuman BDNF blocking antibody (1:1,000, P=0.008, post-hoc Bonferroni test, Figures 5A and 5C).
Figure 5.
Trophic response to cocaine- and amphetamine-regulated transcript (CART) in subventricular zone (SVZ) culture. (A, C) CART treatment increased the proliferation of SVZ progenitor cells in vitro. CART (200 nmol/L) or vehicle was added to the SVZ progenitor cell cultures from DIV1 to DIV7. (A) Administration of CART, as compared with vehicle controls, increased the size of neurospheres (*P<0.05, one-way analysis of variance (ANOVA)). This response was antagonized by cotreatment with anti-brain-derived neurotrophic factor (anti-BDNF). (C) Representative images of the neurospheres in 24-well plates were taken on DIV8. Calibration=50 μm. (B, D) CART treatment enhances the cell migration from the SVZ explants. The cultured SVZ explants were treated with CART (0, 100 nmol/L, 200 nmol/L) on days 1, 2, and 3 and some were cultured with anti-human BDNF blocking antibody (1:1,000) on days 2 to 4. The distance of SVZ cell migration from the explants was examined under microscope from days 2 to 7 after culture. (B) Representative images of SVZ explants. CART treatment increased cell migration from SVZ explants on DIV7. Cotreatment with anti-BDNF antagonized CART-mediated cell migration. There was minimal migration on DIV2. Calibration=1 mm. (D) Treatment with CART dose dependently enhanced the distance and density of migration. (E) Addition of anti-BDNF blocking antibody significantly reduced CART (100 nmol/L)-mediated cell migration. #P<0.05, two-way analysis of variance (ANOVA) + post-hoc Bonferroni test.
Cocaine- and Amphetamine-Regulated Transcript Induces Cell Migration from Subventricular Zone Explants
The CART-induced cell migration from SVZ explants was next examined in Matrigel cultures. The cultured SVZ explants were treated with CART (0, 100 nmol/L, 200 nmol/L) on days 1, 2, and 3 and some were cultured with antihuman BDNF blocking antibody (1:1,000) on days 2 to 4. The distance of SVZ cell migration from the explants was examined under a microscope from days 2 to 7 after culture. There was minimal migration on DIV2 (Figure 5B, top panels). Treatment with CART enhanced the distance of cell migration on DIV7 (Figure 5B lower panels; Figure 5D, P<0.001, F2,15=69.407, two-way ANOVA; P<0.001, post-hoc Bonferroni test). Addition of anti-BDNF blocking antibody (1:1,000) significantly reduced CART enhanced cell migration from SVZ explants (P<0.001, F2,240=7.759, two-way ANOVA; P=0.011, post-hoc Bonferroni test, Figures 5B and 5E). These data further suggest that BDNF is involved in CART-induced SVZ cell migration.
Cocaine- and Amphetamine-Regulated Transcript Induced Neural Repair in Lesioned Cortex
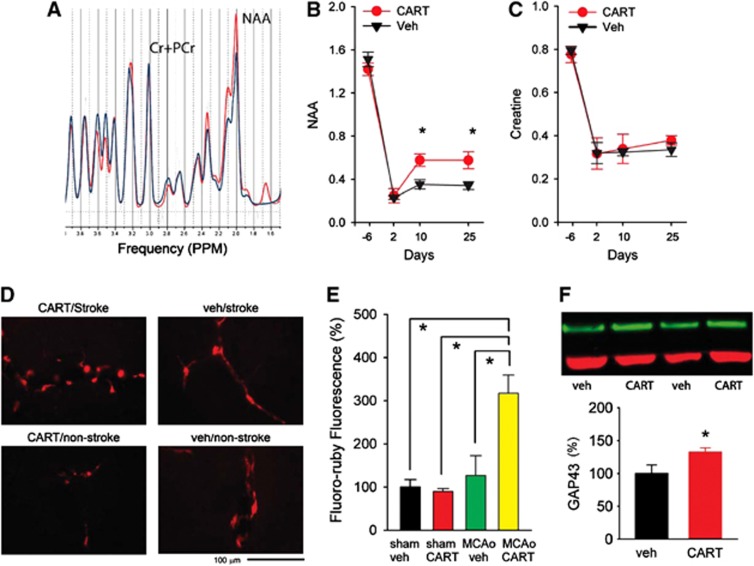
N-acetylaspartate, a marker for mature neurons, in lesioned side cortex was measured by proton magnetic resonance spectroscopy (1H-MRS) 6 days before and 2, 10, and 25 days after MCAo in 12 stroke animals (Figure 6A). The CART or vehicle was administered from day 3 to day 12. The CART treatment significantly increased NAA (Figure 6B, P=0.020, F1,40=5.828, two-way ANOVA). There is a significant interaction between CART and days of treatment (P=0.013, F1,40=4.088, two-way ANOVA). The post-hoc Bonferroni test indicates that CART significantly increased NAA activity in the lesioned cortex on day 10 (P=0.008) and day 25 (P=0.005) after MCAo. The CART treatment did not alter the house-keeping signal, creatine+phosphocreatine (Cr+PCr, Figure 6C), concentration in the lesioned cortex (P=0.796, F1,40=0.0934, two-way ANOVA).
Figure 6.
Cocaine- and amphetamine-regulated transcript (CART)-mediated changes in the lesioned side cortex. (A) Representative neurochemical tracings in the lesioned cortex from animals treated with CART (red tracing) or vehicle (blue tracing) measured by 1H-MRS (proton magnetic resonance spectroscopy) on day 25 after middle cerebral artery occlusion (MCAo). (B, C) In 12 stroke animals studied, treatment with CART significantly increased acetylaspartate (NAA), but not the house-keeping signal creatine+phosphocreatine (Cr+PCr), in the lesioned side cortex on day 10 and 25 after stroke (*P<0.05, two-way analysis of variance (ANOVA)). (D) Representative photomicrographs demonstrate an increase of fluoro-ruby fluorescence in the perilesioned cortex in a stroke rat receiving CART treatment. Fluoro-ruby tracer was administered to the lesioned side cortex on day 14 after MCAo or sham surgery. Fluorescence was examined on day 28 after stroke. (E) In 18 animals studied, intensity of fluorescence was analyzed by ImageJ and was normalized to the mean of control animals receiving vehicle treatment. A significant increase in fluoro-ruby fluorescence was found in stroke animals treated with CART, compared with all other groups (P<0.001, one-way ANOVA). No difference in fluorescence was found in nonstroke animals treated with CART or vehicle. (F) CART treatment significantly enhanced GAP43 immunoreactivity in the lesioned side cortex in 12 stroke rats (P<0.05, t test). GAP43 immunoreactivity was normalized to stroke animals receiving vehicle. Example of GAP43 Western blotting from four stroke rats (upper panel). Green: GAP43; Red: ACT.
To further examine the reinnervation in lesioned side cortex, a total of 18 rats (9 stroke, 9 sham) received intracortical administration (right cortex, AP: −0.23 mm; lateral 3.0 mm, DV: 1.5 mm from brain surface) of fluoro-ruby (207±5 nL) on day 14 after MCAo or sham surgery. Cocaine- and amphetamine-regulated transcript or vehicle was administered from day 3 to day 12. Fluorescence was examined on day 28 poststroke. Fluoro-ruby fluorescence was greatly enhanced in the perilesioned cortex in stroke animals receiving CART treatment (Figure 6D). The intensity of Fluoro-ruby fluorescence in the penumbra cortex or corresponding area was averaged from each animal. Intensity of fluorescence was normalized to the mean of control animals receiving vehicle treatment (Figure 6E). A significant increase in fluoro-ruby fluorescence was found in stroke animals treated with CART, compared with all other groups (Figure 5E, P<0.001, F3,14=11.941, one-way ANOVA; P<0.005, post-hoc Bonferroni test). No difference in fluorescence was found in nonstroke animals treated with CART or vehicle. An additional 12 stroke rats were used for Western blot analysis after 10-day CART or vehicle treatment. GAP43 immunoreactivity was normalized to stroke animals receiving vehicle. Poststroke treatment with CART significantly enhanced growth-associated protein 43 (GAP43) immunoreactivity in the lesioned side cortex (Figure 6F, P=0.048, t test).
Discussion
In this study, we describe a delayed noninvasive poststroke therapeutic approach augmenting functional recovery after stroke. Stroke animals receiving intranasal CART treatment from day 3 after MCAo showed enhanced neural repair by facilitating endogenous NPC proliferation and migration from SVZ, increasing NAA levels in the lesioned cortex, and improving the functional recovery. Our data support a neuroregenerative role of CART in an animal model of stroke.
Many studies have demonstrated a spontaneous behavioral recovery over a period of time in stroke animals. These include neurological scores for posture, step through latency,20 open field locomotor activity,21 and the paw placing test.22 The change in body asymmetry, however, was relatively consistent over 2 weeks after MCAo in rats.4 In this study, behavioral tests were examined in 2 weeks after stroke. Stroke animals that received CART treatment had less body asymmetry and neurological scores as well as an increase in rotarod time.
Previous studies have indicated that maximal cerebral infarction after distal MCAo can be achieved 24 hours after reperfusion.23, 24 The size of lesion here was thus measured by T2W imaging on day 2 after stroke. Since similar sized infarction and behavioral deficits were found in our treatment versus control vehicle groups on day 2 after MCAo while pharmacological treatment was initiated from day 3 after MCAo, the behavioral recovery seen in our study is therefore not related to the changes in the volume of cerebral infarction. These data further suggest that posttreatment with CART induces behavioral improvement in stroke animals.
There are several limitations to deliver drug to brain days after the onset of stroke. Drugs given systemically may not easily cross the blood–brain barrier and can be degraded through first pass metabolism. Intracerebral delivery is not feasible for repeated drug administration and may require chronic cannulation. Previous reports have indicated that CART is a small peptide with molecular weight close to 5.3 kDa, and a recent study indicated that CART peptide, when given peripherally, is able to cross the blood–brain barrier.25 Furthermore, selected peptides26 can reach brain parenchyma noninvasively through intranasal delivery. In our study, we demonstrated a significant elevation of CART levels in the brain after intranasal CART treatment. Our data support that CART can be given repeatedly and efficiently to reach brain parenchyma after nasal delivery in stroke rats.
In this study, multiple doses of CART were given intranasally from day 3 after MCAo. We found that poststroke CART treatment induced neuroregeneration and behavioral improvement. A recent study has demonstrated that intranasal BDNF administration given, as a single dose 2 hours after MCAo, protects against ischemic insults by modulating inflammation.27 Since CART increased BDNF mRNA expression in the brain, it is possible that a similar protective response can be achieved if CART is given intranasally before stroke.
Ischemic brain injury induces SVZ neurogenesis in the adult brain.28, 29 Depending on the stroke model used, the activation of NPC proliferation in the SVZ varies after ischemia.13 We previously examined the kinetics of NPC proliferation in the stroke model used in the current studies. A robust increase of BrdU immunoreactivity in SVZ was found as early as 2 days after MCAo, sustained through 4 days after MCAo. This increase started to decline between day 6 to day 8, and returned to basal levels around day 10.12 In this study, rats were treated with either CART from days 3 to 10 after MCAo. We found that both BrdU and the NPC marker Musashi-1 labeling are enhanced on day 11 in stroke animals receiving CART treatment, suggesting that CART enhanced NPCs proliferation in the SVZ after ischemic insults. The proliferation of these cells from the SVZ was also confirmed by another marker, PCNA, an endogenous protein that is specifically expressed during the cell proliferation cycle. PCNA immunoreactivity in the SVZ was enhanced after CART treatment in stroke rats. CART also increased the size of new neurosphere formation in cultures. These data suggest that the positive effect of CART on NPCs from SVZ is reflected in survival and growth in vitro.
We administered AAV–GFP locally to the contralateral (or nonlesioned side) SVZ to examine migration of SVZ cells. AAV–GFP was not applied to the ipislateral SVZ mainly because the ipsilateral SVZ is close to the area of infarction in the ischemic cerebral cortex as seen in Supplementary Figure 1A. Infection or the expression of AAV–GFP in the ipislateral SVZ or the survival of GFP(+) cells in the ipislateral SVZ may be confounded by the size of lesioning in the ipislateral SVZ after MCAo. We demonstrated that CART treatment facilitates GFP(+) cells migration to the ipsilateral hemisphere in stroke animals. Using the calculated rate of cell migration after CART treatment (Figure 3B), it is reasonable that NPCs in the ipsilateral SVZ can migrate much earlier to the lesioned cortex after CART treatment and induce behavioral improvement as seen on days 7 and 14.
Several trophic factors have been shown to induce neuroregeneration in stroke animals. BMP7, given after stroke, reduced neurological scores and improved locomotor activity through enhancing proliferation of NPCs in SVZ.21, 30 Intracerebral infusion of GDNF promotes neurogenesis after stroke.31 Increasing BDNF expression is associated with behavioral recovery in stroke brain.4 In vitro studies have also demonstrated that BDNF enhanced cell migration from SVZ explants.32 In this study, CART treatment selectively increased BDNF mRNA expression in SVZ from stroke rats. To further define the interaction of CART and BDNF in SVZ for regenerative activity, we demonstrated that CART, similar to BDNF, significantly increased cell migration from SVZ both in vivo and in vitro. The CART-mediated migration response was attenuated by coadministration of an anti-BDNF blocking antibody, suggesting that the CART-induced migration of SVZ cells is mediated through BDNF. Taken together, these data suggest that CART treatment enhanced cell migration from SVZ, which may contribute to neural repair after stroke.
Using 1H-MRS, we also demonstrated that CART increased the signal of a neuronal marker, NAA, in the lesioned cortex. There is also a significant increase in fluoro-ruby fluorescence in the penumbra cortex and GAP43 expression in the lesioned cortex. These data suggest that CART poststroke treatment may enhance reinnervation of the lesioned cortex. More detailed experiments are required to verify this finding.
Several studies have demonstrated that posttreatment with amphetamine induce neuroregeneration in experimental stroke animals. For example, amphetamine improved cognitive performance33, 34 and enhanced long-term improvement in forelimb motor function in stroke rats.35 We also recently demonstrated that poststroke treatment with amphetamine reduced body asymmetry and neurological scores while increasing expression of the axonal proteins synaptophysin and neurofilament in the lesioned cortex.4 However, recent studies have demonstrated that amphetamine did not alter CART expression in stroke4 and nonstroke rodents.3 It is possible that differential mechanisms may be involved in CART and in amphetamine-mediated regeneration in stroke animals.
In this study, we found that poststroke treatment with CART enhances behavioral recovery, increases proliferation and migration of SVZ NPCs as well as neuronal markers in the lesioned cortex. Similarly, a significant correlation between the behavioral outcome and number of surviving BrdU-positive cells migrating to the lesioned cortex has been demonstrated after poststroke treatment with a p53 inhibitor.12 These data support that functional recovery can be associated with neuroregeneration in stroke brain.4
In conclusion, we demonstrated that intranasal delivery of CART, given 3 days after MCAo, increased brain CART level, upregulated BDNF expression, enhanced survival, proliferation and migration of NPCs in SVZ, improved neurological function and facilitated neural regeneration in stroke animals. Our results may provide a new treatment strategy for stroke patients, enabling a noninvasive and longer treatment window, days after stroke occurrence.
Acknowledgments
The authors thank Ms Jenny Chou for technical assistance.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by the National Institute on Drug Abuse, NIH.
Supplementary Material
References
- Goldstein LB. Acute ischemic stroke treatment in 2007. Circulation. 2007;116:1504–1514. doi: 10.1161/CIRCULATIONAHA.106.670885. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, Kristensen P. Cocaine-amphetamine regulated transcript (CART) expression is not regulated by amphetamine. Neuroreport. 2002;13:1215–1218. doi: 10.1097/00001756-200207020-00029. [DOI] [PubMed] [Google Scholar]
- Liu HS, Shen H, Harvey BK, Castillo P, Lu H, Yang Y, et al. Post-treatment with amphetamine enhances reinnervation of the ipsilateral side cortex in stroke rats. Neuroimage. 2011;56:280–289. doi: 10.1016/j.neuroimage.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, Tang-Christensen M, Larsen LK, Kristensen P. Hypothalamic cocaine-amphetamine regulated transcript (CART) is regulated by glucocorticoids. Brain Res. 2003;965:45–50. doi: 10.1016/s0006-8993(02)04064-7. [DOI] [PubMed] [Google Scholar]
- Roh MS, Cui FJ, Ahn YM, Kang UG. Up-regulation of cocaine- and amphetamine-regulated transcript (CART) in the rat nucleus accumbens after repeated electroconvulsive shock. Neurosci Res. 2009;65:210–213. doi: 10.1016/j.neures.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhang W, Klaus J, Young J, Koerner I, Sheldahl LC, et al. Role of cocaine- and amphetamine-regulated transcript in estradiol-mediated neuroprotection. Proc Natl Acad Sci USA. 2006;103:14489–14494. doi: 10.1073/pnas.0602932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Chen X, Zhu W, Luo Y, Hua Z, Xu Y. CART protects brain from damage through ERK activation in ischemic stroke. Neuropeptides. 2008;42:653–661. doi: 10.1016/j.npep.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Mao P, Ardeshiri A, Jacks R, Yang S, Hurn PD, Alkayed NJ. Mitochondrial mechanism of neuroprotection by CART. Eur J Neurosci. 2007;26:624–632. doi: 10.1111/j.1460-9568.2007.05691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Hu S, Yang M, Pan H, Zhu S. CART peptide promotes the survival of hippocampal neurons by upregulating brain-derived neurotrophic factor. Biochem Biophys Res Commun. 2006;347:656–661. doi: 10.1016/j.bbrc.2006.06.117. [DOI] [PubMed] [Google Scholar]
- Abrahams JM, Gokhan S, Flamm ES, Mehler MF. De novo neurogenesis and acute stroke: are exogenous stem cells really necessary. Neurosurgery. 2004;54:150–155. doi: 10.1227/01.neu.0000097515.27930.5e. [DOI] [PubMed] [Google Scholar]
- Luo Y, Kuo CC, Shen H, Chou J, Greig NH, Hoffer BJ, et al. Delayed treatment with a p53 inhibitor enhances recovery in stroke brain. Ann Neurol. 2009;65:520–530. doi: 10.1002/ana.21592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felling RJ, Levison SW. Enhanced neurogenesis following stroke. J Neurosci Res. 2003;73:277–283. doi: 10.1002/jnr.10670. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Hida H, Nishino H. Early assessment of motor dysfunctions aids in successful occlusion of the middle cerebral artery. Neuroreport. 1998;9:3615–3621. doi: 10.1097/00001756-199811160-00012. [DOI] [PubMed] [Google Scholar]
- Chang CF, Morales M, Chou J, Chen HL, Hoffer BJ, Wang Y. Bone morphogenetic proteins are involved in fetal kidney tissue transplantation-induced neuroprotection in stroke rats. Neuropharmacology. 2002;43:418–426. doi: 10.1016/s0028-3908(02)00092-8. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Howard DB, Powers K, Wang Y, Harvey BK. Tropism and toxicity of adeno-associated viral vector serotypes 1,2,5,6,7,8,9 in rat neurons and glia in vitro. Virology. 2008;372:24–34. doi: 10.1016/j.virol.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Wang X, Wu P, Xu C, Zhao M, Morales M, et al. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry. 2009;66:137–145. doi: 10.1016/j.biopsych.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133:363–369. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- Yonemori F, Yamaguchi T, Yamada H, Tamura A. Spatial cognitive performance after chronic focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1999;19:483–494. doi: 10.1097/00004647-199905000-00002. [DOI] [PubMed] [Google Scholar]
- Chang CF, Lin SZ, Chiang YH, Morales M, Chou J, Lein P, et al. Intravenous admininstration of bone morphogenetic protein-7 after ischemia improves motor function in stroke rats. Stroke. 2003;34:558–564. doi: 10.1161/01.str.0000051507.64423.00. [DOI] [PubMed] [Google Scholar]
- Ren J, Kaplan PL, Charette MF, Speller H. Finklestein SP. Time window of intracisternal osteogenic protein-1 in enhancing functional recovery after stroke. Neuropharmacology. 2000;39:860–865. doi: 10.1016/s0028-3908(99)00261-0. [DOI] [PubMed] [Google Scholar]
- Du C, Hu R, Csernansky CA, Hsu CY, Choi DW. Very delayed infarction after mild focal cerebral ischemia: a role for apotosis. J Cereb Blood Flow Metab. 1996;16:195–201. doi: 10.1097/00004647-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lin SZ, Chiou AL, Williams LR, Hoffer BJ. Glial cell line-derived neurotrophic factor protects against ischemia-induced injury in the cerebral cortex. J Neurosci. 1997;17:4341–4348. doi: 10.1523/JNEUROSCI.17-11-04341.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V. Entry of CART into brain is rapid but not inhibited by excess CART or leptin. Am J Physiol. 1999;277:E901–E904. doi: 10.1152/ajpendo.1999.277.5.E901. [DOI] [PubMed] [Google Scholar]
- Dhuria SV, Hanson LR, Frey WH. Intranasal drug targeting of hypocretin-1 (orexin-A) to the central nervous system. J Pharm Sci. 2009;98:2501–2515. doi: 10.1002/jps.21604. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Wei N, Lu T, Zhu J, Xu G, Liu X. Intranasal brain-derived neurotrophic factor protects brain from ischemic insult via modulating local inflammation in rats. Neuroscience. 2011;172:398–405. doi: 10.1016/j.neuroscience.2010.10.054. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Okano H, Sakaguchi M, Ohki K, Suzuki N, Sawamoto K. Regeneration of the central nervous system using endogenous repair mechanisms. J Neurochem. 2007;102:1459–1465. doi: 10.1111/j.1471-4159.2007.04674.x. [DOI] [PubMed] [Google Scholar]
- Chou J, Harvey BK, Chang CF, Shen H, Morales M, Wang Y. Neuroregenerative effects of BMP7 after stroke in rats. J Neurol Sci. 2006;240:21–29. doi: 10.1016/j.jns.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ahlenius H, Thored P, Kobayashi R, Kokaia Z, Lindvall O. Intracerebral infusion of glial cell line-derived neurotrophic factor promotes striatal neurogenesis after stroke in adult rats. Stroke. 2006;37:2361–2367. doi: 10.1161/01.STR.0000236025.44089.e1. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramic M, Emerick AJ, Bollnow MR, O'Brien TE, Tsai SY, Kartje GL. Axonal plasticity is associated with motor recovery following amphetamine treatment combined with rehabilitation after brain injury in the adult rat. Brain Res. 2006;1111:176–186. doi: 10.1016/j.brainres.2006.06.063. [DOI] [PubMed] [Google Scholar]
- Rasmussen RS, Overgaard K, Hildebrandt-Eriksen ES, Boysen G. D-amphetamine improves cognitive deficits and physical therapy promotes fine motor rehabilitation in a rat embolic stroke model. Acta Neurol Scand. 2006;113:189–198. doi: 10.1111/j.1600-0404.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- Papadopoulos CM, Tsai SY, Guillen V, Ortega J, Kartje GL, Wolf WA. Motor recovery and axonal plasticity with short-term amphetamine after stroke. Stroke. 2009;40:294–302. doi: 10.1161/STROKEAHA.108.519769. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.