Abstract
Brain magnetic resonance (MR) study has shown metabolic abnormalities and changes in water distribution of the brain tissue that may relate to the pathogenesis of hepatic encephalopathy (HE). We designed a study to investigate the disturbances in brain water and metabolites during episodic HE using a 3-T MR scanner. Cirrhotic patients with different grades of HE underwent MR during hospitalization (n=18). The MR was repeated at 6 weeks' follow-up (n=14). The results were compared with those of a group of healthy volunteers (n=8). During episodic HE, brain diffusion-weighted imaging showed a high apparent diffusion coefficient (ADC) (12% to 14%) that decreased during follow-up (−1% to −4%). These disturbances were accompanied by high glutamine (581%), low choline (−31%), and low myo-inositol (−86%) peaks on MR spectroscopy. In overt HE, patients showed high glutamine that decreased during follow-up (−22%). In addition, these patients exhibited a rise in plasma S100 beta and enlargement of brain white-matter lesions. In conclusion, several disturbances detected by MR support the presence of impaired brain water homeostasis during episodic HE. Although astrocytes have a major role in this condition, brain edema during episodic HE may be extracellular and does not appear to be directly responsible for the development of neurologic manifestations.
Keywords: acute hepatic encephalopathy, blood–brain barrier, glutamine, leukoaraiosis, spectroscopy
Introduction
Hepatic encephalopathy (HE) is a common complication of cirrhosis that manifests with a wide range of neurologic abnormalities, from subtle cognitive deficits to deep coma.1 Current hypotheses on the pathogenesis of HE are focused on impairment of astrocyte function,2 which determines the integrity of the blood–brain barrier (BBB), the concentration of neurotransmitters in the synaptic cleft, and the metabolites trafficking with the neuron. It has been postulated that excess ammonia and neuroinflammation resulting from liver failure induce astrocyte swelling,3 which can lead to increased BBB permeability to some molecules4 and neuronal dysfunction.5
Certain magnetic resonance (MR) imaging techniques enable study of brain water and metabolites in patients with liver failure.6 Water quantification has shown a trend toward higher percentages of water in the white matter in mild HE.7 This finding is consistent with the results of studies using magnetization transfer, an MR sequence that enables indirect estimate of the accumulation of water in the brain.8 However, contrary to what would be expected from the pathogenetic hypothesis of edema secondary to astrocyte swelling, diffusion-weighted imaging in cirrhosis suggests that the increased water content is located in the extracellular compartment.9 Diffusion imaging analysis using a biexponential approach (in contrast to standard monoexponential analysis) supports the presence of two components that can be ascribed to water bound to membranes (mostly intracellular) or unbound to membranes (mostly extracellular). Although the interpretation is controversial, the results of applying this method in cirrhosis patients are in accordance with an increase in water content in the extracellular compartment.10
Most studies of MR related to HE have been performed in patients with cirrhosis (chronic liver damage) with only minimal HE (no obvious changes in mental status). There are few data in episodic HE with follow-up. Only one study11 has controlled for individual variables by reassessing the same patient during HE and after recovery. This study showed at baseline the characteristic pattern associated with HE: an increase in the Glx peak (a combination of glutamate and glutamine) and a decrease in myo-inositol and choline derivatives. The study by Poveda also showed a decrease in apparent diffusion coefficient (ADC) after improvement of HE, which was interpreted as water flux from extracellular to intracellular compartments and the existence of vasogenic brain edema during HE. However, they could not relate changes in MR spectrum to HE, which may be explained by the use of a 1.5 Tesla (1.5-T) scanner and by the fact that the interval between grading HE and MR imaging (MRI) assessment was up to 24 hours.
The aim of this study was to investigate brain water and metabolite changes in patients with cirrhosis and HE and relate them to the time course and severity of the condition. The MR was performed with a 3-T scanner, which allows specific assessment of brain glutamine,12 a key factor in ammonia-related neurotoxicity.13 The ultimate purpose was to obtain further data related to the pathogenesis of HE and to seek potential diagnostic biomarkers of this condition. In addition, astroglial protein S100 beta concentration, an indicator of glial injury and BBB dysfunction,14, 15 was assessed in serum.
Materials and methods
Design
In this prospective study, clinical and brain MRI characteristics were assessed in a group of cirrhosis patients admitted to the hospital for an episode of overt HE. The patients were clinically stable and had no manifestations of neurologic impairment before the HE episode (within 5 days before admission). All patients underwent daily biochemical analysis, and twice-daily clinical evaluation and grading of HE severity (West Haven criteria) up to the time MRI was performed. All MR studies were performed in the first 5 days after hospital admission, and HE grade was again determined within 30 minutes before the MR examination.
The MR study was repeated 6 weeks later (±1 week) in 14 patients who recovered from HE (2 patients died and 2 patients refused the second MR). At the time of the follow-up MR examination, 12 patients showed no clinical signs of HE and 2 patients still exhibited HE grade 1. The Ethics Committee of Hospital Universitari Vall d'Hebron approved the study, and informed consent was obtained from participants (first by next of kin and later confirmed by the patient).
Patient Characteristics
The study included 18 patients who showed signs of overt HE (grade II (n=6), grade III (n=10), and grade IV (n=2)) at hospital admission. All patients exhibited typical clinical and biochemical parameters of cirrhosis (Table 1); the latter did not differ in relation to the severity of HE (Supplementary Table 1). Patients were treated according to a standard protocol that included correction of potential precipitating factors, administration of intravenous solutions, and initiation of food intake as soon as possible. The precipitating factors included infection (n=5), hyponatremia established on a serum sodium concentration of <130 mEq/L (n=6), and diuretic-induced dehydration (n=9), which was defined as weight loss (>5 kg) <1 month after starting diuretic therapy and a lack of edema and ascites. All patients received lactulose (by rectal, nasogastric, or oral administration) and rifaximin 600 mg b.i.d. (nasogastric or oral route). In many patients, HE improved during the first days of admission; hence, severity grades were lower at the time MR was performed: seven patients recovered consciousness with HE resolution, eight patients showed low-grade HE (grades I and II), and three patients high-grade HE (grades III and IV). Eight healthy volunteers (four men and four women), age-matched (57±8 years) with the patients, were evaluated as a control group.
Table 1. Demographic, clinical, and laboratory characteristics of cirrhosis patients with an acute episode of HE at hospital admittance and 6 weeks after the HE episode (follow-up).
| Study | Admittance | Follow-up |
|---|---|---|
| Number | 18 | 14 |
| Age (years) | 60±10 | 59±11 |
| Male/female | 13/5 | 10/4 |
| Etiology | ||
| Hepatitis C virus | 2 | 2 |
| Alcohol | 8 | 5 |
| Hepatitis C virus+alcohol | 3 | 3 |
| Other | 5 | 4 |
| HE gradea | ||
| 0 | 7 | 12 |
| I | 5 | 2 |
| II | 3 | 0 |
| IV | 2 | 0 |
| Child Pugh A/B/C | 1/4/2 | 0/5/6 |
| Biochemical parametersb | ||
| Prothrombin activity (%) | 53.2±13.9 | 64.0±17.2 |
| Sodium (mEq/L) | 134.6±6.3 | 136.3±3.8 |
| Potassium (mEq/L) | 4.0±0.6 | 4.4±0.3 |
| Creatinine (mg/dL) | 1.1±0.7 | 0.9±0.3 |
| Total bilirubin (mg/dL) | 2.8±1.0 | 2.2±1.1 |
| Conjugated bilirubin (mg/dL) | 1.1±0.4 | 0.9±0.5 |
| Albumin (g/dL) | 3.3±0.6 | 3.3±0.4 |
| Aspartate transaminase (IU/L) | 58.6±48.8 | 68.5±59.3 |
| Alanine transaminase (IU/L) | 36.9±32.3 | 47.7±40.2 |
| Alkaline phosphatase (IU/L) | 118.8±47.0 | 144.9±74.1 |
| Gamma-glutamyl transpeptidase (IU/L) | 96.0±79.1 | 110.2±35.8 |
| Plasma ammonia concentration (μM) | 105.8±53.5 | 89.5±47.8 |
West Haven criteria.
Determined in venous blood samples.
Analytical Procedures
Standard laboratory testing in venous blood samples included a hemogram, reticulocyte count, prothrombin time, and bilirubin, alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase, albumin, sodium, potassium, creatinine, and calcium concentrations. Ammonia was measured in a Cobas Integra analyzer (Roche Diagnostics Indianapolis, IN, USA) using standard methods.
Serum S100 beta protein levels were determined using an ECLIA (electrochemiluminescence immunoassay) on an Elecsys immunoassay system (Roche Diagnostics, Basel, Switzerland). S100 beta concentration is expressed in μg/L (95th percentile value of apparently healthy persons is 0.105 μg/L). The immunoassay is unaffected by jaundice at bilirubin <25 mg/dL or hemolysis at hemoglobin <1 g/dL. All 18 patients had bilirubin levels below the limits of interference; however, 2 patients had hemolytic samples and were not considered in the analysis.
Magnetic Resonance Protocol
The MR studies were performed in a 3.0-T MAGNETOM Trio scanner (Siemens, Erlangen, Germany) equipped with a circular polarized receiver head array coil with the body coil acting as transmitter.
The MR protocol included proton density and T2-weighted fast spin-echo (repetition time (TR)/echo time (TE)/echo train length/acquisitions/turbo factor 2,900 ms/19 to 87 ms/16/2/6) and fast-FLAIR (fluid attenuated inversion recovery) (TR/TE/inversion time/echo train length/acquisitions/turbo factor 9,000 ms/93 ms/2,500 ms/12/1/16). Forty-six contiguous axial slices with a thickness of 3 mm, a pixel size of ∼1 × 1 mm, a 3/4 rectangular field of view of 250 mm, and an acquisition matrix of 256 × 256 were used to record images. T1-weighted images were obtained using magnetization-prepared 180° radio-frequency pulses and rapid gradient-echo (TR/TE/inversion time/acquisitions 2,700 ms/4.32 ms/900 ms/1). A total of 176 contiguous sagittal slices were obtained, with a thickness of 1 mm, a pixel size of ∼1 × 1 mm, a field of view of 256 mm, and an acquisition matrix of 256 × 256.
Diffusion images were acquired using a single-shot echo-planar sequence (TR/TE/acquisitions/turbo factor 4,000 ms/93 ms/6/128) with gradients applied in three directions and four b values (range 0 to 3,000 s/mm2). Images were obtained in 28 axial slices with a slice thickness of 4 mm, an interslice gap of 2 mm, a field of view of 250 mm, and an acquisition matrix of 128 × 128.
Proton MR spectroscopy was performed from a volume of interest localized at the parieto-occipital region and defined by a 20-mm side cube containing mainly white matter. A 90°-180°-180° spin-echo-based pulse sequence was used (TR/TE/acquisitions 3,000 ms/30 ms/80). For water suppression, a chemical shift selective Gaussian pulse was applied. A total of 1,024 data points were collected over a bandwidth of 1,200 Hz.
Magnetic Resonance Analysis
Fast-FLAIR images obtained in the first MR and follow-up scans were used to identify T2 lesions. The lesions were marked on MR films and only focal white-matter lesions at least 3 mm in size located in the brain hemispheres were considered for measurement. Lesions marked on MR films were outlined on the computer image to determine the lesion surface using Jim image analysis software (version 5.0, Xinapse Systems Ltd., Northants, UK, www.xinapse.com). Diffusion imaging data were processed using NUMARIS syngo software, version 4 (Siemens) to calculate the ADC (expressed in μm2/s) in two regions: the parietal white matter and corticospinal tract.
Spectra were analyzed using LCModel software v 6.2-4A (Stephen Provencher Inc., Oakville, ON, Canada) to automatically quantify metabolites (expressed as ratios compared with creatine [Cr]) without water scaling16 because of the potential changes in the amount of water (Supplementary Figure 1). Glutamine, glutamate, NAA compounds (N-acetylaspartate and N-acetyl aspartyl glutamate), choline derivatives (glycerophosphorylcholine and phosphorylcholine), and myo-inositol were analyzed by fitting a linear combination of a basis set of metabolite model spectra to the data (LCModel). The basis set was simulated using the GAMMA library.17
Statistical Analysis
The statistical analysis was performed with the Sigma Stat package (SPSS Inc., Chicago, IL, USA). Values are expressed as the mean±standard deviation. Significant differences between intergroup data were determined with the Student t-test, Mann–Whitney U-test or ANOVA. ANOVA testing was followed by pairwise multiple comparison procedures (Holm-Sidak method or Dunn's method). Comparisons between continuous variables were performed with the paired Student t-test or Wilcoxon W test. Correlations between parameters were performed with the Pearson or Spearman correlation coefficient. P values less than 0.05 were considered as statistically significant.
Results
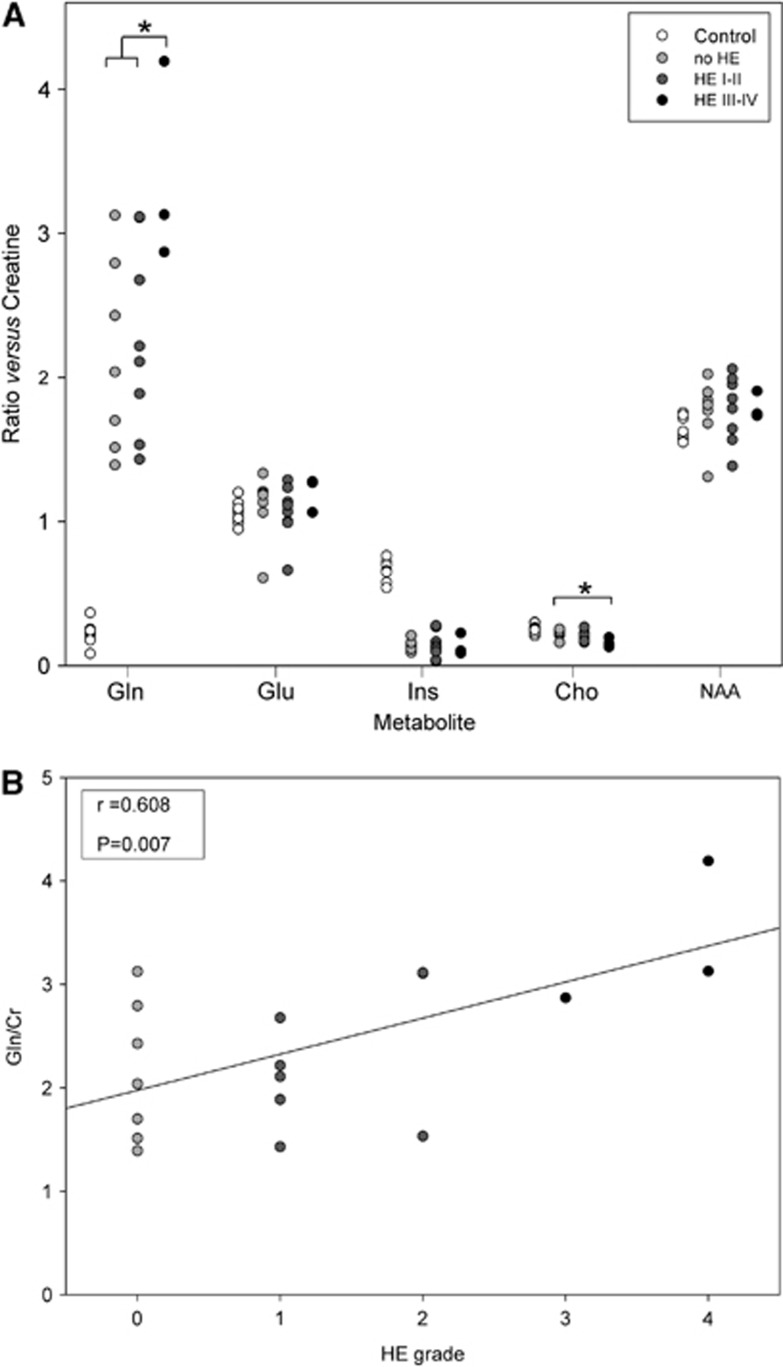
The metabolite profiles obtained by spectroscopy in the first MR study were abnormal in patients with cirrhosis compared with controls: glutamine was higher (Gln/Cr: 2.40±0.78 versus 0.22±0.08, P<0.001) and myo-inositol and choline derivatives were lower (Ins/Cr: 0.14±0.07 versus 0.66±0.07, P<0.001; Cho/Cr: 0.20±0.04 versus 0.26±0.03, P=0.002). However, glutamate and N-acetylaspartate values were not significantly different (Glu/Cr: 1.10±0.20 versus 1.06±0.08, P=0.157 and NAA/Cr: 1.77±0.02 versus 1.64±0.08, P=0.92). The glutamine peak increased significantly in relation to HE severity (P=0.038), whereas the choline derivates peak showed a decrease (P=0.048) (Figure 1A). A positive correlation was found between the glutamine/creatine ratio and HE grade (r=0.608, P=0.007) (Figure 1B).
Figure 1.
(A) Relative metabolite concentrations in patients with cirrhosis (n=18) and healthy controls (n=8). Metabolites: Gln, glutamine; Glu, glutamate; Ins, myo-inositol; Cho, choline derivates; and NAA, N-acetylaspartate. *P<0.050; (B) Correlation between brain glutamine/creatine ratio (Gln/Cr) and the hepatic encephalopathy (HE) grade determined at the time of the first magnetic resonance (MR) examination.
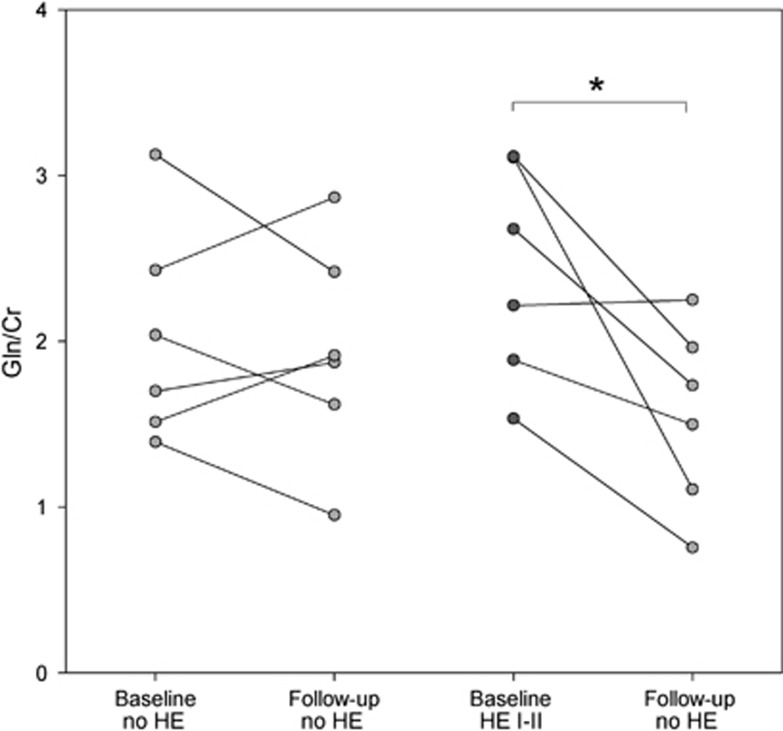
At the follow-up study, the glutamine peak decreased ∼36% in patients who recovered from the HE episode (from 2.42±0.65 to 1.55±0.55, P=0.028). In contrast, glutamine remained stable in patients who were admitted to the hospital with symptoms of HE, but exhibited normal mental status at MR assessment (first MR: 2.03±0.65, 6 weeks' MR: 1.94±0.66, P=0.663) (Figure 2). Brain glutamine correlated with blood ammonia level in the group of 14 patients with a first MR and follow-up data (r=0.526, P=0.004, n=28). Myo-inositol increased in the follow-up study (first: 0.14±0.07, follow-up: 0.21±0.09, P=0.006), but there was no relationship with HE severity. The changes in glutamine at follow-up did not correlate with the myo-inositol changes (r=−0.179, P=0.579). Other metabolites (glutamate, choline, and N-acetylaspartate) remained stable. The same analysis was performed using the absolute signal for each metabolite, and the statistical differences were maintained. Furthermore, the absolute creatine signal did not differ between patients and controls (Supplementary Table 2).
Figure 2.
Follow-up of brain glutamine (Gln/Cr) in relation to the hepatic encephalopathy (HE) severity at first magnetic resonance (MR) study. *P<0.050. Cr, creatine; Gln, glutamine.
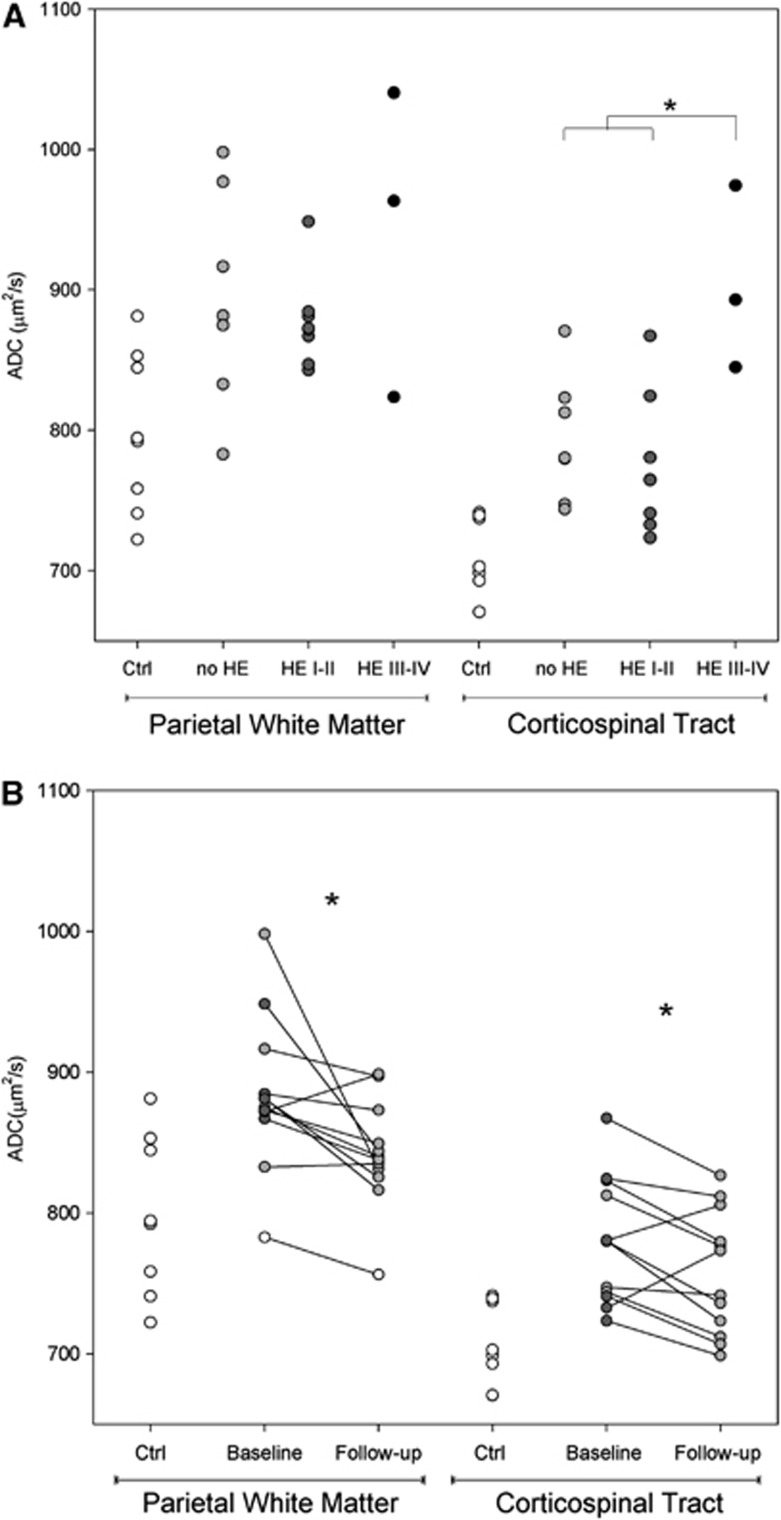
On diffusion-weighted imaging, ADC values were higher in cirrhosis patients than in controls in the corticospinal tract (804±66 μm2/s versus 707±29 μm2/s; P<0.001) and parietal white matter (895±67 μm2/s versus: 798±57 μm2/s; P=0.005). The ADC increase tended to be higher in relation to HE severity (Figure 3A) and reached statistical significance in the corticospinal tract (P=0.006). At follow-up, ADC values in the 12 patients who recovered from the HE episode showed a significant decrease in the corticospinal tract (from 780±44 μm2/s to 758±44 μm2/s, P=0.025) and parietal white matter (from 884±54 μm2/s to 842±38 μm2/s, P=0.016) (Figure 3B). The follow-up ADC values in patients did not differ from those of the controls in parietal white matter, but persisted slightly elevated in the corticospinal tract.
Figure 3.
(A) Apparent diffusion coefficient values (ADC, in μm2/s) in patients with different grades of hepatic encephalopathy (HE) (in gray, n=18) and controls (ctrl, in white, n=8) in two different regions. The mean of each group is marked by a dash. *P<0.050; (B) Follow-up of ADC values (in μm2/s) in patients with cirrhosis (n=12) in two brain regions. At first magnetic resonance (MR) assessment, values in patients without clinical HE are shown as gray circles, whereas patients with low-grade HE (grades I and II) are shown as dark gray circles. Healthy control values are shown to provide a reference for the normal range. *P<0.050.
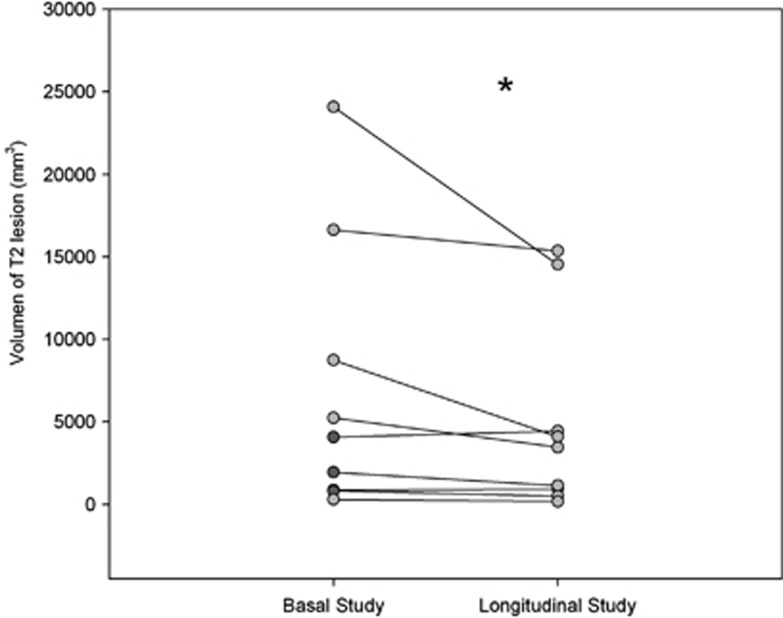
A focal T2 white-matter lesion was detected in 15 patients, 9 of whom were reassessed after HE resolution. Lesion volume decreased after the HE episode (P=0.039) (Figure 4). The comparison between precipitating factors and MR parameters at the first study showed that ADC values in parietal white matter were higher in patients with dehydration (nondehydrated, 861±44 μm2/s versus dehydrated, 928±70 μm2/s; P=0.027). In addition, the myo-inositol/creatine ratio was lower in patients with hyponatremia (0.092±0.05 versus 0.16±0.07; P=0.031).
Figure 4.
Among the 15 patients showing white-matter lesions at first magnetic resonance imaging (MRI), the T2 lesion volume was reassessed at follow-up in 9 patients. *P<0.050.
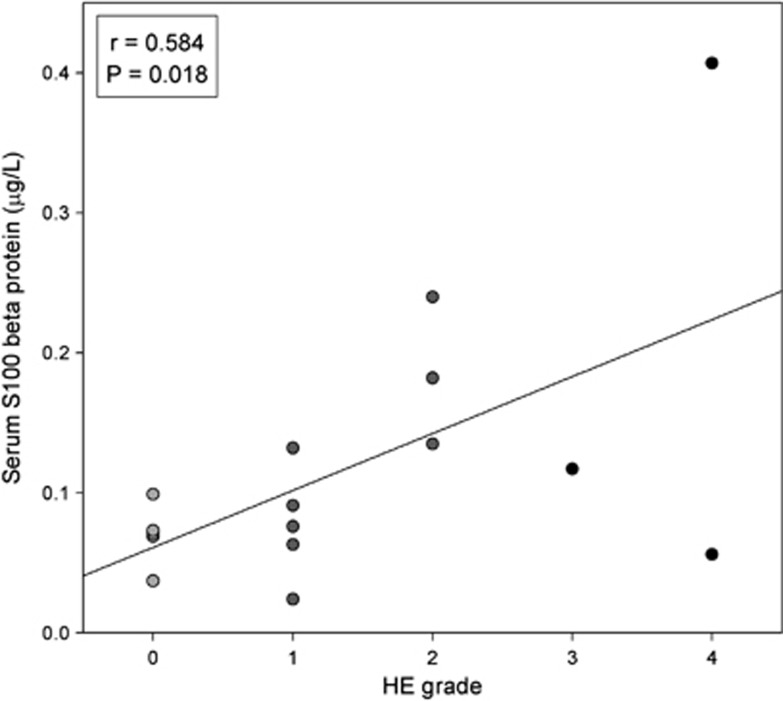
Serum S100 beta concentration at hospital admittance correlated with HE grade (r=0.584, P=0.018, n=16) (Figure 5), but was not associated with other clinical or MR variables.
Figure 5.
Correlation between the serum S100 beta protein concentration and the hepatic encephalopathy (HE) grade at the time of magnetic resonance (MR) examination.
Discussion
In this study, MRI findings showed abnormalities in the brain metabolite profile and in the distribution of water in brain compartments in patients with episodic HE. Among these abnormalities, a high brain tissue glutamine peak appears to be of pathogenic relevance and may be useful for the diagnosis of HE.
Liver failure secondary to cirrhosis impairs the body's capacity to transform ammonia into urea, the metabolite that disposes of waste nitrogen molecules in normal conditions. In patients with cirrhosis, plasma ammonia concentration increases, and this increase together with high levels of inflammatory mediators can be toxic to the brain tissue and lead to the development of neurologic manifestations (HE).
Ammonia reaches the BBB and is rapidly incorporated to glutamine in the astrocytes, the main glutamine synthetase-competent cell. Glutamine has been classically considered as an inert amino acid, but it is suggested to be toxic to the astrocyte by acting as a carrier of ammonia to the interior of the mitochondria.13 Glutamine accumulation may be also related to a decreased capacity of astrocytes to take up glutamate, thus leading to glutamate excitotoxicity.18 Alternatively, glutamine may simply be an indicator of exposure of the brain to ammonia, which may be the key factor in the development of HE.
We observed a high rise in brain glutamine (10-fold compared with controls), which is in the order observed in experimental models.10, 19 The rise in glutamine seen on the first MR study was more marked in patients with more severe HE (grades III and IV). Unfortunately, we were unable to reassess glutamine at follow-up in patients with severe HE. Nevertheless, those with mild HE (grades I and II) at the first MR study showed a >30% decrease in glutamine at recovery (grade 0). In comparison, glutamine remained stable in patients without clinical manifestations of HE at the time MR was performed, and values were similar to those obtained at follow-up in mild HE patients who recovered normal mental status. Other authors have also reported an increased glutamine peak in overt HE20 with a tendency to decrease after therapy.21 The lack of a significant decrease in Glx after HE resolution in a previous study,11 which contrasts with our data, may be related to the lower resolution of the 1.5-T scanner used.
Regardless of its role in the pathogenesis of HE, glutamine level assessed by MR spectroscopy could be a useful biomarker in the diagnosis of difficult cases. Cirrhotic patients can also develop nonhepatic encephalopathy secondary to small-vessel cerebrovascular disease or Alzheimer's disease and in these cases, MR spectroscopy may be of help in the diagnosis. Although there was some overlapping of glutamine values between the various grades of HE, we found that the changes in this metabolite were closely related to the evolution of HE. For this reason, a diagnosis other than HE could be suspected in patients who show discrepancies between brain glutamine changes and the evolution of neurologic manifestations. Further studies are needed to investigate this hypothesis.
Animal models of experimentally induced hyper-acute liver failure show intracellular brain swelling, seen as a low ADC on diffusion tensor MRI, without affecting BBB permeability,10 whereas subacute models are characterized by a mixed pattern of cytotoxic (intracellular) and vasogenic (extracellular) edema.22 Similarly, ADC values are low in patients with acute liver failure (cytotoxic edema),23 but are unaffected in acute-on-chronic liver failure (mixed vasogenic and cytotoxic edema).24
One prevailing theory proposed to explain the mechanism by which brain glutamine originates HE is by inducing swelling of astrocytes with impairment of their function.21 The observation of an ADC decrease (suggestive of intracellular brain swelling) in patients with acute liver failure supports this interpretation.25 However, MR studies in patients with cirrhosis have consistently found a rise in the ADC.9 In other studies investigating clinical situations where brain edema is extracellular (e.g., brain tumors and hypernatremia) an ADC increase has been found, whereas in patients with intracellular edema (hyponatremia) the ADC has been shown to decrease.26, 27 These findings are contrary to the hypothesis that the severity of HE directly depends on astrocyte swelling. Our data show a lack of relationship between neurologic manifestations and the distribution of water between the intracellular and extracellular compartments, in keeping with the results of previous studies.9, 28 We found an increase in ADC values in parietal white matter, which appears to represent an expansion of the extracellular compartment that returned to normal in the 6-week follow-up study. ADC values in the spine, however, did not completely normalized after recovery of HE, in keeping with the results of a previous study that evaluated ADC in the corticospinal tract before and after liver transplantation.29 This finding can be explained by persistent neurologic damage (mild hepatic myelopathy),30 and suggests that the corticospinal tract is more vulnerable to developing liver-induced damage than the parietal white matter.
The ADC values were higher in patients with signs of body dehydration. This intriguing finding may be explained by inhibition of water transport across the BBB caused by diuretics (the main cause of dehydration).31 Interestingly, diuretics are a common factor associated with episodic HE for which no mechanistic explanation has been found. The association between low brain myo-inositol and hyponatremia seen in the present study and reported by other authors32 is another sign of disturbance in brain water homeostasis. Brain myo-inositol is an organic osmolyte with an important regulatory role: the concentration of myo-inositol in astrocytes increases or decreases to balance changes in extracellular osmolality.33 Decreases in myo-inositol are proposed to compensate for an increase in astrocyte osmolality caused by ammonia-induced glutamine synthesis.34, 35, 36 Thus, deficient osmotic compensation can result in an increase in astrocyte water content and explain the development of neurologic manifestations. Nonetheless, we were unable to relate ADC values or myo-inositol level to the severity of HE, which suggests that HE cannot be attributed to brain edema alone.
Our findings are not contrary to the notion that astrocytes participate in the pathogenesis of HE. One possible explanation to reconcile our data with the signs of glial injury seen in HE is the participation of astrocytes in the BBB. In a previous study by our group, white-matter lesions on T2-weighted images compatible with small-vessel cerebrovascular disease were observed in an important percentage of cirrhosis patients with HE.37 More recent studies have shown that these lesions are probably indicative of higher permeability of the BBB.38, 39 It is plausible that during HE, excess ammonia or glutamine cause impairment of astrocyte function, an idea supported by the association between HE severity and increases in serum S100 beta. Astrocyte dysfunction could have two effects that are not directly related: brain edema and neuronal dysfunction. Improvement of astrocyte function with therapy may decrease brain edema and explain the decrease in the volume of white-matter lesions and normalization of ADC,21 but some sequelae in neuronal function may persist.40
In conclusion, the findings of 3-T MR spectroscopy support the participation of brain glutamine in the pathogenesis of HE. Despite the major role astrocytes have in this condition, the brain edema may be mainly extracellular and does not appear to be directly responsible for the development of neurologic manifestations. For this reason, studies on the pathogenesis of HE should avoid the use of water disturbance as a surrogate marker of neurologic manifestations and separate brain edema from HE.
Acknowledgments
The authors are in debt with Dr Provencher for designing a reference spectrum for the study.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This project was supported by grant from Instituto de Salud Carlos III (FIS PI10/01028 and PI11/0954) cofinanced by the European Regional Development Fund (ERDF). CIBEREHD is supported by Instituto de Salud Carlos III. Rita Garcia-Martinez is the recipient of grant CM07/00109.
Supplementary Material
References
- Cordoba J, Minguez B. Hepatic encephalopathy. Semin Liver Dis. 2008;28:70–80. doi: 10.1055/s-2008-1040322. [DOI] [PubMed] [Google Scholar]
- Norenberg MD. Astroglial dysfunction in hepatic encephalopathy. Metab Brain Dis. 1998;13:319–335. doi: 10.1023/a:1020688925901. [DOI] [PubMed] [Google Scholar]
- Zemtsova I, Gorg B, Keitel V, Bidmon HJ, Schror K, Haussinger D. Microglia activation in hepatic encephalopathy in rats and humans. Hepatology. 2011;54:204–215. doi: 10.1002/hep.24326. [DOI] [PubMed] [Google Scholar]
- Nguyen JH, Yamamoto S, Steers J, Sevlever D, Lin W, Shimojima N, et al. Matrix metalloproteinase-9 contributes to brain extravasation and edema in fulminant hepatic failure mice. J Hepatol. 2006;44:1105–1114. doi: 10.1016/j.jhep.2005.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Bidmon HJ, Cremer M, Schleicher A, Kircheis G, Reifenberger G, et al. Neurotransmitter receptor imbalances in motor cortex and basal ganglia in hepatic encephalopathy. Cell Physiol Biochem. 2009;24:291–306. doi: 10.1159/000233254. [DOI] [PubMed] [Google Scholar]
- Mardini H, Smith FE, Record CO, Blamire AM. Magnetic resonance quantification of water and metabolites in the brain of cirrhotics following induced hyperammonaemia. J Hepatol. 2011;54:1154–1160. doi: 10.1016/j.jhep.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Shah NJ, Neeb H, Kircheis G, Engels P, Haussinger D, Zilles K. Quantitative cerebral water content mapping in hepatic encephalopathy. Neuroimage. 2008;41:706–717. doi: 10.1016/j.neuroimage.2008.02.057. [DOI] [PubMed] [Google Scholar]
- Miese FR, Wittsack HJ, Kircheis G, Holstein A, Mathys C, Modder U, et al. Voxel-based analyses of magnetization transfer imaging of the brain in hepatic encephalopathy. World J Gastroenterol. 2009;15:5157–5164. doi: 10.3748/wjg.15.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodi R, Tonon C, Stracciari A, Weiger M, Camaggi V, Iotti S, et al. Diffusion MRI shows increased water apparent diffusion coefficient in the brains of cirrhotics. Neurology. 2004;62:762–766. doi: 10.1212/01.wnl.0000113796.30989.74. [DOI] [PubMed] [Google Scholar]
- Chavarria L, Oria M, Romero-Gimenez J, Alonso J, Lope-Piedrafita S, Cordoba J. Diffusion tensor imaging supports the cytotoxic origin of brain edema in a rat model of acute liver failure. Gastroenterology. 2010;138:1566–1573. doi: 10.1053/j.gastro.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Poveda MJ, Bernabeu A, Concepcion L, Roa E, de ME, Zapater P, et al. Brain edema dynamics in patients with overt hepatic encephalopathy A magnetic resonance imaging study. Neuroimage. 2010;52:481–487. doi: 10.1016/j.neuroimage.2010.04.260. [DOI] [PubMed] [Google Scholar]
- Sawara K, Kato A, Yoshioka Y, Suzuki K. Brain glutamine and glutamate levels in patients with liver cirrhosis: assessed by 3.0-T MRS. Hepatol Res. 2004;30:18–23. doi: 10.1016/j.hepres.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Albrecht J, Norenberg MD. Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology. 2006;44:788–794. doi: 10.1002/hep.21357. [DOI] [PubMed] [Google Scholar]
- Kanner AA, Marchi N, Fazio V, Mayberg MR, Koltz MT, Siomin V, et al. Serum S100beta: a noninvasive marker of blood-brain barrier function and brain lesions. Cancer. 2003;97:2806–2813. doi: 10.1002/cncr.11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapural M, Krizanac-Bengez L, Barnett G, Perl J, Masaryk T, Apollo D, et al. Serum S-100beta as a possible marker of blood-brain barrier disruption. Brain Res. 2002;940:102–104. doi: 10.1016/s0006-8993(02)02586-6. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Smith SA, Levante TO, Meier BH, Ernst RR. Computer simulations in magnetic resonance. an object-oriented programming approach. J Magn Reson Series A. 1994;106:75–105. [Google Scholar]
- Lemberg A, Fernandez MA. Hepatic encephalopathy, ammonia, glutamate, glutamine and oxidative stress. Ann Hepatol. 2009;8:95–102. [PubMed] [Google Scholar]
- Cordoba J, Gottstein J, Blei AT. Glutamine, myo-inositol, and organic brain osmolytes after portocaval anastomosis in the rat: implications for ammonia-induced brain edema. Hepatology. 1996;24:919–923. doi: 10.1002/hep.510240427. [DOI] [PubMed] [Google Scholar]
- Laubenberger J, Haussinger D, Bayer S, Gufler H, Hennig J, Langer M. Proton magnetic resonance spectroscopy of the brain in symptomatic and asymptomatic patients with liver cirrhosis. Gastroenterology. 1997;112:1610–1616. doi: 10.1016/s0016-5085(97)70043-x. [DOI] [PubMed] [Google Scholar]
- Haussinger D, Kircheis G, Fischer R, Schliess F, Schliess F, vom Dahl S. Hepatic encephalopathy in chronic liver disease: a clinical manifestation of astrocyte swelling and low-grade cerebral edema. J Hepatol. 2000;32:1035–1038. doi: 10.1016/s0168-8278(00)80110-5. [DOI] [PubMed] [Google Scholar]
- Cauli O, Lopez-Larrubia P, Rodrigo R, Agusti A, Boix J, Nieto-Charques L, et al. Brain region-selective mechanisms contribute to the progression of cerebral alterations in acute liver failure in rats. Gastroenterology. 2011;140:638–645. doi: 10.1053/j.gastro.2010.10.043. [DOI] [PubMed] [Google Scholar]
- Saksena S, Rai V, Saraswat VA, Rathore RS, Purwar A, Kumar M, et al. Cerebral diffusion tensor imaging and in vivo proton magnetic resonance spectroscopy in patients with fulminant hepatic failure. J Gastroenterol Hepatol. 2008;23 (7 Part 2:e111–e119. doi: 10.1111/j.1440-1746.2007.05158.x. [DOI] [PubMed] [Google Scholar]
- Nath K, Saraswat VA, Krishna YR, Thomas MA, Rathore RK, Pandey CM, et al. Quantification of cerebral edema on diffusion tensor imaging in acute-on-chronic liver failure. NMR Biomed. 2008;21:713–722. doi: 10.1002/nbm.1249. [DOI] [PubMed] [Google Scholar]
- Rai V, Nath K, Saraswat VA, Purwar A, Rathore RK, Gupta RK. Measurement of cytotoxic and interstitial components of cerebral edema in acute hepatic failure by diffusion tensor imaging. J Magn Reson Imaging. 2008;28:334–341. doi: 10.1002/jmri.21438. [DOI] [PubMed] [Google Scholar]
- Brugieres P, Thomas P, Maraval A, Hosseini H, Combes C, Chafiq A, et al. Water diffusion compartmentation at high b values in ischemic human brain. AJNR Am J Neuroradiol. 2004;25:692–698. [PMC free article] [PubMed] [Google Scholar]
- Schwarcz A, Ursprung Z, Berente Z, Bogner P, Kotek G, Meric P, et al. In vivo brain edema classification: New insight offered by large b-value diffusion-weighted MR imaging. J Magn Reson Imaging. 2007;25:26–31. doi: 10.1002/jmri.20789. [DOI] [PubMed] [Google Scholar]
- Kale RA, Gupta RK, Saraswat VA, Hasan KM, Trivedi R, Mishra AM, et al. Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology. 2006;43:698–706. doi: 10.1002/hep.21114. [DOI] [PubMed] [Google Scholar]
- Chavarria L, Alonso J, Garcia-Martinez R, Aymerich FX, Huerga E, Jacas C, et al. Biexponential analysis of diffusion-tensor imaging of the brain in patients with cirrhosis before and after liver transplantation. AJNR Am J Neuroradiol. 2011;32:1510–1517. doi: 10.3174/ajnr.A2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenborn K, Tietge UJ, Bokemeyer M, Mohammadi B, Bode U, Manns MP, et al. Liver transplantation improves hepatic myelopathy: evidence by three cases. Gastroenterology. 2003;124:346–351. doi: 10.1053/gast.2003.50062. [DOI] [PubMed] [Google Scholar]
- Jayakumar AR, Valdes V, Norenberg MD. The Na-K-Cl cotransporter in the brain edema of acute liver failure. J Hepatol. 2011;54:272–278. doi: 10.1016/j.jhep.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Guevara M, Baccaro ME, Torre A, Gomez-Anson B, Rios J, Torres F, et al. Hyponatremia is a risk factor of hepatic encephalopathy in patients with cirrhosis: a prospective study with time-dependent analysis. Am J Gastroenterol. 2009;104:1382–1389. doi: 10.1038/ajg.2009.293. [DOI] [PubMed] [Google Scholar]
- McManus ML, Churchwell KB, Strange K. Regulation of cell volume in health and disease. N Engl J Med. 1995;333:1260–1266. doi: 10.1056/NEJM199511093331906. [DOI] [PubMed] [Google Scholar]
- Cordoba J, Alonso J, Rovira A, Jacas C, Sanpedro F, Castells L, et al. The development of low-grade cerebral edema in cirrhosis is supported by the evolution of (1)H-magnetic resonance abnormalities after liver transplantation. J Hepatol. 2001;35:598–604. doi: 10.1016/s0168-8278(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Tarasow E, Panasiuk A, Siergiejczyk L, Orzechowska-Bobkiewicz A, Lewszuk A, Walecki J, et al. MR and 1H MR spectroscopy of the brain in patients with liver cirrhosis and early stages of hepatic encephalopathy. Hepatogastroenterology. 2003;50:2149–2153. [PubMed] [Google Scholar]
- Verma A, Saraswat VA, Radha KY, Nath K, Thomas MA, Gupta RK. In vivo 1H magnetic resonance spectroscopy-derived metabolite variations between acute-on-chronic liver failure and acute liver failure. Liver Int. 2008;28:1095–1103. doi: 10.1111/j.1478-3231.2007.01648.x. [DOI] [PubMed] [Google Scholar]
- Minguez B, Rovira A, Alonso J, Cordoba J. Decrease in the volume of white matter lesions with improvement of hepatic encephalopathy. AJNR Am J Neuroradiol. 2007;28:1499–1500. doi: 10.3174/ajnr.A0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Cadenas I, Mendioroz M, Domingues-Montanari S, Del Rio-Espinola A, Delgado P, Ruiz A, et al. Leukoaraiosis is associated with genes regulating blood-brain barrier homeostasis in ischaemic stroke patients. Eur J Neurol. 2011;18:826–835. doi: 10.1111/j.1468-1331.2010.03243.x. [DOI] [PubMed] [Google Scholar]
- Topakian R, Barrick TR, Howe FA, Markus HS. Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry. 2010;81:192–197. doi: 10.1136/jnnp.2009.172072. [DOI] [PubMed] [Google Scholar]
- Bajaj JS, Schubert CM, Heuman DM, Wade JB, Gibson DP, Topaz A, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138:2332–2340. doi: 10.1053/j.gastro.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.