Abstract
Glial calcium (Ca2+) waves constitute a means to spread signals between glial cells and to neighboring neurons and blood vessels. These waves occur spontaneously in Bergmann glia (BG) of the mouse cerebellar cortex in vivo. Here, we tested three hypotheses: (1) aging and reduced blood oxygen saturation alters wave activity; (2) glial Ca2+ waves change cerebral oxygen metabolism; and (3) neuronal and glial wave activity is correlated. We used two-photon microscopy in the cerebellar cortexes of adult (8- to 15-week-old) and aging (48- to 80-week-old) ketamine-anesthetized mice after bolus loading with OGB-1/AM and SR101. We report that the occurrence of spontaneous waves is 20 times more frequent in the cerebellar cortex of aging as compared with adult mice, which correlated with a reduction in resting brain oxygen tension. In adult mice, spontaneous glial wave activity increased on reducing resting brain oxygen tension, and ATP-evoked glial waves reduced the tissue O2 tension. Finally, although spontaneous Purkinje cell (PC) activity was not associated with increased glia wave activity, spontaneous glial waves did affect intracellular Ca2+ activity in PCs. The increased wave activity during aging, as well as low resting brain oxygen tension, suggests a relationship between glial waves, brain energy homeostasis, and pathology.
Keywords: aging, astrocytes, energy metabolism, calcium, multiphotonmicroscopy, neuronal–glial interaction
Introduction
Multicellular fast calcium (Ca2+) waves occur in many tissues and correlate with or cause important changes in tissue function.1 In the brain, glial Ca2+ wave propagation involves the release of ATP from glia and depends on the preserved function of glial purinergic (P2Y) receptors.2 Glial Ca2+ wave-like phenomena are observed in vivo in the cerebellar cortex, as well as in the cerebral cortex of transgenic Alzheimer's mice,3 the hippocampus,4 and in the retina.5 In the cerebellum of mice and rats in vivo, radially propagating glial Ca2+ waves form near-ellipsoidal domains spanning dozens of Bergmann glia (BG) processes.2 The wave event rate is significantly increased in awake mice relative to mice under isoflurane anesthesia,6 suggesting that the occurrence of glial waves could correlate with metabolic changes in nonpathologic tissue. However, relationship of glial Ca2+ waves with normal physiologic or pathologic brain function is incompletely understood. Recently, it was reported that BG has a previously unappreciated role in controlling the membrane potential and thereby the activity of adjacent Purkinje cells (PCs) by Ca2+-dependent uptake of extracellular K+.7 This mechanism is expected to have important influence on the overall activity of cerebellar networks given that activated BG within glial waves exhibits highly localized Ca2+ increases and each wave encompasses processes from up to 40 cells.2
In the retina, increased spontaneous glial wave occurrence is observed with age, suggesting that wave generation may be related to pathology.8 Here, we explored in vivo whether spontaneous glial Ca2+ waves increase with age in the cerebellum and examined the physiologic effects of these waves in relation to tissue oxygenation and PC Ca2+ activity. We report that in vivo, the incidence of spontaneous glial Ca2+ waves in murine cerebellum increased with age and hypoxia. Spontaneous glial Ca2+ waves spread around PC bodies with small but significant changes in the simultaneously recorded intracellular Ca2+ levels in the PCs, consistent with increased activity of these cells.7 In contrast, spontaneous PC activity did not influence the activity of neighboring BG. ATP-evoked glial Ca2+ waves triggered transient decreases in resting brain oxygen tension, but had limited influence on the diameter of intracortical blood vessels. This suggested an effect of spontaneous glial Ca2+ waves on brain energetics. We suggest that the generation of spontaneous glial Ca2+ waves in the cerebellum may be a mechanism of brain aging and hypoxia.
Materials and methods
Spontaneous glial Ca2+ waves were examined in mice in vivo using methods reported previously.9 All procedures involving animals were approved by the Danish National Ethics Committee and were performed according to the guidelines set forth in the European Council's Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes. A total of 29 white Naval Medical Research Institute mice (Crl:NMRI(Han)) were used. Adult and aging mice were anesthetized by intraperitoneal injection of a mixture of ketamine (60 mg/kg) and xylazine (10 mg/kg) (Sigma-Aldrich, Broendby, Denmark) and administered supplemental doses of ketamine every 20 minutes. Body temperature was maintained at 37°C using a temperature controller and heating blanket (Model TC-1000 Temperature Controller, CWE, Ardmore, PA, USA). The trachea was intubated for mechanical ventilation with a small-animal ventilator (SAAR-830; CWE) with supplemental oxygen added to the inspiration air to maintain blood oxygen saturation at 96% to 99%. Blood oxygen saturation (sO2) was registered using a Pulse Oximeter mounted on the right hind paw (Kent Scientific, Torrington, CT, USA). A catheter (TYGON S54HL 0.010 × 0.030 mm; VWR International, Herlev, Denmark) was inserted into the left femoral artery for continuous blood pressure monitoring. We did not observe any differences in physiologic variables between adult and old mice, except for the femoral vessels that felt more delicate and frail. A blood sample taken at the beginning of the experiment was used to adjust respiration to obtain physiologic blood gas values (pO2, 95 to 110 mm Hg; pCO2, 35 to 40 mm Hg; pH, 7.35 to 7.45). A custom-made metal plate that allowed cranial access was fixed to the skull with cyanoacrylate gel (Loctite Adhesives, Henkel, Dusseldorf, Germany). A craniotomy (∼4 mm diameter) was made above lobule VI of the medial vermis region of the cerebellum, and the dura was removed. The craniotomy was filled with 1% agarose (Type III-A, low electroendosmosis; Sigma-Aldrich) and was moistened with artificial cerebrospinal fluid (in mmol/L, NaCl 120, KCl 2.8, NaHCO3 22, CaCl2 1.45, Na2HPO4 1, MgCl2 0.876, and glucose 2.55; pH=7.4) at 37°C, bubbled with 95% air/5% CO2. The craniotomy was partly covered with a glass coverslip, which permitted pharmacological interventions and placement of the loading and application pipette and a Clark-like electrode (3 μm diameter, Unisense, Aarhus, Denmark) for measuring the tissue partial pressure of oxygen (tpO2, Figure 1A; Supplementary Information, Supplementary Movie S1).
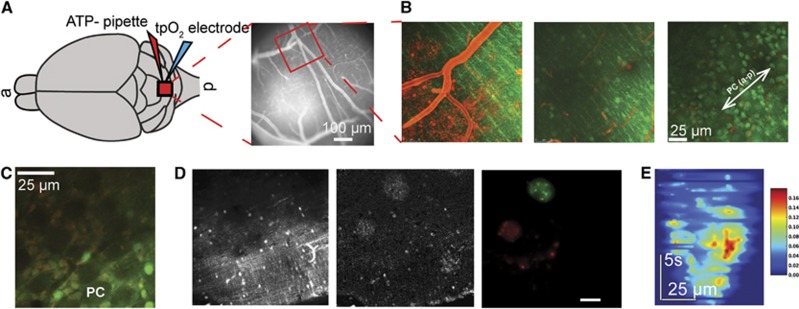
Figure 1.
An overview of the experimental methods and set-up, including representative data examples. (A) Left: schematic showing a top view of the cranial window and placement of the ATP pipette and the tissue partial oxygen tension (tpO2) electrode in lobule VI of the cerebellar cortex. ‘a' and ‘p' indicate anterior and posterior directions of the brain. Right: fluorescent gray-scale image of the cerebellar cortical surface. (B) Two-photon laser scanning microscopy images taken at the surface (0 μm, left panel), at 70 μm depth in the molecular layer (middle panel) and at the Purkinje cell (PC) layer (right panel). TRITC dextran was used to visualize the vessels, while bolus injection of Oregon-green BAPTA-1/AM served as a calcium (Ca2+) indicator and sulforhodamine 101 (SR101) served as an astrocytic marker to label Bergmann glia (BG). The right panel shows the orientation of PC dendritic trees as indicated by the white arrow that coincides with the anterior-posterior (a-p) axis of the brain. (C) A representative high-resolution image from the PC layer showing large ellipsoid PC bodies (one marked PC) and BG cell bodies surrounding each PC body. The few bright green cell bodies that did not take up SR101 may be basket cells. (D) A representative time-averaged gray scale image taken at the level of the molecular layer (left panel), a dF/F0 image showing two glial Ca2+ waves (middle panel), and pseudocoloring of the glial waves extracted from this image sequence using the multiscale vision model (right panel; green (top) and red (left)). (E) Expansion and development of the green-labeled glial wave over time. Scale bar indicates relative change in dF/F0.
Two-Photon Laser Scanning Microscopy
Experiments were performed using a commercial two-photon microscope (SP5 multiphoton/confocal Laser Scanning Microscope; Leica, Ballerup, Denmark), a MaiTai HP Ti:Sapphire laser (Millennia Pro, Spectra Physics, Södertälje, Sweden), and a 20 × 1.0 NA water-immersion objective (Leica). The excitation wavelength was set to 820 nm. The frame size was typically 256 × 256 pixels (207 ms/frame), while 512 × 512 or 2,024 × 2,024 pixel frames were used to obtain overviews.
Bolus loading of the synthetic Ca2+ indicator Oregon Green BAPTA-1/AM (10 mmol/L OGB-1/AM; Invitrogen, Naerum, Denmark) in dimethyl sulfoxide plus 20% Pluronic F-127 (Sigma-Aldrich) diluted in either artificial cerebrospinal fluid or 50 μmol/L sulforhodamine 101 (SR101, Invitrogen) in artificial cerebrospinal fluid yielded a final dye concentration of 0.8 mmol/L. The dye was filtered through Corning Costar Spin-X centrifuge tube filters (0.22 μm, Sigma-Aldrich) and then bolus loaded through a micropipette at 2 to 4 sites at depths of 50 to 100 μm below the surface of the cerebellar cortex (3 to 5 p.s.i., 10 to 50 seconds; Pneumatic Pump; World Precision Instruments) during visual two-photon inspection (Figures 1B and 1C).
Image Analyses
The obtained time sequence frames were processed offline using custom software written in the Python programming language with the use of the SciPy and Matplotlib [Hunter:2007] open source libraries. The custom software is available for download at http://code.google.com/p/image-funcut/ and is distributed under the GNU general public license. Time-lapse fluorescent movies were loaded as series of tiff files. The fluorescence intensity in each pixel was normalized as dF/SD, where dF=FT−F0 represents the difference between the fluorescence at time T (FT) and the average fluorescence during the baseline period (F0), while SD represents the standard deviation during the baseline period. The baseline period was taken as the first 250 frames. Normalization was followed by a smoothing process in which the time series of values in each pixel was smoothed by convolution with a cubic B-spline low-pass filter (Figure 1D, left and middle panels). To evaluate the amplitudes of Ca2+ transients, responses were normalized to the average fluorescence during the baseline period (F0), e.g., when examining the effect of pyridoxalphosphate-6-azophenyl-2',4'-disulfonic acid tetrasodium salt (PPADS, Tocris, Bristol, UK) on Ca2+ transients (Figures 6E and 6G).
The Multiscale Vision Model
The multiscale vision model was used for detection and reconstruction of glial Ca2+ waves. Each frame in a sequence of normalized images was decomposed with the à trous transform, the wavelet coefficients were thresholded at each scale, and the resulting contiguous regions were labeled with interscale connectivity.10 The sets of wavelet coefficients representing individual objects were then used for object reconstruction in a given frame (Figure 1D, right panel). Following this, the overlapping objects in neighboring frames were stitched together to create an XYT 3D object or a time-lapse sequence, corresponding to a single glial wave (Figure 1E).
Cluster Analysis Using a Self-Organizing Map Algorithm
Cluster analysis using a self-organizing map algorithm was used to detect spontaneous activity in both BG and PCs. For cluster analysis of time-lapse imaging data, we applied a self-organizing map algorithm.11 This algorithm maps data patterns onto an n-dimensional grid of units with similar behavior. Number of clusters N=8 was chosen as a minimal number resulting in a meaningful spatial structure (for detailed description, see Supplementary Information).
Correlation Analysis Between Purkinje Cell and Bergmann Glia Calcium Transients
Spontaneous PCs and BG activities were identified in the time-lapse movies using image-funcut (The custom software is available for download at http://code.google.com/p/image-funcut/). Regions of interest (ROIs) were placed in groups of five, one ROI in a central PC and the remaining four ROIs in neighboring BG. The size of the ROI in the PC was 1/2 the diameter of the cell body, which abolished the risk of optical smear from neighboring BG. Positive correlation analysis was performed on time series of 55 seconds that included 15 seconds of baseline activity before the onset of spontaneous activity (OriginPro 8.6, Linear Fit, Northampton, MA, USA).
ATP-Evoked Glial Waves
A pipette (tip diameter 3 to 4 μm) filled with ATP (1.0 mmol/L ATP (Sigma-Aldrich)+15 μmol/L Alexa Fluor 954 hydrazide sodium salt (Invitrogen) in artificial cerebrospinal fluid) was placed in the molecular layer at depths of 25 to 50 μm (Figures 1A and 1B). Injection of ATP with 10 μs 3 to 5 p.s.i. pulses was verified by observation of a red puff at the tip of the pipette and of an evoked glial wave in the red and green channels, respectively.
Measuring Tissue Partial Pressure of Oxygen
Local tpO2 was recorded with a modified Clark-type polarographic oxygen microelectrode (OX-10; Unisense A/S). The small tip size (3 to 5 μm) assured reliable tpO2 measurements, and the built-in guard cathode removed all oxygen from the electrolyte reservoir. This enabled us to measure tpO2 over time under different treatment conditions with excellent long-term stability (signal drift, 0% to 0.5% per hour). The field of sensitivity was a sphere with a diameter that was twice the tip diameter. The oxygen electrodes were calibrated in air-saturated and oxygen-free 0.9% saline before and after each experiment with reproducible oxygen measurements. The tip of the O2 microelectrode was positioned within 20 to 50 μm of the ATP-loaded pipette (Figures 1A and 1B). The oxygen electrode was connected to a high-impedance picoamperometer (PA 2000; Unisense A/S). Signals were A/D converted, recorded at 10 Hz using a Power 1,401 data acquisition interface and Spike 2 software (both from Cambridge Electronic Design, Cambridge, England), and transformed to mm Hg using calibration points in air-saturated and oxygen-free saline. Noise, including heartbeat and mechanical ventilation artifacts, was minimized by smoothing using a time constant of 1 second (Spike 2 software).
Protocols
The spontaneous glial Ca2+ wave phenomenon was examined using (N=45 glial waves from 5 mice) and presented as time-projected images and maximal extent.
Age-dependent changes in spontaneous glial Ca2+ wave activity were investigated in two cohorts of mice: one adult cohort, mean age=10.1 weeks (range: 7.6 to 14.6, N=14) and a second aging cohort, mean age=79.6 weeks (range: 79.6 to 91.3, N=11). The event rate of spontaneous glial waves was monitored using the same laser intensity and digital zoom for both cohorts. The tpO2 electrode was not placed in the tissue because pilot experiments showed that laser light excited the electrode and might elicit glial waves.
The effect of hypoxia on glial wave activity was examined by measuring tpO2 levels in other two groups of mice: an adult cohort, mean age 9.25 weeks (range: 7.6 to 14.6, N=6) and in an aging cohort, mean age 75.9 weeks (range: 61.0 to 91.3, N=5). The effect of reduced oxygen saturation on tpO2 was observed in three adult mice, and the effect of reduced oxygen saturation on spontaneous glial waves was examined in other five adult mice (mean age, 8.8 weeks; range, 8.1 to 11.0).
The relationship between glial waves and oxygen consumption was examined using a tpO2 electrode and an ATP-filled pipette placed in the molecular layer with a tip separation of 25 to 50 μm (mean age 8.9 weeks; range, 7.6 to 14.0, N=7). ATP-evoked glial waves and tpO2 responses were recorded in an interleaved manner as the laser light affected the tpO2 recording. The purinergic P2Y receptor antagonist PPADS (500 μm) was applied topically (8.3 weeks; range, 7.6 to 9.0, N=4) to verify the origin of the responses.
Coupling between the PC and BG activities was investigated in the PC layer, in which PCs and BG cell bodies can be clearly differentiated (mean age 44 weeks; range, 11 to 86, N=4). Regions of interest were placed in PC and BG cell bodies during spontaneous glial waves and PC activity.
Statistics
Post hoc smoothing was applied to all traces originating from ROIs and blood vessel diameter line scans using Savitzky-Golay smoothing (15 points, OriginPro 8.6). Responses are presented as mean values±the standard error of the mean (SEM). One-way and two-way ANOVA and Kolmogorov–Smirnov tests for nonparametric data were used to evaluate significance (α<0.05).
Results
Spontaneous Glial Waves
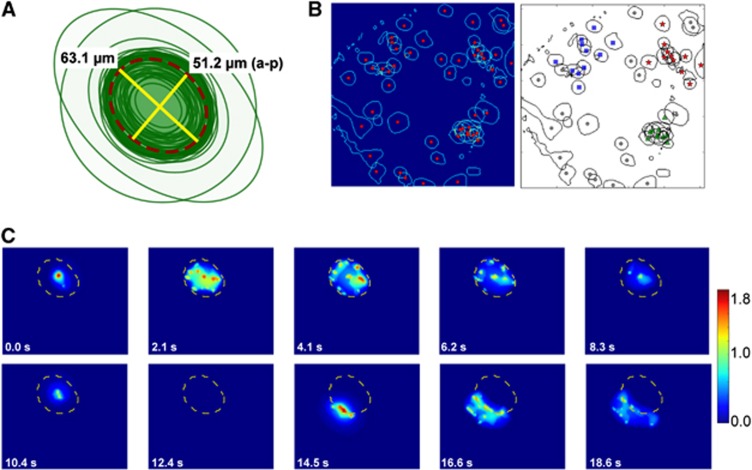
Spontaneous glial Ca2+ waves consist of intracellular Ca2+ increases in BG2, 6 that propagate radially from a central initiation point (Figures 1D and 1E). Glial waves propagated farther in the medio-lateral than in the anterior-posterior directions (63.1±3.1 versus 51.2±2.5 μm, respectively; N=45 waves; Figure 2A), with a ratio between the medio-lateral and anterior-posterior diameters of 1.24±0.03. The characteristics of spontaneous glial waves described here were similar to those reported previously.2 During a 40-minute observation period in which we identified 51 spontaneous glial waves, 60% of the wave centers occurred within a distance of ⩽50 μm of each other, while the remaining 40% were distributed randomly throughout the field of vision (456 × 456 μm2, Figure 2B). Thus, a large fraction of waves clustered, i.e., recurred in similar locations. Additionally, we found that BGs in cortical areas previously involved in a spontaneous glial wave were refractory, i.e., unresponsive to a new one (Figure 2C).
Figure 2.
The characteristics of spontaneous glial calcium (Ca2+) waves. (A) Drawing of the maximal extent of propagation of 45 centered glial Ca2+ waves. Average maximal extent marked with dashed dark red line. The lateral-medial distance was 63.1 μm and anterior-posterior (a-p) distance was 51.2 μm (N=45 waves, 5 mice). These data show that glial Ca2+ waves in the cerebellar cortex extend further mediolaterally than anterioposteriorly. (B) Left: the maximal extent (blue line) and center origin (red dot) of 51 spontaneous glial waves based on time-averaged multiscale vision. Right: black-and-white rendition of data in the left panel showing three identified clusters with 11 (red stars), 10 (green triangles), and 10 (blue squares) waves, respectively, corresponding to 60% of the wavelets. The remaining 40% of the waves were not associated with any clusters. Clusters were identified using a self-organizing map algorithm (see Materials and methods). (C) Time series showing a propagating spontaneous glial wave Ca2+ reaching its maximal extent (dashed yellow line) at 4.1 seconds then receding and finally disappearing at 12.4 seconds. Approximately 2 seconds later, a second spontaneous glial wave began at the border of the previous wave, expanding to both sides without propagating into the area of the first wave, indicating that the area of the first wave was in a refractory state.
Spontaneous Glial Calcium Wave Activity Increased with Age
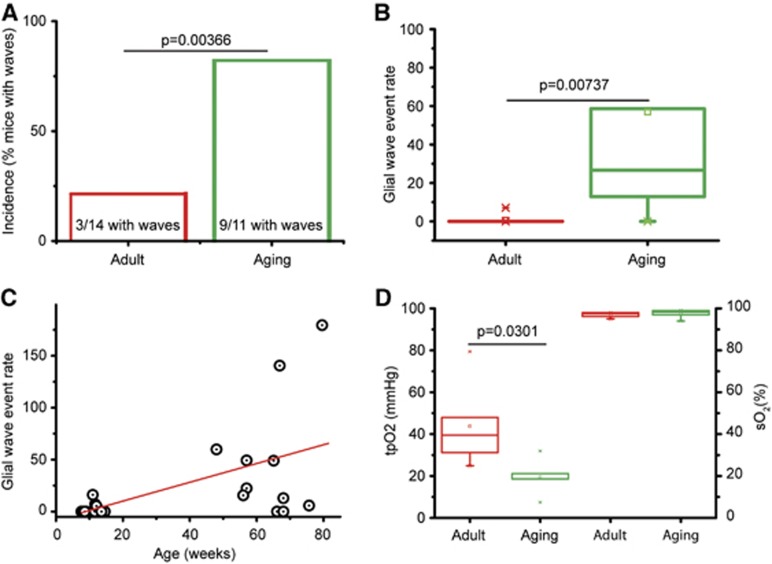
The incidence and event rate of spontaneous glial waves were examined in two cohorts of male NMRI mice: adult mice (N=14) and aging mice (N=11). The same low laser intensity and degree of digital zoom for the two-photon laser-scanning microscope was used for both groups. The number of mice with spontaneous glial Ca2+ waves was significantly different between the two cohorts, as waves were observed in 3 of 14 adult mice (Supplementary Movie S2) and in 9 of 11 aging mice (Supplementary Movie S3, Kolmogorov–Smirnov, P=0.00366; Figure 3A). Spontaneous glial waves were observed throughout the molecular layer as well as in the PC layer. The event rate of spontaneous waves was significantly higher in the aging cohort compared with the adult cohort (2.62 versus 0.13 glial waves/10 minutes per mm2, P=0.00737; Figures 3B and 3C). A significant positive correlation was found between age and wave event rate (slope, 0.276, R2=0.289, P=0.0033, df=23; Figure 3C). Note that eight out of the nine aging mice with glial waves had a glial wave event rate greater than the mean event rate +2SD of adult mice (Figure 3C). Two-photon laser scanning was performed in sequences lasting 5 or 10 minutes for up to 4 hours without observing spontaneous glial waves in adult mice.
Figure 3.
Age-dependent increase in spontaneous glial calcium (Ca2+) wave activity and decrease in basal tissue O2 tension (tpO2). (A) The incidence indicated by % of mice with spontaneous glial Ca2+ waves was significantly higher for aging mice (9 of 11 mice, mean age, 64.3 weeks) compared with adult mice (3 of 14 mice; mean age, 10.1 weeks; Kolmogorov–Smirnov test, P=0.00366). (B) The glial Ca2+ wave event rate (glial waves/10 minutes per mm2) was significantly higher in the aging cohort compared with the adult cohort (ANOVA, P=0.00737). (C) There was a significant positive correlation between age and glial Ca2+ wave event rate when both groups were analyzed together (red line; slope, 0.276, R2=0.289, P=0.0033, df=23). The glial wave event rate was higher in aging mice compared with adult mice (P=0.00112, ANOVA). One adult mouse and eight aging mice had a glial wave event rate greater than the mean+2SD level of the adult group. (D) Basal oxygen tension (tpO2) was significantly lower in the aging mice (N=5) than in the adult mice (ANOVA, P=0.0301; N=6 adult mice; left), but there was no difference in blood oxygen saturation (sO2; ANOVA, P=0.785; right).
Hypoxia Induced Spontaneous Glial Calcium Waves
The tpO2 (mm Hg) was monitored in the molecular layer of the cerebellar cortex in two additional groups of adult (N=6) and aging NMRI mice (N=5) using a Clark-like O2 electrode (tip diameter=3 μm). Resting cortical tpO2 in aging mice was less than half that of adult mice (19.5 versus 43.8 mm Hg; Figure 3D). This finding implies that brain energy homeostasis is more dependent on oxygen levels in aging than in adult mice.
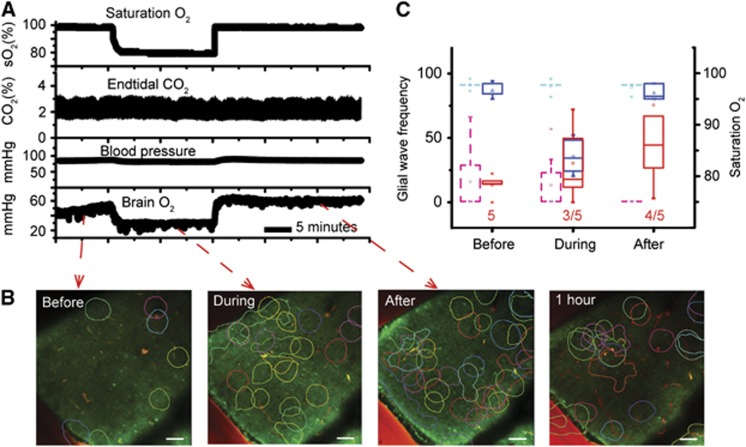
As resting brain oxygen tension and occurrence of spontaneous glial Ca2+ waves appeared to be inversely proportional during the aging process, we tested the hypothesis that brain oxygen tension could influence spontaneous glial wave activity independently of age. Using a group of adult mice (N=5), the tpO2 in the cerebellar cortex was reduced by removing the oxygen supplement given with the inspired atmospheric air. This caused the oxygen saturation of blood (sO2) to decrease significantly (Figure 4A, upper trace) and cortical tpO2 to decrease by 20% to 85% (Figure 4A, lower trace). The reduction in sO2 did not affect end-tidal CO2 (Figure 4A, second trace). These effects persisted until cortical tpO2 normalized after restoration of the oxygen supplement. Importantly while tpO2 was reduced, the event rate of spontaneous glial waves increased (Figure 4B, second panel from the left) and unexpectedly increased further when tpO2 returned to baseline (Figure 4B, second panel from the right); in contrast, the wave event rate gradually decreased after 1 hour of normoxia (Figure 4B, right panel). Figure 4C shows sO2 and spontaneous glial wave activity before and during hypoxia, and at 5 to 25 minutes and 1 hour after return to normoxia.
Figure 4.
Increased spontaneous glial calcium (Ca2+) wave activity induced by acute hypoxia. (A) Raw data showing the induction of acute hypoxia for 20 minutes, as shown by reduced blood O2 saturation (first tracing). Acute hypoxia was characterized by stable end-tidal CO2 (second tracing), stable arterial blood pressure (third tracing), and a reduction in brain tissue O2 tension (tpO2, fourth tracing). (B) Pseudocolored time-averaged images showing the contours of spontaneous glial Ca2+ waves before, during, and after induction of hypoxia. The glial wave event rate increased during acute hypoxia (second panel) and continued to increase after normoxia was reestablished (third panel). One hour after return to normoxia, glial wave activity had decreased but not returned to baseline (fourth panel). Each glial wave is indicated using a different color; the contour lines represent the maximal extent of each wave. (C) Box-plot graph summarizing O2 saturation (blue boxes, time control: dashed light blue boxes) and glial wave event rate (glial waves/10 minutes per mm2, red boxes, time control: dashed magenta boxes) before, during, and immediately after acute hypoxia (N=5). Glial wave event rate increased in three of five mice during hypoxia and increased further in four of five mice 20 minutes after normoxia was reestablished. Time-control recordings were performed during normoxia condition at same time periods as the hypoxia group (N=8).
ATP-Evoked Glial Calcium Waves and Oxygen Consumption
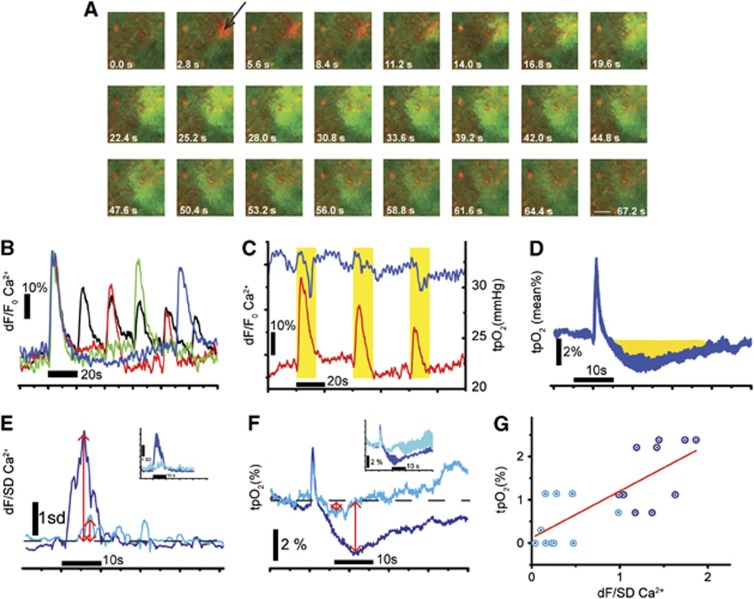
Glial Ca2+ waves can be evoked in cerebellum by intracortical injections of ATP.2 We found that glial waves evoked by ATP (10 μs, 3 to 5 p.s.i.) propagated farther and were longer lasting than spontaneous glial waves (Figure 5A, maximal diameter ∼100 μm at 25.2 to 28.0 seconds). ATP-evoked increases in glial Ca2+ fluorescence (dF/F Ca2+) were reproducible when ATP was injected at intervals of 60 and 90 seconds, but were reduced in amplitude when injected at shorter intervals (Figure 5B), indicating that the tissue was refractory while recovering from the preceding wave.
Figure 5.
Effect of ATP-evoked glial calcium (Ca2+) waves on tissue O2 tension (tpO2). (A) Time series showing how the application of ATP plus Alexa Fluor 954 (red dot indicated with a black arrow) evoked a wave of increased Ca2+ fluorescence (dF/F0) because of activated Bergmann glia. (B) The time course of repetitively evoked glial waves showing that the evoked dF/F0 Ca2+ amplitudes decreased when the application intervals were <60 seconds. Reproducible responses were evoked with injection intervals of 90 seconds (blue trace) and 60 seconds (green trace). The response amplitude declined successively with the number of injections when ATP was administered at intervals of 40 seconds (red trace) and 20 seconds (black trace). (C) Simultaneous recordings of tpO2 (blue trace) and dF/F0 Ca2+ (red trace) during three applications of ATP at 40 seconds intervals. Both responses decreased progressively with the number of applications. Note that during simultaneous recordings of glial wave activity and tpO2, the tpO2 electrode was located outside the two-photon plane of focus, as laser scanning produced high frequency noise. (D) The mean tpO2 response to evoked glial waves (±SEM, N=3). The initial increase in tpO2 is an artifact of ATP injection. The reductions in tpO2 are highlighted in yellow. (E, F) The effect of the P2Y receptor antagonist, PPADS, on the dF/SD Ca2+ and tpO2 responses. ATP-evoked glial waves induced increases in Ca2+ (dF/SD; E) and decreases in tpO2 (F). Both responses (blue traces in E and F) were blocked by topical application of PPADS (500 μmol/L, cyan traces in E and F). The red arrows indicate the responses before (large arrows) and after (small arrows) application of PPADS. Insets in both graphs show the mean responses±SEM (N=4 responses). (G) The tpO2 response (measured as described in F) as a function of the dF/SD Ca2+ amplitude (measured as described in E) evoked before (blue circle) and after application of PPADS (cyan circle). PPADS inhibited both tpO2 and the Ca2+ amplitude of the glial wave concomitantly, resulting in a positive correlation (red line; R2=0.570, P=0.00009, N=4 mice).
As calcium fluxes across glial cell membranes are known to activate glial Na+-K+ ATPase12 that may increase glial energy consumption, we examined brain oxygen tension (tpO2) in the presence of ATP-evoked glial Ca2+ waves. ATP-evoked waves induced time-locked changes in tpO2 (Figure 5C). The mean evoked tpO2 response using 60 seconds application intervals consisted of a short increase in tpO2 lasting 1 to 2 seconds, which was possibly a pressure artifact because of intracortical ATP injection, followed by an ∼20 seconds decrease in tpO2 (Figure 5D, N=3). When ATP was injected at intervals shorter than 60 seconds, the evoked tpO2 reductions diminished in parallel with the glial Ca2+ transients (Figure 5C). The P2Y receptor antagonist PPADS (500 μmol/L) blocked both the evoked tpO2 reductions (Figure 5F) and the glial Ca2+ transients (dF/SD; Figure 5E). A significant positive linear correlation was found between the magnitudes of tpO2 reductions and the glial Ca2+ transients under control conditions and in the presence of PPADS (R2=0.557, P=0.00009, N=4; Figure 5G). This finding suggests that the evoked tpO2 reductions in could be the result of increased ATP-dependent Ca2+-stimulated K+ uptake in BG, from an increase in PC activity (see below), from vasoconstriction in a small proportion of intracortical vessels, or from a combination of these possibilities.
Independence of Glial Waves on Spontaneous Purkinje Cell Activity
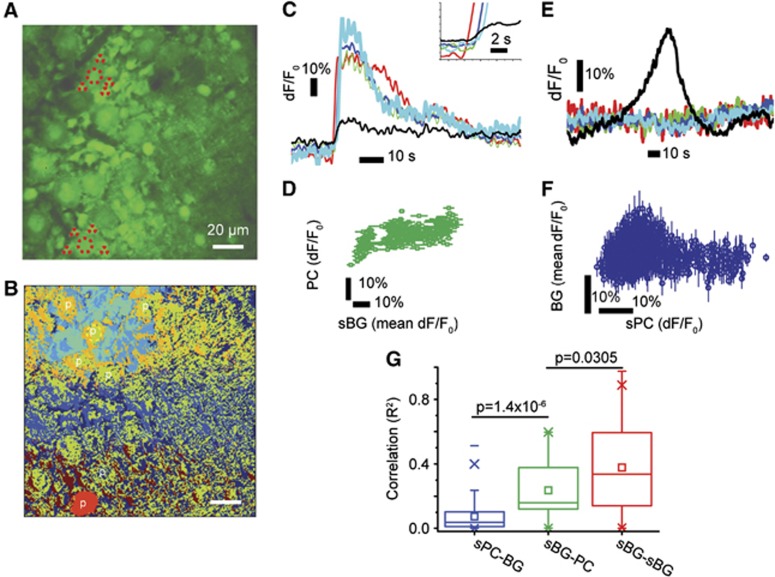
Purkinje cells, the principal output neurons of the cerebellar cortex, and BG, which support glial wave activity, are in close anatomical proximity to each other. The cell bodies of BG encircle PC somata and radially arranged BG fibers wrap around synapses on PC dendrites,13 contributing to glutamate uptake via glutamate transporters.14, 15, 16 The BGs can depolarize PCs and thereby increase their spiking activity via Ca2+-dependent uptake of extracellular K+.12 As PC membrane depolarization evokes PC Ca2+ transients,17 we analyzed our imaging data in more detail to uncover putative correlations between spontaneous Ca2+ activity in BG and PCs. We found that PCs encountered by spontaneous glial waves during propagation (Figure 6B, cyan field) could respond to BG activity, although with small Ca2+ transients compared with those of active BG within the wave (Figure 6C). In contrast, spontaneous PC activity did not spread to surrounding BG (Figure 6B, red area; Figure 6E; Supplementary Movie S4). Thus, a slight but significant correlation between the increases in Ca2+ activity in BG and PCs was found during spontaneous glial wave activity (R2=0.248, P<0.001; Figure 6D), but not during spontaneous PC activity (P>0.05; Figure 6F; Supplementary Movie S4). We also found that glial waves were not triggered by PCs, as PC Ca2+ transients did not precede those of BG (Figure 6C, insert). Figure 6G summarizes the positive correlations (R2) between Ca2+ transients of spontaneously active PCs versus neighboring BG (N=36 events, 4 mice), between Ca2+ transients of spontaneously active BG versus PCs encountered by the same glial wave (N=333 events, 4 mice), and between Ca2+ transients of neighboring spontaneously active BG in the same glial wave (N=336 events, 4 mice). The greatest correlation was found between neighboring BG in the same glial wave (mean R2=0.378±0.013). This correlation was significantly different from that between BG and PCs encountered by the same glial wave (mean R2=0.237±0.026, P=0.0305), indicating a closer interaction between neighboring BG than between BG and PCs during glial wave propagation. The correlation between spontaneously active PC and surrounding BG activities was significantly less than that between active BG and PCs encountered by the same glial wave confirming our finding that spontaneous PC activity did not evoke glial Ca2+ waves (Figure 6G).
Figure 6.
Cluster and correlation analysis of spontaneous Bergmann glia (BG) and Purkinje cell (PC) activity. (A) A representative time-averaged frame obtained from the PC layer of one mouse. Two large PCs are depicted (∼18 μm in diameter), each surrounded by four smaller BG cell bodies (large and small dashed and dotted circles). (B) Pseudocolored clusters in which colors denote areas of clustered activity. A propagating spontaneous glial Ca2+ wave (cyan field) enveloping a PC, as well as a spontaneously active PC (red spot), is visible. The PCs of interest are indicated by ‘p'. (C) dF/F0 traces over time obtained from the spontaneously active BG comprising the glial wave (blue, green, red, and light blue lines) and from the PC the wave enveloped (black line) in (A) (top part, red dashed circles). Purkinje cells encountered by propagating glial Ca2+ wave did not always respond to surrounding BG activity; when they did so, the PC response was only a small fraction of the spontaneous BG activity. Purkinje cells did not initiate waves as BG responded before PCs (insert). (D) A correlation plot shows dF/F0 values from the yellow PC in (A) plotted against dF/F0 values averaged across all yellow BG in (A). There was a small but significant positive correlation (R2=0.29529, P<0.001). (E) dF/F0 traces over time obtained from the spontaneously active PC (black line) and surrounding BG (blue, green, red, and light blue lines) in (A) (bottom, red dotted circles), showing that spontaneous PC activity did not spread into BG. (F) A correlation plot showing dF/F0 values from the red PC in (A) plotted against dF/F0 values averaged across all red BG in (A). No correlation between spontaneous PC activity and surrounding BG activity was found, confirming the finding that spontaneous PC activity did not activate BG (R2=0.002 P=0.259). (G) Box-plot summarizing the correlations (R2) between spontaneously active PCs and surrounding BG (sPC-BG, blue, N=36 interactions from three mice), between spontaneously active BG and PCs encountered by spontaneous glial waves (sBG-PC, green, N=333 interactions from four mice), and between spontaneously active BG (sBG-sBG, red, 336 interactions from 4 mice). While spontaneous PC activity did not spread to surrounding BG, spontaneous glial wave activity consisting of active BG did spread to PCs, although with low dF/F0 amplitude. The positive correlation for sPC→BG was significantly lower than that for sBG→PC (P=1.4 × 10−6) and sBG→PC was significantly lower than that of sBG→sBG (P=0.0305).
Discussion
Intercellular glial Ca2+ waves constitute a coordinated signaling mechanism between glia and can modulate neuronal activity and, to a small extent, blood flow as well.7, 18, 19, 20, 21 Spontaneous glial waves in rodents have been observed in vivo in the cerebellar cortex,2 cerebral cortex,3 retina,8 and hippocampus,4 and have been reported in acute human slices from cerebral cortex,22 showing that glial waves occur globally in the brain. Although radially expanding purine-dependent Ca2+ waves have only been reported to be present in rodent cerebellum2 and retina.8 By imaging glial waves in vivo in the brains of adult and aging mice, we observed age-dependent increases in the event rate of spontaneous glial Ca2+ waves, a lower tpO2 in the brains of aging versus adult mice, and hypoxia-induced increases in spontaneous glial wave activity. In addition, we observed that significant decreases in tpO2 were induced by ATP-evoked glial Ca2+ waves. Finally, spontaneous PC activity had no effect on the initiation of glial Ca2+ waves, although glial wave activity had a small effect on PC activity.
Characterization of Spontaneous Glial Waves
We found that glial Ca2+ waves had a maximal propagation distance and an ellipsoid shape in accord with findings reported previously for the rodent cerebellum.2 We also report that spontaneous glial waves did not invade cortical areas previously occupied by a spontaneous wave, a phenomenon not described before, providing evidence of a BG refractory state after spontaneous glial Ca2+ waves. The present study also confirmed previously reported BG refractory states after ATP-evoked glial waves.2 The BG refractory states after flares have also previously been reported.6 Together, these findings indicate that glial Ca2+ waves do have a physiologic relevance in vivo and in both anesthetized and wake mice.
The Effect of Aging and Hypoxemia on Spontaneous Glial Wave Calcium Activity
Aging mice had a greater incidence of spontaneous glial Ca2+ waves than did adult mice. Age dependence of glial Ca2+ wave activity has been shown previously in the retina.8 In addition to increased wave event rate with increased age, we found that resting tpO2 was reduced in the cerebellar cortex of aging mice. In humans, basal blood flow and cortical O2 consumption undergo disproportionate age-dependent reductions, with blood flow decreasing most prominently, resulting in an increased O2 extraction fraction of blood with age.23 These alterations imply that resting tpO2 decreases with age, which is what we observed in the aging mice in this study. Our finding of both an increased event rate of spontaneous glial waves and reduced resting cortical tpO2 in the aging mouse cohort led us to hypothesize that cortical tpO2 may have a role in inducing glial waves.
The Role of Hypoxemia
We found that lowering tpO2 in the brain resulted in increased glial wave activity. This may be a consequence of an altered NAD(+)/NADH redox state, as increased NADH reflects low tpO2 in brain tissue24 and increased cytosolic NADH results in a pronounced increase in Ca2+ signaling in astrocytes.25 Thus, glial Ca2+ wave activity may occur in response to changes in the NAD(+)/NADH redox state.26 Hypoxia increases the number of plasma membrane Cx43 hemichannels.27 The increase in spontaneous glia activity and hemichannels leads to an elevated release of ATP that triggers glial Ca2+ waves. The subsequent efflux of ATP through the hemichannels leads to the accumulation of its metabolite, adenosine, a potent neuroprotective agent,27 suggesting that increased glial wave activity could be an adaptive process that preconditions the tissue and increases cellular resistance to hypoxia, thereby preserving brain functionality and homeostasis.26
The Contribution of Glial Waves to Oxygen Consumption
ATP-evoked glial waves induced small decreases in the tpO2, suggesting small increases in O2 consumption. This could reflect increases in PC activity resulting from Ca2+-dependent K+ uptake in BG,7, 12 but it is also possible that the reduction in tissue O2 was caused by local vasoconstriction. Both possibilities suggest that cerebellar O2 availability is reduced by glial Ca2+ waves, which may contribute to the low O2 tension in aging mice, and increase tissue sensitivity. Future experiments will address this issue using two-photon laser scanning microscope with piezo-focus.
Bergmann Glia and Purkinje Cell Interactions
We observed that glial wave activity was initiated from a single BG cell from which the wave propagated regeneratively to neighboring BG. Spontaneous activity in BG also propagated to PCs, although with a lower response intensity compared with that of neighboring BG. The correlation between spontaneously active BG and PCs was relatively low, and in some cases the PCs were found not to react at all. This may be related to the reported bistability of PCs,28 in which Ca2+ increases in BG coupled with decreases in extracellular K+ may increase neuronal activity.7 The increased Ca2+ signaling in BG during glial waves was correlated with, time locked to, and preceded increased Ca2+ activity in PCs. In comparison, spontaneous PC Ca2+ transients did not spread to BG in contrast to previous in vitro studies in the hippocampus showing that neuronal activity triggered astrocytic activity indirectly via extracellular K+.7 Thus, we found that glial wave activity was not activated by PC activity but originated in BG.
Conclusion
We present data showing that the aging compared with the adult brain has increased spontaneous glial Ca2+ wave activity and a low resting tpO2 in the cerebellar cortex, suggesting a relationship between glial waves and brain energy. ATP-evoked glial waves were associated with a time-locked reduction in tpO2 implying increased local oxygen consumption. Finally, we report that glial Ca2+ wave activity was triggered independently of principal neuron Ca2+ activity.
Acknowledgments
The authors thank Micael Lønstrup for providing excellent surgical assistance, Sanne Barsballe Jessen for helpful discussions, Krzyzstof Kucharz for assistance with the figures, and Tycho Hoogland for comments and suggestions.
Author contributions
CM designed the study and performed the research; CM and AB developed the analytic tools and analyzed data; and CM, KT, AB and ML wrote the paper.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by the NORDEA Foundation/Center for Healthy Aging, the Lundbeck Foundation via the Lundbeck Foundation Center for Neurovascular Signaling (LUCENS), the NOVO-Nordisk Foundation, the Danish Medical Research Council, and Foundation Leducq.
Supplementary Material
References
- Jaffe LF. Fast calcium waves. Cell Calcium. 2010;48:102–113. doi: 10.1016/j.ceca.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Hoogland TM, Kuhn B, Gobel W, Huang W, Nakai J, Helmchen F, et al. Radially expanding transglial calcium waves in the intact cerebellum. Proc Natl Acad Sci USA. 2009;106:3496–3501. doi: 10.1073/pnas.0809269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323:1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Perea G, Maglio L, Pastor J, Garcia de SR, Araque A.Astrocyte calcium signal and gliotransmission in human brain tissue Cereb Cortexadvance online publication, 10 May 2012 (e-pub ahead of print). [DOI] [PubMed]
- Newman EA. Calcium increases in retinal glial cells evoked by light-induced neuronal activity. J Neurosci. 2005;25:5502–5510. doi: 10.1523/JNEUROSCI.1354-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Mukamel EA, Schnitzer MJ. Motor behavior activates Bergmann glial networks. Neuron. 2009;62:400–412. doi: 10.1016/j.neuron.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Xu Q, Wang W, Takano T, Nedergaard M. Bergmann glia modulate cerebellar Purkinje cell bistability via Ca2+-dependent K+ uptake. Proc Natl Acad Sci USA. 2012;109:7911–7916. doi: 10.1073/pnas.1120380109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth-Nelson ZL, Mishra A, Newman EA. Spontaneous glial calcium waves in the retina develop over early adulthood. J Neurosci. 2009;29:11339–11346. doi: 10.1523/JNEUROSCI.2493-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen C, Caesar K, Thomsen K, Hoogland TM, Witgen BM, Brazhe A, et al. Activity-dependent increases in local oxygen consumption correlate with postsynaptic currents in the mouse cerebellum in vivo. J Neurosci. 2011;31:18327–18337. doi: 10.1523/JNEUROSCI.4526-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijaoui A, Ru F. A multiscale vision model adapted to the astronomical images. Signal Processing. 1995;46:345–362. [Google Scholar]
- Kohonen T. Self-organizing maps. Berlin: Springer; 2001. [Google Scholar]
- Wang F, Smith NA, Xu Q, Fujita T, Baba A, Matsuda T, et al. Astrocytes modulate neural network activity by Ca(2)(+)-dependent uptake of extracellular K(+) Sci Signal. 2012;5:ra26. doi: 10.1126/scisignal.2002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Chan-Palay V.(eds).. Cerebellar cortex, cytology and organization. New York Springer; 1974 [Google Scholar]
- Bergles DE, Dzubay JA, Jahr CE. Glutamate transporter currents in bergmann glial cells follow the time course of extrasynaptic glutamate. Proc Natl Acad Sci USA. 1997;94:14821–14825. doi: 10.1073/pnas.94.26.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BA, Barbour B.Currents evoked in Bergmann glial cells by parallel fibre stimulation in rat cerebellar slices J Physiol 1997502(Pt 2)335–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayasu Y, Iino M, Kakegawa W, Maeno H, Watase K, Wada K, et al. Differential roles of glial and neuronal glutamate transporters in Purkinje cell synapses. J Neurosci. 2005;25:8788–8793. doi: 10.1523/JNEUROSCI.1020-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CY, Hounsgaard J, Midtgaard J. Excitatory synaptic responses in turtle cerebellar Purkinje cells. J Physiol. 1989;409:143–156. doi: 10.1113/jphysiol.1989.sp017489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Mulligan SJ, MacVicar BA. Astrocyte control of the cerebrovasculature. Glia. 2007;55:1214–1221. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci USA. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, MacVicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres A, Wang F, Xu Q, Fujita T, Dobrowolski R, Willecke K, et al. Extracellular Ca(2) acts as a mediator of communication from neurons to glia. Sci Signal. 2012;5:ra8. doi: 10.1126/scisignal.2002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aanerud J, Borghammer P, Chakravarty MM, Vang K, Rodell AB, Jonsdottir KY, et al. Brain energy metabolism and blood flow differences in healthy aging. J Cereb Blood Flow Metab. 2012;32:1177–1187. doi: 10.1038/jcbfm.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasischke KA, Lambert EM, Panepento B, Sun A, Gelbard HA, Burgess RW, et al. Two-photon NADH imaging exposes boundaries of oxygen diffusion in cortical vascular supply regions. J Cereb Blood Flow Metab. 2011;31:68–81. doi: 10.1038/jcbfm.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requardt RP, Hirrlinger PG, Wilhelm F, Winkler U, Besser S, Hirrlinger J. Ca(2)(+) signals of astrocytes are modulated by the NAD(+)/NADH redox state. J Neurochem. 2012;120:1014–1025. doi: 10.1111/j.1471-4159.2012.07645.x. [DOI] [PubMed] [Google Scholar]
- Cerdan S, Rodrigues TB, Sierra A, Benito M, Fonseca LL, Fonseca CP, et al. The redox switch/redox coupling hypothesis. Neurochem Int. 2006;48:523–530. doi: 10.1016/j.neuint.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Lin JH, Lou N, Kang N, Takano T, Hu F, Han X, et al. A central role of connexin 43 in hypoxic preconditioning. J Neurosci. 2008;28:681–695. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen GL, Hockberger PE, Houk JC. Bistability in cerebellar Purkinje cell dendrites modelled with high-threshold calcium and delayed-rectifier potassium channels. Biol Cybern. 1995;73:375–388. doi: 10.1007/BF00199473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.