Abstract
Ischemia, especially pericontusional ischemia, is one of the leading causes of secondary brain damage after traumatic brain injury (TBI). So far efforts to improve cerebral blood flow (CBF) after TBI were not successful because of various reasons. We previously showed that nitric oxide (NO) applied by inhalation after experimental ischemic stroke is transported to the brain and induces vasodilatation in hypoxic brain regions, thus improving regional ischemia, thereby improving brain damage and neurological outcome. As regional ischemia in the traumatic penumbra is a key mechanism determining secondary posttraumatic brain damage, the aim of the current study was to evaluate the effect of NO inhalation after experimental TBI. NO inhalation significantly improved CBF and reduced intracranial pressure after TBI in male C57 Bl/6 mice. Long-term application (24 hours NO inhalation) resulted in reduced lesion volume, reduced brain edema formation and less blood–brain barrier disruption, as well as improved neurological function. No adverse effects, e.g., on cerebral auto-regulation, systemic blood pressure, or oxidative damage were observed. NO inhalation might therefore be a safe and effective treatment option for TBI patients.
Keywords: brain edema, brain trauma, cerebral blood flow, microcirculation, nitric oxide
Introduction
Ischemia is one of the leading causes of secondary brain damage after traumatic brain injury (TBI).1, 2 Typically, cerebral blood flow (CBF) is markedly reduced immediately after and during the first few hours after trauma, especially in the pericontusional region.3 The primary lesion evolving at the time of injury increases in size when CBF in the critically perfused tissue around the contusion (‘traumatic penumbra') gradually decreases below the ischemic threshold giving rise to secondary injury.4 Standard treatment for TBI includes hyperventilation, infusion of hyperosmotic/hyperoncotic solutions, decompressive craniectomy, and barbiturate coma; however, no tools are available to specifically improve perfusion of the traumatic penumbra. Vasodilators would be an obvious choice but are inefficient in saving penumbral tissue because they dilate systemic resistance vessels and have a much stronger effect on vessels in uninjured brain areas as compared with vessels in injured brain. Accordingly, the resulting systemic hypotension and the cerebral steal effect would further lower pericontusional blood flow and further compromise the traumatic penumbra. The development of a vasodilator with a specific action on vessels in hypoxic/ischemic tissue would certainly be a viable strategy for the treatment of pericontusional ischemia; however, such a therapeutic option was not available yet.
Recently we proposed inhaled nitric oxide (NO), the most potent known endogenous vasodilator, to have the properties of an ideal vasodilator for ischemic tissue: when NO, a gas, is given by inhalation after cerebral ischemia, it dilates cerebral resistance vessels selectively in the ischemic penumbra without any effect on systemic blood pressure, thereby increasing CBF and preventing ischemic cell death.5
As we could recently also show that the therapeutic principle of NO inhalation (iNO) does not only apply to cerebral ischemia but also to cardiac ischemia,6 we suggest iNO to be a general therapeutic principle for ischemic conditions where collateral blood flow has an important pathophysiological role. Consequently, the aim of the current study was to evaluate the therapeutic efficacy of iNO after experimental TBI.
Materials and methods
Ethics statement
All animal experiments were performed on male C57 Bl/6 mice (body weight from 24 to 26 g; Charles River, Kisslegg, Germany) and approved by the Ethical Review Board of the Government of Upper Bavaria (protocol #55.2.1.54–2531/06–04). The manuscript was prepared according to the ARRIVE guidelines.7
Husbandry
Mice were kept in groups of maximally four littermates per cage (207 × 140 × 265 mm, 363 cm2, Macrolon II, Ehret Life Science Solutions, Emmendingen, Germany) with cellulose tissue as enrichment at a 12 hour day/night cycle with access to food and water ad libitum. Health screens and hygiene management were performed according to FELASA guidelines and recommendations.8
Randomization
All animals were randomly assigned to the treatment or control group by drawing lots; the surgical preparation and neurological testing was performed by a researcher blinded to the treatment. All data was prepared and analyzed by a researcher blinded to the treatment.
Anesthesia and surgical preparation
Anesthesia was induced in a halothane chamber (4%, 20 seconds, Halocarbon Laboratories, River Edge, NJ, USA) and continued with 1.2% halothane, 30% O2, 69% N2O via face mask for short-term experiments, e.g., trauma application (<20 minutes). For long-term experiments, e.g., determination of cerebral auto-regulation, anesthesia was induced as described above but continued with an intraperitoneal injection of medetomidine (0.5 mg/kg body weight; Domitor, Pfizer, Karlsruhe, Germany), midazolam (5 mg/kg body weight, Ratiopharm, Ulm, Germany) and fentanyl (0.05 mg/kg body weight) in combination with oro-tracheal intubation and mechanical ventilation with 30% O2 and 70% air (Minivent 845, Hugo Sachs Elektronik, March, Hungstetten, Germany). Ventilation was controlled by microcapnometry (Micro Capnograph CI240, Columbus Instruments, Columbus, OH, USA). Systemic blood pressure and blood gases were monitored via a catheter placed in the femoral artery. Regional cerebral blood flow (rCBF) was measured by Laser Doppler Fluxmetry (Periflux 4001 Master, Perimed, Stockholm, Sweden) as previously described.9, 10, 11 Intracranial pressure (ICP) was measured by an intraparenchymal probe (Mammendorfer Institut, Mammendorf, Germany) placed 2 mm right of the bregma. Blood samples (50 μl) were collected before trauma and at the end of the observation period for blood gas analysis.
Trauma induction
Controlled cortical impact (CCI) injury was performed as previously described.3, 9, 12 In brief, after craniotomy on the right parietal cortex an impactor tip (diameter 3 mm) was placed perpendicularly to the surface of the brain on the intact dura. The lesion was induced with a velocity of 8 m/s, with an impact depth of 1 mm and a contact time of 150 ms. The craniotomy was then closed by repositioning and gluing the bone flap (vetbond, 3TM Animal care products, St Paul, MN, USA).
Nitric oxide administration
Nitric oxide gas was supplied in tanks containing 264 mg/m3 NO in N2 (Linde AG, Unterschleissheim, Germany), and was administered at 50 p.p.m. in 30% O2 and 70% air as previously described.5 This dose was chosen based on published data on the use of iNO for selective dilatation of pulmonary vessels (see e.g., Sokol et al13) and our own data showing that iNO induced the most pronounced cerebral vasodilatation at a dose of 50 p.p.m.5
For experiments requiring long-term iNO application (>3 hours), animals were kept in custom-made gas proof glass containers in a 50 p.p.m. NO, 30% oxygen, 70% atmosphere.
NO and NO2 concentrations were continuously monitored by an electrochemical sensor (ITX, Industrial Scientific Corporation, Oakdale, PA, USA).
Experimental groups
Experimental groups are summarized in Table 1. In a first set of experiments we studied possible side effects of iNO in healthy mice. Intracranial pressure was monitored for 30 minutes in iNO and control animals (n=8 per group). Next, we studied iNO effects on cerebral auto-regulation (n=8 per group), expression of NO synthases in brain parenchyma after 24 of iNO (n=8 per group), and oxidative damage as assessed by immunohistochemistry for 3-NT (n=8 per group).
Table 1. Experimental groups—overview.
| Experimental group | Intervention | Measured parameter | Duration of experiment |
|---|---|---|---|
| Possible iNO side effects—ICP | iNO 50 p.p.m. versus control, n=8 each | ICP, CBF, body temperature | 30 Minutes |
| Possible iNO side effects—cerebral auto-regulation | iNO 50 p.p.m. versus control, n=8 each | MAP, CBF, body temperature | 60 Minutes |
| Possible iNO side effects—NOS expression | iNO 50 p.p.m. versus control, n=8 each | eNOS, nNOS, iNOS mRNA expression in brain tissue | 24 Hours |
| Possible iNO side effects—oxidative damage | iNO 50 p.p.m. versus control, n=8 each | Immunohistochemical detection of 3-nitrotyrosine in sequential coronal brain slices | 24 Hours |
| Acute posttraumatic effect of iNO | iNO 50 p.p.m. versus control, n=8 each | ICP, CBF ipsi-, contralateral, MAP, pCO2 | 30 Minutes pre-trauma—90 minutes posttrauma |
| iNO therapeutic window after CCI I | iNO 50 p.p.m., start of iNO 10 minutes, 1, 2 hours after trauma versus 15 minutes and 24 hours control, n=8 each | Lesion volume as determined in Nissl-stained coronal sections 24 hours after trauma | 15 Minutes and 24 hours after trauma |
| iNO therapeutic window after CCI II | iNO 50 p.p.m., sduration of iNO 12, 18, and 24 hours after trauma versus 15 minutes and 24 hours control, n=8 each | Lesion volume as determined in Nissl-stained coronal sections 24 hours after trauma | 15 Minutes and 24 hours after trauma |
| iNO intermittent application | iNO 50 p.p.m. 3 hours on/1 hour off for 24 hours versus 15 and 24 hours control | Lesion volume as determined in Nissl-stained coronal sections 24 hours after trauma | 15 Minutes and 24 hours after trauma |
| iNO effect on brain water content | iNO 50 p.p.m. starting 10 minutes after CCI versus control, n=8 each | Brain water content in each hemisphere 24 hours after trauma | 24 Hours after trauma |
| iNO effect on BBB disruption | iNO 50 p.p.m. starting 10 minutes after CCI versus control, n=8 each | Evans Blue extravasation in brain homogenates 24 hours after CCI | 24 Hours after trauma |
| iNO effect on neurological outcome over 7 days | iNO 50 p.p.m. 10 minutes–24 hours after CCI versus control, n=8 each | Neurological Severity Score points, body weight, lesion volume as determined in Nissl-stained coronal sections 7 days after trauma | 7 days after trauma |
BBB, blood–brain barrier; CBF, cerebral blood flow; eNOS, endothelial nitric oxide synthase; ICP, intracranial pressure; iNO, nitric oxide inhalation; iNOS, inducible nitric oxide synthase; MAP, mean arterial blood pressure; nNOS, neuronal nitric oxide synthase.
In the second part of the study we investigated the effect of iNO after experimental TBI. CBF, ICP, mean arterial blood pressure (MAP) were measured continuously for 90 minutes after trauma in control mice (n=8) or mice ventilated with iNO (n=8; 50 p.p.m. NO 10–90 minutes post trauma). Further we investigated the effect of iNO on lesion size and the therapeutic window of iNO. For this purpose we started iNO at 10 minutes, 1, 2 and 3 hours post trauma (n=8 per group). In the next group of experiments we varied the duration of iNO application, starting the treatment at 10 minutes after trauma and continuing it for 12 or 18 hours (n=8 each). Another group of mice (n=7) received 50 p.p.m. iNO intermittently with a 1-hour break after every 3 hours iNO interval (n=7). To quantify secondary brain damage in control animals, mice were killed 15 minutes (n=8) and 24 hours after trauma (n=8). Control animals were kept at 30% O2/70% air starting 10 minutes after trauma. Another experimental group was used to assess brain water content (n=8 each) and blood–brain barrier (BBB) permeability as assessed by EB extravasation (n=6 each) in animals with or without iNO for 24. Long-term neurological outcome was assessed daily by Neurological Severity Score (NSS) testing performed from 2 days before until 7 days after trauma; lesion volume was then assessed on day 7 after TBI.
Animals were randomly assigned to iNO or control treatment after induction of trauma.
Histology and measurement of contusion volume
Animals were killed by cervical dislocation in deep anesthesia, brains were removed, and immediately frozen in powdered dry ice. Coronal sections (10 μm) were prepared every 500 μm on a Cryostat (Cryostar MH 560, Microm, Walldorf, Germany). Sections were stained according to Nissl and then photographed. The contusion area was measured using a standard image analyzing system (analySIS 3.2 for Olympus DP-soft, Soft Imaging System, Muenster, Germany).
Immunohistochemistry
After 24 hours of NO inhalation, mice were killed by perfusion fixation (4% paraformaldehyde in phosphate-buffered saline). Four-μm-thick coronal brain sections were incubated for 3 hours with a rabbit polyclonal antibody against nitrotyrosine (1:50; Upstate Biotechnology, Dundee, UK) and subsequently with a biotinylated secondary antibody (DAKO, Hamburg, Germany). Visualization was performed with a commercial kit (Vectastain ABC AP Kit, Alexis, Grunberg, Germany). For negative controls, the second antibody was omitted; for positive controls brain sections were treated for 5 minutes with 40 mmol/L peroxynitrite (Calbiochem, Merck Chemical, Nottingham, UK).
NOS gene expression
C57 Bl/6 mice were subjected to 24 hours NO inhalation as described above. Thereafter brains were removed and RNA was extracted from each hemisphere using a standard kit (RNeasy Lipid Tissue Mini Kit 50, Qiagen, Hilden, Germany) followed by DNA digestion (RNase-free DNAse Set, Qiagen). After reverse transcription, quantitative PCR was performed (LightCyler 2.0, Roche, Heidelberg, Germany) using SYBR green (LightCycler–Faststart DNA Master SYBR Green I—Kit Roche, Heidelberg, Germany). Exon spanning primers for endothelial nitric oxide synthase (eNOS), neuronal nitric oxide synthase (nNOS), inducible nitric oxide synthase (iNOS), and aldolase as housekeeping gene (MWG Biotech, Ebersberg, Germany, see Table 2 for sequences) were tested for specificity by conventional PCR. Sample specificity was confirmed by melting curve analysis. Serial log dilutions of cDNA amplificates (107–101) were prepared as a standard for each gene. Each measurement was repeated at least three times. Water control, 105 standard, and calibrator were included into each run. Results were normalized to aldolase.
Table 2. Primers for determination of nitric oxide synthase expression.
| Gene | Primer forward (5′–3′) | Primer reverse (5′–3′) | PCR product |
|---|---|---|---|
| Aldolase | AGCTGTCTGACATCGCTCACCG | CACATACTGGCAGCGCTTCAAG | 571 bp |
| eNOS | CTGGTACATGAGTTCAGAGATTGG | TAGTTGACCATCTCTTGATGGAAG | 337 bp |
| nNOS | CTCGACCAATACTACTCCTCCATTA | TTGACGTGGTTACAGATGTAGTTG | 255 bp |
| iNOS | ATCGACCCGTCCACAGTATGTG | CGACCTGATGTTGCCATTGTTG | 493 bp |
Cerebral blood flow auto-regulation
Cerebral blood flow was measured by laser Doppler fluxmetry in anesthetized and intubated C57 Bl/6 mice at MAP values between 40 and 150 mm Hg. Hypertension was induced by intravenous injection of norepinephrine (Suprarenin 1:1000, Sanofi-Aventis, Frankfurt, Germany), then MAP was lowered at a rate of 0.5 mm Hg/s by hypobaric hypotension as previously described.14 CBF values were plotted against MAP and curves were fitted to the equation y=ax+bx2+cx4+dx6 (Table Curve, V. 4.01, Jandel Scientific, Erkrath, Germany). Auto-regulation was assumed to be intact at CBF values ±15% of baseline.
Assessment of brain water content
Brains were removed after 24 hours and immediately cooled to 4 °C. Optical bulbs and cerebellum were removed, hemispheres separated and the wet weight was assessed. Dry weight was obtained after keeping the hemispheres at 100 °C for 24 hours. Brain water content was calculated as previously described.15
Assessment of blood–brain barrier integrity
Blood–brain barrier integrity was determined by EB as previously described.16 Briefly, animals received 100 μl of a 4% EB solution intravenously 5 minutes after trauma. 24 hours later, animals were transcardially perfused with phosphate-buffered saline. Brains were removed and hemispheres were separated, weighed, and homogenized. Homogenates were centrifuged and EB was measured in the supernatant (excitation wavelength: 620 nm, emission wavelength: 680 nm; Spectrophotometer Model 650–10 S, Perkin-Elmer, Norwalk, OH, USA). Evans Blue concentrations were calculated using a standard curve (50 to 1000 ng/ml) and expressed as mg/g brain tissue.
Neurological severity score
Neurological function was assessed 2 days before trauma, immediately before trauma and on posttraumatic days 1–7 using a modified version of the previously described NSS.9 Ten tasks are performed to assess motor function, balance, and alertness of the animals. Complete failure to successfully perform any of these tasks results in a NSS of 20, healthy mice usually score between 0 and 2 points. Animals unable to perform below 4 points before trauma were excluded from randomization. The NSS was performed by a second researcher blinded to treatment of the animals.
Statistical analysis
All data are given as mean±s.e.m. if not indicated otherwise. For comparisons between groups we used the Mann–Whitney rank-sum test, repeated measurements were analyzed using RM analysis of variance on ranks, comparison of many groups was performed by analysis of variance on ranks. All calculations were performed with a standard statistical software package (Sigma Stat 3.0, Jandel Scientific, Erkrath, Germany). Differences between groups were assumed to be significant when the P-value was smaller or equal to 0.05. Sample size calculations were performed with the same statistical software package (α-error 0.05, β-error 0.2, calculated s.d. 15–20% depending on the parameter investigated, biologically relevant difference 30%).
Results
Evaluation of possible adverse effects of nitric oxide inhalation
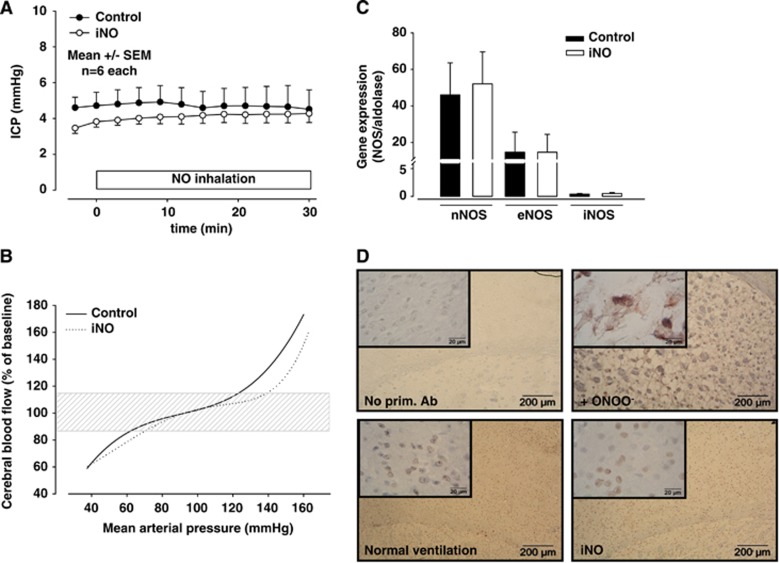
As NO inhalation has been shown to dilate cerebral veins in healthy animals,5 a major concern when planning to use iNO after TBI is whether iNO may leads to an increase in intracranial blood volume and, thus, ICP. NO inhalation, however, did not have any effect on ICP during inhalation of NO for 30 minutes (Figure 1A). Furthermore, iNO (Figure 1B, dashed line) has no major effect on cerebral auto-regulation curves calculated on the bases of ∼3500 CBF-MAP data pairs as compared with normally ventilated mice (solid line). Twenty-four hours of inhaled NO did also not affect cerebral NOS expression (Figure 1C) and did not cause any oxidative damage to the brain of healthy animals as indicated by a lack of protein nitrosylation after iNO (Figure 1D).
Figure 1.
(A) Nitric oxide (NO) inhalation does not increase intracranial pressure in healthy C57 Bl/6 mice during 30 minutes of NO inhalation (50 p.p.m.). (B) Auto-regulation is not changed by NO inhalation (iNO). (C) Expression of NO synthases in brain tissue is not altered by 24 hours of iNO. (D) iNO does not increase protein nitrosylation as assessed by immunohistochemistry. Images: × 40 magnification, inlets: × 100 magnification.
Influence of NO inhalation on pathophysiological processes after traumatic brain injury
Physiological parameters in both iNO and control group were within physiological ranges (Table 3) and did not differ between groups. Throughout the observation period MAP measured in the femoral artery showed no statistically significant differences, indicating that inhalation of NO has no effect on systemic blood pressure (Figure 2A).
Table 3. Physiological parameters before trauma and after 90 minutes of nitric oxide inhalation (iNO).
| pH start | pH end | pO2 start | pO2 end | pCO2 start | pCO2 end | |
|---|---|---|---|---|---|---|
| Control | 7.34±0.04 | 7.29±0.08 | 91±18 | 115±26 | 41±8 | 36±8 |
| iNO | 7.38±0.05 | 7.26±0.01 | 97±20 | 94±4 | 39±1 | 35±0.1 |
Units: pO2, pCO2: mm Hg.
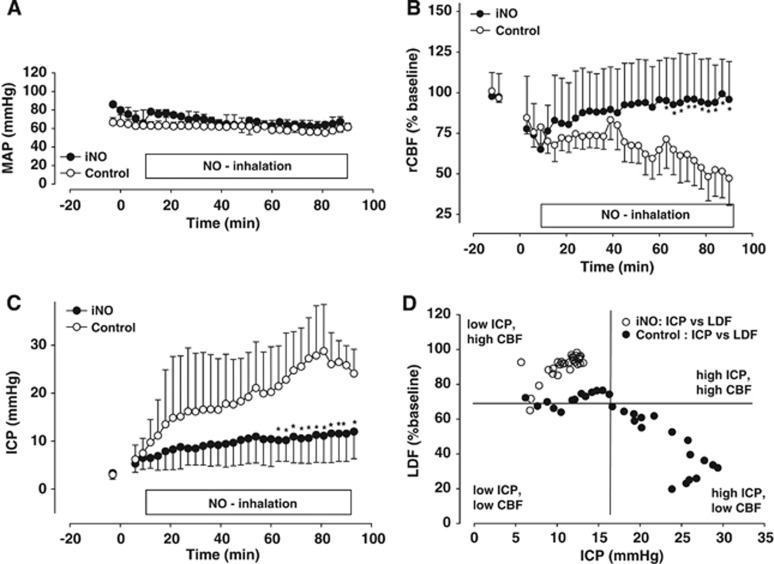
Figure 2.
Nitric oxide (NO) inhalation improves cerebral blood flow and reduces intracranial hypertension after trauma without influencing systemic blood pressure. (A) Mean arterial blood pressure was equal in both groups. (B) Regional cerebral blood after trauma was markedly reduced in both groups. While in controls cerebral blood flow (CBF) remained low, it recovered in NO inhalation (iNO) mice, reaching almost baseline value toward the end of the observation period. (C) After trauma, there was an almost linear intracranial pressure (ICP) increase in untreated controls. Nitric oxide inhalation animals showed the same initial increase in ICP, but it was blunted immediately after the start of the therapy. Maximum ICP was significantly lower than in controls. (D) Posttraumatic ICP values were plotted against corresponding CBF values: Most control animals fall into the low CBF/high ICP group, while most values of iNO animals have high CBF and low ICP. *P<0.05, n=8 each.
Ipsi- and contralateral rCBF decreased rapidly after trauma (Figure 2B). Values decreased to 74±6% of pre-trauma baseline in the iNO and to 75±8% in the control group 6 minutes after trauma. NO inhalation started 10 minutes after trauma led to an almost immediate improvement in rCBF, whereas CBF remained low and decreased further in the control group. Seventy-two minutes after TBI, rCBF in the iNO group nearly recovered to baseline values (93±9% versus 62±15% in controls; P<0.05). This difference was observed until the end of the observation period.
Intracranial pressure values were within the physiological range in both groups before iNO (3.3±0.4 and 3.1±0.3 mm Hg, Figure 2C). After trauma the ICP increased rapidly and in an almost linear manner in the control group; it peaked 78 minutes after trauma at 30±2 mm Hg and remained elevated until the end of the observation period (24±2 mm Hg). Nitric oxide inhalation resulted in a significantly reduced increase of ICP. Peak ICP values in the iNO group were only 12±2 mm Hg (P<0.04) and occurred with a significant delay after TBI, i.e., iNO-treated animals did not develop intracranial hypertension; ICP stayed below the pathological threshold of 20 mm Hg at all times. Plotting posttraumatic ICP values against their corresponding rCBF value reveals that most control animals fall into the prognostically unfavorable ‘high ICP, low CBF' category (Figure 2D). However, the majority of iNO-treated animals shows low ICP values, while CBF is satisfactory. Furthermore, our results allow the calculation of the threshold between compensated and uncompensated intracranial hypertension in mice: when ICP increases above 17 mm Hg CBF decreases, i.e., above 17 mm Hg the intracranial compliance is exhausted and rCBF decreases linearly to the increase of ICP.
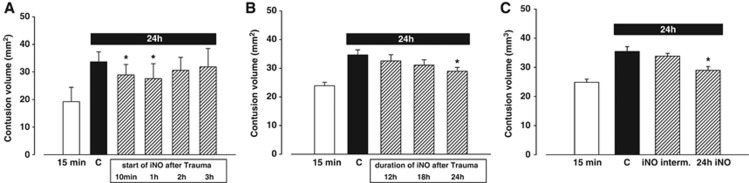
Effect of nitric oxide inhalation on lesion volume after 24 hours
In the control group, the primary lesion grew from 19.2±1.5 mm3 (at 15 minutes) to 33.6±1.2 mm3 within the first 24 hours after TBI (Figure 3A). Nitric oxide inhalation treatment resulted in a significant reduction of secondary lesion growth when started 10 minutes or 1 hour after trauma, i.e., to only 28.9±1.3 and 28.3±1.9 mm3, respectively (P<0.04 versus control; Figure 3A). If NO inhalation was started 2 or 3 hours after trauma, lesion volume showed only a tendency to decrease (2 hours: 30.6±1.7 mm3; 3 hours: 29.8±2.5 mm3). If NO inhalation was discontinued 12 or 18 hours after TBI, contusion volume was 32.5±2.2 and 31.1±1.8 mm3, respectively (NS). Intermittent application of NO over 24 hours (Figure 3C) to detect a putative tachyphylactic response did not result in any effect of iNO (33.9±0.9 versus 35.5±1.6 mm3 in controls).
Figure 3.
Nitric oxide (NO) inhalation is neuroprotective after trauma in a dose-dependent way. (A) If initiated within 1 hour after trauma, NO inhalation (iNO) significantly reduces contusion volume at 24 hours. (B) For this neuroprotective effect it is necessary to conduct iNO over a period of 24 hours: If performed for 12 or 18 hours after trauma, lesion volume tends to be smaller, but the changes did not reach statistical significance. (C) Intermittent application of iNO (3 hours iNO, 1 hour break) could not reduce contusion volume in a significant way as continuous administration did. *P<0.05, n=8 each.
Effect of nitric oxide inhalation on posttraumatic brain edema formation
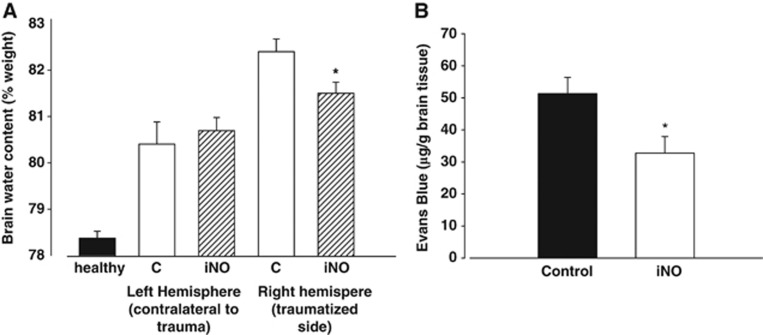
Brain edema formation in the traumatized hemisphere of animals receiving 50 p.p.m. iNO for 24 hours was significantly reduced as compared with the control group (Figure 4A; 81.5±0.7% versus 82.4±0.8% P<0.02).
Figure 4.
(A) Posttraumatic edema formation was significantly reduced after 24 hours of nitric oxide inhalation (iNO). *P<0.02, n=8 each. (B) Evans Blue extravasation in the traumatized hemisphere was significantly lower in the iNO group than in controls (n=6 each, *P<0.05).
Effect of nitric oxide inhalation on blood–brain barrier dysfunction
Evans Blue extravasation, a measure of BBB disruption, was significantly lower in the NO inhalation group (51.3±5.1 μg EB/g versus 32.8±5.1 μg EB/g brain tissue, n=6 each group, P<0.05).
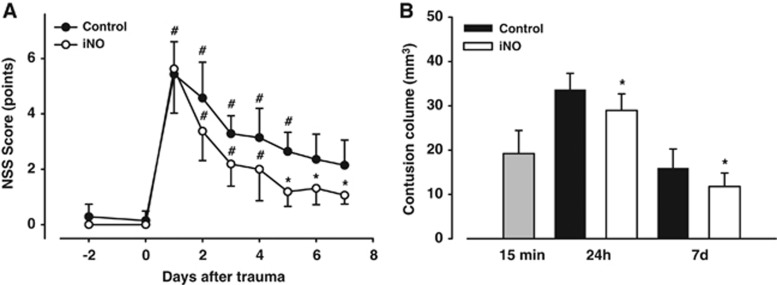
Neurological outcome and histopathological effect of nitric oxide inhalation 7 days after traumatic brain injury
All animals achieved NSS scores below 2 points before trauma. All animals survived the 7-day observation period and were included into the analysis. On the first day after trauma, performance in controls deteriorated significantly compared with pre-trauma values (5.4±1.3 versus 0.3±0.5, P<0.001, Figure 5A). Performance gradually improved over the following 7 days, but there was a clear neurological deficit at the end of the observation period in untreated mice (2.1±1.1, P<0.01). In iNO-treated animals, NSS scores also deteriorated on day 1 (5.6±1.6 versus 0 on day 1, P<0.001), but animals recovered significantly better as compared with untreated controls during the following days, and from day 5 on until the end of the observation period iNO-treated mice reached significantly better scores than control animals (Figure 5A).
Figure 5.
Neurological outcome over 7 days after trauma is better in animals receiving nitric oxide inhalation (iNO) for the first 24 hours after trauma. (A) Performance in Neurological Severity Score (NSS) testing significantly deteriorated in both groups compared with pre-trauma values. Controls recovered slowly and still presented a marked neurological deficit at day 7, iNO animals regained neurological functions quicker than untreated mice; from day 5 on they achieved significantly better NSS results than controls. n=8 each, *P<0.05 versus control, #P<0.05 compared with corresponding pre-trauma score. (B) iNO significantly reduced contusion volume after 7 days.*P<0.05 versus control, #P<0.001 versus corresponding pre-trauma value, n=8 each.
Control animals had a lesion volume of 16.0±4.3 mm3 on day 7 after trauma, while iNO-treated mice had significantly smaller contusions (11.8±3.1 mm3, P<0.05, Figure 5B).
Discussion
This is the first study to use inhaled NO for the therapy of TBI. In the first part of the present study we showed that iNO does not increase ICP in healthy animals. Cerebral auto-regulation and endogenous NO production are not altered by NO inhalation, nor did prolonged iNO exposure lead to increased oxidative damage in healthy animals. After experimental TBI, iNO significantly improves CBF, reduces brain edema formation, and ICP. As a result, secondary contusion expansion was significantly reduced and neurological outcome significantly improved in iNO-treated mice up to 7 days after TBI.
The CCI model of TBI in mice has been widely used to study pathophysiology of TBI. As published previously,3, 15 the impact induces a highly reproducible contusion, which grows in the posttraumatic period, reaching a maximum after 24 hours. Also, posttraumatic sequels of human closed head injury like decrease of cerebral perfusion, intracranial hypertension, and brain edema formation can be reliably monitored after CCI injury in mice. Together with the randomized and blinded design of the study we believe that all experiments of the current study were performed with the most possible care to avoid any sources of bias or experimental artifacts.
Nitric oxide has a major role in the pathophysiology of TBI. After trauma, a very short-lasting peak of NO concentration as well as a transient rise in constitutive, i.e., eNOS and nNOS activity was described to occur in animals that underwent CCI trauma.17, 18, 19, 20, 21 Within seconds after the insult, both NO concentration and cNOS activity decrease significantly and remain suppressed for up to 7 days.17, 18, 19, 20, 22, 23 NOS downregulation is most prominent in the tissue adjacent to the lesion.17 It has been shown that remaining eNOS-derived NO is neuroprotective after experimental TBI24 and that attenuating the local NO depletion, e.g., by local application of direct NO precursor L-arginine, improves CBF and reduces ICP.24, 25, 26 Topical application of NO donors, however, is not feasible in a clinical context. Systemic application of NO donors, on the other hand, is known to induce systemic hypotension, a condition which leads to a decrease of cerebral perfusion, which is associated with higher mortality and worse outcome after TBI.27, 28
As shown previously5 iNO induces an increase of CBF specifically in regions of low or critical perfusion without influencing normally perfused brain regions or systemic blood pressure, thereby redistributing blood from well-nourished tissue to tissue in need. Therefore, this inverse steal phenomenon was also termed, ‘Robin Hood Effect'. The effects seen in the current study are well in line with the ‘Robin Hood' concept. Improvement of CBF after traumatic brain injury attenuates ischemic cell death, thereby reducing cytotoxic edema and, thus, lesion volume and neurological outcome. In addition, the observed beneficial effect of iNO after trauma may be—at least in part—a result of local posttraumatic NO depletion and eNOS downregulation (see above); some authors even suggest that iNO-mediated effects on the vasculature are present in NO-depleted vascular beds only.29, 30
Furthermore, iNO could also have a direct effect on the formation of vasogenic brain edema. In this case, interstitial fluid accumulation is brought about by pathological vasodilatation, leading to the increased hydrostatic pressure; at the same time there is an increase of cerebrovascular permeability because of BBB dysfunction, which changes osmotic gradients.31 The role of NO in regulating microvascular permeability has not yet been fully elucidated. While some studies suggest that NO decreases vascular and BBB permeability,32, 33, 34 others found the opposite.35, 36 Administration of NO precursor L-arginine after trauma led to decreased edema formation,37 but no impact on ICP was found.19, 25, 26 In this study, iNO therapy had a significant effect on BBB dysfunction as measured by EB extravasation, so the shown reduction of brain water content is most probably because of a combination of reduction of vasogenic and cytotoxic edema formation. Furthermore, NO inhalation has been shown to exert anti-platelet activity38, 39, 40 and to act anti-inflammatorily.29, 41 We previously showed that microthrombus formation occurs in our model of murine CCI and further deteriorates CBF;42 in the same study leukocyte-platelet aggregates were detected in cerebral venules. Therefore, it is possible that NO inhalation also furthers pericontusional perfusion by reduction of these phenomena, further studies are needed to evaluate the impact of these factors.
Continuous systemic application of NO-containing drugs like glycerolnitrate leads to tachyphylaxy within 24 hours, therefore making treatment longer than 8 hours ineffective.43, 44 The reasons for this phenomenon are not entirely clear yet.44 The current experience with iNO therapy, however, suggests that there is no loss of drug effect even after prolonged treatment,45, 46 on the contrary, iNO concentrations can be reduced in the course of treatment without losing effectiveness.46, 47 In our study, iNO-induced neuroprotection could not be improved by intermittent application of the therapy, suggesting that—as evidenced by other groups—tachyphylaxy does not occur during NO inhalation therapy.
In terms of the clinical use of iNO after TBI, the observed short therapeutic window of only 1 hour may be a matter of concern. One has, however, to take into consideration that (1) in humans secondary brain damage is known to occur over much longer periods of time than in mice, i.e., several hours or even days, and (2) iNO may be initiated already during the pre-hospital phase by respective emergency personnel, as it is easy to apply, has no obvious adverse effects, and a clinically proven safety record in thousands of patients receiving iNO for pulmonary disorders. Although further studies—ideally in large animal models of TBI—will need to be performed to define the therapeutic window of iNO after TBI, our data indicate that the fundamental principle of iNO has a clinical potential.
In conclusion, the current experimental study shows that NO inhalation effectively reduces brain damage and improves neurological function after TBI. Underlying mechanisms are, as already shown for cerebral ischemia, a selective dilation of resistance vessels with a concomitant increase of CBF in the traumatic penumbra. NO inhalation may therefore represent a promising and safe novel treatment strategy for TBI.
Acknowledgments
This work was supported by the GEMI Fund (NP). Parts of the present study are part of the PhD thesis of NT.
The authors declare no conflict of interest.
References
- Bouma GJ, Muizelaar JP, Stringer WA, Choi SC, Fatouros P, Young HF. Ultra-early evaluation of regional cerebral blood flow in severely head-injured patients using xenon-enhanced computerized tomography. J Neurosurg. 1992;77:360–368. doi: 10.3171/jns.1992.77.3.0360. [DOI] [PubMed] [Google Scholar]
- Graham DI, Adams JH, Doyle D. Ischaemic brain damage in fatal non-missile head injuries. J Neurol Sci. 1978;39:213–234. doi: 10.1016/0022-510x(78)90124-7. [DOI] [PubMed] [Google Scholar]
- Engel DC, Mies G, Terpolilli NA, Trabold R, Loch A, De Zeeuw CI, et al. Changes of cerebral blood flow during the secondary expansion of a cortical contusion assessed by 14C-iodoantipyrine autoradiography in mice using a non-invasive protocol. J Neurotrauma. 2008;25:739–753. doi: 10.1089/neu.2007.0480. [DOI] [PubMed] [Google Scholar]
- Menon DK. Procrustes, the traumatic penumbra, and perfusion pressure targets in closed head injury. Anesthesiology. 2003;98:805–807. doi: 10.1097/00000542-200304000-00002. [DOI] [PubMed] [Google Scholar]
- Terpolilli NA, Kim SW, Thal SC, Kataoka H, Zeisig V, Nitzsche B, et al. Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ Res. 2012;110:727–738. doi: 10.1161/CIRCRESAHA.111.253419. [DOI] [PubMed] [Google Scholar]
- Neye N, Enigk F, Shiva S, Habazettl H, Plesnila N, Kuppe H, et al. Inhalation of NO during myocardial ischemia reduces infarct size and improves cardiac function. Intensive Care Med. 2012;38:1381–1391. doi: 10.1007/s00134-012-2605-1. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillen J. FEALSA Guidelines and Recommendations. J Am Assoc Lab Anim Sci. 2012;51:311–321. [PMC free article] [PubMed] [Google Scholar]
- Terpolilli NA, Zweckberger K, Trabold R, Schilling L, Schinzel R, Tegtmeier F, et al. The novel nitric oxide synthase inhibitor 4-amino-tetrahydro-L-biopterine prevents brain edema formation and intracranial hypertension following traumatic brain injury in mice. J Neurotrauma. 2009;26:1963–1975. 10. doi: 10.1089/neu.2008.0853. [DOI] [PubMed] [Google Scholar]
- von Baumgarten L, Trabold R, Thal S, Back T, Plesnila N. Role of cortical spreading depressions for secondary brain damage after traumatic brain injury in mice. J Cereb Blood Flow Metab. 2008;28:1353–1360. doi: 10.1038/jcbfm.2008.30. [DOI] [PubMed] [Google Scholar]
- Plesnila N, Zinkel S, Le D, Amin-Hanjani S, Wu Y, Qui J, et al. BID mediates neuronal cel death after oxygen/ glucose deprivation and focal cerebral ischemia. Proc Natl Acad Sci USA. 2001;98:15318–15323. doi: 10.1073/pnas.261323298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweckberger K, Stoffel M, Baethmann A, Plesnila N. Effect of decompression craniotomy on increase of contusion volume and functional outcome after controlled cortical impact in mice. J Neurotrauma. 2003;20:1307–1314. doi: 10.1089/089771503322686102. [DOI] [PubMed] [Google Scholar]
- Sokol J, Jacobs SE, Bohn D. Inhaled nitric oxide for acute hypoxic respiratory failure in children and adults: a meta-analysis. Anesth Analg. 2003;97:989–998. doi: 10.1213/01.ANE.0000078819.48523.26. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Thoren P, Villringer A, Sixt G, Them A, Einhaupl KM. Global forebrain ischaemia in the rat: controlled reduction of cerebral blood flow by hypobaric hypotension and two-vessel occlusion. Neurol Res. 1993;15:128–130. doi: 10.1080/01616412.1993.11740122. [DOI] [PubMed] [Google Scholar]
- Zweckberger K, Eros C, Zimmermann R, Kim SW, Engel D, Plesnila N. Effect of early and delayed decompressive craniectomy on secondary brain damage after controlled cortical impact in mice. J Neurotrauma. 2006;23:1083–1093. doi: 10.1089/neu.2006.23.1083. [DOI] [PubMed] [Google Scholar]
- Vakili A, Kataoka H, Plesnila N. Role of arginine vasopressin V1 and V2 receptors for brain damage after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:1012–1019. doi: 10.1038/sj.jcbfm.9600097. [DOI] [PubMed] [Google Scholar]
- Wada K, Chatzipanteli K, Busto R, Dietrich WD. Role of nitric oxide in traumatic brain injury in the rat. J Neurosurg. 1998;89:807–818. doi: 10.3171/jns.1998.89.5.0807. [DOI] [PubMed] [Google Scholar]
- Wada K, Chatzipanteli K, Busto R, Dietrich WD. Effects of L-NAME and 7-NI on NOS catalytic activity and behavioral outcome after traumatic brain injury in the rat. J Neurotrauma. 1999;16:203–212. doi: 10.1089/neu.1999.16.203. [DOI] [PubMed] [Google Scholar]
- Cherian L, Robertson CS. L-arginine and free radical scavengers increase cerebral blood flow and brain tissue nitric oxide concentrations after controlled cortical impact injury in rats. J Neurotrauma. 2003;20:77–85. doi: 10.1089/08977150360517209. [DOI] [PubMed] [Google Scholar]
- Cherian L, Hlatky R, Robertson CS. Nitric oxide in traumatic brain injury. Brain Pathol. 2004;14:195–201. doi: 10.1111/j.1750-3639.2004.tb00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto KI, Fujisawa H, Koizumi H, Tsuchida E, Ito H, Sadamitsu D, et al. Effects of mild hypothermia on nitric oxide synthesis following contusion trauma in the rat. J Neurotrauma. 1997;14:349–353. doi: 10.1089/neu.1997.14.349. [DOI] [PubMed] [Google Scholar]
- Cherian L, Goodman JC, Robertson CS. Brain nitric oxide changes after controlled cortical impact injury in rats. J Neurophysiol. 2000;83:2171–2178. doi: 10.1152/jn.2000.83.4.2171. [DOI] [PubMed] [Google Scholar]
- Hlatky R, Furuya Y, Valadka AB, Goodman JC, Robertson CS. Comparison of microdialysate arginine and glutamate levels in severely head-injured patient. Acta Neurochir Suppl. 2002;81:347–349. doi: 10.1007/978-3-7091-6738-0_88. [DOI] [PubMed] [Google Scholar]
- Hlatky R, Lui H, Cherian L, Goodman JC, O'Brien WE, Contant CF, et al. The role of endothelial nitric oxide synthase in the cerebral hemodynamics after controlled cortical impact injury in mice. J Neurotrauma. 2003;20:995–1006. doi: 10.1089/089771503770195849. [DOI] [PubMed] [Google Scholar]
- Cherian L, Chacko G, Goodman JC, Robertson CS. Cerebral hemodynamic effects of phenylephrine and L-arginine after cortical impact injury. Crit Care Med. 1999;27:2512–2517. doi: 10.1097/00003246-199911000-00031. [DOI] [PubMed] [Google Scholar]
- Liu H, Goodman JC, Robertson CS. The effects of L-arginine on cerebral hemodynamics after controlled cortical impact injury in the mouse. J Neurotrauma. 2002;19:327–334. doi: 10.1089/089771502753594891. [DOI] [PubMed] [Google Scholar]
- Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Barton CW, Hemphill JC, Morabito D, Manley G. A novel method of evaluating the impact of secondary brain insults on functional outcomes in traumatic brain-injured patients. Acad Emerg Med. 2005;12:1–6. doi: 10.1197/j.aem.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Fox-Robichaud A, Payne D, Hasan SU, Ostrovsky L, Fairhead T, Reinhardt P, et al. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. J Clin Invest. 1998;101:2497–2505. doi: 10.1172/JCI2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon RO, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, et al. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest. 2001;108:279–287. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterberg AW, Stover J, Kress B, Kiening KL. Edema and brain trauma. Neuroscience. 2004;129:1021–1029. doi: 10.1016/j.neuroscience.2004.06.046. [DOI] [PubMed] [Google Scholar]
- Filep JG, Foldes-Filep E, Sirois P. Nitric oxide modulates vascular permeability in the rat coronary circulation. Br J Pharmacol. 1993;108:323–326. doi: 10.1111/j.1476-5381.1993.tb12803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver JA. Endothelium-derived relaxing factor contributes to the regulation of endothelial permeability. J Cell Physiol. 1992;151:506–511. doi: 10.1002/jcp.1041510309. [DOI] [PubMed] [Google Scholar]
- Wong D, Dorovini-Zis K, Vincent SR. Cytokines nitric oxide, and cGMP modulate the permeability of an in vitro model of the human blood-brain barrier. Exp Neurol. 2004;190:446–455. doi: 10.1016/j.expneurol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- He P, Liu B, Curry FE. Effect of nitric oxide synthase inhibitors on endothelial [Ca2+]i and microvessel permeability. Am J Physiol. 1997;272 (1 Pt 2:H176–H185. doi: 10.1152/ajpheart.1997.272.1.H176. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. Nitric oxide donor-induced increase in permeability of the blood-brain barrier. Brain Res. 2000;866:101–108. doi: 10.1016/s0006-8993(00)02254-x. [DOI] [PubMed] [Google Scholar]
- Lundblad C, Bentzer P. Effects of l-arginine on cerebral blood flow, microvascular permeability, number of perfused capillaries, and brain water content in the traumatized mouse brain. Microvasc Res. 2007;74:1–8. doi: 10.1016/j.mvr.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Samama CM, Diaby M, Fellahi JL, Mdhafar A, Eyraud D, Arock M, et al. Inhibition of platelet aggregation by inhaled nitric oxide in patients with acute respiratory distress syndrome. Anesthesiology. 1995;83:56–65. doi: 10.1097/00000542-199507000-00007. [DOI] [PubMed] [Google Scholar]
- Kermarrec N, Zunic P, Beloucif S, Benessiano J, Drouet L, Payen D. Impact of inhaled nitric oxide on platelet aggregation and fibrinolysis in rats with endotoxic lung injury. Role of cyclic guanosine 5'-monophosphate. Am J Respir Crit Care Med. 1998;158:833–839. doi: 10.1164/ajrccm.158.3.9709097. [DOI] [PubMed] [Google Scholar]
- Hogman M, Frostell C, Arnberg H, Sandhagen B, Hedenstierna G. Prolonged bleeding time during nitric oxide inhalation in the rabbit. Acta Physiol Scand. 1994;151:125–129. doi: 10.1111/j.1748-1716.1994.tb09728.x. [DOI] [PubMed] [Google Scholar]
- El Kebir D, Hubert B, Taha R, Troncy E, Wang T, Gauvin D, et al. Effects of inhaled nitric oxide on inflammation and apoptosis after cardiopulmonary bypass. Chest. 2005;128:2910–2917. doi: 10.1378/chest.128.4.2910. [DOI] [PubMed] [Google Scholar]
- Schwarzmaier SM, Kim SW, Trabold R, Plesnila N. Temporal profile of thrombogenesis in the cerebral microcirculation after traumatic brain injury in mice. J Neurotrauma. 2010;27:121–130. doi: 10.1089/neu.2009.1114. [DOI] [PubMed] [Google Scholar]
- Parker JO. Nitrate tolerance. A problem during continuous nitrate administration. Eur J Clin Pharmacol. 1990;38 (Suppl 1:S21–S25. doi: 10.1007/BF01417561. [DOI] [PubMed] [Google Scholar]
- Bode-Boger SM, Kojda G. Organic nitrates in cardiovascular disease. Cell Mol Biol (Noisy -le-grand) 2005;51:307–320. [PubMed] [Google Scholar]
- Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993;328:399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- Roberts JD, Fineman JR, Morin FC, Shaul PW, Rimar S, Schreiber MD, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N Engl J Med. 1997;336:605–610. doi: 10.1056/NEJM199702273360902. [DOI] [PubMed] [Google Scholar]
- Kinsella JP, Neish SR, Ivy DD, Shaffer E, Abman SH. Clinical responses to prolonged treatment of persistent pulmonary hypertension of the newborn with low doses of inhaled nitric oxide. J Pediatr. 1993;123:103–108. doi: 10.1016/s0022-3476(05)81551-3. [DOI] [PubMed] [Google Scholar]