Abstract
In light of the relevance of therapeutic hypothermia to stroke treatment, we investigated whether 5′-adenosine monophosphate (AMP)-dependent cooling affords protection from ischemic brain injury. We show that hypothermia by AMP is because of adenosine A1 receptor (A1R) activation and is not invariantly associated with hypotension. Inhibition of ecto-5′-nucleotidase-dependent constitutive degradation of brain extracellular AMP by methylene-ADP (AMPCP) also suffices to prompt A1R-dependent hypothermia without hypotension. Both intraischemic and postischemic hypothermia by AMP or AMPCP reduce infarct volumes and mortality of mice subjected to transient middle cerebral artery occlusion. Data disclose that AMP-dependent hypothermia is of therapeutic relevance to treatment of brain ischemia.
Keywords: A1R, AMP, hypothalamus, hypothermia, stroke
Introduction
Although trials of therapeutic hypothermia in patients experiencing stroke, neonatal hypoxia or cardiac arrest proved promising,1, 2, 3 current cooling devices experience a substantial degree of invasiveness and a considerable delay in inducing the hypothermic effect.4, 5 Indeed, tools that selectively prompt transient cooling of body temperature (Tb) and avoid negative side effects or suspended animation are not available in humans.6 In this respect, injection of the endogenous nucleotide 5′-adenosine monophosphate (AMP) induces profound and transient hypothermia in rodents.7, 8 Recently, we showed that AMP prompts hypothermia by activating adenosine (Ado) A1 receptors (A1Rs) on temperature-insensitive hypothalamic neurons of mice, thereby suppressing the thermoregulatory responses that maintain Tb.9 We also show that AMP-dependent hypothermia reduces prostaglandin-induced fever in mice, having no effect on peripheral hyperthermia induced by dioxymethamphetamine (ecstasy) overdose.9 Theoretically, AMP-dependent cooling might be harnessed to therapeutic hypothermia of ischemic brain injury. In rats undergoing middle cerebral artery occlusion (MCAo), however, AMP increases infarct size probably because of concomitant hypotension and hyperglycemia. Of note, in that study very high doses of AMP have been used (1.39 g/kg equal to 415 mg/rat),8 possibly predisposing to deleterious side effects. Thus, whether AMP affords ischemic neuroprotection under appropriate dosage regimens remains an open question. We therefore sought to better elucidate the effects of cooling by AMP in an experimental stroke model.
Materials and methods
C57/Bl6 male mice of 20 to 25 g were used (Harlan Nossan, Udine, Italy). Because rectal and temporalis muscle temperature similarly decreased on AMP or AMPCP treatment, a rectal probe (Harvard Apparatus, Holliston, MA, USA) was routinely used to measure Tb in mice.10 Coordinates for microinjections (1 μL) into the hypothalamic preoptic area were P+0.10, L+0.80, and V−4.50 mm from bregma. Intracerebroventricular injections (3 μL) were performed in the lateral ventricle according to standard procedures. A BP-2000 series II blood pressure analysis system (Visitech System, Napa Place, Apex, NC, USA) was used. Animal procedures were conducted according to the European Community Guidelines for Animal Care. Mice were randomized and subjected to MCAo as previously described.11 Mice were anesthetized with 2% isoflurane and maintained on 1.5% isoflurane in 70% nitrous oxide and 30% oxygen. Regional cerebral blood flow was measured by Laser-Doppler (PF2B; Perimed, Stockholm, Sweden). In randomly selected animals, the left femoral artery was cannulated with a PE-10 polyethylene tube for blood gas determination. Rectal temperature was maintained between 36.5 and 37.5°C with a homeothermic blanket (Frederick Haer and Co., Brunswick, ME, USA). Middle cerebral artery was occluded for 1 hour using the classical intraluminal filament technique. 5′-Adenosine monophosphate was injected at time of artery occlusion or at different times after reperfusion. Animals were killed at different times after reperfusion and the brains snap frozen in N2 vapor for cryostat sectioning. Infarction areas were quantitated by a blind evaluator by means of MCID M4 image analysis software (Imaging Research Inc., St Catharine's, ON, Canada) on hematoxylin eosin-stained sections. To account for and eliminate swelling/edema, infarction volume was calculated using an indirect measurement by summing the volumes of each section using the following formula: contralateral hemisphere (mm3)−undamaged ipsilateral hemisphere (mm3). Data are expressed as mean±s.e.m. Statistical significance was evaluated using paired two-tailed Student's t-test or ANOVA plus Tukey's post hoc test. Differences were considered significant at P<0.05.
Results
Effect of 5′-Adenosine Monophosphate or AMPCP on Body Temperature and Blood Pressure in Mice
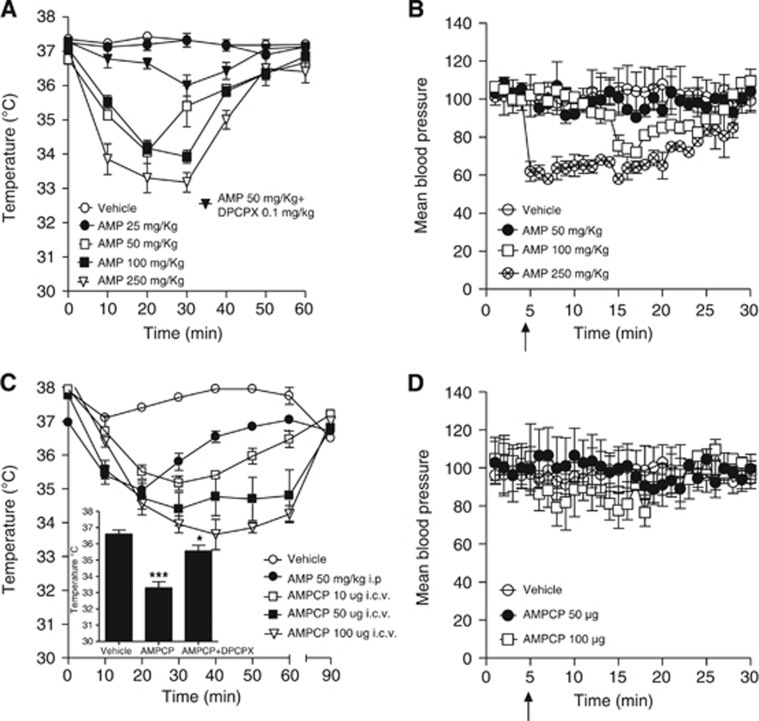
We first attempted to clarify the dose-effect relationship of AMP to Tb cooling. We found that AMP started reducing Tb dose dependently in mice when injected at 50 mg/kg intraperitoneally (i.e., a dose eightfold lower than that previously used in rats).8 In keeping with prior work from our group,9 AMP-dependent cooling was almost completely abrogated by pretreatment (5 minutes) with the A1R antagonist 8-Cyclopentyl-t,3-dipropylxanthine (DPCPX, 0.1 mg/kg, intraperitoneally) (Figure 1A). Of note, hypothermia by AMP was transient, and Tb completely recovered after 1 hour (Figure 1A). We next investigated whether blood pressure was altered by AMP injected at doses comprised in the range of 50 to 250 mg/kg. Remarkably, hypothermia induced by 50 mg/kg AMP was not accompanied by hypotension. Transient hypotension was only observed at doses equal or higher than 100 mg/kg (Figure 1B). Notably, the cooling effect of AMP is reproduced by preventing its degradation into Ado within the brain intersynaptic space with the ecto-5′-nucleotidase inhibitor AMPCP.9 We therefore hypothesized that AMPCP can also be harnessed to hypothermic treatment of stroke. Again, we first conducted dose-finding experiments showing that AMPCP intracerebroventricularly dose dependently reduced Tb in mice, causing Tb loss comparable to that of AMP intraperitoneally but of prolonged duration (Figure 1C). Even in the case of AMPCP, cooling was counteracted by the A1R antagonist DPCPX administered intraperitoneally (Figure 1C, inset), indicating that AMPCP reduced Tb indirectly by elevating brain extracellular AMP. As shown in Figure 1D, intracerebroventricular injections of hypothermic doses of AMPCP (50 to 100 μg) did not affect blood pressure.
Figure 1.
5′-Adenosine monophosphate (AMP) induces hypothermia in mice by activating hypothalamic adenosine A1 receptor (A1R). (A) Effects of different doses of AMP on body temperature (Tb). The effect of DPCPX (0.1 mg/kg intraperitoneally, 5 minutes pretreatment) on hypothermia by 50 mg/kg AMP is also shown. (B) Effects of AMP on blood pressure in mice. (C) Effects of the 5′-nucleotidase inhibitor AMPCP injected intracerebroventricularly on Tb of mice. The hypothermic effect or AMP (50 mg/kg intraperitoneally) is shown for comparison. (C, inset) Effect of DPCPX (0.1 mg/kg intraperitoneally) on hypothermia by AMPCP (50 μg intracerebroventricularly) 40 minutes after drug injection. (D) Effects of AMPCP on blood pressure in mice. Drugs were injected at T=0 (A, C) or T=5 minutes (B, D arrow). For all graphs, each point/column represents the mean±s.e.m., at least eight animals per group were used. *P<0.05, ***P<0.001 versus vehicle.
Hypothermia Induced by 5′-Adenosine Monophosphate Reduces Ischemic Brain Injury and Mortality in Mice
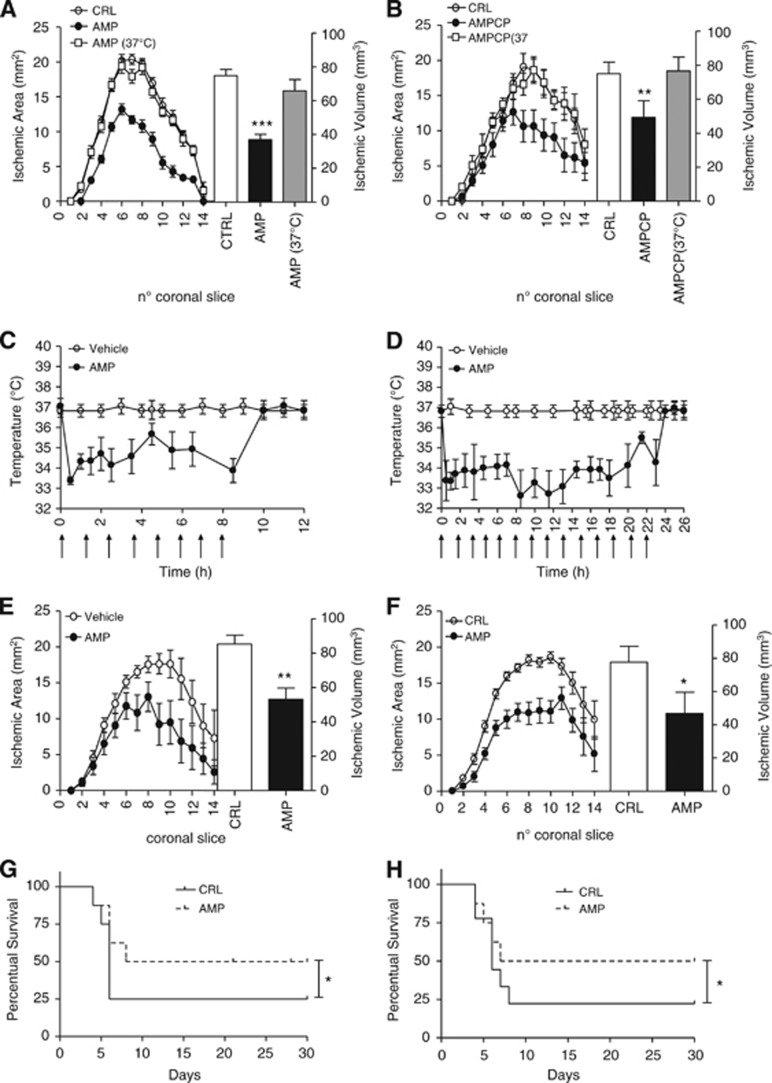
We next investigated whether AMP- or AMPCP-dependent cooling afford protection from experimental stroke. In mice subjected to 1 hour MCAo/24 hours reperfusion, intraischemic AMP injection reduced infarct volumes. Neuroprotection was lost in AMP-treated mice artificially kept at 37°C during MCAo (Figure 2A), indicating that the sole hypothermic effect underlined AMP-dependent neuroprotection. Importantly, intraischemic intracerebroventricular injections of AMPCP also prompted hypothermia-dependent neuroprotection (Figure 2B). We then adopted prolonged reperfusion times concomitant to posttreatment protocols to rule out temporary neuroprotection and strengthen clinical significance of AMP to stroke therapy. We found that in mice subjected to 1 hour MCAo, tolerance to the hypothermic effects of AMP did not occur with protocols of postischemic hypothermia/reperfusion of 10 hours/24 hours or 24 hours/72 hours (Figures 2C and 2D). Notably, both hypothermic protocols afforded protection from ischemic brain injury (Figures 2E and 2F). Further strengthening relevance of AMP-dependent hypothermia to stroke therapy, either intraischemic or postischemic treatment with AMP increased survival of mice (Figures 2G and 2H).
Figure 2.
Effect of 5′-adenosine monophosphate (AMP) and AMPCP on ischemic brain injury and survival in mice. Effect of intraischemic injection of AMP (50 mg/kg intraperitoneally) on brain infarct areas and volumes (A) of mice subjected to 1 hour middle cerebral artery occlusion (MCAo)/23 hours reperfusion. Ischemic neuroprotection is lost in mice receiving AMP and artificially kept at 37°C. Effect of intraischemic injection of AMPCP (50 μg intracerebroventricularly) on brain infarct areas and volumes (B) of mice subjected to 1 hour MCAo/23 hours reperfusion. Ischemic neuroprotection is lost in mice receiving AMPCP and artificially kept at 37°C. Body temperature (Tb) of mice subjected to 1 hour MCAo and posttreatment protocols of 10 hours hypothermia/24 hours reperfusion (C) or 24 hours hypothermia/72 hours reperfusion (D) obtained with AMP injections (arrows) of 50 mg/kg intraperitoneally every 90 minutes. The effect of posttreatment protocols of 10 hours hypothermia/24 hours reperfusion or 24 hours hypothermia/72 hours reperfusion on ischemic areas and volumes is shown in (E) and (F), respectively. Effects of protocols of intraischemic (G) or postischemic (24 hours) (H) hypothermia by AMP on survival of mice subjected to 1 hour MCAo. (A, B) Each point/column represents the mean±s.e.m., of at least eight animals per group. **P<0.01, ***P<0.001 versus control (CRL). (E, F) Each point/column represents the mean±s.e.m., of at least eight animals per group. (G, H) Each line represents survival of groups of 10 ischemic mice. *P<0.05, **P<0.01 versus control (CRL).
Discussion
The present study shows that the endogenous nucleotide AMP affords ischemic neuroprotection in an hypothermia-dependent manner. Data showing that hypothermia is counteracted by the A1R antagonist DPCPX confirm that AMP-dependent hypothermia is because of activation of A1Rs.9 Accordingly, A1R negatively regulates central thermogenesis12 and very recent evidence indicates that AMP is a bona fide A1R agonist.13 Our findings also indicate that inhibition of ongoing degradation of AMP into Ado within the brain extracellular space by targeting ecto-5′-nucleotidase with AMPCP suffices to prompt Tb cooling in an A1R-dependent manner, and protects from experimental stroke. Thus, AMP-dependent hypothermia, obtained either by direct nucleotide injection or by inhibition of endogenous nucleotide degradation, could be exploited for innovative cooling strategies of relevance to ischemic neuroprotection. This assumption is strengthened by the finding that AMP-dependent cooling is not invariantly paralleled by harmful hypotension (Figures 1B and 1D) or changes of blood parameters including severe hyperglycemia.9 Evidence that repetitive injections of AMP do not prompt tolerance to the hypothermic effects, and are well tolerated in mice subjected to brain ischemia, a condition that per se sensitizes animals to changes of cardiovascular parameters, corroborates the translational potential of AMP-dependent cooling to stroke therapy. Interestingly, the ability of A1R agonists to prevent shivering14 suggests that therapeutic hypothermia by AMP or A1R activation might not be compromised by this autonomic thermoregulatory reflex that contrasts cooling efficacy.15
In conclusion, at variance with prior work,8 the present study indicates that hypothermia by AMP can be obtained at doses not compromising blood pressure but still able to afford ischemic neuroprotection. Sensitivity of a given species to the hypothermic effects of AMP appears inversely related to the hypothalamic expression of the AMP-degrading enzyme 5′-ectonucleotidase.9 Accordingly, hypothalamic 5′-ectonucleotidase activity is higher in rats than in mice,9 and the former undergo AMP-dependent hypothermia only in the presence of 5′-ectonucleotidase inhibition9 or high doses of AMP.8 Therapeutic hypothermia induced by AMP could be harnessed to treatment of victims of stroke, cardiac arrest or neonatal hypoxia, and studies on sensitivity to AMP of gyrencephalic animals and humans might be worth pursuing.
The authors declare no conflict of interest.
Footnotes
This work was supported by The Health Program 2009 of the Region of Tuscany and AstraZeneca.
References
- Yenari MA, Hemmen TM. Therapeutic hypothermia for brain ischemia: where have we come and where do we go. Stroke. 2010;41:S72–S74. doi: 10.1161/STROKEAHA.110.595371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PS. Hypothermia: a systematic review and meta-analysis of clinical trials. Semin Fetal Neonatal Med. 2010;15:238–246. doi: 10.1016/j.siny.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13:267–278. doi: 10.1038/nrn3174. [DOI] [PubMed] [Google Scholar]
- Diller KR, Zhu L. Hypothermia therapy for brain injury. Annu Rev Biomed Eng. 2009;11:135–162. doi: 10.1146/annurev-bioeng-061008-124908. [DOI] [PubMed] [Google Scholar]
- Testori C, Sterz F, Behringer W, Spiel A, Firbas C, Jilma B. Surface cooling for induction of mild hypothermia in conscious healthy volunteers—a feasibility trial. Crit Care. 2011;15:R248. doi: 10.1186/cc10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CC. Is human hibernation possible. Annu Rev Med. 2008;59:177–186. doi: 10.1146/annurev.med.59.061506.110403. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kaasik K, Blackburn MR, Lee CC. Constant darkness is a circadian metabolic signal in mammals. Nature. 2006;439:340–343. doi: 10.1038/nature04368. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wang S, Luo Y, Ji X, Nemoto EM, Chen J. When hypothermia meets hypotension and hyperglycemia: the diverse effects of adenosine 5′-monophosphate on cerebral ischemia in rats. J Cereb Blood Flow Metab. 2009;29:1022–1034. doi: 10.1038/jcbfm.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzi M, Blasi F, Masi A, Coppi E, Traini C, Felici R, et al. Neurological basis of AMP-dependent thermoregulation and its relevance to central and peripheral hyperthermia J Cereb Blood Flow Metabadvance online publication, 24 October 2012; doi: 10.1038/jcbfm.2012.157(in press). [DOI] [PMC free article] [PubMed]
- Muzzi M, Felici R, Cavone L, Gerace E, Minassi A, Appendino G, et al. Ischemic neuroprotection by TRPV1 receptor-induced hypothermia. J Cereb Blood Flow Metab. 2012;32:978–982. doi: 10.1038/jcbfm.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi J, Harada J, Chiarugi A, Moskowitz MA. STAT-1 is activated in neurons after ischemia and contributes to ischemic brain injury. J Cereb Blood Flow Metab. 2002;22:1311–1318. doi: 10.1097/01.WCB.0000034148.72481.F4. [DOI] [PubMed] [Google Scholar]
- Drew KL, Buck CL, Barnes BM, Christian SL, Rasley BT, Harris MB. Central nervous system regulation of mammalian hibernation: implications for metabolic suppression and ischemia tolerance. J Neurochem. 2007;102:1713–1726. doi: 10.1111/j.1471-4159.2007.04675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittiner JE, Korboukh I, Hull-Ryde EA, Jin J, Janzen WP, Frye SV, et al. AMP is an adenosine A1 receptor agonist. J Biol Chem. 2012;287:5301–5309. doi: 10.1074/jbc.M111.291666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse SY, Wei ET. Inhibition of the shake response in rats by adenosine and 2-chloroadenosine. Psychopharmacology (Berl) 1986;90:322–326. doi: 10.1007/BF00179184. [DOI] [PubMed] [Google Scholar]
- Sessler DI. Defeating normal thermoregulatory defenses: induction of therapeutic hypothermia. Stroke. 2009;40:e614–e621. doi: 10.1161/STROKEAHA.108.520858. [DOI] [PubMed] [Google Scholar]