Abstract
Context
Many diets can produce weight loss over the short term, but the biological effects of dietary composition during weight loss maintenance have not been well studied.
Objective
To examine three diets differing widely in macronutrient composition and glycemic load following weight loss.
Design and Setting
Controlled feeding study with a three-way cross-over design conducted in major metropolitan area (June 2006 to June 2010), with recruitment by newspaper advertisements and postings.
Participants
Overweight and obese young adults (n=21).
Interventions
After achieving 10 to 15% weight loss on a run-in diet, participants consumed low-fat (LF; 60% of energy from carbohydrate, 20% fat, 20% protein; high glycemic load), low-glycemic index (LGI; 40%-40%-20%; moderate glycemic load), and very-low-carbohydrate (VLC; 10%-60%-30%; low glycemic load) diets in random order, each for 4 weeks.
Main Outcome Measures
Resting energy expenditure (REE, primary outcome), total energy expenditure (TEE), hormones, and metabolic syndrome components.
Results
The decline in REE (mean [95% CI]) with weight loss was greatest with the LF diet (−205 [−265 to −144] kcal/d), intermediate with the LGI diet (−166 [−227 to −106] kcal/d), and least with the VLC diet (−138 [−198 to −77] kcal/d; P=0.03, P for trend by glycemic load=0.009). The decline in TEE showed a similar pattern (LF: −423 [−606 to −239] kcal/d; LGI:−297 [−479 to −115] kcal/d); VLC: −97 [−281 to +86] kcal/d; P=0.003, P for trend=0.0009). Hormones and metabolic syndrome components also varied during weight maintenance by diet: leptin (P=0.0006); 24-hour urinary cortisol (P=0.005); indexes of peripheral (P=0.02) and hepatic (P=0.03) insulin sensitivity; HDL cholesterol (P<0.0001); non-HDL cholesterol (P=0.0005); triglycerides (P<0.0001); PAI-1 (P for trend=0.04); and CRP (P for trend=0.05).
Conclusions
During isocaloric feeding following weight loss, declines in resting and total energy expenditure varied by dietary glycemic load and were least with the VLC diet, intermediate with the LGI diet, and greatest with the LF diet.
Trial Registration
ClinicalTrials.gov Identifier NCT00315354
Many people can lose weight for a few months, but most have difficulty maintaining clinically significant weight loss over the long term. According to data from the National Health and Nutrition Examination Survey (1999–2006), only 1 in 6 overweight and obese adults report ever having maintained weight loss of at least 10% for 1 year.1 Among dietary weight loss trials, in which reporting bias can be eliminated, the long-term success rates may be even lower.2 One explanation for the poor long-term outcome of weight loss diets relates to behavior, in that the motivation to adhere to restrictive regimens typically diminishes with time. An alternative explanation is that weight loss elicits biological adaptations – specifically a decline in energy expenditure (adaptive thermogenesis) and an increase in hunger – that promote weight regain.3, 4 These two possibilities can be examined within the conceptual framework of thermodynamic theory.
According to the first law of thermodynamics, energy cannot be created nor destroyed. From this perspective, a calorie is a calorie,5 and no weight reducing diet has inherent superiority to any other. Rather, obesity treatment should emphasize behavioral methods to foster and maintain decreased energy intake. In support of this possibility, several recent clinical trials indicate a direct relationship between dietary adherence and weight loss, regardless of dietary treatment group assignment.6–8 However, the second law of thermodynamics recognizes that irreversible chemical processes tend to be inefficient and increase entropy. Because metabolic pathways vary in energetic efficiency, dietary composition could affect energy expenditure directly, by virtue of macronutrient differences, or indirectly, through hormonal responses to diet that regulate metabolic pathways.9, 10
Diets that aim to attenuate the rise in blood glucose after eating – specifically, low-glycemic index (LGI, emphasizing carbohydrate source)11 and very-low-carbohydrate (VLC, focusing on carbohydrate restriction)12 – have been hypothesized to confer such a “metabolic advantage.” Both of these diets have a low glycemic load (the mathematical product of GI and total carbohydrate), which comprises the single best predictor of how typical foods or meals affect postprandial glycemia.13, 14 Acutely, a low glycemic load diet may elicit hormonal changes that improve the availability of metabolic fuels in the late postprandial period, and thereby decrease hunger and voluntary food intake.10, 15 Chronically, a low glycemic load diet may attenuate the fall in resting energy expenditure that occurs during weight loss.16, 17
In light of debate regarding dietary composition, we conducted a controlled feeding study to evaluate the biological effects of three weight loss maintenance diets, encompassing prevailing ranges of macronutrient composition and glycemic load. The low-fat (LF) diet, consistent with conventional recommendations, had a high glycemic load due to high carbohydrate content; the LGI diet had a moderate glycemic load, obtained by emphasizing sources of carbohydrate with a low GI; and the VLC diet had a low glycemic load achieved through carbohydrate restriction, consistent with the Atkins Diet.12
METHODS
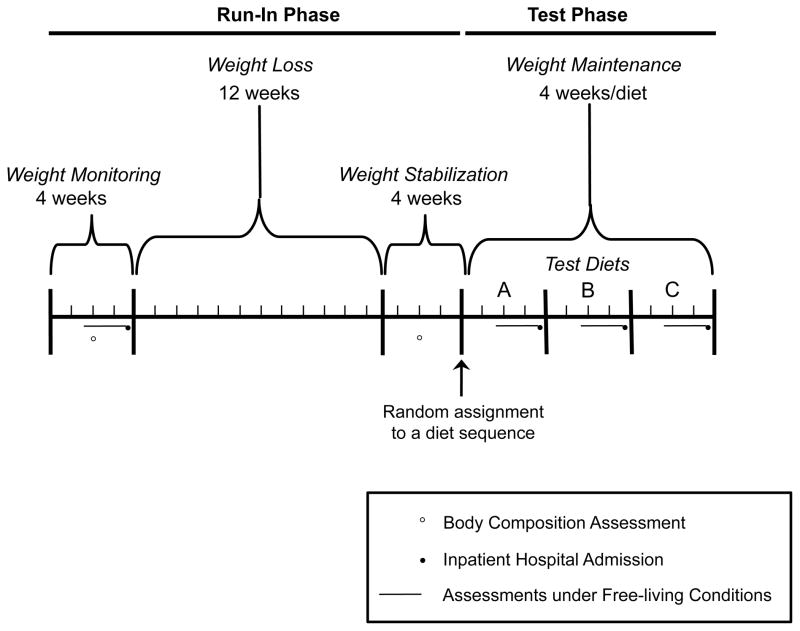
The study comprised run-in and test phases, as shown in Figure 1. During the run-in phase, we obtained baseline data for study outcomes, restricted energy intake of participants to achieve a 12.5% decrease in body weight, and then established energy requirements for stabilizing weight at the reduced level. We assessed body composition by dual-energy x-ray absorptiometry (DXA) before and after weight loss. During the test phase, we used a three-way crossover design to evaluate test diets (LF, LGI, and VLC) in random order, under conditions of weight maintenance. We measured study outcomes during an inpatient hospital admission and under free-living conditions at baseline and the end of each test diet period. Data were collected at Children’s Hospital Boston and Brigham and Women’s Hospital in Boston, Massachusetts between June 2006 and June 2010. Stable isotope analysis for assessing total energy expenditure (TEE) was conducted at Baylor College of Medicine in Houston, Texas. The institutional review boards at all participating institutions approved the study protocol, and participants provided written informed consent. Methodological detail can be found in the eSupplement.
Figure 1. Study Design.
Body composition was assessed during the weight monitoring period of the run-in phase and following weight loss. Assessments during inpatient hospital admissions and under free living conditions occurred during the weight monitoring period and at the end of each test diet period. Immediately prior to the 3-day inpatient hospital admission, the assessments under free living conditions were conducted over 14 (total energy expenditure) or 7 (physical activity) days. There were 6 possible diet sequences to which each subject could be randomly assigned, as described in the eSupplement.
Participants
Participants included men and women aged 18 to 40 years, with a BMI of 27 kg/m2 or above. To compensate participants for their effort, we provided $500 at the end of the run-in phase, following at least 10% weight loss, and an additional $2,000 upon completion of the final inpatient hospital admission.
Dietary Interventions
We aimed to design test diets that: 1) would encompass a broad range of macronutrient composition and glycemic load, 2) have been commonly recommended for obesity treatment, and 3) could be physiologically sustainable for long periods of time. To avoid bias, we formulated menus with healthful components inherent to typical prescriptions for respective diets. In view of the mechanistic nature of this study, relying on a feeding protocol, we did not design the diets for long-term practicality.
Table 1 summarizes the composition of the run-in and test diets. The run-in diet was consistent with the Acceptable Macronutrient Distribution Range (AMDR) specified by the Institute of Medicine,18 with protein intake at the upper end of the range to enhance satiety during weight loss.19 The LF diet, which had a high glycemic load, was designed to reflect conventional recommendations to reduce dietary fat, emphasize whole grain products, and include a variety of vegetables and fruits.20 The LGI diet aimed to achieve a moderate glycemic load by replacing some grain products and starchy vegetables with sources of healthful fat and low-GI vegetables, legumes, and fruits. The LF and LGI diets had similar protein and fiber contents. The VLC diet was modeled on the Atkins Diet, and had a low glycemic load due to more severe restriction of carbohydrate. We provided 3 grams of fiber with each meal (Metamucil, Procter & Gamble, Cincinnati, OH) during the VLC diet, as recommended.12 To ensure micronutrient adequacy and minimize the influence of micronutrient differences among test diets, we gave each participant a daily multi-vitamin and mineral supplement.
Table 1.
Composition of the Run-in and Test Diets (per 2,000 kcal).
| Nutrient | Run-in Diet * | Test Diets During Weight Maintenance **
|
||
|---|---|---|---|---|
| LF | LGI | VLC | ||
| Targeted Macronutrient Distribution – % energy | ||||
| Carbohydrate | 45 | 60 | 40 | 10 |
| Fat | 30 | 20 | 40 | 60 |
| Protein | 25 | 20 | 20 | 30 |
|
| ||||
| Dietary Intake – Mean ± SD | ||||
| Carbohydrate – g/day | 229.5 ± 9.1 | 310.4 ± 1.7 | 205.1 ± 3.3 | 50.1 ± 1.2 |
| Glycemic Index | 52.6 ± 5.9 | 67.7 ± 2.5 | 32.9 ± 3.2 | 28.4 ± 9.0 |
| Glycemic Load – g/day | 68.9 ± 13.1 | 185.1 ± 8.6 | 51.1 ± 6.3 | 3.9 ± 2.2 |
|
| ||||
| Fat – g/day | 68.6 ± 2.7 | 46.5 ± 0.3 | 90.2 ± 4.3 | 133.4 ± 2.7 |
| Saturated | 15.0 ± 2.0 | 12.8 ± 0.5 | 22.4 ± 3.7 | 47.8 ± 8.4 |
| Monounsaturated | 27.1 ± 4.4 | 15.3 ± 2.2 | 40.0 ± 5.8 | 47.7 ± 7.1 |
| Polyunsaturated | 16.6 ±3.8 | 15.7 ± 2.4 | 22.3 ± 6.3 | 22.0 ± 7.4 |
|
| ||||
| Protein – g/day | 126.9 ± 5.6 | 104.8 ± 0.6 | 105.5 ± 2.0 | 151.5 ± 1.1 |
|
| ||||
| Fiber – g/day | 27.1 ± 3.4 | 30.3 ± 2.8 | 32.8 ± 1.8 | 11.2 ± 2.0 |
|
| ||||
| Cholesterol – mg/day | 216.4 ± 47.5 | 140.3 ± 12.2 | 280.1 ± 173.1 | 978.1 ± 329.7 |
|
| ||||
| Sodium – mg/day | 2363 ± 604 | 2546 ± 379 | 2647 ± 329 | 2646 ± 718 |
Abbreviations: LF, low-fat; LGI, low-glycemic index; VLC, very-low-carbohydrate
The diet for the weight loss and weight stabilization periods of the run-in phase provided 60% and 100% of estimated energy requirements, respectively.
The energy content of diets throughout the test phase remained constant, at the level required for weight stabilization at the end of the run-in phase.
Study Outcomes
Assessments conducted during inpatient hospital admissions included resting energy expenditure (REE, the primary outcome) by indirect calorimetry, hormones (leptin, thyroid stimulating hormone [TSH], triiodothyronine [T3], free urinary cortisol), insulin sensitivity (indexes derived from an oral glucose tolerance test21), other metabolic syndrome components (high-density lipoprotein [HDL] cholesterol, total cholesterol, triglycerides, plasminogen activator inhibitor-1 activity [PAI-1], high sensitivity C-reactive protein [CRP], blood pressure), and participant ratings of hunger and well-being. Assessments conducted under free-living conditions included TEE by doubly-labeled water (DLW) and physical activity by accelerometry.
Statistical Analyses
This crossover trial was designed to provide over 80% power to detect a difference of 80 kcal/d in REE between diets, as observed in our prior study.17 The order of diets in the test phase was randomly assigned for each participant. We followed the intention-to-treat principle, ascribing the assigned diet to each measure regardless of compliance.
Analytic procedures were based on methods for crossover trials described by Senn.22 For each outcome, we fitted a repeated-measures mixed-effects model with measurement period as independent variable (baseline, LF, LGI, VLC), adjusting for sex; age; weight after run-in; sequence of diets; mean weight during measurement period; order of measurement period (baseline always first; test-phase diets second, third, or fourth); within-subject covariance among measurement periods; and, where applicable, correlation among three daily measures within period. Variables with skewed distribution were log-transformed for analysis. One variable with extreme skew (CRP) was rank-transformed for analysis.23
We tested the ‘overall’ null hypothesis of equal mean in the three test-phase periods (H0: LF=LGI=VLC) using a two-sided criterion of P<0.05. Whenever this hypothesis was rejected, we performed pairwise comparisons with a Bonferroni-adjusted criterion of P<0.05/3. We also constructed a test for linear trend across diets, proceeding from highest to lowest glycemic load. We applied an outlier-deletion algorithm with optimal properties, equivalent to ‘robust’ regression.24 As missing values were uncommon (typically 1 per outcome), we did not perform any imputation, relying on the unbiasedness of mixed-effects regression when data are missing at random.25 We used SAS software (version 9.2, Cary, NC) for all computations. Data are presented as mean [95% CI] unless otherwise noted.
RESULTS
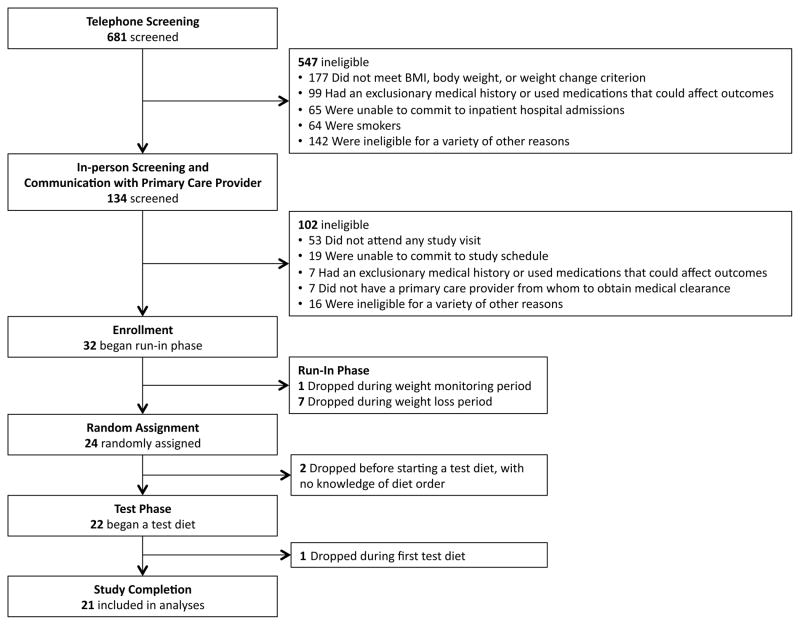
We enrolled 32 participants, including 17 males and 15 females. Of these, 11 participants did not complete the study, as summarized in Figure 2. Baseline characteristics for the 21 participants who completed the study are presented in Table 2. Non-completers did not differ from completers with respect to any of these characteristics. During the run-in phase, participants lost 14.3 ± 0.9 kg (mean ± SD), corresponding to 13.6% of baseline body weight. Percent body fat by DXA decreased from mean 33.6 [30.0 to 37.2]% at baseline to 29.1 [25.1 to 33.1]% after weight loss. Energy intake during the test diet phase was 2626 ± 686 kcal/d (mean ± SD). Body weight did not differ significantly among the three diets (LF: 91.5 [87.4 to 95.6] kg; LGI: 91.1 [87.0 to 95.2] kg; VLC: 91.2 [87.1 to 95.3] kg; P=0.80).
Figure 2. Participant Flow Chart.
Table 2.
Baseline Characteristics of the Study Participants (n=21).
| Continuous Variable | Mean ± SD |
|---|---|
| Age – yr * | 30.3 ± 5.7 |
| Height – m | 174.3 ± 11.3 |
| Weight – kg | 105.0 ± 20.1 |
| BMI – kg/m2 ** | 34.4 ± 4.9 |
| Waist Circumference – cm † | 103.5 ± 12.9 |
| Categorical Variable | N (%) |
| Sex – no. (%) | |
| Male | 13 (62) |
| Female | 8 (38) |
| Race – no. (%) ‡ | |
| White | 4 (19) |
| Black | 8 (38) |
| Asian | 4 (19) |
| Other | 5 (24) |
| Ethnicity – no. (%) ‡ | |
| Hispanic | 4 (19) |
Age was calculated from date of birth and date of baseline hospital admission.
Body mass index was calculated as weight in kg divided by height in m2.
Waist was measured at the midpoint between the lower rib and iliac crest.
We asked participants to self-report race and ethnicity.
Energy Expenditure (Table 3, Figure 3)
Table 3.
Study Outcomes.
| Variable | Mean (95% Confidence Interval)
|
P *
|
||||
|---|---|---|---|---|---|---|
| Baseline | Test Diets During Weight Maintenance
|
Overall (LF=LGI =VLC) | Trend (LF–LGI –VLC) | |||
| LF | LGI | VLC | ||||
| Energy Metabolism | ||||||
|
| ||||||
| REE – kcal/day | 1781 (1737 to 1824) | 1576 (1528 to 1624)a | 1614 (1566 to 1662)a,b | 1643 (1595 to 1691)b | 0.03 | 0.009 |
| REE – kcal/kg FFM/day | 27.4 (26.6 to 28.5) | 24.4 (23.6 to 25.2)a | 25.0 (24.2 to 25.8)a,b | 25.5 (24.7 to 26.4)b | 0.04 | 0.01 |
|
| ||||||
| Resting RQ | 0.901 (0.884 to 0.918) | 0.905 (0.894 to 0.924) | 0.861 (0.845 to 0.875) | 0.826 (0.817 to 0.848) | <0.0001 | <0.0001 |
|
| ||||||
| TEE – kcal/day | ||||||
| Using calculated FQ | 3234 (3081 to 3388) | 2812 (2599 to 3024)a | 2937 (2730 to 3145)a | 3137 (2926 to 3348) | 0.003 | 0.0009 |
| Using measured RQ | 3235 (3082 to 3389) | 2767 (2564 to 2970)a | 2926 (2729 to 3124)a,b | 3013 (2811 to 3216)b | 0.02 | 0.007 |
| TEE – kcal/kg FFM/day | ||||||
| Using calculated FQ | 49.8 (46.6 to 52.9) | 43.7 (40.3 to 47.1)a | 45.8 (42.4 to 49.1)a,b | 47.6 (44.2 to 51.0)b | 0.008 | 0.003 |
| Using measured RQ | 49.7 (46.5 to 52.8) | 42.9 (39.4 to 46.4)a | 45.2 (41.8 to 48.7)a,b | 46.6 (43.0 to 50.1)b | 0.02 | 0.005 |
|
|
|
|
||||
| Physical Activity | ||||||
| Total counts – thousands | 299 (259 to 339) | 301 (258 to 344) | 314 (271 to 358) | 287 (245 to 330) | 0.20 | 0.33 |
| MVPA – min/day** | 13.5 (10.2 to 18.0) | 15.8 (10.9 to 22.8) | 14.7 (10.3 to 20.9) | 11.7 (8.2 to 16.6) | 0.18 | 0.08 |
|
| ||||||
| Hormones | ||||||
|
|
|
|
||||
| Leptin – ng/mL** | 29.2 (24.3 to 35.1) | 14.9 (12.1 to 18.4) | 12.7 (10.3 to 15.6)b | 11.2 (9.1 to 13.8)b | 0.0006 | 0.0002 |
|
|
|
|
||||
| Urinary Cortisol – μg/day** | 58 (47 to 73) | 50 (41 to 60)a | 60 (49 to 73)a,b | 71 (58 to 86)b | 0.005 | 0.001 |
|
|
|
|
||||
| Thyroid Function | ||||||
| TSH – μIU/mL** | 1.15 (0.97 to 1.37) | 1.27 (1.01 to 1.60)a | 1.22 (0.97 to 1.54)a,b | 1.11 (0.88 to 1.40)b | 0.04 | 0.01 |
| T3 – ng/dL** | 137 (127 to 149) | 121 (108 to 135)a | 123 (110 to 137)a | 108 (96 to 120) | 0.006 | 0.007 |
|
| ||||||
| Components of the Metabolic Syndrome | ||||||
|
| ||||||
| Insulin Sensitivity Indexes† | ||||||
| Peripheral | 0.24 (0.11 to 0.59) | 0.53 (0.24 to 0.83)a | 0.87 (0.56 to 1.18)a,b | 0.93 (0.63 to 1.22)b | 0.02 | 0.008 |
| Hepatic ** | 0.56 (0.41 to 0.78) | 0.93 (0.71 to 1.23)a | 1.04 (0.78 to 1.37)a,b | 1.24 (0.94 to 1.63)b | 0.03 | 0.01 |
|
|
|
|
||||
| Cholesterol – mg/dL | ||||||
| HDL | 46 (41 to 50) | 40 (35 to 45) | 45 41 to 50) | 48 (44 to 53) | <0.0001 | <0.0001 |
| Non-HDL | 131 (121 to 142) | 109 (95 to 122)a | 111 (98 to 124)a | 127 (114 to 140) | 0.0005 | 0.0003 |
|
|
|
|
||||
| Triglycerides – mg/dL ** | 116 (93 to 144) | 107 (87 to 131) | 87 (71 to 106) | 66 (54 to 81) | <0.0001 | <0.0001 |
|
|
|
|
||||
| Blood Pressure – mm Hg | ||||||
| Systolic | 116 (114 to 119) | 110 (107 to 113) | 109 (107 to 112) | 111 (109 to 114) | 0.34 | 0.32 |
| Diastolic | 67 (64 to 70) | 61 (59 to 64) | 62 (59 to 65) | 63 (61 to 66) | 0.35 | 0.16 |
|
| ||||||
| PAI-1 – ng/mL** | 3.90 (2.54 to 5.98) | 1.39 (0.94 to 2.05) | 1.15 (0.78 to 1.71) | 1.01 (0.68 to 1.49) | 0.11 | 0.04 |
|
| ||||||
| CRP – mg/L ‡ | 1.75 (0.44 to 4.61) | 0.78 (0.38 to 1.92) | 0.76 (0.50 to 2.20) | 0.87 (0.57 to 2.69) | 0.13 | 0.05 |
Abbreviations: LF, low-fat; LGI, low-glycemic index; VLC, very-low-carbohydrate; RQ, respiratory quotient; REE, resting energy expenditure; TEE, total energy expenditure; FFM, fat-free mass; FQ, food quotient; MVPA, moderate-to vigorous-intensity physical activity; TSH, thyroid stimulating hormone; T3, triiodothyronine; HDL-C, high-density lipoprotein cholesterol; PAI-1, plasminogen activator inhibitor-1; CRP, C-reactive protein.
From repeated-measures analysis of variance modeling variation among the four measurement periods, adjusted for sex, age, order of diets, baseline weight, and mean weight during each period as well as covariance among periods within subject and covariance among three measurement days within period. P overall tests the hypothesis that mean outcome was equal in the three test diet periods (LF, LGI, VLC). P for trend tests the hypothesis of linear increase in mean outcome from LF to LGI to VLC, assuming equal spacing.
A common superscript a or b indicates that two diet-phase means for a particular outcome were not significantly different as judged by Bonferroni-adjusted comparison (P>0.017) following significant overall test of H0: LF=LGI=VLC (P<0.05).
Log-transformed for analysis; adjusted mean and 95% confidence interval retransformed to natural units.
In these formulas, glucose is expressed in mg/dL and insulin in μIU/mL. In the table, hepatic insulin sensitivity is scaled up by 103 for readability.
Rank-transformed for analysis; entries are median and 95% confidence limits in natural units.
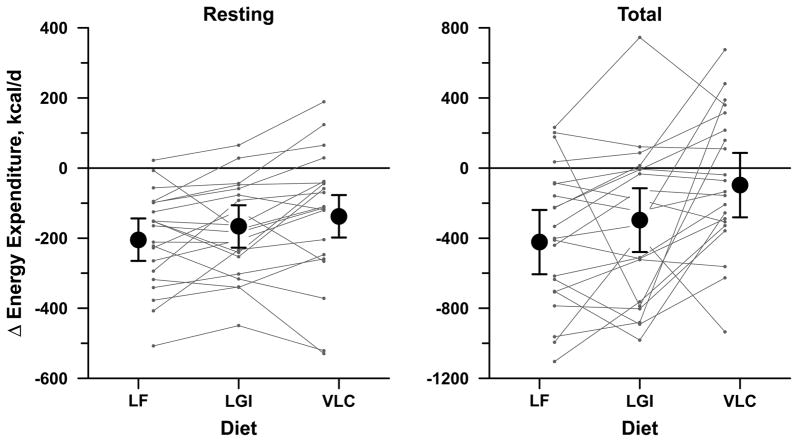
Figure 3. Changes in Energy Expenditure.
Resting energy expenditure (left) and total energy expenditure (right) during three test diets for weight-loss maintenance: low-fat (LF), low-glycemic index (LGI), and very low carbohydrate (VLC). Each symbol with error bars indicates mean change from a common baseline period preceding weight loss, with 95% confidence interval, obtained from analysis of cross-over experiment and adjusted for sex, age, order of diets, baseline weight, and mean weight during the 4-wk diet period. Connected lines indicate individual outcomes for the 21 subjects. Both resting and total energy expenditure showed a significant linear trend in mean change from LF to LGI to VLC, P< 0.01.
Energy expenditure during weight loss maintenance differed significantly among the diets. The decline in REE from pre-weight loss levels, measured by indirect calorimetry in the fasting state, was greatest for the LF diet (mean relative to baseline, −205 [−265 to −144] kcal/d), intermediate with the LGI diet (−166 [−227 to −106] kcal/d), and least for the VLC diet (−138 [−198 to −77] kcal/d; P=0.03, P for trend by glycemic load=0.009). The decline in TEE, as assessed using DLW methodology, also differed significantly by diet (LF: −423 [−606 to −239] kcal/d; LGI:−297 [−479 to −115] kcal/d; VLC: −97 [−281 to +86] kcal/d; P=0.003, P for trend=0.0009). This result was not materially changed when substituting measured RQ for calculated FQ. Neither total physical activity nor time spent in moderate-to-vigorous physical activity differed among the diets.
Hormones and Components of the Metabolic Syndrome (Table 3)
Serum leptin was highest with the LF diet (14.9 [12.1 to 18.4] ng/mL), intermediate with the LGI diet (12.7 [10.3 to 15.6] ng/mL) and lowest with the VLC diet (11.2 [9.1 to 13.8] ng/mL; P=0.0006). Cortisol excretion measured with a 24-hour urine collection (LF: 50 [41 to 60] μg/d; LGI: 60 [49 to 73] μg/d; VLC: 71 [58 to 86] μg/d; P=0.005) and serum TSH (LF: 1.27 [1.01 to 1.60] μIU/mL; LGI: 1.22 [0.97 to 1.54] μIU/mL; VLC: 1.11 [0.88 to 1.40] μIU/mL; P=0.04) also differed in a linear fashion by glycemic load. Serum T3 was lower with the VLC diet compared to the other two diets (LF: 121 [108 to 135] ng/dL; LGI: 123 [110 to 137] ng/dL; VLC: 108 [96 to 120] ng/dL; P=0.006).
Regarding components of the metabolic syndrome, indexes of peripheral (P=0.02) and hepatic (P=0.03) insulin sensitivity were lowest with the LF diet. Serum HDL-cholesterol (LF: 40 [35 to 45] mg/dL; LGI: 45 [41 to 50] mg/dL; VLC: 48 [44 to 53] mg/dL; P<0.0001), triglycerides (LF: 107 [87 to 131] mg/dL; LGI: 87 [71 to 106] mg/dL; VLC: 66 [54 to 81] mg/dL; P<0.0001), and PAI-1 (LF: 1.39 [0.94 to 2.05] ng/mL; LGI: 1.15 [0.78 to 1.71] ng/mL; VLC: 1.01 [0.68 to 1.49] ng/mL; P for trend=0.04) were most favorable with the VLC diet and least favorable with the LF diet. However, CRP (median [95% CI]) tended to be higher with the VLC diet (LF: 0.78 [0.38 to 1.92] mg/L; LGI: 0.76 [0.50 to 2.20] mg/L; VLC: 0.87 [0.57 to 2.69] mg/L; P for trend=0.05). Blood pressure did not differ among the diets.
Hunger and Well-being
Using a 10-cm scale visual analog scale, ratings of subjective hunger (LF: 5.7 [4.6 to 6.8] cm; LGI: 5.4 [4.4 to 6.5] cm; VLC: 5.8 [4.8 to 6.9] cm; P=0.62) and well-being (LF: 6.1 [5.2 to 7.0] cm; LGI: 6.9 [6.0 to 7.8] cm; VLC: 6.3 [5.3 to 7.2] cm; P=0.21) obtained prior to breakfast did not differ significantly among the diets.
COMMENT
The results of this study challenge the notion that a calorie is a calorie from a metabolic perspective. During isocaloric feeding following weight loss, REE was 67 kcal/d greater with the VLC diet compared to the LF diet. TEE differed by about 300 kcal/d between these two diets, an effect corresponding to the amount of energy typically expended in 1 hour of moderate-intensity physical activity.
The physiological basis for the differences in REE and TEE remains subject to speculation. T3 was lowest with the VLC diet, consistent with previously reported effects of carbohydrate restriction;26 thus, changes in thyroid hormone concentration cannot account for the higher energy expenditure on this diet. The thermic effect of food (TEF, the increase in energy expenditure arising from digestive and metabolic processes) dissipates in the late postprandial period and would not affect REE measured in the fasting state. Because TEF tends to be greater for carbohydrate than fat27, 28 it would also not explain the lower TEE on the LF diet. Although protein has a high TEF,19 the content of this macronutrient was the same for the LF and LGI diets and contributed only 10% more to total energy intake with the VLC diet compared to the other two diets. Furthermore, physical activity as assessed by accelerometry did not change throughout the study. Alternative explanations for the observed differences in REE and TEE may involve intrinsic effects of dietary composition on the availability of metabolic fuels16, 17 or metabolic efficiency; changes in hormones (other than thyroid) or autonomic tone affecting catabolic or anabolic pathways; and (for TEE) skeletal muscle efficiency, as regulated by leptin.29–32 Regarding the last possibility, the ratio of energy expenditure to leptin concentration has been proposed as a measure of leptin sensitivity,33 and this ratio varied as expected in our study (VLC > LGI > LF).
Although the VLC diet produced the greatest improvements in most metabolic syndrome components examined here, we identified two potentially deleterious effects of this diet. Twenty-four hour urinary cortisol excretion, a hormonal measure of stress, was highest with the VLC diet. Consistent with this finding, Stimson et al34 reported increased whole-body regeneration of cortisol by 11β-HSD1 and reduced inactivation of cortisol by 5α-and 5β-reductases over 4 weeks on a VLC vs. a moderate-carbohydrate diet. Higher cortisol levels may promote adiposity, insulin resistance, and cardiovascular disease, as observed in epidemiological studies.35–37 In a 6-year prospective population-based study of older adults in Italy, individuals in the highest vs. lowest tertile of 24-hour cortisol excretion, with or without preexisting cardiovascular disease, had a 5-fold increased risk of cardiovascular mortality.38 CRP also tended to be higher on the VLC diet in our study, consistent with the findings of Rankin and Turpyn.39 Other studies also have found reductions in measures of chronic inflammation, including CRP with a low-GI diet.40–42
A main strength of this study was use of a controlled feeding protocol to establish weight stability following weight loss. Other strengths include a cross-over design to allow for within-individual comparisons, examination of three physiologically sustainable diets spanning a wide range of prevailing macronutrient compositions, control for dietary protein between the LF and LGI diets, state-of-the-art methods to assess TEE under free-living conditions, collection of other study outcomes under direct observation during inpatient hospital admissions to a metabolic ward, and use of observed respiratory quotient (RQ) by indirect calorimetry to verify macronutrient differences among the diets.
Main study limitations are the relatively short duration of the test diets and the difficulty extrapolating findings from a feeding study to a more natural setting, in which individuals consume self-selected diets. The VLC diet, in particular, involved more severe carbohydrate restriction than would be feasible for many individuals over the long term. Therefore, the study may overestimate the magnitude of effects that could be obtained by carbohydrate restriction in the context of a behavioral intervention. In addition, participants in the study were selected for ability to comply with the rigors of a 7-month feeding protocol and may not represent overweight and obese individuals in the general population. While we could not assess compliance during the outpatient phases of the study, good maintenance of weight loss throughout the test phase provides some reassurance on this point.
A methodological issue in cross-over feeding studies involves the possibility of carryover effects between test diets. However, random assignment of participants to a diet sequence and statistical control for order effects would diminish this possibility. In addition, we used compartmental modeling for analysis of TEE, to correct for residual tracer and possible variations in dilution spaces and water kinetics among study periods. Another limitation relating to TEE measurement involves reliance on several assumptions, including the food quotient (FQ) of the test diets. However, sensitivity analysis demonstrated that our results would withstand plausible inaccuracies in estimates of FQ, and qualitatively similar results were obtained when substituting measured RQ for calculated FQ. Finally, we did not assess physiological differences among participants, for example involving insulin secretion,43, 44 that might influence individual responses to the test diets.
In summary, this study demonstrates that commonly consumed diets can affect metabolism and components of the metabolic syndrome in markedly different ways during weight loss maintenance, independent of energy content. The LF diet produced changes in energy expenditure and serum leptin45–47 that would predict weight regain. In addition, this conventionally-recommended diet had unfavorable effects on most of the metabolic syndrome components studied here. In contrast, the VLC diet had the most beneficial effects on energy expenditure and several metabolic syndrome components, but this restrictive regimen may increase cortisol excretion and CRP. The LGI diet appears to have qualitatively similar, though smaller, metabolic benefits to the VLC diet, possibly without the deleterious effects on physiological stress and chronic inflammation. These findings suggest that a strategy to reduce glycemic load, rather than dietary fat, may be advantageous for weight loss maintenance and cardiovascular disease prevention. Ultimately, successful weight loss maintenance will require behavioral and environmental interventions to facilitate long-term dietary adherence. But such interventions will be most effective if they promote a dietary pattern that ameliorates the adverse biological changes accompanying weight loss.
Supplementary Material
Acknowledgments
We thank Michael Leidig, RD and Carolyn Walsh, MD for organizing daily study operations; Karen Yee, MS, RD, Rachel Froelich, MS, RD, and Lisa Bielak, MS, RD for their roles in developing and delivering the dietary interventions; Robert Markowitz, MD for help with hospital admissions and blood sample collections; and Sarah Kalil, Hope Forbes, MA and Elizabeth Scarola, MA for assistance with data collection and management. Mr Leidig, Ms Yee, Ms Froelich, Ms Bielak, Dr Markowitz, Ms Kahil, and Ms Forbes received compensation for their work in the form of salary support.
Role of Sponsors: The funding organizations had no role in the preparation, review, or approval of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases, National Center for Research Resources, the National Institutes of Health, or the United States Department of Agriculture.
Funding/Support: This study was supported by grants R01 DK072428 and K24DK082730 from the National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda, Md); grants MO1-RR02172 from the National Center for Research Resources, National Institutes of Health, to the Children’s Hospital Boston General Clinical Research Center; grant M01-02635 from the National Center for Research Resources, National Institutes of Health, to the Brigham and Women’s Hospital General Clinical Research Center; grant UL1 RR025758-01 from the National Center for Research Resources, National Institutes of Health, to the Harvard Catalyst Clinical and Translational Science Center, and a grant from the New Balance Foundation.
Footnotes
Financial Disclosures: Dr. Ludwig reported receiving grants from the National Institutes of Health and foundations for obesity-related research, mentoring, and patient care; and royalties from a book about childhood obesity. No other authors report conflicts of interest.
Author Contributions: As principal investigator, Dr Ludwig had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ebbeling, Swain, Ludwig
Acquisition of data: Ebbeling, Swain, Wong, Garcia-Lago, Ludwig
Analysis and interpretation of data: Ebbeling, Feldman, Wong, Hachey, Ludwig
Drafting of manuscript: Ebbeling, Ludwig
Critical revision of the manuscript for important intellectual content: Swain, Feldman, Wong, Hachey, Garcia-Lago
Statistical expertise: Feldman
Obtained funding: Ebbeling, Ludwig
Administrative, technical, or material support: Garcia-Lago
Supervision: Ebbeling, Swain, Ludwig
Contributor Information
Cara B. Ebbeling, Email: cara.ebbeling@childrens.harvard.edu.
Janis F. Swain, Email: jswain@partners.org.
Henry A. Feldman, Email: henry.feldman@childrens.harvard.edu.
William W. Wong, Email: wwong@bcm.edu.
David L. Hachey, Email: david.l.hachey@vanderbilt.edu.
Erica Garcia-Lago, Email: egarcialago@gmail.com.
David S. Ludwig, Email: david.ludwig@childrens.harvard.edu.
References
- 1.Kraschnewski JL, Boan J, Esposito J, et al. Long-term weight loss maintenance in the United States. Int J Obes (Lond) 2010;34:1644–1654. doi: 10.1038/ijo.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douketis JD, Macie C, Thabane L, Williamson DF. Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes (Lond) 2005;29:1153–1167. doi: 10.1038/sj.ijo.0802982. [DOI] [PubMed] [Google Scholar]
- 3.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 4.Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597–1604. doi: 10.1056/NEJMoa1105816. [DOI] [PubMed] [Google Scholar]
- 5.Buchholz AC, Schoeller DA. Is a calorie a calorie? Am J Clin Nutr. 2004;79:899S–906S. doi: 10.1093/ajcn/79.5.899S. [DOI] [PubMed] [Google Scholar]
- 6.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53. doi: 10.1001/jama.293.1.43. [DOI] [PubMed] [Google Scholar]
- 7.Foster GD, Wyatt HR, Hill JO, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153:147–157. doi: 10.1059/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feinman RD, Fine EJ. Thermodynamics and metabolic advantage of weight loss diets. Metab Syndr Relat Disord. 2003;1:209–219. doi: 10.1089/154041903322716688. [DOI] [PubMed] [Google Scholar]
- 10.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins DJ, Wolever TM, Taylor RH, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 12.Atkins RC. Atkins for Life. New York: St. Martin’s Griffin; 2004. [Google Scholar]
- 13.Salmeron J, Ascherio A, Rimm EB, et al. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care. 1997;20:545–550. doi: 10.2337/diacare.20.4.545. [DOI] [PubMed] [Google Scholar]
- 14.Brand-Miller JC, Thomas M, Swan V, Ahmad ZI, Petocz P, Colagiuri S. Physiological validation of the concept of glycemic load in lean young adults. J Nutr. 2003;133:2728–2732. doi: 10.1093/jn/133.9.2728. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig DS, Majzoub JA, Al-Zahrani A, Dallal GE, Blanco I, Roberts SB. High glycemic index foods, overeating, and obesity. Pediatrics. 1999;103:E26. doi: 10.1542/peds.103.3.e26. [DOI] [PubMed] [Google Scholar]
- 16.Agus MS, Swain JF, Larson CL, Eckert EA, Ludwig DS. Dietary composition and physiologic adaptations to energy restriction. Am J Clin Nutr. 2000;71:901–907. doi: 10.1093/ajcn/71.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. 2004;292:2482–2490. doi: 10.1001/jama.292.20.2482. [DOI] [PubMed] [Google Scholar]
- 18.Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids. Washington (DC): The National Academies Press; 2002. [DOI] [PubMed] [Google Scholar]
- 19.Halton TL, Hu FB. The effects of high protein diets on thermogenesis, satiety and weight loss: a critical review. J Am Coll Nutr. 2004;23:373–385. doi: 10.1080/07315724.2004.10719381. [DOI] [PubMed] [Google Scholar]
- 20.Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies. A statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Am J Clin Nutr. 2004;80:257–263. doi: 10.1093/ajcn/80.2.257. [DOI] [PubMed] [Google Scholar]
- 21.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care. 2007;30:89–94. doi: 10.2337/dc06-1519. [DOI] [PubMed] [Google Scholar]
- 22.Senn S. Cross-over Trials in Clinical Research. 2. New York: Chichester, UK: John Wiley & Sons; 2002. [Google Scholar]
- 23.Iman RL, Conover WJ. The use of the rank transform in regression. Technometrics. 1979;21:499–509. [Google Scholar]
- 24.Rousseeuw PJ, Leroy AM. Robust Regression and Outlier Detection. New York: John Wiley & Sons, Inc; 1987. [Google Scholar]
- 25.Gadbury GL, Coffey CS, Allison DB. Modern statistical methods for handling missing repeated measurements in obesity trial data: beyond LOCF. Obes Rev. 2003;4:175–184. doi: 10.1046/j.1467-789x.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 26.Sims EA. Experimental obesity, dietary-induced thermogenesis, and their clinical implications. Clin Endocrinol Metab. 1976;5:377–395. doi: 10.1016/s0300-595x(76)80027-8. [DOI] [PubMed] [Google Scholar]
- 27.Tentolouris N, Alexiadou K, Kokkinos A, et al. Meal-induced thermogenesis and macronutrient oxidation in lean and obese women after consumption of carbohydrate-rich and fat-rich meals. Nutrition. 2011;27:310–315. doi: 10.1016/j.nut.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Labayen I, Forga L, Martinez JA. Nutrient oxidation and metabolic rate as affected by meals containing different proportions of carbohydrate and fat, in healthy young women. Eur J Nutr. 1999;38:158–166. doi: 10.1007/s003940050057. [DOI] [PubMed] [Google Scholar]
- 29.Rosenbaum M, Vandenborne K, Goldsmith R, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol. 2003;285:R183–192. doi: 10.1152/ajpregu.00474.2002. [DOI] [PubMed] [Google Scholar]
- 30.Goldsmith R, Joanisse DR, Gallagher D, et al. Effects of experimental weight perturbation on skeletal muscle work efficiency, fuel utilization, and biochemistry in human subjects. Am J Physiol Regul Integr Comp Physiol. 2010;298:R79–88. doi: 10.1152/ajpregu.00053.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baldwin KM, Joanisse DR, Haddad F, et al. Effects of weight loss and leptin on skeletal muscle in human subjects. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1259–1266. doi: 10.1152/ajpregu.00397.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenbaum M, Goldsmith R, Bloomfield D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lustig RH, Sen S, Soberman JE, Velasquez-Mieyer PA. Obesity, leptin resistance, and the effects of insulin reduction. Int J Obes Relat Metab Disord. 2004;28:1344–1348. doi: 10.1038/sj.ijo.0802753. [DOI] [PubMed] [Google Scholar]
- 34.Stimson RH, Johnstone AM, Homer NZ, et al. Dietary macronutrient content alters cortisol metabolism independently of body weight changes in obese men. J Clin Endocrinol Metab. 2007;92:4480–4484. doi: 10.1210/jc.2007-0692. [DOI] [PubMed] [Google Scholar]
- 35.Adam TC, Hasson RE, Ventura EE, et al. Cortisol is negatively associated with insulin sensitivity in overweight Latino youth. J Clin Endocrinol Metab. 2010;95:4729–4735. doi: 10.1210/jc.2010-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holt HB, Wild SH, Postle AD, et al. Cortisol clearance and associations with insulin sensitivity, body fat and fatty liver in middle-aged men. Diabetologia. 2007;50:1024–1032. doi: 10.1007/s00125-007-0629-9. [DOI] [PubMed] [Google Scholar]
- 37.Purnell JQ, Kahn SE, Samuels MH, Brandon D, Loriaux DL, Brunzell JD. Enhanced cortisol production rates, free cortisol, and 11beta-HSD-1 expression correlate with visceral fat and insulin resistance in men: effect of weight loss. Am J Physiol Endocrinol Metab. 2009;296:E351–357. doi: 10.1152/ajpendo.90769.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogelzangs N, Beekman AT, Milaneschi Y, Bandinelli S, Ferrucci L, Penninx BW. Urinary cortisol and six-year risk of all-cause and cardiovascular mortality. J Clin Endocrinol Metab. 2010;95:4959–4964. doi: 10.1210/jc.2010-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rankin JW, Turpyn AD. Low carbohydrate, high fat diet increases C-reactive protein during weight loss. J Am Coll Nutr. 2007;26:163–169. doi: 10.1080/07315724.2007.10719598. [DOI] [PubMed] [Google Scholar]
- 40.Gogebakan O, Kohl A, Osterhoff MA, et al. Effects of weight loss and long-term weight maintenance with diets varying in protein and glycemic index on cardiovascular risk factors: the Diet, Obesity, and Genes (DiOGenes) Study: a randomized, controlled trial. Circulation. 2011 doi: 10.1161/CIRCULATIONAHA.111.033274. [DOI] [PubMed] [Google Scholar]
- 41.Kelly KR, Haus JM, Solomon TP, et al. A low-glycemic index diet and exercise intervention reduces TNF(alpha) in isolated mononuclear cells of older, obese adults. J Nutr. 2011;141:1089–1094. doi: 10.3945/jn.111.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brand-Miller J, Dickinson S, Barclay A, Celermajer D. The glycemic index and cardiovascular disease risk. Curr Atheroscler Rep. 2007;9:479–485. doi: 10.1007/s11883-007-0064-x. [DOI] [PubMed] [Google Scholar]
- 43.Ebbeling CB, Leidig MM, Feldman HA, Lovesky MM, Ludwig DS. Effects of a low-glycemic load vs low-fat diet in obese young adults: a randomized trial. JAMA. 2007;297:2092–2102. doi: 10.1001/jama.297.19.2092. [DOI] [PubMed] [Google Scholar]
- 44.Chaput JP, Tremblay A, Rimm EB, Bouchard C, Ludwig DS. A novel interaction between dietary composition and insulin secretion: effects on weight gain in the Quebec Family Study. Am J Clin Nutr. 2008;87:303–309. doi: 10.1093/ajcn/87.2.303. [DOI] [PubMed] [Google Scholar]
- 45.Erez G, Tirosh A, Rudich A, et al. Phenotypic and genetic variation in leptin as determinants of weight regain. Int J Obes (Lond) 2011;35:785–792. doi: 10.1038/ijo.2010.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mavri A, Stegnar M, Sabovic M. Do baseline serum leptin levels predict weight regain after dieting in obese women? Diabetes Obes Metab. 2001;3:293–296. doi: 10.1046/j.1463-1326.2001.00134.x. [DOI] [PubMed] [Google Scholar]
- 47.Crujeiras AB, Goyenechea E, Abete I, et al. Weight regain after a diet-induced loss is predicted by higher baseline leptin and lower ghrelin plasma levels. J Clin Endocrinol Metab. 2010;95:5037–5044. doi: 10.1210/jc.2009-2566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.