Abstract
Primary hyperparathyroidism, a common endocrine disorder, is traditionally defined by hypercalcemia and elevated levels of parathyroid hormone (PTH). A newer presentation of primary hyperparathyroidism has been described over the past decade, in which PTH is elevated but serum calcium is consistently normal, in the absence of secondary causes of hyperparathyroidism, such as renal disease or vitamin D deficiency. Recognition of this phenotype of primary hyperparathyroidism, normocalcemic primary hyperparathyroidism, supports a biphasic chronological time course in some individuals in which PTH levels are first elevated but serum calcium is normal, followed by the development of frank hypercalcemia. This review focuses on the available literature regarding this newly described phenotype of primary hyperparathyroidism.
Key Terms: Normocalcemic Primary Hyperparathyroidism, Parathyroid Hormone, PTH(1–84)
Introduction
Primary hyperparathyroidism is a disorder characterized traditionally by hypercalcemia and elevated levels of parathyroid hormone (PTH). It is one of the most common endocrine disorders with an estimated prevalence in the United States between 0.1 and 0.5%. Prior to the advent of the multichannel autoanalyzer in the 1970s, classical primary hyperparathyroidism most often presented as a symptomatic disorder, with bone loss and kidney stones. However, with serum calcium values routinely available as a biochemical screening test result, the clinical profile of primary hyperparathyroidism has evolved into a disorder that presents most commonly asymptomatically [1, 2]. In these patients, biochemical screening test leads to the discovery of hypercalcemia when there was no obvious rationale for checking the serum calcium. Despite the fact that most patients are discovered incidentally, bone mass is typically reduced when it is measured by dual energy X-ray absorptiometry (DXA). The densitometric profile of primary hyperparathyroidism favors reductions at the distal 1/3 forearm, a site that is enriched in cortical bone [3, 4]. The characteristic densitometric findings of primary hyperparathyroidism are usually noted at the time of diagnosis.
A newer clinical presentation of primary hyperparathyroidism has been described over the past decade [5–8]. It is characterized by normal total and ionized serum calcium concentrations and consistently elevated PTH levels. These patients have no obvious causes for secondary elevations of PTH, such as renal disease or vitamin D deficiency. Recognition of this new phenotype of primary hyperparathyroidism supports a concept, first proposed by Rao et al., that describes a biphasic chronology of its clinical development [7]. During the first phase, PTH levels are elevated but the serum calcium is normal. Until recently, this first phase was a subclinical one because PTH levels were rarely measured when the serum calcium concentration was normal. The second phase is the one that has traditionally been recognized because hypercalcemia surfaces.
The discovery of these individuals raises the question: Why would the PTH be measured when the serum calcium is normal? The answer can be found in the fact that many osteoporosis and metabolic bone diseases units have become more proactive in their comprehensive approach to biochemical evaluation of the skeleton. Now, PTH levels are often measured even in patients whose serum calcium is normal. It is in this context that most individuals have been discovered with normocalcemic primary hyperparathyroidism.
While normocalcemic primary hyperparathyroidism was first formally recognized at the time of the Third International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism in 2008 [9], the entity remains incompletely described, particularly regarding its epidemiology, natural history, and management. In this report, we summarize what is known about normocalcemic primary hyperparathyroidism and present approaches to gain greater insights into this phenotypic variant.
Pathophysiology
Maruani et al. [5] suggested that normocalcemic primary hyperparathyroidism may be due to target organ resistance to the actions of PTH. After matching normocalcemic and hypercalcemic subjects with primary hyperparathyroidism with regard to age, gender, and PTH concentration, normocalcemic subjects demonstrated inadequate suppression of PTH in response to an oral calcium load. Fasting bone turnover markers and net skeletal calcium release, assessed by fasting calcium/creatinine, were lower in the normocalcemic group. In comparison to the hypercalcemic cohort, renal tubular calcium reabsorption was lower, as was the ability of PTH to reduce tubular phosphate reabsorption and to stimulate 1,25-dihydroxyvitamin D synthesis. In other studies, estrogen has been noted to protect against the bone-resorbing effects of PTH [10], and estrogen replacement in postmenopausal women with primary hyperparathyroidism significantly decreases serum and urine calcium [11, 12]. Maruani et al. [5], therefore, also proposed that the development of estrogen deficiency in postmenopausal women plays a role in the unmasking of hypercalcemia in these subjects. This formulation is consistent with the demographics of hypercalcemic primary hyperparathyroidism, demonstrating its peak incidence in the first decade after the menopause [1, 2]. While the hypothesis of Maruani et al. is an attractive one, it does not explain the fact that normocalcemic primary hyperparathyroidism occurs most commonly in postmenopausal, estrogen deficient women. PTH resistance, if a valid pathogenetic factor in this disease, would not be explained by the masking effect of estrogen on hypercalcemia in the majority of individuals with normocalcemic primary hyperparathyroidism.
Diagnosis
In some patients with traditional primary hyperparathyroidism, the classical biochemical hallmark of hypercalcemia in not always present. While these patients may have normal serum and ionized calcium levels at times during their disease course, they are hypercalcemic the majority of the time. It is important to distinguish these patients from those with normocalcemic primary hyperparathyroidism, in which the serum calcium is always normal over the entire course of monitoring. Some cohorts previously reported with normocalcemic primary hyperparathyroidism are more likely to be explained by the occasional normal calcium value that is seen in hypercalcemic individuals [13]. In addition to normal total serum calcium concentration, normocalcemic primary hyperparathyroidism is also characterized by normal ionized calcium concentrations. Reports that have described normocalcemic individuals with elevated ionized calcium levels would, therefore, not qualify as having normocalcemic primary hyperparathyroidism [14]. The diagnostic criteria for normocalcemic primary hyperparathyroidism, therefore, should include consistently normal albumin-adjusted total serum calcium and normal ionized calcium.
Additionally, secondary causes of an elevated PTH level must be ruled out. The following conditions should be excluded in order to make a diagnosis of normocalcemic primary hyperparathyroidism:
Vitamin D deficiency. There is an inverse relationship between PTH and 25-hydroxyvitamin D. At some reduced level of 25-hydroxyvitamin D, the parathyroid glands are signaled to increase PTH secretion. Exactly what the threshold value for 25-hydroxyvitamin that leads to an increase in PTH is controversial. The Institute of Medicine report [15] states that there is no conclusive evidence that levels of 25-hydroxyvitamin D ≥20 ng/ml are regularly associated with increases in PTH levels in population sampling. However, these studies are confounded by the lack of any prospective data that would track an individual’s PTH level as the 25-hydroxyvitamin D levels is increased from 20 ng/mL to 30 ng/mL. For example, an individual with a “normal” PTH level of 40 pg/mL when the 25-hydoxyvitamin D level is 20 ng/mL might show a reduction to a PTH level 25 pg/mL when the 25-hydroxyvitamin D level is raised to 30 ng/mL. Both PTH levels are within the normal range but for the given individual, it would vary within that normal population range as a function of the 25-hydroxyvitamin D level. To be confident in the diagnosis of normocalcemic primary hyperparathyroidism, it would seem advisable to ensure that the 25-hydroxyvitamin D level is greater than 30 ng/mL. There is another reason for requiring the 25-hydroxyvitamin D level to be truly sufficient. Occasionally normocalcemic patients who demonstrate high PTH levels will become hypercalcemic when 25-hydroxyvitamin D levels are raised to over 30 ng/mL. In these situations, the correct diagnosis is traditional hypercalcemic primary hyperparathyroidism that was masked by the vitamin D deficiency.
Reduced creatinine clearance. Martinez et al. [16–18] demonstrated that PTH begins to rise with a GFR <60 cc/min. While it is true that these determinations are based upon large population surveys such as NHANES III, one cannot know in a given situation whether a mild reduction in creatinine clearance is the explanation for the increased PTH level. It is also noteworthy that Walker et al. [19] have recently shown that in hypercalcemic primary hyperparathyroidism, the reduction in creatinine clearance to <60 cc/min is associated with increased parameters of bone resorption as determined by dynamic histomorphometric analysis of bone biopsies. Thus, it seems reasonable to require that GFR be greater than 60 cc/min if the diagnosis of normocalcemic primary hyperparathyroidism is to be substantiated.
Medications. Hydrochlorothiazide [20] and lithium [21] have both been associated with increased PTH levels and thus should be considered as an etiological cause for increased PTH levels in subjects on these medications. If the abnormality persists after these drugs are withdrawn for several months, the diagnosis can once again be entertained.
Hypercalciuria. Hypercalcuria as a primary renal abnormality can be associated with a secondary rise in PTH levels [22].
Gastrointestinal disorders associated with calcium malabsorption [23, 24]. Usually, but not always, a malabsorption syndrome is clinically obvious. Gluten enteropathy, for example, can be present in individuals who give no obvious symptoms of gastrointestinal tract disease. A low normal serum calcium concentration, along with vitamin D deficiency and low urinary calcium excretion, can be clues to the diagnosis.
Clinical presentation
Reports of normocalcemic primary hyperparathyroidism have largely come from referral centers in which subjects were evaluated for a metabolic bone disease. Our original cohort [25] of 37 individuals with normocalcemic primary hyperparathyroidism came from patients referred to the Columbia University Metabolic Bone Diseases Unit. The group consisted of 29 postmenopausal women, 6 premenopausal women, and 2 men, aged 58 ± 2 years (mean ± SEM; range 32–78). All subjects had normal renal function and 25-hydroxyvitamin D levels >20 ng/ml (65% had levels >30 ng/ml); none used thiazide diuretics or lithium or demonstrated significant hypercalciuria. The reasons for referral included elevated PTH discovered during the evaluation of low bone mass (n=27), recent fragility fracture (n=4), nephrolithiasis (n=2), or other (n=4). At the time of diagnosis, 57% had osteoporosis by BMD, 11% had documented fragility fractures, and 14% had nephrolithiasis. PTH was elevated, while albumin-corrected serum calcium, serum phosphorus, alkaline phosphatase activity, urinary calcium, and N-telopeptide were all in the mid-normal range.
Other cohorts described in the literature [26–29] are summarized in Table 1. These patients had been identified among symptomatic individuals referred for further evaluation or treatment of hyperparathyroidism. As a result, the phenotype was not seen incidentally but in a referral population. Of particular interest is the report of Charopoulos et al. [30] in which high resolution peripheral quantitative computed tomography was used to assess the skeleton in normocalcemic primary hyperparathyroidism in comparison to those with “hypercalcemic” primary hyperparathyroidism. Of the 52 postmenopausal women studied, 24 had normocalcemic primary hyperparathyroidism. The mean age of subjects with normocalcemic primary hyperparathyroidism was 60 ± 9 years (± SD). Catabolic effects were noted in both groups but more pronounced in hypercalcemic subjects than in normocalcemic subjects. Cortical geometric properties were also adversely affected in subjects with normocalcemic primary hyperparathyroidism; however, trabecular properties were preserved.
Table 1.
Summary of cohorts with normocalcemic primary hyperparathyroidism described in the literature.
| Study | Cohort Size | Age (years) | Female (%) | Osteoporosis (%) | Nephrolithiasis (%) | Comments |
|---|---|---|---|---|---|---|
| Symptomatic cohorts | ||||||
| Lowe et al. [23] | 37 | 58 ± 12 | 95 | 57a | 14 | Ionized calcium not available for all |
| Tordjman et al. [24] | 32 | 61 ± 11 | 84 | 36 | 9 | Six with hypercalciuria not responding to hydrochlorothiazide, 3 with vitamin D deficiency although hyperparathyroidism persisted despite vitamin D repletion |
| Amaral et al. [25] | 33 | 64 ± 14 | 79 | 15b | 18 | Ionized calcium not measured |
| Cakir et al. [26] | 18 | 50 ± 10 | 47 | 47 | 11 | Ionized calcium not measured Aim of investigating glucose and lipid metabolism; no differences between patients and age-, sex-, and BMI-matched controls with respect to indicators of insulin resistance |
| Wade et al. [27] | 8 | 60 | 63 | 25c | 25 | Surgical cohort: Five subjects had single gland disease and 3 multiple glands |
| Asymptomatic cohort | ||||||
| Garcia-Martin et al. [29] | 6 | 56 ± 3 | 100d | 0 | 0 | Ionized calcium not measured Population-based cohort |
Mean ± SD
Body Mass Index, BMI
11% with fragility fracture
Only fracture history available
13% with fragility fracture
Study design
Virtually all populations that have been described with normocalcemic primary hyperparathyroidism are referral cohorts. The observation that they have skeletal involvement is, therefore, not surprising. This brings up the possibility that there are individuals with normocalcemic primary hyperparathyroidism who are free-living community members without known metabolic bone disease. The two-phase hypothesis of the evolution of primary hyperparathyroidism suggests, therefore, that there is another cohort of asymptomatic normocalcemic subjects (Figure 1) that exists and would be discovered through screening of large community-dwelling populations, unselected in any way. Population-based studies that are unselected but designed to follow patients over time for the appearance of health conditions would be ideal cohorts to examine in this regard. Thus, they have the potential to identify asymptomatic individuals with normocalcemic primary hyperparathyroidism. The first step in this quest was taken by Garcia-Martin et al. [31], who identified 6 out of 100 healthy postmenopausal women (prevalence 6%) with high PTH and normal albumin-adjusted serum calcium after excluding renal disease, vitamin D deficiency (25-hydroxyvitamin D <30 ng/dL), and malnutrition. One caveat is that ionized calcium values were not performed. Women identified with normocalcemic primary hyperparathyroidism were compared to those with secondary hyperparathyroidism. Both groups had normal bone turnover markers and bone mass as measured by quantitative ultrasound. There were no differences in biochemical or clinical indices between these two groups except that the 25-hydroxyvitamin D level was lower in those with secondary hyperparathyroidism.
Figure 1.
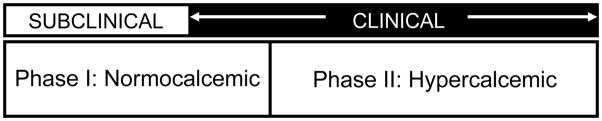
The two-phase hypothesis of the evolution of primary hyperparathyroidism, from asymptomatic normocalcemic to symptomatic hypercalcemic disease.
Epidemiology
The epidemiology of normocalcemic primary hyperparathyroidism has been investigated in a number of populations but interpretation of the data is confounded by differing methods used to exclude secondary hyperparathyroidism among the various studies (Table 2). Lundgren et al. [32] identified 28 subjects with elevated PTH levels and normal serum and ionized calcium levels from a cohort of 5202 (prevalence 0.5%) Swedish postmenopausal women aged 55–75 years. However, secondary etiologies of hyperparathyroidism, such as vitamin D deficiency, were not excluded. Data from the population-based National Health and Nutrition Examination Survey (NHANES) cohort [33], sampling both men and women >45 years of age, showed a prevalence estimate of 1% after excluding significant kidney disease (GFR <60 cc/min) and vitamin D deficiency (25-hydroxyvitamin D <30 ng/dL). Data from the population-based Canadian Multicentre Osteoporosis Study (CaMos) [34], sampling both men and women age 19–-97 years, identified 312 of 1871 individuals (prevalence 16.7%) using a cutoff of 25-hydroxyvitamin D <20 ng/dL but not excluding renal insufficiency. The population-based study by Garcia-Martin et al. [31], described in further detail above, identified 6 out of 100 healthy Spanish postmenopausal women (prevalence 6%). The wide range of prevalence figures undoubtedly reflects the differing methodologies by which normocalcemic primary hyperparathyroidism was defined and inconsistency across these studies in ruling out secondary hyperparathyroid states.
Table 2.
Prevalence of normocalcemic primary hyperparathyroidism in various populations.
| Study | Population | Prevalence | Comments |
|---|---|---|---|
| Lundgren et al. [30] | Postmenopausal women 55–75 years, Sweden | 0.5% | Secondary etiologies of hyperparathyroidism not excluded |
| Misra et al. [31] | Men and women 345 years, US (NHANES) | 1% | Excluding renal failure (GFR <60 cc/min) and vitamin D deficiency (25-hydroxyvitamin D <30 ng/dL) |
| Berger et al. [32] | Men and women 19–97 years, Canada (CaMos) | 16.7% | Excluding vitamin D deficiency (25-hydroxyvitamin D <20 ng/dL) |
| Garcia-Martin et al. [29] | Postmenopausal women, Spain | 6% | Excluding renal disease, vitamin D deficiency (25-hydroxyvitamin D <30 ng/dL), and malnutrition |
National Health and Nutrition Examination Survey, NHANES; Canadian Multicentre Osteoporosis Study, CaMos
Natural history
Data are limited regarding the natural history of the disease. In the cohort of Tordjman et al. [26], 12 patients with positive localization studies underwent successful parathyroidectomy, with operative findings of a single adenoma or hyperplasia. Twenty patients who did not undergo surgery were followed for a mean of 4.1 ± 3 years, without significant change in serum calcium or development of hypercalcemia. In the cohort described by Garcia-Martin et al. [31], after 1 year of follow-up, PTH continued to remain elevated in all 6 women defined as having normocalcemic primary hyperparathyroidism. None of these asymptomatic women, however, developed hypercalcemia, nephrolithiasis, or fracture in their very short follow-up period.
In the Columbia cohort [25], a symptomatic population at diagnosis, 40% of the 37 individuals developed further signs of primary hyperparathyroidism during the mean follow-up period of 3.1 ± 0.3 years (maximum 8 years; median 3). Hypercalcemia developed in 19% of these individuals. The subjects who became hypercalcemic tended to be older, had higher baseline serum calcium levels, and higher baseline urinary calcium excretion. Three of the patients who developed hypercalcemia and 4 additional patients with persistently normal serum calcium levels underwent successful parathyroidectomy, with operative findings of a single adenoma or hyperplastic disease. Pathological examination revealed findings similar to those found in typical primary hyperparathyroidism. After surgery, PTH levels normalized.
Management
Normocalcemic primary hyperparathyroidism was first formally recognized at the Third International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism in 2008 [9]. At that time, however, the expert panel stated that since so little is known about this form of the disease, the guidelines for the hypercalcemic form of primary hyperparathyroidism could not be applied with confidence. Moreover, it was premature to suggest other guidelines for the normocalcemic variant.
Our approach is to monitor subjects with normocalcemic primary hyperparathyroidism in the same way we monitor those with asymptomatic hypercalcemic primary hyperparathyroidism. Annual serum calcium, PTH and bone mineral density determinations seem reasonable. If the disease evolves into the hypercalcemic form, then the published guidelines from the Third International Workshop would be reasonable to follow. Progression of the disease in other ways, such as worsening bone density, a fracture, or a kidney stone would signal a more proactive surgical approach to the disease, even if patients continue to be normocalcemic.
Conclusions
The historical view of primary hyperparathyroidism describes two distinct entities marked by two eras. Prior to the advent of the multichannel autoanalyzer in the 1970s, classical primary hyperparathyroidism commonly presented with marked hypercalcemia and symptomatic bone and stone disease. The presentation shifted in the 1970’s to a disorder characterized by mild hypercalcemia without classical symptomatic features. We appear to have entered a third era in the history of this disease in which patients are being discovered with normal total and ionized serum calcium concentrations but with PTH levels that are consistently elevated. The majority of the cohorts described in the literature have been symptomatic and were discovered during the evaluation for an underlying metabolic bone disease. Population-based studies, that are not preselected and in other ways are not influenced by referral bias, have the potential to identify asymptomatic individuals with normocalcemic primary hyperparathyroidism. Even the limited data available today suggests that there is no single time line for the evolution from normocalcemic to hypercalcemic disease, and that there may be a subset of patients who will remain persistently normocalcemic. With so much that is unknown, management guidelines await more definitive characterization of the disease and its clinical course.
Acknowledgments
Funding source: This work was supported in part by National Institutes of Health grants DK32333, DK074457, and DK095944.
Footnotes
Disclosures: Dr. Bilezikian is a consultant for Amgen, Eli Lilly, Radius, NPS Pharmaceuticals, Merck, Warner Chilcott, and GSK, and receives research support from NPS Pharmaceuticals and Amgen. No conflicts of interest reported for the remaining authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bilezikian JP, Silverberg SJ. Primary Hyperparathyroidism. In: Rosen C, editor. Primer on Metabolic Bone Diseases and Disorders of Mineral Metabolism. 7. Washington, D.C: American Society for Bone and Mineral Research; 2009. [Google Scholar]
- 2.Bilezikian JP, Marcus R, Levine MA. The Parathyroids: Basic and Clinical Concepts. 2001. [Google Scholar]
- 3.Silverberg SJ, Shane E, Jacobs TP, et al. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. N Engl J Med. 1999;341:1249–55. doi: 10.1056/NEJM199910213411701. [DOI] [PubMed] [Google Scholar]
- 4.Rubin MR, Bilezikian JP, McMahon DJ, et al. The natural history of primary hyperparathyroidism with or without parathyroid surgery after 15 years. J Clin Endocrinol Metab. 2008;93:3462–70. doi: 10.1210/jc.2007-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maruani G, Hertig A, Paillard M, Houillier P. Normocalcemic primary hyperparathyroidism: evidence for a generalized target-tissue resistance to parathyroid hormone. J Clin Endocrinol Metab. 2003;88:4641–8. doi: 10.1210/jc.2002-021404. [DOI] [PubMed] [Google Scholar]
- 6.Silverberg SJ, Bilezikian JP. “Incipient” primary hyperparathyroidism: a “forme fruste” of an old disease. J Clin Endocrinol Metab. 2003;88:5348–52. doi: 10.1210/jc.2003-031014. [DOI] [PubMed] [Google Scholar]
- 7.Rao DS, Wilson RJ, Kleerekoper M, Parfitt AM. Lack of biochemical progression or continuation of accelerated bone loss in mild asymptomatic primary hyperparathyroidism: evidence for biphasic disease course. J Clin Endocrinol Metab. 1988;67:1294–8. doi: 10.1210/jcem-67-6-1294. [DOI] [PubMed] [Google Scholar]
- 8.Bilezikian JP, Silverberg SJ. Normocalcemic primary hyperparathyroidism. Arq Bras Endocrinol Metabol. 2010;54:106–9. doi: 10.1590/s0004-27302010000200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilezikian JP, Khan AA, Potts JT., Jr Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab. 2009;94:335–9. doi: 10.1210/jc.2008-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosman F, Shen V, Xie F, et al. Estrogen protection against bone resorbing effects of parathyroid hormone infusion. Assessment by use of biochemical markers. Ann Intern Med. 1993;118:337–43. doi: 10.7326/0003-4819-118-5-199303010-00003. [DOI] [PubMed] [Google Scholar]
- 11.Gallagher JC, Nordin BE. Treatment with with oestrogens of primary hyperparathyroidism in post-menopausal women. Lancet. 1972;1:503–7. doi: 10.1016/s0140-6736(72)90173-0. [DOI] [PubMed] [Google Scholar]
- 12.Selby PL, Peacock M. Ethinyl estradiol and norethindrone in the treatment of primary hyperparathyroidism in postmenopausal women. N Engl J Med. 1986;314:1481–5. doi: 10.1056/NEJM198606053142304. [DOI] [PubMed] [Google Scholar]
- 13.Wills MR, Pak CY, Hammond WG, Bartter FC. Normocalcemic primary hyperparathyroidism. Am J Med. 1969;47:384–91. doi: 10.1016/0002-9343(69)90222-8. [DOI] [PubMed] [Google Scholar]
- 14.Monchik JM, Gorgun E. Normocalcemic hyperparathyroidism in patients with osteoporosis. Surgery. 2004;136:1242–6. doi: 10.1016/j.surg.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 15.Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Dietary Reference Intakes. Washington, D.C: The National Academies Press; 2011. Dietary Reference Intakes for Calcium and Vitamin D. [PubMed] [Google Scholar]
- 16.Martinez I, Saracho R, Montenegro J, Llach F. The importance of dietary calcium and phosphorous in the secondary hyperparathyroidism of patients with early renal failure. Am J Kidney Dis. 1997;29:496–502. doi: 10.1016/s0272-6386(97)90330-9. [DOI] [PubMed] [Google Scholar]
- 17.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–201. [PubMed] [Google Scholar]
- 18.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009:S1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 19.Walker MD, Dempster DW, McMahon DJ, et al. Effect of renal function on skeletal health in primary hyperparathyroidism. J Clin Endocrinol Metab. 2012;97:1501–7. doi: 10.1210/jc.2011-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paloyan E, Farland M, Pickleman JR. Hyperparathyroidism coexisting with hypertension and prolonged thiazide administration. JAMA. 1969;210:1243–5. [PubMed] [Google Scholar]
- 21.Mallette LE, Khouri K, Zengotita H, et al. Lithium treatment increases intact and midregion parathyroid hormone and parathyroid volume. J Clin Endocrinol Metab. 1989;68:654–60. doi: 10.1210/jcem-68-3-654. [DOI] [PubMed] [Google Scholar]
- 22.Coe FL, Canterbury JM, Firpo JJ, Reiss E. Evidence for secondary hyperparathyroidism in idiopathic hypercalciuria. J Clin Invest. 1973;52:134–42. doi: 10.1172/JCI107156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balsa JA, Botella-Carretero JI, Peromingo R, et al. Role of calcium malabsorption in the development of secondary hyperparathyroidism after biliopancreatic diversion. J Endocrinol Invest. 2008;31:845–50. doi: 10.1007/BF03346429. [DOI] [PubMed] [Google Scholar]
- 24.Selby PL, Davies M, Adams JE, Mawer EB. Bone loss in celiac disease is related to secondary hyperparathyroidism. J Bone Miner Res. 1999;14:652–7. doi: 10.1359/jbmr.1999.14.4.652. [DOI] [PubMed] [Google Scholar]
- 25.Lowe H, McMahon DJ, Rubin MR, et al. Normocalcemic primary hyperparathyroidism: further characterization of a new clinical phenotype. J Clin Endocrinol Metab. 2007;92:3001–5. doi: 10.1210/jc.2006-2802. [DOI] [PubMed] [Google Scholar]
- 26.Tordjman KM, Greenman Y, Osher E, et al. Characterization of normocalcemic primary hyperparathyroidism. Am J Med. 2004;117:861–3. doi: 10.1016/j.amjmed.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 27.Amaral LM, Queiroz DC, Marques TF, et al. Normocalcemic versus Hypercalcemic Primary Hyperparathyroidism: More Stone than Bone? J Osteoporos. 2012;2012:128352. doi: 10.1155/2012/128352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cakir I, Unluhizarci K, Tanriverdi F, et al. Investigation of insulin resistance in patients with normocalcemic primary hyperparathyroidism. Endocrine. 2012 doi: 10.1007/s12020-012-9627-x. [DOI] [PubMed] [Google Scholar]
- 29.Wade TJ, Yen TW, Amin AL, Wang TS. Surgical management of normocalcemic primary hyperparathyroidism. World J Surg. 2012;36:761–6. doi: 10.1007/s00268-012-1438-y. [DOI] [PubMed] [Google Scholar]
- 30.Charopoulos I, Tournis S, Trovas G, et al. Effect of primary hyperparathyroidism on volumetric bone mineral density and bone geometry assessed by peripheral quantitative computed tomography in postmenopausal women. J Clin Endocrinol Metab. 2006;91:1748–53. doi: 10.1210/jc.2005-2102. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Martin A, Reyes-Garcia R, Munoz-Torres M. Normocalcemic primary hyperparathyroidism: one-year follow-up in one hundred postmenopausal women. Endocrine. 2012 doi: 10.1007/s12020-012-9694-z. [DOI] [PubMed] [Google Scholar]
- 32.Lundgren E, Rastad J, Thrufjell E, et al. Population-based screening for primary hyperparathyroidism with serum calcium and parathyroid hormone values in menopausal women. Surgery. 1997;121:287–94. doi: 10.1016/s0039-6060(97)90357-3. [DOI] [PubMed] [Google Scholar]
- 33.Misra B, McMahon DJ, Silverberg SJ, Bilezikian JP. New data on the impact of renal function on the relationship between 25-hydroxyvitamin D and Parathyroid Hormone. Program of the 30th Annual Meeting of the American Society of Bone and Mineral Research; Montreal, Canada. 2008. p. 1031. [Google Scholar]
- 34.Berger C, Langsetmo L, Hanley D, et al. Relative Prevalence of Normocalcemic and Hypercalcemic Hyperparathryoidism in a Community-Dwelling Cohort. Program of the 33rd Annual Meeting of the American Society of Bone and Mineral Research; San Diego, California. 2011. p. SU0173. [Google Scholar]


