Abstract
Context
Non-dystrophic myotonias (NDM) are rare diseases caused by mutations in skeletal muscle ion channels. Patients experience delayed muscle relaxation causing functionally-limiting stiffness and pain. Mexiletine-induced sodium channel blockade reduced myotonia in case studies and one single blind trial. As is common in rare diseases, larger studies of safety and efficacy have not previously been considered feasible.
Objective
To determine the effects of mexiletine for symptoms and signs of myotonia in NDM.
Design, Setting, and Participation
Fifty-nine patients with NDM participated in a randomized, double-blind, placebo-controlled two-period crossover study conducted between December 23, 2008 and March 30, 2011 at 7 neuromuscular referral centers in 4 countries, as part of the NIH-funded Rare Disease Clinical Research Network.
Intervention
Oral 200 mg mexiletine or placebo capsules three times daily for 4 weeks, followed by the opposite intervention for 4 weeks, with 1 week wash-out between periods.
Main Outcome Measures
Patient-reported stiffness recorded on an interactive voice response diary (IVR) was the primary endpoint (1 ‘minimal’ to 9 ‘worst ever experienced’). Secondary endpoints included IVR-reported changes in pain, weakness, and tiredness, clinical myotonia assessment, quantitative grip myotonia, Individualized Neuromuscular Quality of Life (INQoL, percent of maximal detrimental impact), SF-36, electrophysiological exercise testing, and needle EMG.
Results
Mexiletine significantly improved patient-reported stiffness on the IVR. Because of a statistically significant interaction between treatment and period for this outcome, primary endpoint is presented by period (period 1 means were mexiletine 2.53 versus placebo 4.21, difference −1.68, 95% Confidence Interval [CI] −2.66, −0.706, P<0.001; period 2 means were mexiletine 1.60 versus placebo 5.27, difference −3.68, 95% CI −3.85, −0.139, P=0.04). Mexiletine improved the INQoL QOL score (mexiletine 14.0, placebo 16.7, difference −2.69, 95% CI −4.07, −1.30, P<0.001) and decreased handgrip myotonia on clinical exam (seconds: mexiletine 0.164, placebo 0.494, difference −0.330, 95% CI −0.633, −0.142, P<0.001). The most common adverse effect was gastrointestinal (9 mexiletine, 1 placebo). Two participants experienced transient cardiac effects that did not require stopping the study (1 placebo, 1 mexiletine). One serious adverse event was determined to be not study-related.
Conclusion
In this preliminary study of patients with NDM, the use of mexiletine compared with placebo resulted in improved patient-reported stiffness over 4 weeks of treatment, despite some concern about the maintenance of blinding.
Trial Registration
Clinicaltrials.gov identifier: NCT 00832000
INTRODUCTION
The non-dystrophic myotonias (NDM) are rare disorders (prevalence 1:100,0001) caused by mutations in skeletal muscle chloride and sodium channels with the common clinical feature of myotonia without muscle wasting2. Myotonia causes functionally limiting stiffness, pain, fatigue and weakness. Data on treatment of NDM is largely anecdotal, consisting of case series and a single blind controlled trial of quinine3, procainamide3,4, phenytoin4, tocainide5, and mexiletine6,7. A 2006 Cochrane review concluded there was not sufficient data to consider any treatment safe and effective for myotonia8.
Mexiletine is a class 1b antiarrhythmic medication with a high affinity for muscle sodium channels. In vitro and animal models suggest mexiletine reduces muscle fiber excitability caused by common NDM mutations9–12. A recent randomized controlled crossover study showed mexiletine to be effective for reducing myotonia in patients with myotonic dystrophy type 113. A major impediment to randomized controlled trials in NDM is its rarity. The NIH-funded Rare Disease Clinical Research Network (RDCRN) was designed to provide centralized infrastructure for investigations of rare diseases. In a natural history study we used a novel interactive voice response(IVR) diary of patient symptoms and found stiffness was the most common and severe symptom reported in NDM regardless of mutation14. Here we report a phase II international randomized, placebo-controlled crossover study of mexiletine in NDM utilizing the RDCRN and patient reported stiffness on the IVR as the primary outcome.
METHODS
Trial Design
We conducted a randomized, double-blind, placebo-controlled, two-period cross-over trial at 7 centers in 4 countries. Treatment periods were 4 weeks in duration separated by a 1 week washout period. The trial was approved by institutional review boards and written and informed consent was obtained from all participants. The National Institutes of Health established a Data Safety Monitoring Board which met every 6 months.
Participants
Eligible participants were at least 16 years of age, had clinical symptoms or signs of NDM, and myotonic potentials on electromyography. Participants were either enrolled in the CINCH NDM Natural History Study, or a new patient with genetically confirmed NDM, or with clinical features of NDM but negative myotonic dystrophy DNA testing. Patients taking anti-myotonic agents were required to discontinue medications for a wash-out period equal to 7 times the half-life of elimination prior to their baseline visit. Participants were ineligible if they has specific contraindications to taking mexiletine (cardiac conduction defects, hepatic or renal disease, or heart failure).
The trial was registered with clinicaltrials.gov (NCT 00721942) in July 2008. Due to a duplicate registration number, records were consolidated in January 2009 (NCT 00832000). The study was conducted between December 23, 2008 and March 30, 2011 (first patient enrolled December 23, 2008) at the following RDCRN/CINCH sites: University of Kansas Medical Center, University of Rochester Medical Center, Brigham and Women’s Hospital, University of Texas Southwestern, London Health Sciences Center, MRC Centre for Neuromuscular Diseases UCL Institute of Neurology, and the University of Milan IRCCS Policlinico San Donato.
Interventions
Participants were randomized to mexiletine 200 mg capsules three times a day (TID) or placebo 200 mg capsules TID for 4 weeks. After a 1 week wash-out period, they were placed on the opposite intervention for 4 weeks.
Mexiletine was purchased from TEVA Pharmaceutical. The placebo was Microcrystalline Cellulose (Avicel PH 102). The mexiletine and placebo were encapsulated at the University of Iowa Research Pharmacy with Swedish Orange Capsule. A Qualified Person from Brecon inspected TEVA and the University of Iowa Research Pharmacy for the purpose of the European Directive. Mexiletine drug level testing was performed at Mayo Medical Laboratory. Random drug levels were collected prior to study visits at baseline, the end of weeks 4, 5, and 9.
Outcomes
Baseline characteristics included gender, age, and self-reported race and ethnicity. For the Interactive Voice Response Diary (IVR) calls were made daily for the entire 9 week study. All other outcomes measurements were performed at baseline, the end of each treatment period, and the end of washout.
Primary Outcome Measure
The primary endpoint was defined as the severity score of stiffness reported by participants during the 3rd and 4th week of each treatment period via the IVR. Participants called in to report symptom severity on a 1–9 scale, 1 being minimal and 9 the worst ever experienced (no symptom = 0 for analysis, eFigure 1)14.
Secondary Outcome Measures
1) Participant-assessed pain, weakness, and tiredness as measured by the IVR from daily calls made over the last two weeks of each period14. 2) Clinical myotonia bedside assessment: participants were asked to squeeze their eyes closed for 5 seconds then rapidly open them; and make a tight fist for 5 seconds then rapidly open. Five trials of each maneuver were performed in sequence at each visit and the time measured on a stopwatch. 3) A quantitative measure of handgrip myotonia was obtained using a commercially available grip dynamometer and computerized capture system. Maximum voluntary contractions following forced right hand grip were recorded and the time to relax from 90% to 5% of maximal force was determined using automated analysis software15,16. 4) The maximal post-exercise decrement in compound muscle action potential (CMAP) after short and long exercise was determined as previously described17,18. 5) Myotonia on needle electromyography was graded on a 1+ to 3+ scale in the right abductor digiti minimi and right tibialis anterior19. 6) Patients filled out the SF-36 and the Individualized Quality of Life questionnaire for neuromuscular disorders (INQoL).20–22 The INQoL is comprised of 10 sections (muscle locking, weakness, pain, fatigue, activities, social relationships, independence, emotions, body image, and effects of treatment) and a summary quality of life score.
Sample Size
The sample size goal was set to 54 participants with available primary endpoint measurements for both treatment periods. This sample size, determined by computer simulation, provided at least 93% power to detect an effect size of one-quarter of a standard deviation (within participant) in the primary endpoint with a 2-sided hypothesis test and an alpha level to 0.05. The variation in power was due to varying the degree of between-participant standard deviation; larger standard deviations lowered the power since the effect in the active treatment period for low severity scores cannot be less than 0. The simulations were based on 500 Monte Carlo realizations, a mean for the placebo group of 3, a within-participant standard deviation of 1.5 and a between-participant standard deviation ranging from 1.5 to 3.0. The effect size of one-quarter of a standard deviation was chosen to be conservative given the tentative assumptions in the simulation, to compensate for the unknown degree of participant compliance to treatment, and the smaller sample size available for the secondary IVR endpoints where some participants do not have the symptom.
Randomization and Blinding
Participants were randomly assigned the order of the two treatments in a 1:1 ratio, stratified by institution. Randomization was done centrally at the Data Management Coordinating Center (University of South Florida) using a computer generated permuted block structure, initially with a block size of four then towards the end of the trial, switching to a block size of two. Each participant was assigned a ‘Kit’ number. In this kit, there were only two bottles of medication (‘A’ for period 1 and ‘B’ for period 2). Only one bottle was dispensed at a time. Participants, physicians, and evaluators were blinded to medication assignment.
Statistical Analysis
This study utilized the intention-to-treat principle modified to remove missing values. Missing values were assumed to be missing at random. All treatment effect analysis employed the linear mixed-effects model (random effect for participant, independent and identically distributed random errors within participant) in order to adjust for any period effect and include data from dropouts23–25. One assumption required to produce valid Wald Tests is that the residuals be normally distributed. To fulfill this assumption the daily reported IVR severity scores (involving the four endpoints: stiffness, pain, tiredness, and weakness) were replaced with the weekly means and QQ plots confirmed that this assumption was satisfied. Another assumption when modeling crossover study data and including only the main effects for period and treatment is that the treatment effect is the same across periods. The lack of consistency is often referred to as a “carryover” effect, although this term can be a misnomer26. For the primary endpoint the Wald test of the treatment-sequence group variable (treatment group) was significant (estimate: 0.997, P = 0.04). This result does not necessarily indicate that the second period data are invalid and should be ignored25,27. However, it may indicate that the treatment effect in the period 2 is biased and that the additive model may yield biased estimates. A fair presentation of the results is to include an interaction term for period 2 and treatment, in order to present the treatment effect estimates separately by period. The test for “carryover” effect was considered significant if the P < 0.1024. Significance was detected for 4 of the subscales of the SF-36, specifically, Vitality, Emotional Role, Mental Health, and Mental Composite. Thus these results and stiffness are displayed by period. The significance level displayed for period 2 is from the Wald test associated with the interaction term of period 2 and mexiletine and not the entire treatment effect while the significance level displayed for period 1 is from the test of the main effect term for treatment variable. Most of the confidence intervals displayed in Table 2 were computed in the usual way using the standard error of the estimate taken from the model results; the exceptions were the endpoints requiring a log transformation for which a boot-strap confidence interval was computed. The effect size, displayed in Table 2, was the treatment effect estimate divided by the within-participant standard deviation.
Table 2.
Mixed Model Results including mean estimate under both treatments, the difference (mexiletine minus placebo) with 95% confidence interval, Effect Size and Significance Level from the Wald Test.
| Endpoint – Period (No. of Participants) | Mean Mexiletine Treatment (95% Confidence Interval)* | Mean Placebo Treatment (95% Confidence Interval)* | Treatment Effect Estimate △ (95% Confidence Interval) | Effect Size◆ | P-value† |
|---|---|---|---|---|---|
| IV: Stiffness – First (57)‡ | 2.53 (1.80, 3.17) | 4.21 (3.40, 5.20) | −1.68 (−2.66, −0.706) | −1.36 | <0.001 |
| IVR: Stiffness - Second (57)‡ | 1.60 (1.04, 2.20) | 5.27 (4.44, 6.27) | −3.68 (−3.85, −0.139) | −2.97 | 0.04 |
| IVR: Pain – Overall (48)§ | 1.54 (0.924, 2.13) | 3.17 (2.43, 3.93) | −1.63 (−2.00, −1.26) | −1.36 | <0.001 |
| IVR: Weakness – Overall (44) § | 1.96 (1.42, 2.63) | 3.22 (2.52, 3.98) | −1.26 (−1.67, −0.861) | −0.994 | <0.001 |
| IVR: Tiredness – Overall (49) § | 2.9 (2.12, 3.68) | 3.82 (3.03, 4.53) | −0.918 (−1.30, −0.532) | −0.709 | <0.001 |
| Short Exercise – Overall (% baseline; 56) | 83.1 (77.5, 88.4) | 78.6 (71.9, 84.7) | 4.54 (−0.680, 9.75) | 0.347 | 0.09 |
| Prolonged Exercise – Overall (% baseline; 56) | 81.8 (76.8, 87.0) | 80.1 (74.7, 86.4) | 1.69 (−3.34, 6.73) | 0.134 | 0.50 |
| Needle EMG: RADM – Overall (56) | 2.05 (1.75, 2.33) | 2.62 (2.39, 2.86) | −0.568 (−0.812, −0.325) | −0.947 | <0.001 |
| Needle EMG: RTA – Overall (56) | 2.07 (1.73, 2.37) | 2.54 (2.28, 2.76) | −0.464 (−0.675, −0.254) | −0.900 | <0.001 |
| SF36: Physical Function – Overall (57) | 42.8 (40.1, 46.1) | 37.8 (34.9, 41.3) | 5.00 (2.81, 7.20) | .904 | <0.001 |
| SF36: Role Physical – Overall (57) | 46.5 (43.6, 49.2) | 39.2 (35.7, 42.6) | 7.23 (4.55, 9.92) | 1.07 | <0.001 |
| SF36: Bodily Pain – Overall (57) | 49.8 (46.4, 52.6) | 42.0 (38.6, 45.5) | 7.78 (5.08, 10.5) | 1.14 | <0.001 |
| SF36: General Health – Overall (57) | 45.5 (41.9, 48.7) | 44.5 (41, 47.7) | 0.977 (−0.659, 2.61) | 0.240 | 0.24 |
| SF36: Vitality – First (57) | 45.5 (41.1, 49.6) | 43.7 (39.7,48.1) | 1.76 (−4.34, 7.85) | 0.211 | 0.57 |
| SF36: Vitality – Second (57) | 51.9 (48.1, 55.5) | 40.0 (35.1, 45.0) | 11.9 (−0.307, 20.5) | 1.43 | 0.06 |
| SF36: Social Function – Overall (57) | 47.1 (44.4, 49.8) | 41.9 (38.5, 44.9) | 5.27 (2.69, 7.85) | 0.809 | <0.001 |
| SF36: Role Emotional – First (57) | 46.2 (42.0, 50.3) | 45.5 (41.2, 49.4) | 0.764 (−5.68, 7.21) | 0.102 | 0.81 |
| SF36: Role Emotional – Second (57) | 49.9 (46.2, 53.1) | 39.1 (33.5, 45.0) | 10.8 (−1.51, 21.6) | 1.45 | 0.09 |
| SF36: Mental Health – First (57) | 47.3 (43.6, 51.0) | 47.3 (43.7, 50.6) | 0.016 (−5.24,5.27) | 0.00258 | 0.99 |
| SF36: Mental Health – Second (57) | 53.3 (50.2, 56.2) | 44.4 (39.8, 48.7) | 8.84 (−0.572, 18.2) | 1.42 | 0.07 |
| SF36: Physical Composite – Overall (57) | 44.8 (41.9, 47.4) | 39.2 (35.9, 41.9) | 5.58 (3.44, 7.72) | 1.04 | <0.001 |
| SF36: Mental Composite – First (57) | 47.4 (44.0, 50.2) | 47.7 (44.2, 51.3) | −0.351 (−5.87, 5.17) | −0.0539 | 0.90 |
| SF36: Mental Composite – Second (57) | 53.1 (50.3, 55.8) | 42.7 (36.8, 48.3) | 10.4 (0.941, 20.6) | 1.60 | 0.03 |
| INQoL: Weakness – Overall (35) | 45.7 (37.7, 52.6) | 49.3 (41.7, 57.3) | −3.56 (−9.54, 2.43) | −0.290 | 0.24 |
| INQoL: Muscle Locking – Overall (43) | 40.0 (33.1, 46.7) | 53.8 (46.4, 61.1) | −13.7 (−20.4, −7.03) | −0.888 | <0.001 |
| INQoL: Pain – Overall (32) | 39.9 (30.6, 49.0) | 48.2 (39.2, 57.1) | −8.32 (−13.8, −2.87) | −0.782 | 0.004 |
| INQoL: Fatigue – Overall (35) | 48.4 (40.9, 56.6) | 58.3 (50.6, 66.0) | −9.96 (−17.0, −2.93) | −0.678 | 0.007 |
| INQoL: Activity – Overall (51) | 34.2 (26.7, 43.0) | 47.1 (40.1, 55.5) | −12.9 (−18.3, −7.43) | −0.950 | <0.001 |
| INQoL: Independence – Overall (51) | 17.8 (12.3, 23.3) | 22.5 (17.2, 28.1) | −4.74 (−8.14, −1.35) | −0.561 | 0.007 |
| INQoL: Social Relations – Overall (51) | 18.9 (13.5, 24.5) | 25.9 (18.0, 35.2) | −7.02 (−13.4, −0.671) | −0.440 | 0.03 |
| INQoL - Emotions – Overall (51) | 27.7 (22.0, 34.4) | 33.8 (27.1, 41.5) | −6.13 (−10.1, −2.15) | −0.619 | 0.003 |
| INQoL: Body Image – Overall (51) | 24.2 (17.3, 31.0) | 29.4 (22.0, 36.5) | −5.27 (−10.4, −0.105) | −0.408 | 0.05 |
| INQoL: QOL – Overall (51) | 14.0 (11.6, 16.5) | 16.7 (14.0, 19.4) | −2.69 (−4.07, −1.30) | −0.780 | <0.001 |
| INQoL: Perceived Rx Effect – Overall (51) | 36.6 (27.1, 45.8) | 21.7 (12.7, 31.1) | 14.9 (7.43, 22.3) | 0.797 | <0.001 |
| INQoL: Expected Rx Effect – Overall (51) | 36.1 (26.9, 47.0) | 23.1 (14.5, 33.6) | 13.0 (4.18, 21.8) | 0.585 | 0.005 |
| Clinical Assessment: Eye Closure – Overall (seconds; 57) € | 0.161 (0.0704, 0.314) | 0.474 (0.261, 0.871) | −0.313 (−0.602, −0.149) | −0.888 | <0.001 |
| Clinical Assessment: Hand Grip – Overall (seconds; 57) ¤ | 0.164 (0.0858, 0.294) | 0.494 (0.281, 0.872) | −0.330 −0.633, −0.142) | −0.748 | <0.001 |
| QMA Hand Grip – Overall (seconds; 54) ¤ | 0.321 (0.274, 0.370) | 0.429 (0.365, 0.517) | −0.109 (−0.177, −0.0560) | −0.518 | <0.001 |
The confidence intervals for the predicted treatment group means are boot strap confidence intervals. These confidence intervals reflect the precision of the estimates without exploiting the correlated nature of the data unlike the treatment effect confidence intervals.
All treatment effect estimates and confidence intervals are extracted from mexiletine treatment variable of the fitted mixed model.
The effect size is the treatment effect estimate divided by the within-participant standard deviation.
Significance level of the Wald Test associated with the Mexiletine Effect from the additive model, when no carryover effect was detected. When a carryover effect was detected, the significance level associated with the additive portion of the Mexiletine Effect (labeled period 1) followed in the next row by the level associated with the interaction of Mexiletine and period 2 (labeled period 2). The exceptions are to two needle EMG tests where the Wilcoxon test was substituted because of the outcome is not a continuous variable and therefore normality of the residuals is not satisfied.
Primary outcome: 52 participants contributed to the both periods while 5 only contributed to period 1.
Only participants that experienced this symptom were included.
The treatment-specific group mean is a geometric mean estimate. The treatment effect estimate is the difference between the treatment-specific group means.
The treatment-specific group mean is a geometric-like mean estimate using the log(t + 0.1) “normalizing” transformation. The treatment effect estimate is the difference between the treatment-specific group means.
In order to test whether the overall treatment effect varies within mutation class, we employed the log likelihood test contrasting the model with versus without the treatment and mutation class interaction terms as a homogeneity test.
For the electrographic myotonia assessment the score was converted to a numeric value as follows: absent = 0, 1+ = 1, 2+ = 2, and 3+ = 3. The endpoint was the sum of the numerical scores of the two muscles. Although the mixed model was used to provide mean estimates, the Paired Wilcoxon test was used to test the treatment effect hypothesis. To fulfill the normality assumption for the clinical handgrip and eye closure times we applied the following transformation: log (ti + 0.1). Similarly, Quantitative handgrip myometry required a log (ti) transformation; the model included a linear term for grip sequence number and a nested random effect for trial number. All p-values are two-sided, and 0.05 is considered the threshold of statistical significance for all tests except for the carryover effect. Since this trial identified a primary endpoint, all other p-values presented were for secondary endpoints and are not adjusted for multiple testing. Analysis was performed using TIBCO Spotfire S+ version 8.1.
RESULTS
Participant Flow
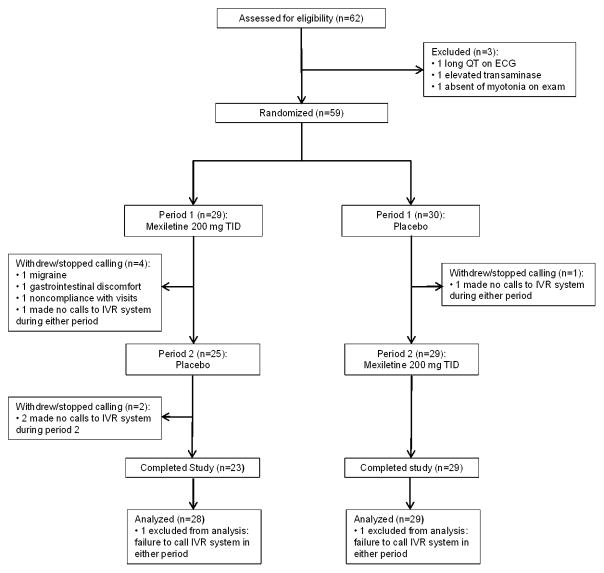
Eligible participants were recruited between December 23, 2008 and January 25, 2011. Of 62 participants recruited, 3 were ineligible: 1 had a prolonged QTc at screening visit, 1 had an elevated transaminase, and 1 had no clinical myotonia on examination. Fifty-nine participants were randomized to receive study medication or placebo. Two particpants did not make expected phone calls to the IVR system during weeks 3–4 of either period. There were 3 dropouts: 1 secondary to migraine headaches, 1 secondary to gastric discomfort, and 1 for failure to comply with study visits. An additional 2 participants did not make calls to the IVR system during weeks 3–4 of the second period, so only provided data for period 1 (Figure 1).
Figure 1.
Study design and disposition of patients. Sixty-two participants screened, 59 randomized, 2 made no calls to IVR system in both periods, 3 drop outs, and 2 made no calls to IVR system in period 2.
Baseline Data
We studied 33 men and 26 women, mean age 42.9 years (16 to 68 years). Participants were predominately white (57/59) and non-Hispanic (46/59). Thirty-four participants had chloride channel mutations, 21 had sodium channel mutations, and 4 had no mutation identified. Seventeen participants were taking medications for myotonia prior to the start of the study, including 13 (22%) taking mexiletine (Table 1). Randomization between groups was balanced with the exception of more men in the placebo followed by mexiletine group.
Table 1.
Screening Characteristics
| Baseline Characteristics# | Treatment Sequence | |
|---|---|---|
| Mexiletine then Placebo N=29 | Placebo then Mexiletine N=30 | |
| Age in Years - Mean (Range) | 41.1 (16–66) | 44.7 (22–68) |
| Gender: Male - No. (%) | 13 (44.8) | 20 (66.7) |
| Race: White - No. (%)◆ | 28 (96.6) | 29 (100.0) |
| Ethnicity: Hispanic - No. (%) | 4 (13.8) | 9 (30.0) |
| Medication: Mexiletine - No. (%) | 7 (24.1) | 6 (20.0) |
| Medication: Other - No. (%) | 3 (10.3) | 1 (3.3) |
| IVR – Stiffness - Mean (SD)¶ | 3.89 (2.39) | 4.63 (2.99) |
| SF-36 – Physical Norm-Based - Mean (SD) | 38.7 (9.65) | 40.8 (11.0) |
| SF-36 Mental Component - Mean (SD) | 44.5 (13.3) | 47.6 (9.8) |
| INQol – QOL Score - Mean (SD) € | 14.0 (9.03) | 15.9 (12.5) |
| Clinical Hand Opening Time in Seconds – Geometric-like Mean (pseudo SD)¥ | 1.11 (0.898, 3.48) | 0.605 (0.510, 1.84) |
| Clinical Eye opening Time in Seconds - Geometric-like Mean (pseudo SD)¥ | 0.507 (0.486, 2.42) | 0.466 (0.455, 2.31) |
| Abductor Digiti Minimi EMG grade 3+ - No. (%)¤ | 18 (62.1) | 18 (62.1) |
| Short Exercise Test (% of Baseline) - Mean (SD) £ | 78.7 (24.5) | 80.8 (28.7) |
| Tibialis Anterior EMG grade 3+ - No. (%)¤ | 20 (69.0) | 19 (65.5) |
| Quantitative Handgrip Myotonia in Seconds - Geometric-like Mean (pseudo SD)¥ | 0.651 (0.288, 0.518) | 0.507 (0.211, 0.361) |
| Mutation – Chloride - No. (%) | 17 (58.6) | 17 (56.7) |
| Mutation – Sodium - No. (%) | 10 (34.5) | 11 (36.7) |
| Mutation – No Identified Mutation – No. (%) | 2 (6.9) | 2 (6.7) |
SD = standard deviation
No. = number of participants
Reference ranges for the scales used in this study are as follows: for the interactive voice response diary (IVR) 0=no symptom, 1=’minimal, 9= ‘worst ever experienced14; SF36 Physical and Mental composite employs a linear T-score transformation 0–100 scale with US mean = 50, lower = larger impact22; the Individualized Neuromuscular Quality of Life scores are presented as a percentage of the maximum detrimental impact, a higher score indicates greater impact, with the exception of treatment effects, where a higher score indicates perceived effectiveness20; clinical hand opening time and eye opening time increase with increasing myotonia; the EMG grade ranges from 0 for no myotonia, 1+ for meeting minimal electrographic criteria for myotonia to 3+ for myotonia in every needle position19; the % of baseline on short exercise testing will decrease with increasing myotonia18; quantitative handgrip myotonia evaluation is expected to increase with increasing myotonia15.
1 participant declined reporting their race
Very few (8) participants had a true baseline report of stiffness severity. Consequently, if unreported, day 1 report was used (40) and if that was unreported, day 2 report was used (10).
1 participant missing Abductor Digiti Minimi EMG grade and Tibialis Anterior EMG grade
1 participant missing short exercise test results
1 participant missing QOL score
geometric-like mean is the inverse transformation (exp[y]−0.1) of the mean of transformed (log[t+0.1]) times. The pseudo standard deviations are the widths of the inverse transformed interval between the mean and plus/minus one standard deviation from the mean, these being calculated on the transformed scale. 8 participants did not have baseline Quantitative Handgrip Myotonia test. None were missing for the clinical tests.
Numbers Analyzed and Drug Levels
Data from 57 participants who made calls to the IVR in weeks 3–4 of period 1 or 2 were included in analysis (Figure 1). Compliance for the primary endpoint, stiffness on the IVR, was 74.3% of possible calls (78.6% in period 1, and 70% in period 2).
Pill compliance was similar between treatments (means for the ratio of the number of pills ‘taken’ to the number of pills distributed were for period 1: mexiletine 90.2%, placebo 92.7%; period 2: mexiletine 93.0%, placebo 92.7%). Mexiletine levels at baseline, the end of wash-out, and the end of both placebo arms were not detectible. The mean mexiletine level at the end of mexiletine treatment periods was 0.54 μg/mL, SD 0.35 (reference anti-arrhythmic therapeutic range for 600–1200 mg/day: 0.5–2.0 μg/mL).
Outcomes and Estimations
Mexiletine was associated with significantly improved stiffness as reported on the IVR in both treatment periods. As explained in the Methods section, we estimated the treatment effect for each period separately: period 1 mexiletine 2.53 versus placebo 4.21 (difference −1.68, 95% confidence interval [CI] −2.66, −0.706, P<0.001); period 2 mexiletine 1.60 versus placebo 5.27 (difference −3.68, 95% CI −3.85, −0.139, P=0.04) (Table 2, Figure 2A).
Figure 2.
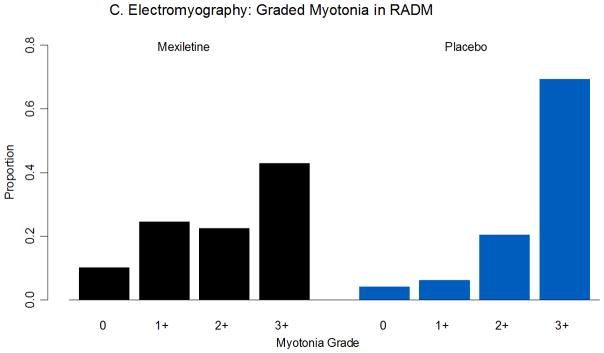
Outcome measures. A. IVR stiffness severity by week, mexiletine followed by placebo (left, n=28), and placebo followed by mexiletine (right, n=29). B. Clinical evaluation of handgrip myotonia, mexiletine followed by placebo (left, n=28), and placebo followed by mexiletine (right, n=29). C. Graded myotonia on electromyography for right abductor digiti minimi (n=56). RADM = right abductor digiti minimi.
There were significant improvements with mexiletine in almost all other outcomes in the study, including patient-reported outcomes, quality of life scales, and quantitative measures of myotonia (Table 2). Mexiletine improved the SF-36 physical composite score (mexiletine 44.8, placebo 39.2, difference 5.58, 95% CI 3.44, 7.72, P<0.001) and INQoL summary QOL score (mexiletine 14.0, placebo 16.7, difference −2.69, 95% CI −4.07, −1.30, P<0.001).
Mexiletine improved myotonia as measured on clinical exam (hand grip seconds: mexiletine 0.164, placebo 0.494, difference −0.330, 95% CI −0.608, −0.124, P<0.001, Figure 2B), and quantitative handgrip 90% to 5% relaxation times (seconds: mexiletine 0.321, placebo 0.429, difference −0.109, 95% CI −0.177, −0.0560, P<0.001). Electrophysiological measures of myotonia showed a mixed response. Mexiletine significantly improved the severity of graded myotonia on electromyography (abductor digiti minimi: difference −0.568, 95% CI −0.812, −0.325, P<0.001, Figure 2C). There was no statistically significant association with mexiletine and electrophysiological exercise testing.
Ancillary Analyses
The reduction in the severity of stiffness was more pronounced for participants with chloride mutations than sodium mutations in period 2 (chloride −4.18, 95% CI −5.25, −3.12; sodium −2.67, 95% CI −3.84, −1.51, P=0.003, eTable 1) but the reverse in period 1 (chloride −1.67, 95% CI −2.73, −0.614; sodium −2.11, 95% CI −3.28, −0.933). In addition the decrease in the clinical handgrip myotonia assessment was greater for participants with chloride mutations than sodium mutations (seconds: chloride −1.24, 95% CI −1.77, −0.711; sodium −0.355, 95% CI −1.03, 0.316, P=0.04).
Safety
There was one serious adverse event determined to be not study related (narcotic withdrawal). The most common adverse event was gastrointestinal in (9 mexiletine, 1 placebo, Table 3). There were 2 reported cardiac adverse events both found incidentally on EKG at the end of week 4: one patient had bradycardia (mexiletine) that resolved on follow up EKG; the other had premature ventricular complexes (placebo). Neither necessitated stopping the study.
Table 3.
Adverse Events.
| Category | Mexiletine | Placebo |
|---|---|---|
| Cardiac | 1 | 1 |
| Constitutional | 3 | 0 |
| Dermatology/Skin | 1 | 2 |
| Gastrointestinal | 9 | 1 |
| Infection | 1 | 3 |
| Lymphatics | 0 | 1 |
| Musculoskeletal/Soft Tissue | 0 | 2 |
| Neurologic | 5 | 1 |
| Pain | 4 | 0 |
| Total | 24 | 11 |
Survey
A survey performed after the completion of each study period asked participants to guess their treatment allocation during the preceding period. The number of participants that guessed correctly was: period 1 mexiletine 18 (64%) and placebo 20 (69%); period 2 mexiletine 23 (79%) and placebo 20 (80%).
DISCUSSION
This study provides preliminary evidence of the effectiveness of mexiletine for symptoms and signs of myotonia in NDM. There was a significant increase in IVR treatment effect for stiffness in period 2 compared with period 1. This so called “carryover” effect is contrary to usual definition of ‘the persistence of a treatment applied in one period in a subsequent period of treatment’27. There was no evidence for a lingering effect of mexiletine into period 2. Wash-out of mexiletine was effective (drug levels zero or not detectible after washout). Nor was there evidence of an unbalanced effect based on group assignment. The aggregate within participant difference between mexiletine and placebo was similar whether participants received mexiletine followed by placebo (−2.55) or placebo followed by mexiletine (−2.62). It is possible that unintentional unblinding of participants was associated with this increase. The cause-effect mechanism can be explained in one of two ways: 1) unintentional unblinding was due to a true treatment effect which suggests that additional benefit detected in the second period is attributable to mexiletine; or 2) the side effects of mexiletine (or the absence of side effects for those receiving placebo) in the second period lead to exaggerating the score to a lower (or higher) value. It is not possible to tease out from the data which explanation is correct. The effect for period 1 confirms its significance (P<0.001) and represents the lower bound of the treatment effect in this trial. The fairest interpretation we can propose is that the treatment effect lies somewhere between the estimates from period 1 (−1.68) and 2 (−3.68).
The clinical significance of the improvement in stiffness on the IVR is supported by the broad improvement in clinical, quantitative, and electrophysiological measures of myotonia. Although patient-reported outcomes might be susceptible to exaggeration by participants who had guessed their treatment assignment, quantitative measures are not: mexiletine decreased myotonia on both quantitative handgrip testing and electromyography. Overall most effect sizes were greater than 0.5, which in the literature corresponds to moderate responsiveness, and greater than 0.8, which corresponds to large responsiveness, for many outcomes (stiffness, weakness, and pain on the IVR, SF-36 physical composite score, clinical eye closure myotonia, and electrophysiological myotonia grades, Table 2)28–31. Many studies have suggested that statistically an effect size of 0.5 corresponds well to minimally clinically important differences in health-related quality of life instruments32–35.
Mexiletine was well tolerated in this study. Gastrointestinal discomfort was the most common adverse event, and there were no serious study-related adverse events.
Limitations to our study include the short duration of treatment, limited power for detecting adverse events, and the inclusion of participants with both chloride and sodium channel mutations in a single group to obtain necessary study power. Although there was an indication mexiletine resulted in greater improvement in stiffness for chloride participants versus sodium in period 2, the opposite was true in period 1. The clinical implications for this are not clear. Both groups appear to have improved with mexiletine, and the study is not powered to determine relative effectiveness by mutation.
In conclusion this study provides preliminary evidence of the effectiveness of mexiletine for patients with myotonia. The RDCRN provided common data elements and the centralized infrastructure necessary for such a broad international collaboration, and serves as a model for future rare diseases research.
Supplementary Material
Acknowledgments
Funding Support:
This study was supported by the FDA Orphan Products Division- FDA-OPD RO1 FD 003454 and the National Center for Research Resources and the National Institutes of Health through Grant Number U54 NS059065-05S1. Additional funding was provided in part by the University of Kansas Medical Center CTSA grant UL1 RR 033179, the University of Rochester CTSA grant UL1 RR 024160, University College London MRC Centre grant G0601943, and the University of Texas Southwestern CTSA grant UL1 RR 024982 NCRR/NIH.
Dr. Rayan is a MRC Clinical Training Fellow. Dr. Statland is funded by the NIH Experimental Therapeutics in Neurological Disorders grant # T32 NS07338-20.
Role of the sponsor:
The funding sources had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
APPENDIX
Consortium for Clinical Investigation of Neurologic Channelopathies
University of Kansas Medical Center: Investigators: Richard J. Barohn, MD (Principal Investigator, Steering Committee, Manuscript Preparation), Yunxia Wang, MD (Co-Principal Investigator, Steering Committee, Manuscript Preparation), Jeffrey Statland, MD (Steering Committee, Manuscript Preparation—first author), Mazen Dimachkie, MD; Project Manager/Study Coordinator/Clinical Evaluator: Laura Herbelin CCRP (Steering Committee, Manuscript Preparation); JoAnn Miller, BA, CCRP (regulatory program manager); Central Cardiologist: Rhea Pimental, MD
University of Texas Southwestern Medical Center: Investigators: Jaya Trivedi, MD (Manuscript Preparation); Study Coordinator: Nina Gorham, CCRP, Clinical Evaluator: Rhonda McLin, PTA, Vivian Gonzales.
Brigham and Women’s Hospital: Investigators: Mohammad Kian Salajegheh, MD (Steering Committee, Manuscript Preparation), Anthony A. Amato, MD; Study Coordinator: Kristen Roe BS; Clinical Evaluator: Samantha Chused, MSPT, Essa Kayd, RT.
London Health Science Center (London Ontario Canada): Investigators: Shannon Venance, MD (Steering Committee, Manuscript Preparation), Angelica Hahn, MD; Study Coordinators/Clinical Evaluators: Wilma Koopman, Jennifer Verheyden, Ashley Ten Haaf, Christine Piechowicz.
University of Rochester: Investigators: Robert C. Griggs, MD (Steering Committee, Manuscript Preparation), Emma Ciafaloni, MD (Manuscript Preparation), Paul Twydell, DO; Study Coordinators: Katherine Aronson, Kimberly Hart (Steering Committee), Patricia Smith, Barbara Herr, Kate Eichinger, Shree Pandya, Stephen Bean, Araya Puwanant, Quing Ke, Jill Scheltz, and Laura Whitesell.
Institute of Neurology (London, England): Investigators: Michael Hanna, MD (Steering Committee, Manuscript Preparation), Dipa L. Raja Rayan, MRCP (Manuscript Preparation), Emma Matthews, MRCP (Manuscript Preparation); Study Coordinators/Clinical Evaluator: Gisela Barreto, Veronica Tan, James Burge, Elizabeth Dewar, Daleen Lopez-Begum; Genetic testing: Richa Sud, Andrea Haworth, Samuel McCall.
University of Milan (Milan, Italy): Investigators: Valeria Sansone, MD (Manuscript Preparation), Giovanni Meola, MD (Manuscript Preparation), Alice Zanolini, MD, and Matteo Ciocca, MD.
University of South Florida (DMCC): Statistician: Brian Bundy, PhD (Steering Committee, Data Analysis, Manuscript Preparation); Jeffrey Krischer, PhD, Holly Ruhlig, Joseph Gomes, Rachel Richesson, Renee Leduc, Jennifer Pilger.
Footnotes
DSMB Members:
John W. Day, MD; Ph.D. (Standford School of Medicine, Stanford, CA), Robert T. Leshner, MD (UC San Diego, San Diego, CA), Todd A. Mackenzie, Ph.D. (Dartmouth Hitchcock Medical Center, Hanover, NH), and Kathryn Wagner, MD, Ph.D. (The Johns Hopkins School of Medicine, Baltimore, MD)
NINDS Staff:
Joanne C. Odenkirchen, MPH (Clinical Research Project Manager, NINDS, Bethesda, MD), and Randy Stewart, Ph.D. (Program Director, NINDS, Bethesda, MD)
Additional contributions:
We thank the study participants and their families for their commitment and generosity—they are the impetus for this international collaboration.
Author contributions:
Dr. Barohn had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Statland, Bundy, Trivedi, Wang, Barohn, Hanna, and Griggs.
Acquisition of data: Wang, Rayan, Trivedi, Sansone, Salajegheh, Venance, Ciafaloni, Matthews, Meola, Herbelin, Griggs, and Barohn.
Analysis and interpretation of data: Statland, Bundy, Trivedi, Salajegheh, Griggs, and Barohn.
Drafting of the manuscript: Statland, Bundy, Wang, Herbelin, Salajegheh, Griggs, and Barohn.
Critical revision of the manuscript for important intellectual content: Statland, Bundy, Wang, Rayan, Trivedi, Sansone, Salajegheh, Venance, Ciafaloni, Matthews, Meola, Herbelin, Griggs, Barohn, and Hanna.
Statistical analysis: Bundy.
Obtained funding: Statland, Wang, Herbelin, Griggs, and Barohn.
Administrative, technical, or material support: Bundy, Trivedi, Salajegheh, Griggs, and Herbelin.
Study supervision: Wang, Rayan, Trivedi, Sansone, Salajegheh, Venance, Ciafaloni, Matthews, Meola, Herbelin, Griggs, Barohn, and Hanna.
Financial disclosures:
The authors have completed the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Venance reports receiving reimbursement for travel from Genzyme and royalties from Wiley Publishing for her role as co-editor Neurology in Practice: Neuromuscular Disorders. Dr. Meola is supported in part by AFM. Dr. Griggs serves as Chair of Executive Committee of the Muscle Study Group, which receives support from pharmaceutical companies; has served on scientific advisory boards for The National Hospital Queen Square and PTC Therapeutics, Inc.; serves on the editorial boards of NeuroTherapeutics and Current Treatment Opinions in Neurology; receives royalties from the publication of Andreoli and Carpenter’s Cecil Essentials of Medicine, Eighth Edition (W.B. Saunders Company, 2000, 2004, 2007, and 2010) and Cecil Textbook of Medicine, 24th Edition (Saunders, 2000, 2004, 2008, and 2010 [in press]); and has received research support from TaroPharma. Dr. Barohn receives support from Grifols and Genzyme Speakers Bureau, and is a consultant for Pfizer, MedImmune, Novartis, and NuFactor.
References
- 1.Emery AE. Population frequencies of inherited neuromuscular diseases--a world survey. Neuromuscul Disord. 1991;1(1):19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- 2.Matthews E, Fialho D, Tan SV, et al. The non-dystrophic myotonias: molecular pathogenesis, diagnosis and treatment. Brain. 2010 Jan;133(Pt 1):9–22. doi: 10.1093/brain/awp294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leyburn P, Walton JN. The treatment of myotonia: a controlled clinical trial. Brain. 1959 Mar;82(1):81–91. doi: 10.1093/brain/82.1.81. [DOI] [PubMed] [Google Scholar]
- 4.Griggs RC, Davis RJ, Anderson DC, Dove JT. Cardiac conduction in myotonic dystrophy. Am J Med. 1975 Jul;59(1):37–42. doi: 10.1016/0002-9343(75)90319-8. [DOI] [PubMed] [Google Scholar]
- 5.Streib EW. Paramyotonia congenita: successful treatment with tocainide. Clinical and electrophysiologic findings in seven patients. Muscle Nerve. 1987 Feb;10(2):155–162. doi: 10.1002/mus.880100209. [DOI] [PubMed] [Google Scholar]
- 6.Jackson CE, Barohn RJ, Ptacek LJ. Paramyotonia congenita: abnormal short exercise test, and improvement after mexiletine therapy. Muscle Nerve. 1994 Jul;17(7):763–768. doi: 10.1002/mus.880170710. [DOI] [PubMed] [Google Scholar]
- 7.Kwiecinski H, Ryniewicz B, Ostrzycki A. Treatment of myotonia with antiarrhythmic drugs. Acta Neurol Scand. 1992 Oct;86(4):371–375. doi: 10.1111/j.1600-0404.1992.tb05103.x. [DOI] [PubMed] [Google Scholar]
- 8.Trip J, Drost G, van Engelen BG, Faber CG. Drug treatment for myotonia. Cochrane Database Syst Rev. 2006;(1):CD004762. doi: 10.1002/14651858.CD004762.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Luca A, Pierno S, Liantonio A, et al. New potent mexiletine and tocainide analogues evaluated in vivo and in vitro as antimyotonic agents on the myotonic ADR mouse. Neuromuscul Disord. 2004 Jul;14(7):405–416. doi: 10.1016/j.nmd.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Desaphy JF, De Luca A, Tortorella P, De Vito D, George AL, Jr, Conte Camerino D. Gating of myotonic Na channel mutants defines the response to mexiletine and a potent derivative. Neurology. 2001 Nov 27;57(10):1849–1857. doi: 10.1212/wnl.57.10.1849. [DOI] [PubMed] [Google Scholar]
- 11.Mohammadi B, Jurkat-Rott K, Alekov A, Dengler R, Bufler J, Lehmann-Horn F. Preferred mexiletine block of human sodium channels with IVS4 mutations and its pH-dependence. Pharmacogenet Genomics. 2005 Apr;15(4):235–244. doi: 10.1097/01213011-200504000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Wang GK, Russell C, Wang SY. Mexiletine block of wild-type and inactivation-deficient human skeletal muscle hNav1.4 Na+ channels. J Physiol. 2004 Feb 1;554(Pt 3):621–633. doi: 10.1113/jphysiol.2003.054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logigian EL, Martens WB, Moxley RTt, et al. Mexiletine is an effective antimyotonia treatment in myotonic dystrophy type 1. Neurology. 2010 May 4;74(18):1441–1448. doi: 10.1212/WNL.0b013e3181dc1a3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Statland JM, Wang Y, Richesson R, et al. An interactive voice response diary for patients with non-dystrophic myotonia. Muscle Nerve. 2011 Jul;44(1):30–35. doi: 10.1002/mus.22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logigian EL, Blood CL, Dilek N, et al. Quantitative analysis of the “warm-up” phenomenon in myotonic dystrophy type 1. Muscle Nerve. 2005 Jul;32(1):35–42. doi: 10.1002/mus.20339. [DOI] [PubMed] [Google Scholar]
- 16.Moxley RT, 3rd, Logigian EL, Martens WB, et al. Computerized hand grip myometry reliably measures myotonia and muscle strength in myotonic dystrophy (DM1) Muscle Nerve. 2007 Sep;36(3):320–328. doi: 10.1002/mus.20822. [DOI] [PubMed] [Google Scholar]
- 17.Fournier E, Arzel M, Sternberg D, et al. Electromyography guides toward subgroups of mutations in muscle channelopathies. Ann Neurol. 2004 Nov;56(5):650–661. doi: 10.1002/ana.20241. [DOI] [PubMed] [Google Scholar]
- 18.Tan SV, Matthews E, Barber M, et al. Refined exercise testing can aid DNA-based diagnosis in muscle channelopathies. Ann Neurol. 2011 Feb;69(2):328–340. doi: 10.1002/ana.22238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Streib EW. AAEE minimonograph #27: differential diagnosis of myotonic syndromes. Muscle Nerve. 1987 Sep;10(7):603–615. doi: 10.1002/mus.880100704. [DOI] [PubMed] [Google Scholar]
- 20.Vincent KA, Carr AJ, Walburn J, Scott DL, Rose MR. Construction and validation of a quality of life questionnaire for neuromuscular disease (INQoL) Neurology. 2007 Mar 27;68(13):1051–1057. doi: 10.1212/01.wnl.0000257819.47628.41. [DOI] [PubMed] [Google Scholar]
- 21.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993 Mar;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Ware J E. GB, and I.P. Group. The SF-36 Health Survey: Development and Use in Mental Health Research and IQOLA Project. International Journal of Mental Health. 1994;23(2):49–73. [Google Scholar]
- 23.Brown BW., Jr The crossover experiment for clinical trials. Biometrics. 1980 Mar;36(1):69–79. [PubMed] [Google Scholar]
- 24.Grizzle JE. The Two-Period Change-over Design an Its Use in Clinical Trials. Biometrics. 1965 Jun;21:467–480. [PubMed] [Google Scholar]
- 25.Willan AR, Pater JL. Carryover and the two-period crossover clinical trial. Biometrics. 1986 Sep;42(3):593–599. [PubMed] [Google Scholar]
- 26.Piantadosi S. Clinical Trials: A Methodological Perspective. 2. Hoboken, NJ: John Wiley & Sons, Inc; 2005. Crossover Designs; pp. 515–529. [Google Scholar]
- 27.Senn S. Statistical Issues in Drug Development. 2. Hoboken, NJ: John Wiley & Sons, Inc; 1997. Cross Over Trials; pp. 237–248. [Google Scholar]
- 28.de Vet HC, Terwee CB, Ostelo RW, Beckerman H, Knol DL, Bouter LM. Minimal changes in health status questionnaires: distinction between minimally detectable change and minimally important change. Health Qual Life Outcomes. 2006;4:54. doi: 10.1186/1477-7525-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Husted JA, Cook RJ, Farewell VT, Gladman DD. Methods for assessing responsiveness: a critical review and recommendations. J Clin Epidemiol. 2000 May;53(5):459–468. doi: 10.1016/s0895-4356(99)00206-1. [DOI] [PubMed] [Google Scholar]
- 30.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Med Care. 1989 Mar;27(3 Suppl):S178–189. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 31.Liang MH, Larson MG, Cullen KE, Schwartz JA. Comparative measurement efficiency and sensitivity of five health status instruments for arthritis research. Arthritis Rheum. 1985 May;28(5):542–547. doi: 10.1002/art.1780280513. [DOI] [PubMed] [Google Scholar]
- 32.Cella D, Eton DT, Fairclough DL, et al. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) Questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. J Clin Epidemiol. 2002 Mar;55(3):285–295. doi: 10.1016/s0895-4356(01)00477-2. [DOI] [PubMed] [Google Scholar]
- 33.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003 May;41(5):582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 34.Wyrwich KW, Nienaber NA, Tierney WM, Wolinsky FD. Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Med Care. 1999 May;37(5):469–478. doi: 10.1097/00005650-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol. 1999 Sep;52(9):861–873. doi: 10.1016/s0895-4356(99)00071-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.