Abstract
Skin is complex and comprised of distinct layers, each layer with unique architecture and immunologic functions. Cells within these layers produce differing amounts of antimicrobial peptides and lipids (sphingoid bases and sebaceous fatty acids) that limit colonization of commensal and opportunistic microorganisms. Furthermore, antimicrobial peptides and lipids have distinct, concentration-dependent ancillary innate and adaptive immune functions. At 0.1–2.0 µM, antimicrobial peptides induce cell migration and adaptive immune responses to coadministered antigens. At 2.0–6.0 µM, they induce cell proliferation and enhance wound healing. At 6.0–12.0 µM, they can regulate chemokine and cytokine production and at their highest concentrations of 15.0–30.0 µM, antimicrobial peptides can be cytotoxic. At 1–100 nM, lipids enhance cell migration induced by chemokines, suppress apoptosis, and optimize T cell cytotoxicity, and at 0.3–1.0 µM they inhibit cell migration and attenuate chemokine and pro-inflammatory cytokine responses. Recently, many antimicrobial peptides and lipids at 0.1–2.0 µM have been found to attenuate the production of chemokines and pro-inflammatory cytokines to microbial antigens. Together, both the antimicrobial and the anti-inflammatory activities of these peptides and lipids may serve to create a strong, overlapping immunologic barrier that not only controls the concentrations of cutaneous commensal flora but also the extent to which they induce a localized inflammatory response.
Key Words: Antimicrobial peptides, Antimicrobial lipids, Skin, Innate immunity, Bactericidal, Anti-inflammatory activity, Sphingoid bases, Fatty acids
Introduction
Skin is a tough and durable physical barrier composed of cells and intercellular matrix. It is a two-way barrier with several overlapping functions, which include protecting the body from noxious external stimuli and helping to maintain critical homeostatic conditions by containing and protecting the underlying tissues. To these ends, the skin covers and protects the underlying tissues against mechanical injury, ultraviolet irradiation, and environmental contamination; it maintains body temperature by varying the caliber of the underlying capillaries to control heat loss or by producing sweat to dissipate heat via evaporation; and it impedes the transcutaneous loss of water and electrolytes. The skin also protects the underlying tissues from infection by environmental microorganisms, commensal microorganisms that colonize the skin surface, and opportunistic pathogenic microorganisms.
The structure of the skin is complex and contains several distinct layers including (from outermost to innermost): stratum corneum, epidermis, dermis, and underlying subcutaneous fat. The stratum corneum provides the main barrier function of the skin. Structurally, the stratum corneum was first described by Michaels et al. [1] as a ‘brick and mortar’ model, comprised of fully differentiated keratinocytes (corneocytes) embedded in a continuous lipid matrix. The keratinocytes provide mechanical strength, while the lipids serve as the primary barrier of the skin, affecting water transport, movement of electrolytes, and penetration of drugs and xenobiotics. Three primary groups of lipids comprise the intercellular domain of the stratum corneum in a consistent ratio: ceramides (50%), cholesterol (25%), and free fatty acids (10–20%). Underneath the stratum corneum is the avascular viable epidermis, approximately 50–100 µm thick, followed by the dermis, which contains the pilosebaceous units, sweat glands, and microvasculature of the skin.
Based on its complex structure and our improved understanding of its functions, the skin is now recognized as much more than just a simple cover for underlying tissues. The components of the skin structure provide a unique environment to protect the body against insult, and particularly against infection from microorganisms. The average surface pH of the skin (commonly referred to as the ‘acid mantle’ of the stratum corneum) ranges from about 4.2 to 5.6, which is thought to be related to the free fatty acids in the lipid milieu, secretions from eccrine and sebaceous glands, and proton pumps [2, 3]. This acidic pH on the surface is particularly important, as it is thought to be a component of the cutaneous antimicrobial defense. The skin also has a strong immunologic role [4]. It is a very responsive tissue and keratinocytes and underlying nonkeratinocytes produce antimicrobial peptides and antimicrobial lipids that control the concentrations of cutaneous commensal flora and the extent to which they may induce a localized inflammatory response. Here, we briefly review the types of antimicrobial peptides and antimicrobial lipids, their sites of production, their antimicrobial activities for commensal and opportunistic microorganisms, and their roles in regulating locally produced chemokines and cytokines, the latter function being of importance for maintaining normal tissue homeostasis.
The Abundance of Cutaneous Microorganisms
The skin is continually challenged via exposure to high concentrations of environmental microorganisms in air, dust, soil, and water as well as by exposure to the commensal Gram-positive bacteria, Gram-negative bacteria, and fungi that colonize its surface [5]. Here, the composition and concentrations of the commensal flora vary from 102 to 106 colony-forming units/cm2 depending upon the anatomical location and the moisture content [5, 6]. Interestingly, almost all skin bacteria fall into the Actinobacteria, Firmicutes, Bacteroidetes, and Proteobacteria phyla [5]. Bacteria in these four dominant phyla are also found in the oral cavity and gastrointestinal tract. Occasionally, these microorganisms cause skin infections in immunocompromised individuals, unsuspecting travelers, or individuals with specific occupations [7, 8, 9]. However, in the majority of individuals under normal circumstances, exposure to environmental or commensal microorganisms is well tolerated without either infection or inflammation.
The Abundance of Antimicrobial Peptides and Antimicrobial Lipids
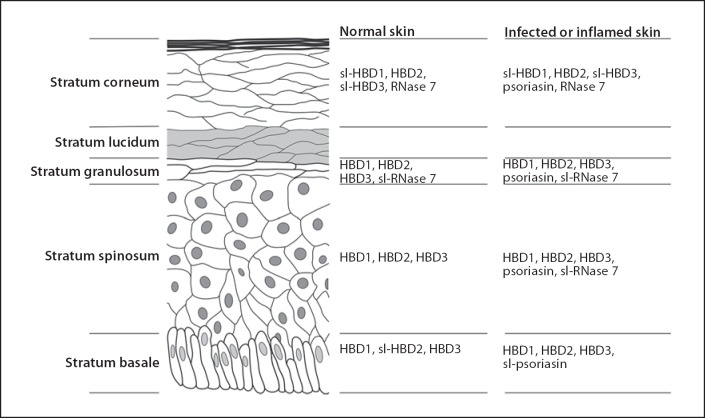
Tissue composition and architecture are important in the host's effort to counter the abundance of microorganisms on cutaneous surfaces. The different layers in the skin produce differing types and amounts of antimicrobial peptides and antimicrobial lipids, thus forming an overlapping antimicrobial and anti-inflammatory barrier. It is worth noting that some antimicrobial peptides are constitutively expressed and are detected in abundance without difference among healthy, infected, or chronically inflamed epithelium (e.g. human β-defensin (HBD) 1 and RNase 7) whereas other antimicrobial peptides are absent in healthy epithelium but detected in abundance in infected or chronically inflamed epithelium (e.g. psoriasin S100A7, HBD2, and HBD3; fig. 1). Other antimicrobial peptides are produced locally within follicles and glands. Lysozyme is found in pilosebaceous follicle cells, dermcidin is found in eccrine gland cells, and LL-37 is found in the eccrine glands and duct cells and in ductal epithelium. Neutrophil products, LL-37 and human neutrophil peptide (HNP)-1–3, are also found in some areas of the skin if neutrophils are present.
Fig. 1.
Immunohistochemical detection of antimicrobial peptides in epidermal layers of skin from normal subjects or subjects with infected or inflamed skin (compiled from other studies) [23, 24]. sl = Slight immunohistochemical detection. Note that some peptides are detected by immunohistochemistry in the stratum corneum, stratum granulosum, and stratum spinosum layers and less so in the stratum basale (e.g. HBD2, psoriasin, and RNase 7), whereas others are detected by immunohistochemistry to a lesser extent in the stratum corneum, but more so in the stratum granulosum, stratum spinosum, and in the stratum basale layers (e.g. HBD1).
Lipids are a major constituent of the skin and include phospholipids, cholesterol, glycolipids, free fatty acids, free ceramides, and acylceramides [10, 11, 12, 13]. The ceramides are the sources of epidermal long-chain bases and consist of normal fatty acids, α-hydroxyacids or ω-hydroxyacids, amide-linked to sphingosines, dihydrosphingosines, phytosphingosines, and 6-hydroxysphingosines [10, 14]. The free fatty acids are saturated, straight chains of 20–28 carbons in length. Lipids are also a major constituent of gland secretions. Squalene, wax monoesters, triglycerides, and lesser amounts of cholesterol and cholesterol esters are secreted from dermal sebaceous glands to the skin surface. Secreted triglycerides undergo hydrolysis by acid lipases secreted from the lamellar granules and to a lesser extent by commensal bacterial lipases. The product of hydrolysis is a series of fatty acids ranging from 7 to 22 carbons [15, 16]. These are mostly saturated or monounsaturated and include lauric acid (C12:0) and sapienic acid (C16:1Δ6). Of the sebaceous fatty acids, antimicrobial activities are associated with lauric acid, sapienic acid and some of the very short, odd-numbered carbon-chain species (e.g. C7:0, C9:0, C11:0, and C13:0).
Activities of Antimicrobial Peptides and Lipids
Antimicrobial Peptides
The major groups of antimicrobial peptides present in the skin include the defensins, cathelicidins, dermcidin, psoriasin (S100A15), neuropeptides and peptide hormones, antimicrobial chemokines, and antimicrobial proteins [4, 17]. The skin is overly redundant in the number and unique types of antimicrobial peptides it produces. Some peptides appear to have a primary antimicrobial function and a secondary innate and adaptive immune function, whereas other peptides appear to have a primary innate immune function, an adaptive immune function, or a neurologic function (e.g. chemokines, cytokines, neuropeptides, and peptide hormones) and a secondary antimicrobial function. Together, all these peptides, if present, have overlapping spectra of antimicrobial activity, which provides good coverage against both the quantity and diversity of microorganisms found on the skin surface. Table 1 shows the four main phyla found on human skin and the reported susceptibility of their members to antimicrobial peptides and lipids. Often, microorganisms within phyla are resistant to one antimicrobial peptide yet susceptible to another. Also, within some phyla there are often both susceptible and resistant microorganisms, yet in other phyla there are not. It is worthy to note that there is much more information on the minimal inhibitory concentrations of defensins and LL-37 than other skin-derived antimicrobial peptides. The latter data are more often reflected as killing kinetics in the literature rather than traditional minimal inhibitory concentrations. The lack of minimal inhibitory concentration data does not reflect a lack of interest in these peptides or a lack of their activity.
Table 1.
Minimal inhibitory concentrations (µM) of antimicrobial peptides and antimicrobial lipids commonly found in the skin for the four major phyla of bacteria commonly found in the skin microbiome [5]
| Agent | Actinobacteria | Firmicutes | Bacteroidetes | Proteobacteria | Fungi | |
|---|---|---|---|---|---|---|
| Antimicrobial peptides1 | ||||||
| HBD1 | 12.5–25.1 (n = 3) | 12.7 (n =1) | 12.5–25.4 (n = 6) | 1.8 (n = 1) | ||
| HBD2 | 1.9–3.2 (n = 6) | 0.9–>57.7 (n = 14)2 | 8.0–>57.7 (n = 5) | 0.9–>57.7 (n = 7) | 0.9–>57.7 (n = 16) | |
| HBD3 | 0.8–3.0 (n = 7) | 0.5–>48.5 (n = 35) | 1.1–>48.5 (n = 18) | 0.6–>48.5 (n = 25) | 0.3–>48.5 (n = 18) | |
| HNP | 0.2–11.5 (n = 16) | 2.9–145.1 (n = 5) | 0.2–>145.1 (n = 30) | >72.6 (n = 1) | ||
| LL–37 | 31.3 (n = 1) | 0.2–>27.8 (n = 41) | 3.5–>27.8 (n = 12) | 0.02–44.5 (n = 34) | >55.7 (n = 1) | |
| Dermcidin | 2.1–>42.5 (n = 5) | 6.4–>42.5 (n = 3) | ||||
| Adrenomedullin | 0.0002–2.1 (n = 3) | 2.1 (n = 4) | 0.0002–0.1 (n = 2) | 0.1–2.1 (n = 2) | ||
| Histone H4 | 1.1 (n = 1) | |||||
| Antimicrobial lipids3 | ||||||
| Sphingosine | 4.3–21.6 (n = 3) | 3.3–17.3 (n = 8) | 28.0–>1,663.9 (n = 5) | 20.0–31.3 (n = 3) | ||
| Dihydrosphingosine | 3.3–51.7 (n = 3) | 3.3–>331.7 (n = 10) | 103.8–>1,658.3 (n = 5) | >331.7 (n = 3) | ||
| Phytosphingosine | 16.4–629.9 (n = 6) | 4.1–629.9 (n = 9) | 0.6 (n = 1) | 12.3–>1,574.8 (n = 6) | 18.9–24.6 (n = 3) | |
| Sapienic acid | >1,965.4 (n = 3) | 117.9–>1,965.4 (n = 4) | 18.1 (n = 1) | >1,965.4 (n = 3) | ||
| Lauric acid | 20.0–2,080.4 (n = 7) | 5.0–2,486.3 (n = 13) | >2,496.3 (n = 3) | 499.3–2,496.3 (n = 3) | ||
It is worthy to note that there is much more information on the minimal inhibitory concentrations of defensins and the cathelicidin LL-37 than other skin-derived antimicobial peptides. The latter data is more often reflected as killing kinetics rather than traditional minimal inhibitory concentrations.
The minimal inhibitory concentrations (µM) of antimicrobial peptides were compiled from other studies [37, 45, 50, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145].
Represents the range from 0.9 to greater than 57.7 µM (n = 14).
Defensins
The human defensin family contains HBD, HNP α-defensins, and θ-defensins. Defensins differ in the number of residues, the location of the cysteine residues, and the ordering of disulfide bonds. All members generally have broad-spectrum antimicrobial activity against bacteria, fungi, and enveloped viruses. However, within each phylum there appears to be some resistant microorganisms (table 1).
HBD1, HBD2, and HBD3 contain 36–45 amino acid residues with a +5 to +11 net cationic charge and monoisotopic masses of 3,931.8–5,157.7 Da. They are very active innate and adaptive immune mediators; they are chemotactic for T cells, dendritic cells, and mast cells [18]; they stimulate mast cells to release histamine or generate PGD2 [19, 20]; and they induce keratinocytes to produce a variety of chemokines and cytokines (fig. 2) [4]. For example, HBD2, HBD3, and HBD4 (not included above) induce keratinocytes to produce cytokines IL-6, IL-10, and IL-18 and chemokines CXCL10 (IP-10), CCL2 (MCP-1), CCL20 (MIP-3α), and CCL5 (RANTES) [21, 22].
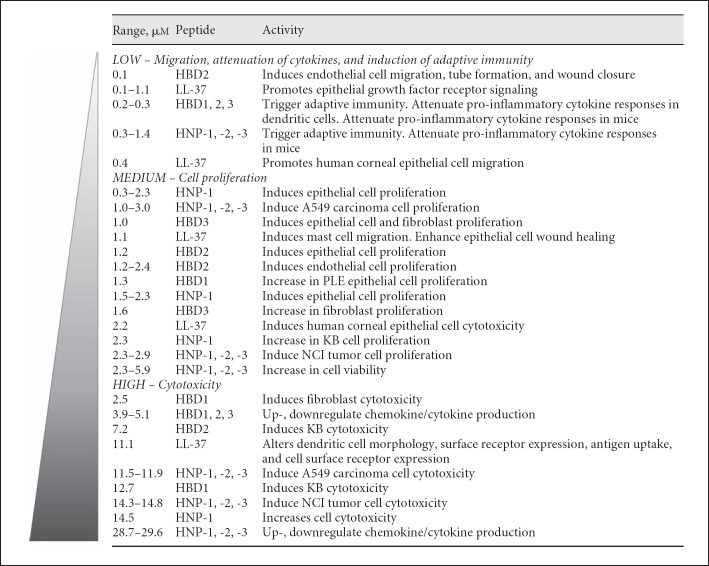
Fig. 2.
Various dose-dependent innate and adaptive immune functions of antimicrobial peptides. The specific concentrations and activities of antimicrobial peptides were compiled from other studies [33, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86].
In the epidermis, the β-defensins are differentially expressed (fig. 1). HBD1 transcription occurs in epithelium and HBD1 peptide can be detected by immunohistochemistry to a lesser extent in the stratum corneum (shown as slight or sl-HBD1 in fig. 1), but more so in the stratum granulosum, stratum spinosum, and in the stratum basale layers [23]. Generally, there is no difference in transcription or immunohistochemical staining patterns between infected wounds and healthy epithelium [23].
HBD2 transcription occurs in epithelium and HBD2 peptide can be detected by immunohistochemistry in the stratum corneum, stratum granulosum, and stratum spinosum layers and less so in the stratum basale [23]. HBD2 transcription is generally low in healthy epithelium but elevated in infected wounds [23] and in chronic wound margins [24]. HBD2 is present in the superficial epidermis of all subjects with psoriasis, but decreased in acute and chronic lesions from subjects with atopic dermatitis [25].
HBD3 transcription occurs in epithelium and, like HBD1, can be detected by immunohistochemistry to a lesser extent in the stratum corneum but more so in the stratum granulosum, stratum spinosum, and in the stratum basale layers [23]. HBD3 transcription is generally low in healthy epithelium but elevated in infected wounds [23]. Interestingly, there are no reported major differences in immunohistochemical staining patterns between healthy epithelium and infected or chronic wounds [23, 24]. HBD3 is lower in lesions from subjects with atopic dermatitis compared with other inflammatory skin diseases [26].
HNP α-defensins are a little smaller and less charged than the β-defensins; HNP-1, HNP-2, and HNP-3 contain 29–30 amino acid residues with a +2 to +3 net cationic charge and monoisotopic masses of 3,374.5–3,489.6 Da. They are present in the azurophil granules of human neutrophils (HNP α-defensins 1–4), macrophages, mucosal crypt cells, and Paneth cells (e.g. human defensins 5 and 6). HNPs are generally not detected in the epidermis, but are expressed by neutrophils in tissue infiltrates.
Cathelicidins
Cathelicidins are a diverse group of antimicrobial peptides differing greatly in sequence, structure, and size. They all have a common N-terminal preproregion of about 100 residues that is homologous to that of the cysteine protease inhibitor cathelin and a highly variable C-terminus containing the cationic antimicrobial domain. LL-37, the only known cathelicidin in humans, contains 37 amino acid residues with a +6 net cationic charge and a monoisotopic mass of 4,490.6 Da [27, 28]. It is active against Gram-positive bacteria, Gram-negative bacteria, and fungi (table 1). LL-37 can permeate membranes and induce pore formation/membrane disintegration and inhibit bacterial macromolecular synthesis, especially RNA and protein synthesis, without binding to microbial DNA or RNA [29].
LL-37 is a very active peptide [27, 30, 31, 32]. In addition to its potent antimicrobial activity, it is chemotactic for blood leukocytes and mast cells [19, 33], binds and inactivates endotoxin, activates epithelial cells, releases histamine from mast cells, induces angiogenesis, modulates gene expression, aids in the reepithelialization of skin, and regulates dendritic cell differentiation. LL-37 induces keratinocytes to produce IL-18, induces neutrophils to produce IL-8, and induces both mRNA expression and protein release of HNP-1–3 [21, 34].
In normal human skin, the production of LL-37 is negligible. LL-37 accumulates in chronic facial skin inflammatory diseases of subjects with psoriasis and rosacea but not in chronic lesions of subjects with atopic dermatitis [25, 35]. It is also lower in the lesional skin of subjects with atopic dermatitis compared to those with other inflammatory skin diseases [26]. LL-37 is present in high levels in the facial skin of subjects with rosacea, and the proteolytically processed forms of these peptides are different from those present in normal individuals. These findings suggest that it has a role in skin inflammatory responses, possibly an exacerbated innate immune response in the pathogenesis of these diseases [36].
Dermcidin
Dermcidin is a unique family of peptides with about 14 members. There are 13 different congeners in eccrine sweat, each congener derived by postsecretory proteolytic processing from the C-terminus. Another congener, derived from the N-terminus, is called YDP-42 [37, 38]. The parent peptide DCD-1L has no homology to other known antimicrobial peptides. Dermcidin contains 47 amino acid residues with a −2 net anionic charge and a monoisotopic mass of 4,702.5 Da [4, 39]. Dermcidin is active against Gram-positive and Gram-negative bacteria at about 0.2 µM, and against fungi at about 2.1 µM [4, 39]. Dermcidin-derived peptides do not induce pore formation in bacterial membranes but have time-dependent bactericidal activity, which is followed by bacterial membrane depolarization. Dermcidin induces keratinocytes to produce pro-inflammatory chemokines like CXCL8 (IL-8) and CCL20 (MIP-3α) and pro-inflammatory cytokines like TNF-α [40].
Psoriasin S100A7
Psoriasin contains 101 amino acid residues with a −1 net charge and a monoisotopic mass of 11,449.6 Da. It is active against Gram-positive and Gram-negative bacteria and is chemotactic for T cells and neutrophils. Psoriasin stimulates normal keratinocytes [41]; it activates neutrophils to produce IL-6, CXCL8 (IL-8), TNF-α, CCL3 (MIP-1α), CCL4 (MIP-1β), and CCL20 (MIP-3α) [42]; it induces phosphorylation of mitogen-activated protein kinase p38 and extracellular signal-regulated kinase (ERK) but not c-Jun N-terminal kinase (JNK) [42]; and it enhances mRNA expression and induces the extracellular release of HNP-1–3 [42]. Secreted psoriasin in vaginal fluids accounts for approximately 2.5–3.0% of the total protein where it also enhances the expression and release of HNP-1–3 [4, 43].
Psoriasin S100A7 transcription occurs in epithelium and psoriasin can be detected by immunohistochemistry in the stratum corneum, stratum granulosum, and stratum spinosum layers [23] (fig. 1). Transcription and immunohistochemical staining patterns are generally low in healthy epithelium but elevated in inflamed wounds [23] and in chronic wound margins [24].
RNase 7
RNase 7 is a member of the RNase A superfamily [44]. It is a 156-amino acid residue protein with a monoisotopic mass of 17,459.9 Da. It has potent antimicrobial activity against Gram-positive and Gram-negative bacteria and kills Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Enterococcus faecium, Propionibacterium acnes, and Candida albicans in 3 h. E. coli and S. aureus incubated with 4 µM RNase 7 for 4 h both show evidence of ultrastructural damage at the cell surface [44].
RNase 7 is expressed in epithelium and can be detected by immunohistochemistry in the stratum corneum and to a lesser extent in the stratum granulosum [24] (fig. 1). Generally, there is no difference in immunohistochemical staining patterns between chronic wound margins and healthy epithelium.
Histone H4
Histone H4 is a major component of the antimicrobial activity of sebocyte extracts [45]. It contains 103 amino acid residues with a monoisotopic mass of 11,360.4 Da. Recombinant histone H4 has antimicrobial activity against S. aureus (2.2 µM) and P. acnes (1.1 µM) [45], thus providing an additional source of antimicrobial activity in defense against infection (table 1).
Antimicrobial Neuropeptides and Peptide Hormones
Many neuropeptides and peptide hormones are very similar to antimicrobial peptides in their amino acid composition, amphipathic design, cationic charge, and size [46]. These peptides are present in the peripheral nerves of human skin and in epidermal cells, i.e. Merkel cells, mast cells, dendritic cells, Langerhans cells, and epidermal keratinocytes [47, 48, 49]. Here, they likely play important roles in innate defense and as mediators in local inflammation. For example, adrenomedullin is a 6,027.9-Da peptide with activity against Gram-positive and Gram-negative bacteria, but generally not yeast [50]. Substance P is a 1,347.7-Da peptide belonging to the tachykinin family of neuropeptides, with activity against S. aureus, and neuropeptide Y is a 4,107.0-Da peptide occurring throughout the central and peripheral nervous systems with activity against S. aureus[51].
Antimicrobial Chemokines and Cytokines
The majority of antimicrobial peptides produced in the skin induce cells to produce a wide spectrum of chemokines and cytokines. It is interesting to note, however briefly, that some IFN-γ-inducible CXC chemokines and CXCL-1 chemokine have secondary antimicrobial activity [52]. The extent to which these chemotaxic peptides synergize with other antimicrobial peptides is not yet known. However, they do contribute to the overall sum of antimicrobial activity, which gives the skin additional coverage against both the quantity and diversity of microorganisms found on the skin surface.
Antimicrobial Proteins
The skin contains large proteins with antimicrobial and anti-inflammatory activities. Human lactoferrin is expressed in a variety of glandular epithelium and skin epidermal keratinocytes [53]. It has antimicrobial activity, regulates immune functions, enhances keratinocyte proliferation, stimulates keratinocyte migration, and protects cells from apoptosis. Human lysozyme is expressed in the cytoplasm of epidermal cells in the skin, pilosebaceous follicles, and eccrine glands and has antimicrobial activity against Gram-positive and Gram-negative bacteria.
Antimicrobial Lipids
There is current interest in a variety of natural and synthetic lipids with antimicrobial activity [54, 55, 56]. Often, Gram-positive bacteria and yeasts are more susceptible than Gram-negative bacteria [57, 58]. It is not surprising, therefore, that many of the structural lipids found in the outer layers of the skin also have antimicrobial activity that appears to be both lipid-specific and microorganism specific [59, 60]. Among the most prominent are the long-chain bases and sebaceous fatty acids. Free sphingoid bases are present in the stratum corneum in a concentration of 5 mg/g of dry stratum corneum [59]. It is thought that they may occur as a gradient throughout the epidermis with higher concentrations in the stratum corneum. This concentration gradient may regulate cell division and cell differentiation in the skin and may allow communication between the stratum corneum and the viable epidermis. More importantly it provides a potent antimicrobial barrier. Also present are two specific fatty acids derived from sebaceous triglycerides: sapienic acid and lauric acid, likely released when triglycerides undergo hydrolysis [59].
Among the lipids, phospholipids and sphingolipids, other substances such as mixed galacto-cerebrosides, phosphatidic acid, dioleoylphosphatidic acid-monomethylester, phosphatidylethanolamine, β-oleoyl-γ-palmitoyl-phosphatidylethanolamine, phosphatidylcholine, D-sphingosine, D,L-dihydrosphingosine, 4-D-hydroxysphinganine, oleoyl-sphingosine, N,N-dimethylsphingosine, and stearylamine have antimicrobial activity against Gram-positive bacteria and fungi but only moderate or no activity against Gram-negative bacteria [61].
Sphingolipids
Sphingosine, dihydrosphingosine, and 6-hydroxysphingosine in the stratum corneum have antimicrobial activities [59, 61, 62, 63, 64], and the antimicrobial activities of sphingosine and phytosphingosine are generally more potent than those of dihydrosphingosine. Free sphingosine bases are antimicrobial for Gram-positive bacteria [63, 64]. Sphingosine, dihydrosphingosine (e.g. sphinganine), dimethylsphingosine, phytosphingosine, and stearyl amine are antimicrobial for C. albicans[62]. The exact mechanisms of antimicrobial activity are not known but one target may be the cell wall [62]. Dihydrosphingosine-treated S. aureus has multiple lesions in the cell wall, evaginations in the plasma membrane, and a loss of ribosomes in the cytoplasm. It is possible that the cell wall lesions may be sequelae of an altered plasma membrane. Sphingosine and dihydrosphingosine also interfere with cell wall synthesis [62].
Sphingosine 1-phosphate, a polar sphingolipid metabolite, is a potent extracellular and intracellular messenger regulating cell signaling pathways [65]. As little as 5 µM sphingosine 1-phosphate stimulates antimicrobial activity in human macrophages that leads to the intracellular killing of Mycobacterium smegmatis and Mycobacterium tuberculosis[66].
Fatty Acids
In 1942, cutaneous lipid extracts were initially reported to kill S. aureus [67]. It was speculated that the activity was due to free fatty acids, but this proposition was not directly tested until recently. Shortly after the initial demonstration of the antimicrobial activity of skin surface lipids, a remarkable series of studies was published [68]. It was observed that children who became infected with ringworm of the scalp (tinea capitis), which is caused by Microsporum audouini, had recurring disease until they reached puberty and sebum secretion became elevated. At this point, the condition spontaneously resolved and did not recur. It was speculated that some component of the sebum had antimicrobial activity against this organism. It was then shown that whole sebum was active against M. audouini in vitro, and the fatty acid fraction contained the active component. The fatty acids were then separated according to chain length by fractional distillation. Fractions included chain lengths of 7–22 carbons. Some fractions contained mixtures of saturated and monounsaturated fatty acids, which were separated by crystallizing the saturated species while leaving the monounsaturated species in solution. Each individual fatty acid was tested for activity against M. audouini. Notably, the activity was attributable to the 7-, 9-, 11-, and 13-carbon saturated species. This group had earlier demonstrated that a short fatty acid found in human sweat, i.e. undecylenic acid (C11:1Δ10), had antifungal activity. Undecylenic acid is the active component in some preparations used to treat foot fungus. We now know that fatty acids are antimicrobial against Gram-positive bacteria, Gram-negative bacteria, and C. albicans but exceptions occur among them [54, 57, 60]. Fatty acids are antimicrobial for oral microorganisms [69]; lauric acid is antimicrobial for P. acnes, S. aureus, C. albicans, and Staphylococcus epidermidis but not group A Streptococcus and group B Streptococcus [70], and sapienic acid is antimicrobial for S. aureus[69]. Other saturated and unsaturated fatty acids and their derivatives (e.g. 10–18 carbons long) also have antimicrobial activity. In other studies, 11 fatty acids and derivatives had antimicrobial activity for Actinobacteria (MIC range 12.3–487.2 µg/ml, n = 23); 14 fatty acids and derivatives had antimicrobial activity for Firmicutes (MIC range 0.1–1,222.6 µg/ml, n = 95); 7 fatty acids and derivatives had antimicrobial activity for Proteobacteria (MIC range 50.0 to >339.0 µg/ml, n = 13); and 11 fatty acids and derivatives had antimicrobial activity for fungi (MIC range 12.5–1,000.0 µg/ml, n = 14) [55, 56, 71, 72].
The exact mechanisms of antimicrobial activity are not known but there are some clues [73]. Fatty acids do not necessarily disrupt the overall integrity of the bacterial or fungal cell. Often, there are no visible effects on bacterial cell walls, seen by either scanning electron microscopy or in thin sections examined by transmission electron microscopy. Rather, the site of action appears to be the plasma membrane, which is often partially dissolved or missing. Group B Streptococcus treated with 10 mM monocaprin for 30 min are killed by disintegration of the cell membrane, leaving the bacterial cell wall intact [58]. The plasma membrane is gone and there are no visible effects of monocaprin on the bacterial cell wall directly. Similarly, C. albicans treated with capric acid has a disintegrated plasma membrane with a disorganized and shrunken cytoplasm [57]. Again, no visible changes are seen in either the shape or size of the cell wall. Whether there is a general fluidizing effect resulting in leakage of cellular contents or a more specific interaction with membrane components is not yet known.
Synergy among Antimicrobial Peptides and Antimicrobial Lipids
Antimicrobial peptides and antimicrobial lipids are produced in close proximity to each other within the outer epidermal layers and it would be logical to assume that they have synergistic antimicrobial activity. Unfortunately, there are only a few examples of synergy in the literature. Minimal inhibitory concentrations of sphingosine are significantly lower for E. coli, S. aureus, methicillin-resistant S. aureus, and C. albicans when a subinhibitory concentration of LL-37 is added to sphingosine [74, 75]. Synergy also occurs among histone H4 and free fatty acids from human sebum [45]. The antimicrobial activity of 2.2-µM histone H4 for S. aureus is halved in low 7.5-µM concentrations of lauric acid or oleic acid and S. aureus growth is completely inhibited by histone H4 in high 300-µM concentrations of lauric acid.
Anti-Inflammatory Activities of Antimicrobial Peptides and Lipids
Antimicrobial peptides and lipids have a long list of ancillary functions in innate and adaptive immunity. In figure 2, the specific concentrations and activities of antimicrobial peptides were compiled from other studies [33, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86]. At 0.1–2.0 µM, antimicrobial peptides induce cell migration and adaptive immune responses to coadministered antigens. At 2.0–6.0 µM, they induce cell proliferation and enhance wound healing. At 6.0–12.0 µM, antimicrobial peptides can up- and down-regulate chemokine and cytokine production and at their highest concentrations (e.g. 15.0–30.0 µM), antimicrobial peptides can be cytotoxic [87].
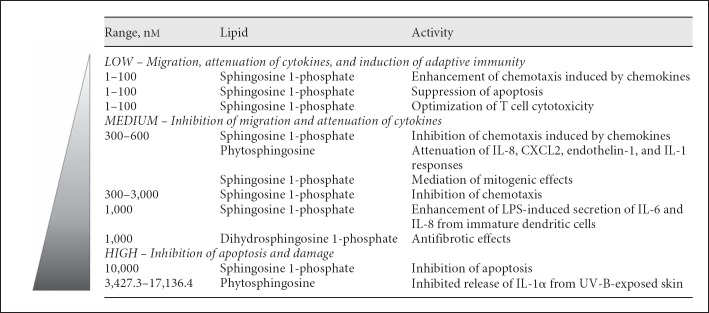
Sphingoid base signaling molecules can regulate cell cycle arrest, proliferation, differentiation, and apoptosis [88]. In figure 3, the specific concentrations and activities of antimicrobial lipids were also compiled from other studies [65, 88, 89, 90, 91, 92, 93, 94, 95, 96]. At 1–100 nM, lipids enhance cell migration induced by chemokines, suppress apoptosis, and optimize T cell cytotoxicity. At 0.3–1.0 µM, lipids inhibit cell migration and attenuate chemokine and pro-inflammatory cytokine responses. At 3.0–17.0 µM, lipids can inhibit apoptosis and slow cell damage.
Fig. 3.
Various dose-dependent innate and adaptive immune functions of antimicrobial lipids. The specific concentrations and activities of antimicrobial lipids were compiled from other studies [65, 88, 89, 90, 91, 92, 93, 94, 95, 96].
Many antimicrobial peptides at 0.1–2.0 µM and antimicrobial lipids at 0.3–0.6 µM have anti-inflammatory activities and can attenuate the production of chemokines and pro-inflammatory cytokines to microbial antigens. Antimicrobial peptides that have the ability to attenuate the production of chemokines and pro-inflammatory cytokines to microbial antigens include salivary gland-derived peptides [97], LL-37 [98, 99, 100], lactoferrin [101, 102], HNP α-defensins [103, 104, 105], and HBDs [84, 106, 107]. Human neutrophils dying by apoptosis or necrosis release HNPs that inhibit the secretion of multiple pro-inflammatory cytokines from macrophages [105]. In our work we have found that HBD3 attenuates the IL-6, IL-10, GM-CSF, and TNF-α responses of human myeloid dendritic cells to the recombinant hemagglutinin B from the periodontal pathogen Porphyromonas gingivalis[84].
Epidermal layers and sebaceous secretions are known to contain lipids with anti-inflammatory activity [108]. Sebaceous gland secretions have direct anti-inflammatory properties and can also produce lipids with anti-inflammatory properties [109]. For example, sphingoid bases can downregulate the expression of chemokines and pro-inflammatory cytokines expressed by primary epidermal keratinocytes [88].
Linked with antimicrobial activity, anti-inflammatory activity of antimicrobial peptides and lipids may be paramount to maintaining normal tissue homeostasis, first by controlling the concentrations of cutaneous commensal flora, and second by regulating the extent to which they may induce a localized inflammatory response.
Possible Implications for Transdermal Drug Delivery
As already mentioned, the skin possesses a number of unique structural and biochemical properties including (but not limited to) the brick and mortar structure, antimicrobial peptides and lipids, structural integrity provided by keratinocytes, and an acidic surface pH. These properties create effective permeability and antimicrobial barriers while also posing challenging hurdles with regard to transdermal drug delivery, in which systemic drug delivery is achieved by applying a drug patch to the skin, thus allowing the active drug moiety to passively diffuse through the skin into the circulation. Despite being introduced to the US market over 30 years ago with the approval of the scopolamine patch, the current transdermal drug market only encompasses approximately 20 drug molecules that can be passively delivered via this route. Multiple attempts have been made to overcome the skin's barrier function in order to expand the array of drug molecules that can be delivered transdermally. These efforts to disrupt the skin barrier include the use of chemical solvents/permeation enhancers (i.e. azone, alcohols, terpenes, etc.), various physical enhancement techniques (i.e. iontophoresis, ultrasound, microdermabrasion, microneedles, etc.), and combinations of chemical and physical disruption methods [110, 111, 112]. All of these methods are effective to varying degrees for overcoming the skin's barrier, and a great deal of work has been devoted towards understanding the skin's healing processes following acute disruption, specifically how restoration of the skin's barriers could be accelerated and improved by understanding the pathways involved.
Coregulation of the Antimicrobial and Permeability Barriers
The skin's antimicrobial defenses and permeability barrier function are not isolated properties; rather, it appears that these processes are regulated in parallel. Following acute disruption, reestablishment of the permeability barrier leads to restoration of the antimicrobial skin barrier and vice versa [113, 114]. For example, disruptions in the physical skin barrier in mice (tape stripping or acetone treatment) result in the induction of mBD3 (a murine homolog of HBD2) and CRAMP, a murine cathlicidin [115]; barrier function impaired by psychological stress decreases the levels of mBD3 and CRAMP [116]; and upregulation of mBD-1, mBD-3 and mBD-14 following acute disruption of the barrier has also been demonstrated [117]. These processes are observed regardless of how the permeability disruption is achieved (solvent, physical, metabolic, or psychological stress). Following acute disruption, restoring the barrier artificially via occlusion moderately inhibits the increased expression of the mBDs, suggesting the interdependence of the permeability and antimicrobial barriers [117]. Looking from the other direction, CRAMP knockout mice display subtle alterations in the permeability barrier [115], suggesting that at least this one AMP is necessary for maintaining normal permeability function. In human dermatologic diseases, the altered AMP skin profiles correlate predictably with the barrier abnormalities. Subjects with atopic dermatitis display a decreased expression of HBD2 and LL-37 [25] and these subjects have a consistently compromised permeability barrier demonstrated by increased transepidermal water loss [113]. Conversely, these AMPs are upregulated in psoriasis [25] and it is thought that this may contribute to the observed inflammation.
Restoration of the Skin Barrier(s) following Acute Disruption
Recovery of permeability barrier function following disruption includes the restoration of ion gradients (primarily calcium) and the release of lipid precursors from lamellar bodies (secretory bilayer organelles specific to the skin that release lipids, enzymes, and antimicrobial peptides). A calcium gradient exists in the unperturbed epidermis, such that high concentrations of extracellular calcium are found in the upper epidermis. Following physical barrier disruption, increased water movement within the stratum corneum dissipates the gradient [118], and this change is one of the primary signals for the induction of lamellar body secretion [119, 120]. The formation and release of lamellar body contents is also a major pathway in the process of restoring the permeability barrier [121]. Acute disruption of the barrier stimulates a characteristic sequence of recovery events that contributes to restoration of normal function. There is an initial burst (0–30 min) of release of preformed lamellar body contents (lipids, proteases, antiproteases, other lipid processing enzymes, and antimicrobial peptides) followed by the upregulation of lamellar body synthesis [119, 120, 121, 122, 123, 124, 125, 126].
Given the interdependence between the permeability and antimicrobial barriers, from a transdermal drug delivery perspective it is important to understand what effect the disruption of the permeability barrier also has on the antimicrobial barrier, and how the skin restores homeostasis. In addition to the lipids and lipid-processing enzymes, lamellar body contents also include LL-37 and HBD2, supporting the close coregulation of the permeability and antimicrobial barriers of the skin following disruption [114, 115, 121, 127]. Increased levels of mRNA for several pro-inflammatory factors and cytokines (TNF-α, IL-1α, IL-1β, and IL-1 receptor antagonist) have been demonstrated following acute barrier disruption via solvent or physical disruption (acetone treatment or tape stripping, respectively) [128, 129]. Furthermore, modulation of the calcium gradient by iontophoresis and sonophoresis at energies that do not alter the barrier also leads to increased levels of epidermal TNF-α and IL-1α cytokine expression [130].
It would be very interesting to further explore how the increased levels of these inflammatory markers correlate with the expression of skin antimicrobial peptides and lipids, specifically in the context of barrier disruption to enhance drug delivery. As already described, antimicrobial peptides and lipids can be key players in maintaining normal tissue homeostasis, and their anti-inflammatory activities can attenuate the production of chemokines and pro-inflammatory cytokines to microbial antigens. However, it is also known that HBD2 is activated by IL-1α [121, 127, 131, 132]. The balance of these processes and the specific roles that they play following physical or chemical barrier disruption remain to be elucidated. This would provide a clearer understanding of how the skin coregulates the permeability and antimicrobial barriers, and how it maintains a necessary balance of pro- and anti-inflammatory mediators under various conditions (e.g. microbial vs. physical challenges).
Modulation of Barrier Repair
A predictable delay in permeability barrier recovery is observed when the skin's normal restoration processes are blocked. For example, if the change in the calcium gradient is prevented then increased lamellar body secretion does not occur and the process of initiating barrier repair is delayed [118]. Additionally, decreases in synthesis of any of the primary skin lipids or their precursors lead to a delay in barrier recovery due to malformation of lamellar bodies [122, 133]. Topical HMG CoA reductase inhibitors have been shown to cause a delay in barrier recovery when applied to the barrier-disrupted skin of adult hairless mice (HMG CoA reductase is the integral enzyme in the cholesterol synthesis pathway) [125]. These findings were part of mechanistic studies to elucidate the role of ion gradients and skin lipids in permeability barrier recovery and have yet to be applied for therapeutic purposes. We propose that understanding these processes and the coregulation with the antimicrobial skin barrier might allow for unique opportunities to attenuate the healing process on a short-term basis. In the context of transdermal delivery, this could allow for drug delivery for a longer time frame following barrier disruption with physical or chemical enhancement methods. The ideal would be to achieve a 7-day delivery system, which would be comparable to other transdermal patches that deliver drugs via passive diffusion through the skin. While modulation of the calcium gradient or lipid synthesis pathways has not been specifically explored in a drug delivery realm, other literature has explored the effect of topical anti-inflammatory therapies following disruption of the permeability barrier. The application of diclofenac sodium (a nonspecific cyclooxygenase inhibitor) has been shown to delay micropore closure following one-time microneedle application in hairless guinea pigs and in human subjects [134]. The authors hypothesized that a local, subclinical inflammatory response contributes to rapid rates of micropore healing, and that the observed delay in micropore closure kinetics was likely due to the anti-inflammatory effect of diclofenac. However, the authors did not investigate any potential effects on antimicrobial properties of the skin or how the balance between the permeability and antimicrobial barriers was affected. It has been demonstrated previously that diclofenac has some ability in vitro to modulate immune function in the skin via the reduction of type 2 dendritic cell polarization [135], but effects on the skin with regard to cytokine/chemokine release, antimicrobial peptides, or lipids have not been explored in vivo. Further characterization of these effects in both healthy and diseased skin (which differ in their baseline characteristics) might help elucidate a more specific mechanism of action and allow for more selective and targeted therapies to help enhance transdermal delivery techniques. This rationale would apply not only to anti-inflammatory techniques, but could be explored in the context of other targets for modulating physical enhancement techniques including the calcium gradient or lamellar body formation and release.
Acknowledgements
We thank Patricia J. Conrad for preparing figures 1, 2, 3. This work was supported by funds from the National Institute of Dental and Craniofacial Research, National Institutes of Health (R01 DE014390 and R01 DE018032).
References
- 1.Michaels AS, Chandrasekaran SK, Shaw JE. Drug permeation through human skin: theory and in vitro experimental measurement. AIChE J. 1975;21:985–996. [Google Scholar]
- 2.Schmid-Wendtner MH, Korting HC. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol. 2006;19:296–302. doi: 10.1159/000094670. [DOI] [PubMed] [Google Scholar]
- 3.Rippke F, Schreiner V, Schwanitz HJ. The acidic milieu of the horny layer: new findings on the physiology and pathophysiology of skin pH. Am J Clin Dermatol. 2002;3:261–272. doi: 10.2165/00128071-200203040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Schroder JM, Harder J. Antimicrobial skin peptides and proteins. Cell Mol Life Sci. 2006;63:469–486. doi: 10.1007/s00018-005-5364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9:244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tannock G. Normal Microflora. New York, Chapman & Hall. 1995 [Google Scholar]
- 7.Bratt CL, Wertz P, Drake D, Dawson DV, Brogden KA, Antimicrobial lipids of the skin and tear film . Lipids and Essential Oils as Antimicrobial Agents. In: Thormar H, editor. Chichester, Wiley. 2011. pp. pp 99–121. [Google Scholar]
- 8.Hochedez P, Caumes E. Common skin infections in travelers. J Travel Med. 2008;15:252–262. doi: 10.1111/j.1708-8305.2008.00206.x. [DOI] [PubMed] [Google Scholar]
- 9.Harries MJ, Lear JT. Occupational skin infections. Occup Med (Lond) 2004;54:441–449. doi: 10.1093/occmed/kqh096. [DOI] [PubMed] [Google Scholar]
- 10.Wertz PW, Downing DT. Ceramidase activity in porcine epidermis. FEBS Lett. 1990;268:110–112. doi: 10.1016/0014-5793(90)80985-r. [DOI] [PubMed] [Google Scholar]
- 11.Yada Y, Higuchi K, Imokawa G. Purification and biochemical characterization of membrane-bound epidermal ceramidases from guinea pig skin. J Biol Chem. 1995;270:12677–12684. doi: 10.1074/jbc.270.21.12677. [DOI] [PubMed] [Google Scholar]
- 12.Gray GM, Yardley HJ. Different populations of pig epidermal cells: isolation and lipid composition. J Lipid Res. 1975;16:441–447. [PubMed] [Google Scholar]
- 13.Law S, Wertz PW, Swartzendruber DC, Squier CA. Regional variation in content, composition and organization of porcine epithelial barrier lipids revealed by thin-layer chromatography and transmission electron microscopy. Arch Oral Biol. 1995;40:1085–1091. doi: 10.1016/0003-9969(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 14.Ponec M, Weerheim A, Lankhorst P, Wertz P. New acylceramide in native and reconstructed epidermis. J Invest Dermatol. 2003;120:581–588. doi: 10.1046/j.1523-1747.2003.12103.x. [DOI] [PubMed] [Google Scholar]
- 15.James AT, Wheatley VR. Studies of sebum. 6. The determination of the component fatty acids of human forearm sebum by gas-liquid chromatography. Biochem J. 1956;63:269–273. doi: 10.1042/bj0630269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haahti F. Major lipid constituents of human skin surface with special reference to gas-chromatographic methods. Scand J Clin Lab Invest. 1961;59((suppl)):1–108. [PubMed] [Google Scholar]
- 17.Niyonsaba F, Ogawa H. Protective roles of the skin against infection: implication of naturally occurring human antimicrobial agents beta-defensins, cathelicidin LL-37 and lysozyme. J Dermatol Sci. 2005;40:157–168. doi: 10.1016/j.jdermsci.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Niyonsaba F, Iwabuchi K, Matsuda H, Ogawa H, Nagaoka I. Epithelial cell-derived human beta-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int Immunol. 2002;14:421–426. doi: 10.1093/intimm/14.4.421. [DOI] [PubMed] [Google Scholar]
- 19.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human beta-defensins-1/-2 and LL-37 on histamine release and prostaglandin D(2) production from mast cells. Eur J Immunol. 2001;31:1066–1075. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Niyonsaba F, Ushio H, Hara M, Yokoi H, Matsumoto K, Saito H, Nagaoka I, Ikeda S, Okumura K, Ogawa H. Antimicrobial peptides human beta-defensin (hBD)-3 and hBD-4 activate mast cells and increase skin vascular permeability. Eur J Immunol. 2007;37:434–444. doi: 10.1002/eji.200636379. [DOI] [PubMed] [Google Scholar]
- 21.Niyonsaba F, Ushio H, Nagaoka I, Okumura K, Ogawa H. The human beta-defensins (−1, −2, −3, −4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J Immunol. 2005;175:1776–1784. doi: 10.4049/jimmunol.175.3.1776. [DOI] [PubMed] [Google Scholar]
- 22.Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, Nagaoka I, Okumura K, Ogawa H. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation and production of proinflammatory cytokines and chemokines. J Invest Dermatol. 2007;127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 23.Kesting MR, Stoeckelhuber M, Holzle F, Mucke T, Neumann K, Woermann K, Jacobsen F, Steinstraesser L, Wolff KD, Loeffelbein DJ, Rohleder NH. Expression of antimicrobial peptides in cutaneous infections after skin surgery. Br J Dermatol. 2010;163:121–127. doi: 10.1111/j.1365-2133.2010.09781.x. [DOI] [PubMed] [Google Scholar]
- 24.Dressel S, Harder J, Cordes J, Wittersheim M, Meyer-Hoffert U, Sunderkotter C, Glaser R. Differential expression of antimicrobial peptides in margins of chronic wounds. Exp Dermatol. 2010;19:628–632. doi: 10.1111/j.1600-0625.2009.01030.x. [DOI] [PubMed] [Google Scholar]
- 25.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 26.Hata TR, Gallo RL. Antimicrobial peptides, skin infections, and atopic dermatitis. Semin Cutan Med Surg. 2008;27:144–150. doi: 10.1016/j.sder.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bucki R, Leszczynska K, Namiot A, Sokolowski W. Cathelicidin LL-37: a multitask antimicrobial peptide. Arch Immunol Ther Exp. 2010;58:15–25. doi: 10.1007/s00005-009-0057-2. [DOI] [PubMed] [Google Scholar]
- 28.Agerberth B, Gunne H, Odeberg J, Kogner P, Boman HG, Gudmundsson GH. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci USA. 1995;92:195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senyurek I, Paulmann M, Sinnberg T, Kalbacher H, Deeg M, Gutsmann T, Hermes M, Kohler T, Gotz F, Wolz C, Peschel A, Schittek B. Dermcidin-derived peptides show a different mode of action than the cathelicidin LL-37 against Staphylococcus aureus. Antimicrob Agents Chemother. 2009;53:2499–2509. doi: 10.1128/AAC.01679-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomasinsig L, Zanetti M. The cathelicidins – structure, function and evolution. Curr Protein Pept Sci. 2005;6:23–34. doi: 10.2174/1389203053027520. [DOI] [PubMed] [Google Scholar]
- 31.Yang D, Oppenheim JJ, Multiple functions of antimicrobial peptides in host immunity . Mammalian Host Defense Peptides. In: Devine DA, Hancock REW, editors. Cambridge, Cambridge University Press. 2004. pp. pp 39–68. [Google Scholar]
- 32.Burton MF, Steel PG. The chemistry and biology of LL-37. Nat Prod Rep. 2009;26:1572–1584. doi: 10.1039/b912533g. [DOI] [PubMed] [Google Scholar]
- 33.Niyonsaba F, Iwabuchi K, Someya A, Hirata M, Matsuda H, Ogawa H, Nagaoka I. A cathelicidin family of human antibacterial peptide LL-37 induces mast cell chemotaxis. Immunology. 2002;106:20–26. doi: 10.1046/j.1365-2567.2002.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Y, Niyonsaba F, Ushio H, Nagaoka I, Ikeda S, Okumura K, Ogawa H. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br J Dermatol. 2007;157:1124–1131. doi: 10.1111/j.1365-2133.2007.08196.x. [DOI] [PubMed] [Google Scholar]
- 35.Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, Dorschner RA, Bonnart C, Descargues P, Hovnanian A, Morhenn VB, Gallo RL. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 36.Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci. 2009;55:77–81. doi: 10.1016/j.jdermsci.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steffen H, Rieg S, Wiedemann I, Kalbacher H, Deeg M, Sahl HG, Peschel A, Gotz F, Garbe C, Schittek B. Naturally processed dermcidin-derived peptides do not permeabilize bacterial membranes and kill microorganisms irrespective of their charge. Antimicrob Agents Chemother. 2006;50:2608–2620. doi: 10.1128/AAC.00181-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rieg S, Seeber S, Steffen H, Humeny A, Kalbacher H, Stevanovic S, Kimura A, Garbe C, Schittek B. Generation of multiple stable dermcidin-derived antimicrobial peptides in sweat of different body sites. J Invest Dermatol. 2006;126:354–365. doi: 10.1038/sj.jid.5700041. [DOI] [PubMed] [Google Scholar]
- 39.Schittek B, Hipfel R, Sauer B, Bauer J, Kalbacher H, Stevanovic S, Schirle M, Schroeder K, Blin N, Meier F, Rassner G, Garbe C. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat Immunol. 2001;2:1133–1137. doi: 10.1038/ni732. [DOI] [PubMed] [Google Scholar]
- 40.Niyonsaba F, Suzuki A, Ushio H, Nagaoka I, Ogawa H, Okumura K. The human antimicrobial peptide dermcidin activates normal human keratinocytes. Br J Dermatol. 2009;160:243–249. doi: 10.1111/j.1365-2133.2008.08925.x. [DOI] [PubMed] [Google Scholar]
- 41.Niyonsaba F, Hattori F, Maeyama K, Ogawa H, Okamoto K. Induction of a microbicidal protein psoriasin (S100A7), and its stimulatory effects on normal human keratinocytes. J Dermatol Sci. 2008;52:216–219. doi: 10.1016/j.jdermsci.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Zheng Y, Niyonsaba F, Ushio H, Ikeda S, Nagaoka I, Okumura K, Ogawa H. Microbicidal protein psoriasin is a multifunctional modulator of neutrophil activation. Immunology. 2008;124:357–367. doi: 10.1111/j.1365-2567.2007.02782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mildner M, Stichenwirth M, Abtin A, Eckhart L, Sam C, Glaser R, Schroder JM, Gmeiner R, Mlitz V, Pammer J, Geusau A, Tschachler E. Psoriasin (S100A7) is a major Escherichia coli-cidal factor of the female genital tract. Mucosal Immunol. 2010;3:602–609. doi: 10.1038/mi.2010.37. [DOI] [PubMed] [Google Scholar]
- 44.Torrent M, Badia M, Moussaoui M, Sanchez D, Nogues MV, Boix E. Comparison of human RNase 3 and RNase 7 bactericidal action at the Gram-negative and Gram-positive bacterial cell wall. FEBS J. 2010;277:1713–1725. doi: 10.1111/j.1742-4658.2010.07595.x. [DOI] [PubMed] [Google Scholar]
- 45.Lee DY, Huang CM, Nakatsuji T, Thiboutot D, Kang SA, Monestier M, Gallo RL. Histone H4 Is a major component of the antimicrobial action of human sebocytes. J Invest Dermatol. 2009;129:2489–2496. doi: 10.1038/jid.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brogden KA, Guthmiller JM, Salzet M, Zasloff M. The nervous system and innate immunity: the neuropeptide connection. Nat Immunol. 2005;6:558–564. doi: 10.1038/ni1209. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi K, Nakanishi S, Imamura S. Direct effects of cutaneous neuropeptides on adenylyl cyclase activity and proliferation in a keratinocyte cell line: stimulation of cyclic AMP formation by CGRP and VIP/PHM, and inhibition by NPY through G protein-coupled receptors. J Invest Dermatol. 1993;101:646–651. doi: 10.1111/1523-1747.ep12371670. [DOI] [PubMed] [Google Scholar]
- 48.Kapas S, Tenchini ML, Farthing PM. Regulation of adrenomedullin secretion in cultured human skin and oral keratinocytes. J Invest Dermatol. 2001;117:353–359. doi: 10.1046/j.0022-202x.2001.01426.x. [DOI] [PubMed] [Google Scholar]
- 49.Lambert RW, Campton K, Ding W, Ozawa H, Granstein RD. Langerhans cell expression of neuropeptide Y and peptide YY. Neuropeptides. 2002;36:246–251. doi: 10.1016/s0143-4179(02)00020-3. [DOI] [PubMed] [Google Scholar]
- 50.Allaker RP, Zihni C, Kapas S. An investigation into the antimicrobial effects of adrenomedullin on members of the skin, oral, respiratory tract and gut microflora. FEMS Immunol Med Microbiol. 1999;23:289–293. doi: 10.1111/j.1574-695X.1999.tb01250.x. [DOI] [PubMed] [Google Scholar]
- 51.Hansen CJ, Burnell KK, Brogden KA. Antimicrobial activity of Substance P and Neuropeptide Y against laboratory strains of bacteria and oral microorganisms. J Neuroimmunol. 2006;177:215–218. doi: 10.1016/j.jneuroim.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 52.Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, Strieter RM. Cutting edge: IFN-inducible ELR-CXC chemokines display defensin-like antimicrobial activity. J Immunol. 2001;167:623–627. doi: 10.4049/jimmunol.167.2.623. [DOI] [PubMed] [Google Scholar]
- 53.Tang L, Wu JJ, Ma Q, Cui T, Andreopoulos FM, Gil J, Valdes J, Davis SC, Li J. Human lactoferrin stimulates skin keratinocyte function and wound re-epithelialization. Br J Dermatol. 2010;163:38–47. doi: 10.1111/j.1365-2133.2010.09748.x. [DOI] [PubMed] [Google Scholar]
- 54.Kabara JJ, Vrable R. Antimicrobial lipids: natural and synthetic fatty acids and monoglycerides. Lipids. 1977;12:753–759. doi: 10.1007/BF02570908. [DOI] [PubMed] [Google Scholar]
- 55.Kabara JJ, Swieczkowski DM, Conley AJ, Truant JP. Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother. 1972;2:23–28. doi: 10.1128/aac.2.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raychowdhury MK, Goswami R, Chakrabarti P. Effect of unsaturated fatty acids in growth inhibition of some penicillin-resistant and sensitive bacteria. J Appl Bacteriol. 1985;59:183–188. doi: 10.1111/j.1365-2672.1985.tb03319.x. [DOI] [PubMed] [Google Scholar]
- 57.Bergsson G, Arnfinnsson J, Steingrimsson O, Thormar H. In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrob Agents Chemother. 2001;45:3209–3212. doi: 10.1128/AAC.45.11.3209-3212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bergsson G, Arnfinnsson J, Steingrimsson O, Thormar H. Killing of Gram-positive cocci by fatty acids and monoglycerides. APMIS. 2001;109:670–678. doi: 10.1034/j.1600-0463.2001.d01-131.x. [DOI] [PubMed] [Google Scholar]
- 59.Drake DR, Brogden KA, Dawson DV, Wertz PW. Thematic review series: skin lipids. Antimicrobial lipids at the skin surface. J Lipid Res. 2008;49:4–11. doi: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- 60.Thormar H, Hilmarsson H. The role of microbicidal lipids in host defense against pathogens and their potential as therapeutic agents. Chem Phys Lipids. 2007;150:1–11. doi: 10.1016/j.chemphyslip.2007.06.220. [DOI] [PubMed] [Google Scholar]
- 61.Bibel DJ, Aly R, Shinefield HR. Antimicrobial activity of sphingosines. J Invest Dermatol. 1992;98:269–273. doi: 10.1111/1523-1747.ep12497842. [DOI] [PubMed] [Google Scholar]
- 62.Bibel DJ, Aly R, Shah S, Shinefield HR. Sphingosines: antimicrobial barriers of the skin. Acta Derm Venereol. 1993;73:407–411. doi: 10.2340/0001555573407411. [DOI] [PubMed] [Google Scholar]
- 63.Bibel DJ, Aly R, Shinefield HR. Topical sphingolipids in antisepsis and antifungal therapy. Clin Exp Dermatol. 1995;20:395–400. doi: 10.1111/j.1365-2230.1995.tb01356.x. [DOI] [PubMed] [Google Scholar]
- 64.Payne CD, Ray TL, Downing DT. Cholesterol sulfate protects Candida albicans from inhibition by sphingosine in vitro. J Invest Dermatol. 1996;106:549–552. doi: 10.1111/1523-1747.ep12344007. [DOI] [PubMed] [Google Scholar]
- 65.Martino A. Sphingosine 1-phosphate as a novel immune regulator of dendritic cells. J Biosci. 2007;32:1207–1212. doi: 10.1007/s12038-007-0122-0. [DOI] [PubMed] [Google Scholar]
- 66.Garg SK, Volpe E, Palmieri G, Mattei M, Galati D, Martino A, Piccioni MS, Valente E, Bonanno E, De Vito P, Baldini PM, Spagnoli LG, Colizzi V, Fraziano M. Sphingosine 1-phosphate induces antimicrobial activity both in vitro and in vivo. J Infect Dis. 2004;189:2129–2138. doi: 10.1086/386286. [DOI] [PubMed] [Google Scholar]
- 67.Burtenshaw JM. The mechanisms of self-disinfection of the human skin and its appendages. J Hyg (Lond) 1942;42:184–209. doi: 10.1017/s0022172400035373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rothman S, Lorincz AL. Defense mechanisms of the skin. Annu Rev Med. 1963;14:215–242. doi: 10.1146/annurev.me.14.020163.001243. [DOI] [PubMed] [Google Scholar]
- 69.Fischer CL, Drake DR, Dawson DV, Blanchette DR, Brogden KA, Wertz PW. Antibacterial activity of sphingoid bases and fatty acids against Gram-positive and Gram-negative bacteria. Antimicrob Agents Chemother. 2012;56:1157–1161. doi: 10.1128/AAC.05151-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakatsuji T, Kao MC, Fang JY, Zouboulis CC, Zhang L, Gallo RL, Huang CM. Antimicrobial property of lauric acid against Propionibacterium acnes: its therapeutic potential for inflammatory acne vulgaris. J Invest Dermatol. 2009;129:2480–2488. doi: 10.1038/jid.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Galbraith H, Miller TB, Paton AM, Thompson JK. Antibacterial activity of long chain fatty acids and the reversal with calcium, magnesium, ergocalciferol and cholesterol. J Appl Bacteriol. 1971;34:803–813. doi: 10.1111/j.1365-2672.1971.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 72.Kabara JJ. Fatty acids and derivatives as antimicrobial agents: a review; in Kabara JJ (ed): The Pharmacological Effect of Lipids. Champaign, The American Oil Chemists' Society. 1978:pp 1–14. [Google Scholar]
- 73.Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 2010;85:1629–1642. doi: 10.1007/s00253-009-2355-3. [DOI] [PubMed] [Google Scholar]
- 74.Robertson E, Brogden K, Wertz P, Dawson D, Drake D. Antimicrobial activity of human skin lipids. 83rd General Session IADR meeting, Baltimore. 2005 [Google Scholar]
- 75.Robertson E, Burnell K, Qian F, Brogden KA, Wertz P, Drake D. Synergistic activity of human skin lipids and LL-37. 84th General Session IADR meeting, Orlando. 2006 [Google Scholar]
- 76.Aarbiou J, Ertmann M, van Wetering S, van Noort P, Rook D, Rabe KF, Litvinov SV, van Krieken JH, de Boer WI, Hiemstra PS. Human neutrophil defensins induce lung epithelial cell proliferation in vitro. J Leukoc Biol. 2002;72:167–174. [PubMed] [Google Scholar]
- 77.Baroni A, Donnarumma G, Paoletti I, Longanesi-Cattani I, Bifulco K, Tufano MA, Carriero MV. Antimicrobial human beta-defensin-2 stimulates migration, proliferation and tube formation of human umbilical vein endothelial cells. Peptides. 2009;30:267–272. doi: 10.1016/j.peptides.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 78.Boniotto M, Jordan WJ, Eskdale J, Tossi A, Antcheva N, Crovella S, Connell ND, Gallagher G. Human beta-defensin 2 induces a vigorous cytokine response in peripheral blood mononuclear cells. Antimicrob Agents Chemother. 2006;50:1433–1441. doi: 10.1128/AAC.50.4.1433-1441.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brogden KA, Heidari M, Sacco RE, Palmquist D, Guthmiller JM, Johnson GK, Jia HP, Tack BF, McCray PB. Defensin-induced adaptive immunity in mice and its potential in preventing periodontal disease. Oral Microbiol Immunol. 2003;18:95–99. doi: 10.1034/j.1399-302x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 80.Davidson DJ, Currie AJ, Reid GS, Bowdish DM, MacDonald KL, Ma RC, Hancock RE, Speert DP. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J Immunol. 2004;172:1146–1156. doi: 10.4049/jimmunol.172.2.1146. [DOI] [PubMed] [Google Scholar]
- 81.Kohlgraf KG, Ackermann A, Lu X, Burnell K, Belanger M, Cavanaugh JE, Xie H, Progulske-Fox A, Brogden KA. Defensins attenuate cytokine responses yet enhance antibody responses to Porphyromonas gingivalis adhesins in mice. Future Microbiol. 2010;5:115–125. doi: 10.2217/fmb.09.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lillard JW, Jr, Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc Natl Acad Sci USA. 1999;96:651–656. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nishimura M, Abiko Y, Kurashige Y, Takeshima M, Yamazaki M, Kusano K, Saitoh M, Nakashima K, Inoue T, Kaku T. Effect of defensin peptides on eukaryotic cells: primary epithelial cells, fibroblasts and squamous cell carcinoma cell lines. J Dermatol Sci. 2004;36:87–95. doi: 10.1016/j.jdermsci.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 84.Pingel LC, Kohlgraf KG, Hansen CJ, Eastman CG, Dietrich DE, Burnell KK, Srikantha RN, Xiao X, Belanger M, Progulske-Fox A, Cavanaugh JE, Guthmiller JM, Johnson GK, Joly S, Kurago ZB, Dawson DV, Brogden KA. Human beta-defensin 3 binds to hemagglutinin B (rHagB), a non-fimbrial adhesin from Porphyromonas gingivalis, and attenuates a pro-inflammatory cytokine response. Immunol Cell Biol. 2008;86:643–649. doi: 10.1038/icb.2008.56. [DOI] [PubMed] [Google Scholar]
- 85.van Wetering S, Mannesse-Lazeroms SP, van Sterkenburg MA, Hiemstra PS. Neutrophil defensins stimulate the release of cytokines by airway epithelial cells: modulation by dexamethasone. Inflamm Res. 2002;51:8–15. doi: 10.1007/pl00000282. [DOI] [PubMed] [Google Scholar]
- 86.Yin J, Yu FS. LL-37 via EGFR transactivation to promote high glucose-attenuated epithelial wound healing in organ-cultured corneas. Invest Ophthalmol Vis Sci. 2010;51:1891–1897. doi: 10.1167/iovs.09-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bowdish DM, Davidson DJ, Hancock RE. A re-evaluation of the role of host defence peptides in mammalian immunity. Curr Protein Pept Sci. 2005;6:35–51. doi: 10.2174/1389203053027494. [DOI] [PubMed] [Google Scholar]
- 88.Klee SK, Farwick M, Lersch P. The effect of sphingolipids as a new therapeutic option for acne treatment. Basic Clin Dermatol. 2007;40:155–156. [Google Scholar]
- 89.Bu S, Asano Y, Bujor A, Highland K, Hant F, Trojanowska M. Dihydrosphingosine 1-phosphate has a potent antifibrotic effect in scleroderma fibroblasts via normalization of phosphatase and tensin homolog levels. Arthritis Rheum. 2010;62:2117–2126. doi: 10.1002/art.27463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 91.Goetzl EJ, Graler MH. Sphingosine 1-phosphate and its type 1 G protein-coupled receptor: trophic support and functional regulation of T lymphocytes. J Leukoc Biol. 2004;76:30–35. doi: 10.1189/jlb.1103567. [DOI] [PubMed] [Google Scholar]
- 92.Graeler M, Goetzl EJ. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. FASEB J. 2002;16:1874–1878. doi: 10.1096/fj.02-0548com. [DOI] [PubMed] [Google Scholar]
- 93.Oz-Arslan D, Ruscher W, Myrtek D, Ziemer M, Jin Y, Damaj BB, Sorichter S, Idzko M, Norgauer J, Maghazachi AA. IL-6 and IL-8 release is mediated via multiple signaling pathways after stimulating dendritic cells with lysophospholipids. J Leukoc Biol. 2006;80:287–297. doi: 10.1189/jlb.1205751. [DOI] [PubMed] [Google Scholar]
- 94.Pavicic T, Wollenweber U, Farwick M, Korting HC. Anti-microbial and -inflammatory activity and efficacy of phytosphingosine: an in vitro and in vivo study addressing acne vulgaris. Int J Cosmet Sci. 2007;29:181–190. doi: 10.1111/j.1467-2494.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- 95.Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Spiegel S, Cuvillier O, Edsall LC, Kohama T, Menzeleev R, Olah Z, Olivera A, Pirianov G, Thomas DM, Tu Z, Van Brocklyn JR, Wang F. Sphingosine-1-phosphate in cell growth and cell death. Ann NY Acad Sci. 1998;845:11–18. doi: 10.1111/j.1749-6632.1998.tb09658.x. [DOI] [PubMed] [Google Scholar]
- 97.Mathison RD, Davison JS, Befus AD, Gingerich DA. Salivary gland derived peptides as a new class of anti-inflammatory agents: review of preclinical pharmacology of C-terminal peptides of SMR1 protein. J Inflamm (Lond) 2010;7:49. doi: 10.1186/1476-9255-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scott MG, Davidson DJ, Gold MR, Bowdish D, Hancock RE. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J Immunol. 2002;169:3883–3891. doi: 10.4049/jimmunol.169.7.3883. [DOI] [PubMed] [Google Scholar]
- 99.Mookherjee N, Brown KL, Bowdish DM, Doria S, Falsafi R, Hokamp K, Roche FM, Mu R, Doho GH, Pistolic J, Powers JP, Bryan J, Brinkman FS, Hancock RE. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J Immunol. 2006;176:2455–2464. doi: 10.4049/jimmunol.176.4.2455. [DOI] [PubMed] [Google Scholar]
- 100.Molhoek EM, den Hertog AL, de Vries AM, Nazmi K, Veerman EC, Hartgers FC, Yazdanbakhsh M, Bikker FJ, van der Kleij D. Structure-function relationship of the human antimicrobial peptide LL-37 and LL-37 fragments in the modulation of TLR responses. Biol Chem. 2009;390:295–303. doi: 10.1515/BC.2009.037. [DOI] [PubMed] [Google Scholar]
- 101.Baveye S, Elass E, Mazurier J, Spik G, Legrand D. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med. 1999;37:281–286. doi: 10.1515/CCLM.1999.049. [DOI] [PubMed] [Google Scholar]
- 102.Caccavo D, Pellegrino NM, Altamura M, Rigon A, Amati L, Amoroso A, Jirillo E. Antimicrobial and immunoregulatory functions of lactoferrin and its potential therapeutic application. J Endotoxin Res. 2002;8:403–417. doi: 10.1179/096805102125001000. [DOI] [PubMed] [Google Scholar]
- 103.Shi J, Aono S, Lu W, Ouellette AJ, Hu X, Ji Y, Wang L, Lenz S, van Ginkel FW, Liles M, Dykstra C, Morrison EE, Elson CO. A novel role for defensins in intestinal homeostasis: regulation of IL-1beta secretion. J Immunol. 2007;179:1245–1253. doi: 10.4049/jimmunol.179.2.1245. [DOI] [PubMed] [Google Scholar]
- 104.Kohlgraf KG, Pingel LC, Dietrich DE, Brogden KA. Defensins as anti-inflammatory compounds and mucosal adjuvants. Future Microbiol. 2010;5:99–113. doi: 10.2217/fmb.09.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miles K, Clarke DJ, Lu W, Sibinska Z, Beaumont PE, Davidson DJ, Barr TA, Campopiano DJ, Gray M. Dying and necrotic neutrophils are anti-inflammatory secondary to the release of alpha-defensins. J Immunol. 2009;183:2122–2132. doi: 10.4049/jimmunol.0804187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Semple F, Macpherson H, Webb S, Cox SL, Mallin LJ, Tyrrell C, Grimes GR, Semple CA, Nix MA, Millhauser GL, Dorin JR. Human beta-defensin 3 affects the activity of pro-inflammatory pathways associated with MyD88 and TRIF. Eur J Immunol. 2011;41:3291–3300. doi: 10.1002/eji.201141648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Semple F, Webb S, Li HN, Patel HB, Perretti M, Jackson IJ, Gray M, Davidson DJ, Dorin JR. Human beta-defensin 3 has immunosuppressive activity in vitro and in vivo. Eur J Immunol. 2010;40:1073–1078. doi: 10.1002/eji.200940041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol. 2004;22:360–366. doi: 10.1016/j.clindermatol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 109.Zouboulis CC, Baron JM, Bohm M, Kippenberger S, Kurzen H, Reichrath J, Thielitz A. Frontiers in sebaceous gland biology and pathology. Exp Dermatol. 2008;17:542–551. doi: 10.1111/j.1600-0625.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 110.Sinha VR, Kaur MP. Permeation enhancers for transdermal drug delivery. Drug Dev Ind Pharm. 2000;26:1131–1140. doi: 10.1081/ddc-100100984. [DOI] [PubMed] [Google Scholar]
- 111.Choi EH, Lee SH, Ahn SK, Hwang SM. The pretreatment effect of chemical skin penetration enhancers in transdermal drug delivery using iontophoresis. Skin Pharmacol Appl Skin Physiol. 1999;12:326–335. doi: 10.1159/000029894. [DOI] [PubMed] [Google Scholar]
- 112.Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park KY, Kim DH, Jeong MS, Li K, Seo SJ. Changes of antimicrobial peptides and transepidermal water loss after topical application of tacrolimus and ceramide-dominant emollient in patients with atopic dermatitis. J Korean Med Sci. 2010;25:766–771. doi: 10.3346/jkms.2010.25.5.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Elias PM. The skin barrier as an innate immune element. Semin Immunopathol. 2007;29:3–14. doi: 10.1007/s00281-007-0060-9. [DOI] [PubMed] [Google Scholar]
- 115.Aberg KM, Man MQ, Gallo RL, Ganz T, Crumrine D, Brown BE, Choi EH, Kim DK, Schroder JM, Feingold KR, Elias PM. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008;128:917–925. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aberg KM, Radek KA, Choi EH, Kim DK, Demerjian M, Hupe M, Kerbleski J, Gallo RL, Ganz T, Mauro T, Feingold KR, Elias PM. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007;117:3339–3349. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ahrens K, Schunck M, Podda GF, Meingassner J, Stuetz A, Schroder JM, Harder J, Proksch E. Mechanical and metabolic injury to the skin barrier leads to increased expression of murine beta-defensin-1, −3, and −14. J Invest Dermatol. 2011;131:443–452. doi: 10.1038/jid.2010.289. [DOI] [PubMed] [Google Scholar]
- 118.Feingold KR. Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J Lipid Res. 2007;48:2531–2546. doi: 10.1194/jlr.R700013-JLR200. [DOI] [PubMed] [Google Scholar]
- 119.Lee SH, Elias PM, Proksch E, Menon GK, Mao-Quiang M, Feingold KR. Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J Clin Invest. 1992;89:530–538. doi: 10.1172/JCI115617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mao-Qiang M, Mauro T, Bench G, Warren R, Elias PM, Feingold KR. Calcium and potassium inhibit barrier recovery after disruption, independent of the type of insult in hairless mice. Exp Dermatol. 1997;6:36–40. doi: 10.1111/j.1600-0625.1997.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 121.Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol. 2005;125:183–200. doi: 10.1111/j.0022-202X.2005.23668.x. [DOI] [PubMed] [Google Scholar]
- 122.Menon GK, Feingold KR, Elias PM. Lamellar body secretory response to barrier disruption. J Invest Dermatol. 1992;98:279–289. doi: 10.1111/1523-1747.ep12497866. [DOI] [PubMed] [Google Scholar]
- 123.Harris IR, Farrell AM, Grunfeld C, Holleran WM, Elias PM, Feingold KR. Permeability barrier disruption coordinately regulates mRNA levels for key enzymes of cholesterol, fatty acid, and ceramide synthesis in the epidermis. J Invest Dermatol. 1997;109:783–787. doi: 10.1111/1523-1747.ep12340962. [DOI] [PubMed] [Google Scholar]
- 124.Menon GK, Feingold KR, Mao-Qiang M, Schaude M, Elias PM. Structural basis for the barrier abnormality following inhibition of HMG CoA reductase in murine epidermis. J Invest Dermatol. 1992;98:209–219. doi: 10.1111/1523-1747.ep12555880. [DOI] [PubMed] [Google Scholar]
- 125.Feingold KR, Man MQ, Proksch E, Menon GK, Brown BE, Elias PM. The lovastatin-treated rodent: a new model of barrier disruption and epidermal hyperplasia. J Invest Dermatol. 1991;96:201–209. doi: 10.1111/1523-1747.ep12461153. [DOI] [PubMed] [Google Scholar]
- 126.Elias PM, Tsai J, Menon GK, Holleran WM, Feingold KR. The potential of metabolic interventions to enhance transdermal drug delivery. J Investig Dermatol Symp Proc. 2002;7:79–85. doi: 10.1046/j.1523-1747.2002.19632.x. [DOI] [PubMed] [Google Scholar]
- 127.Oren A, Ganz T, Liu L, Meerloo T. In human epidermis, beta-defensin 2 is packaged in lamellar bodies. Exp Mol Pathol. 2003;74:180–182. doi: 10.1016/s0014-4800(02)00023-0. [DOI] [PubMed] [Google Scholar]
- 128.Wood LC, Elias PM, Calhoun C, Tsai JC, Grunfeld C, Feingold KR. Barrier disruption stimulates interleukin-1 alpha expression and release from a pre-formed pool in murine epidermis. J Invest Dermatol. 1996;106:397–403. doi: 10.1111/1523-1747.ep12343392. [DOI] [PubMed] [Google Scholar]
- 129.Wood LC, Jackson SM, Elias PM, Grunfeld C, Feingold KR. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90:482–487. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Choi EH, Kim MJ, Yeh BI, Ahn SK, Lee SH. Iontophoresis and sonophoresis stimulate epidermal cytokine expression at energies that do not provoke a barrier abnormality: lamellar body secretion and cytokine expression are linked to altered epidermal calcium levels. J Invest Dermatol. 2003;121:1138–1144. doi: 10.1046/j.1523-1747.2003.12566.x. [DOI] [PubMed] [Google Scholar]
- 131.Huh WK, Oono T, Shirafuji Y, Akiyama H, Arata J, Sakaguchi M, Huh NH, Iwatsuki K. Dynamic alteration of human beta-defensin 2 localization from cytoplasm to intercellular space in psoriatic skin. J Mol Med (Berl) 2002;80:678–684. doi: 10.1007/s00109-002-0373-z. [DOI] [PubMed] [Google Scholar]
- 132.Liu AY, Destoumieux D, Wong AV, Park CH, Valore EV, Liu L, Ganz T. Human beta-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J Invest Dermatol. 2002;118:275–281. doi: 10.1046/j.0022-202x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- 133.Grubauer G, Feingold KR, Harris RM, Elias PM. Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res. 1989;30:89–96. [PubMed] [Google Scholar]
- 134.Banks SL, Paudel KS, Brogden NK, Loftin CD, Stinchcomb AL. Diclofenac enables prolonged delivery of naltrexone through microneedle-treated skin. Pharm Res. 2011;28:1211–1219. doi: 10.1007/s11095-011-0372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Toebak MJ, de Rooij J, Moed H, Stoof TJ, von Blomberg BM, Bruynzeel DP, Scheper RJ, Gibbs S, Rustemeyer T. Differential suppression of dendritic cell cytokine production by anti-inflammatory drugs. Br J Dermatol. 2008;158:225–233. doi: 10.1111/j.1365-2133.2007.08297.x. [DOI] [PubMed] [Google Scholar]
- 136.Krishnakumari V, Rangaraj N, Nagaraj R. Antifungal activities of human beta-defensins HBD-1 to HBD-3 and their C-terminal analogs Phd1 to Phd3. Antimicrob Agents Chemother. 2009;53:256–260. doi: 10.1128/AAC.00470-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dhople V, Krukemeyer A, Ramamoorthy A. The human beta-defensin-3, an antibacterial peptide with multiple biological functions. Biochim Biophys Acta. 2006;1758:1499–1512. doi: 10.1016/j.bbamem.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 138.Joly S, Maze C, McCray PB, Jr, Guthmiller JM. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol. 2004;42:1024–1029. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zou G, de Leeuw E, Li C, Pazgier M, Zeng P, Lu WY, Lubkowski J, Lu W. Toward understanding the cationicity of defensins. Arg and Lys versus their noncoded analogs. J Biol Chem. 2007;282:19653–19665. doi: 10.1074/jbc.M611003200. [DOI] [PubMed] [Google Scholar]
- 140.Scudiero O, Galdiero S, Cantisani M, Di Noto R, Vitiello M, Galdiero M, Naclerio G, Cassiman JJ, Pedone C, Castaldo G, Salvatore F. Novel synthetic, salt-resistant analogs of human beta-defensins 1 and 3 endowed with enhanced antimicrobial activity. Antimicrob Agents Chemother. 2010;54:2312–2322. doi: 10.1128/AAC.01550-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Miyasaki KT, Bodeau AL, Ganz T, Selsted ME, Lehrer RI. In vitro sensitivity of oral, gram-negative, facultative bacteria to the bactericidal activity of human neutrophil defensins. Infect Immun. 1990;58:3934–3940. doi: 10.1128/iai.58.12.3934-3940.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ouhara K, Komatsuzawa H, Yamada S, Shiba H, Fujiwara T, Ohara M, Sayama K, Hashimoto K, Kurihara H, Sugai M. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, {beta}-defensins and LL37, produced by human epithelial cells. J Antimicrob Chemother. 2005;55:888–896. doi: 10.1093/jac/dki103. [DOI] [PubMed] [Google Scholar]
- 143.Diamond G, Beckloff N, Ryan LK. Host defense peptides in the oral cavity and the lung: similarities and differences. J Dent Res. 2008;87:915–927. doi: 10.1177/154405910808701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ji S, Hyun J, Park E, Lee BL, Kim KK, Choi Y. Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils. J Periodontal Res. 2007;42:410–419. doi: 10.1111/j.1600-0765.2006.00962.x. [DOI] [PubMed] [Google Scholar]
- 145.Turner J, Cho Y, Dinh NN, Waring AJ, Lehrer RI. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42:2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Saito H, Tomioka H, Yoneyama T. Growth of group IV mycobacteria on medium containing various saturated and unsaturated fatty acids. Antimicrob Agents Chemother. 1984;26:164–169. doi: 10.1128/aac.26.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kitahara T, Koyama N, Matsuda J, Aoyama Y, Hirakata Y, Kamihira S, Kohno S, Nakashima M, Sasaki H. Antimicrobial activity of saturated fatty acids and fatty amines against methicillin-resistant Staphylococcus aureus. Biol Pharm Bull. 2004;27:1321–1326. doi: 10.1248/bpb.27.1321. [DOI] [PubMed] [Google Scholar]
- 148.Bratt CL, Walters K, Dawson DV, Drake D, Brogden KA, Wertz P. Susceptibility of Porphyromonas gingivalis to oral lipids and ultrastructural damage. J Dent Res. 2011;90((Spec Issue A)):286. [Google Scholar]