Abstract
Purpose of review
New research on the mechanisms of neurodegeneration highlights parallels between prion disease pathogenesis and other, more common disorders not typically thought to be infectious. This involves propagation of protein misfolding from cell to cell by templated conformational change. This review focuses on the cell biology that underlies propagation of protein aggregation between cells, including a discussion of protein biochemistry and relevant mouse models.
Recent findings
Like the prion protein, several other proteins exhibit self-propagating fibrillar conformations in vitro. Multiple cellular studies have now implicated endocytic mechanisms in the uptake of aggregates into cells. Aggregates that enter cells somehow escape endocytic vesicles to contact cytosolic protein. The mechanism of release of protein monomers and aggregates from cells is not well understood. Animal models have confirmed that brain lysates and purified protein can accelerate brain disorder in a manner similar to prions.
Summary
Aggregate flux in and out of cells likely contributes to the progression of neuropathology in neurodegenerative diseases. A better understanding of these mechanisms is emerging and can help explain local spread of protein aggregation and the role of neural networks in disease. This will also inform new therapeutic strategies aimed at blocking this process.
Keywords: cell–cell propagation, networks, neurodegeneration, prion, templated conformational change
INTRODUCTION
Fundamental links between prion-based and conventional neurodegenerative diseases have inspired a new paradigm to understand the progression of protein aggregation disorder. Pathologically folded prion protein (PrP) corrupts the conformation of normal PrP through templated conformational change. Prion disorder relentlessly propagates among cells in the brain, whether the initial cause is an infectious inoculum, or, more commonly, the spontaneous development of PrP aggregates. Likewise, it is now very clear that other proteins associated with common neurodegenerative diseases have the same characteristics. Similarities between prion diseases and Alzheimer’s disease have previously generated speculation that Alzheimer’s disease is a prion-like syndrome [1,2], and early studies in nonhuman primates documented evidence for [3,4] and against [5] the induction of β-amyloid disorder based on Alzheimer’s disease brain inoculation. In the last several years, new molecular, cellular, and animal studies strongly suggest that transcellular propagation of protein misfolding occurs for a variety of aggregation-prone proteins, including tau, synuclein, superoxide disumutase (SOD)-1, huntingtin, and TAR DNA-binding protein-43, whereby protein aggregates formed in vitro or in a cell can seed further aggregation in recipient cells (Table 1). This transcellular propagation may underlie the progression of disorder in diseased brains [7–9], with one affected region communicating the disorder to connected regions via neural networks. In the following review, we discuss cell and molecular mechanisms of seeded aggregation and transcellular propagation, and the animal models used to investigate this phenomenon.
Table 1.
Prion phenomena in neurodegeneration
| Seeded aggregation |
Transcelluar propagation |
Inducible disorder |
|||||
|---|---|---|---|---|---|---|---|
| Protein | In vitro | In culture | In culture | In mice | Brain lysate | Synthetic seed | Controls |
| PrP | yes | yes | yes | yes | yes1 | yes2 |
1PrP immundepletion 2Monomeric rPrP |
| Tau | yes | yes | yes | yes | yes1 | n.d. | 1tau immundepletion |
| α-synuclein | yes | yes | yes | yes | yes1 | yes2 |
1Healthy brain lysate 2PBS |
| β-amyloid | yes | yes | yes | yes | yes1 | yes |
1Healthy brain lysate, Aβ immundepletion, formic acid denaturation |
| Huntingtin | yes | yes | yes | n.d. | n.d. | n.d. | N/A |
| SOD-1 | yes | yes | yes | n.d. | n.d. | n.d. | N/A |
| TDP-43 | yes | yes [6] | yes [6] | n.d. | n.d. | n.d. | N/A |
Summary of current evidence for prion-like mechanisms in neurodegeneration. Seeded aggregation is the process by which protein aggregates induce misfolding of the cognate monomer in vitro. Transcellular propagation is the process by which protein aggregates escape one cell, enter a neighboring cell and induce misfolding of the cognate monomer. Inducible disorder is the ability of brain lysates or synthetic seeds to induce misfolding in vivo. Controls refer to negative control conditions employed in the inducible disorder experiments to ensure specificity of the inoculum, n.d., not determined; PrP, prion protein; SOD, superoxide dismutase; TDP-43; TAR DNA-binding protein-43.
SEEDED AGGREGATION AND TRANSCELLULAR PROPAGATION
Templated conformational change refers to the process by which a normally folded protein is converted to a new conformation via direct contact with a differently folded species. This is commonly referred to as aggregate ‘seeding ’ or ‘seeded’ polymerization, and propagates the new conformation. For example, PrP is known to adopt multiple pathological conformations. These unique conformers underlie distinct pathological phenotypes in mice and humans [10–12]. Like PrP, tau fibrils exhibit conformational diversity and stable propagation of these conformations in vitro [13], and self-propagating fibril conformers have also been described for β-amyloid [14] and α-synuclein [15]. Such conformational diversity might underlie the phenotypic diversity of age-related neurodegeneration syndromes such as the tauopathies, although this has not been explicitly tested.
Importantly, the misfolded state can propagate from the outside to the inside of the cell via fibrillar ‘seeds’. Frost et al. [16] initially described the cellular events that could explain a propagation phenomenon. First, recombinant tau fibrils, but not monomer, are directly internalized by cultured cells and colocalize with dextran, a fluid-phase marker of endocytosis. The internalized tau aggregates induce the fibrillization of full-length intracellular tau. Recently, direct evidence has been presented for true transcellular propagation of tau protein misfolding. Tau aggregates from one cell population-induced fibrillization of intracellular tau in naïve recipient cells via direct protein–protein contact [17▪▪], strongly supporting a role for templated conformational change. This work further clarified that tau aggregates are released directly into the extracellular space (as opposed to being contained in exosomes), because an anti-tau antibody was identified that blocks cell uptake, and can be used to purify fibrillar species from conditioned medium [17▪▪]. These findings have yet to be confirmed for other pathological proteins but suggest a basic mechanism by which aggregates move between cells, and implicate antibodies as potentially potent therapies for neurodegeneration.
UPTAKE AND RELEASE OF AGGREGATES
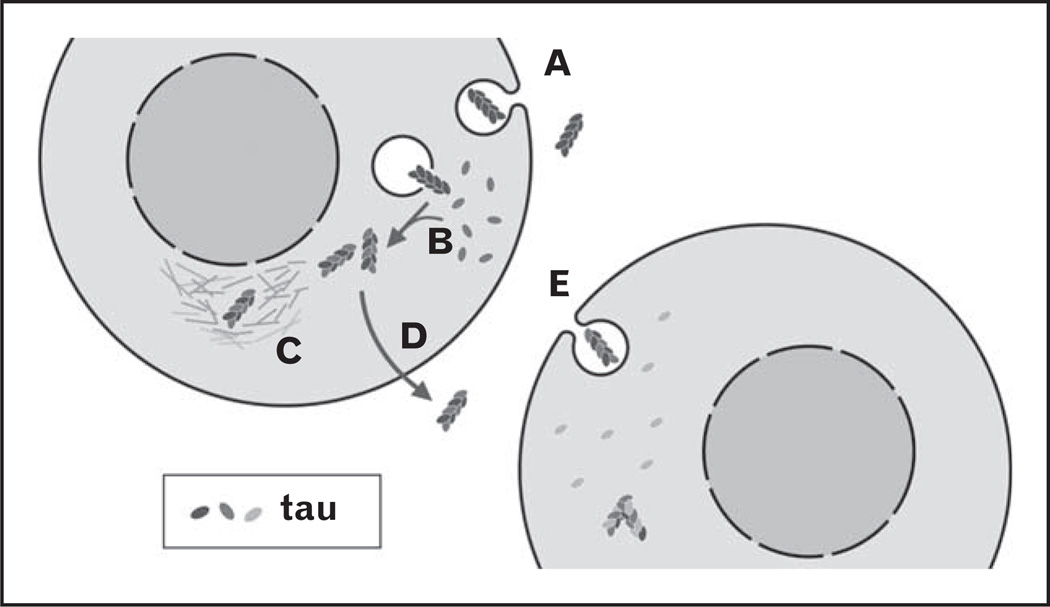
Like most proteins associated with neurodegenerative disease, tau, α-synuclein, huntingtin, and SOD-1 each aggregate inside cells. To transfer to other cells and propagate a conformational change, the aggregated protein must cross at least two membrane barriers. First, newly formed intracellular aggregates must be released into the extracellular space to initiate the cycle. Extracellular aggregates must then be internalized. If internalization occurs via endocytosis, which seems likely, aggregates must then escape the vesicular lumen to access the cytosol, contact cognate monomer, and seed aggregation. Finally, the newly formed aggregates must be released into the extracellular space to reinitiate the cycle (Fig. 1).
FIGURE 1.
Transcellular propagation of protein misfolding. A: Extracellular aggregates are internalized into cells via unconventional endocytosis (i.e., macropinocytosis). B: Endocytosed aggregates escape the vesicular lumen to reach the cytosol and seed aggregation of cognate monomer. C: Newly formed endogenous aggregates are associated with aggresomes. D: Endogenous aggregates are released by the cell into the extracellular space via an unknown mechanism. E: A neighboring cell internalizes the released aggregate and reinitiates the cycle.
Aggregate internalization is best defined for α-synuclein. Fibrillar α-synuclein uptake requires physiological temperatures and dynamin-1 activity [18,19], characteristics of endocytosis. Additionally, pretreatment of cells with proteinase K inhibits α-synuclein fibril uptake, suggesting the role for a protein-based receptor [18]. Internalization of α-synuclein is increased by the presence of wheat germagglutinin, a lectin that binds N-acetyl-glucosamine and sialic acids at the cell surface and induces adsorptive endocytosis [20]. Thus, glycoproteins may play a critical role in the binding and internalization of α-synuclein fibrils.
Cellular internalization of synthetic polyglutamine fibrils was previously proposed to occur via direct penetration of the lipid bilayer [21]. However, recent work from the same group suggests a role for cell surface structures in the binding and internalization of both synthetic polyglutamine (K2Q44K2) and huntingtin exon 1 (Htt exon1Q4444) fibrils [22▪]. As for α-synuclein, polyglutamine fibril binding was strongly reduced by pretreatment of cells with trypsin, suggesting involvement of protein receptors. Importantly, the authors also probed the effect of net charge on polyglutamine fibril binding and internalization. Cationic polyglutamine fibrils (K2Q44K2) efficiently bound to mammalian cells, whereas anionic fibril (D2Q44D2) binding was significantly reduced. Indeed, basic residues are critical mediators of protein interactions with cell surface structures such as glycosaminoglycans. It seems likely that sulfated glycosaminoglycans could be the cell surface structures responsible for amyloid binding and internalization [20,22▪], although this has not been explicitly tested. Such interactions have previously been documented for PrP rods and Aβ42 monomer [23–25], and may extend to other pathogenic aggregates including α-synuclein, huntingtin, and tau.
What is the fate of internalized aggregates, and how might they contact and seed aggregation of a cytosolic protein? A critical role for macropinocytosis, an energy-dependent nonadsorptive endocytosis characterized by membrane ruffling, has been proposed for SOD-1 [26▪▪]. Macropinosomes are known to be leaky and are often exploited by viruses to gain access to the cytosol, a process enhanced by acidic pH [27–30]. It is currently unclear whether fibrillar proteins can escape macropinosomes in the manner of viruses. Santa-Maria et al. [31▪] recently demonstrated that mammalian cells internalize Alzheimer’s disease patient-derived paired helical filaments (PHFs) by an endocytic mechanism, with tau aggregates subsequently observed in aggresomes. Aggresomes are perinuclear inclusion bodies that form in response to the presence of a high load of abnormal protein within the cytosol [32]. Although the presence of exogenously derived tau in the aggresome does not prove leakage from an endocytic vesicle, the data provide circumstantial evidence that endocytosed tau fibrils could seed intracellular aggregation in the cytosol. Interestingly, internalized tau had no association with lysosomes, autophagosomes, or the Golgi apparatus, suggesting that a nonclassical endocytic pathway is responsible for internalization and cellular transport of PHFs [31▪].
The mechanism for release of intracellular aggregates into the extracellular space is unknown. Additionally, several studies have highlighted the release of monomeric tau and α-synuclein, which may occur via distinct mechanisms. An analysis of brain interstitial fluid (ISF) tau levels within the hippocampus of mice revealed significant amounts of tau monomer [33▪]. ISF tau was also detectable in wild-type mice, suggesting that tau is released into the ISF in the absence of aggregation or neurodegeneration. ISF tau monomer dramatically decreased following the onset of tau disorder, and injection of tau fibrils into the hippocampus rapidly reduced soluble ISF tau, presumably by coaggregation. Thus, ISF tau monomer is probably in equilibrium with extracellular tau aggregates [33▪]. Work from cell culture models supports these findings, although it remains unclear by which mechanism tau is being released [34–38]. For example, one study shows that tau is constitutively released at low levels by an active, unconventional secretion process, and is not vesicle associated. By using human neurons differentiated from induced pluripotent stem cells, the investigators confirmed that this was not due to overexpression artifact or cell lysis [38]. Other studies have implicated exosomes in release [36,37,39,40]; however, the data are mostly circumstantial, without definitive copurification of tau and exosomes. Indeed, in the setting of intracellular aggregation, tau aggregates are found free within conditioned cell medium [17▪▪]. In summary, it is not yet clear to what extent protein release correlates with intracellular aggregation, and whether the same mechanisms apply to release of monomer versus aggregate.
MOUSE STUDIES: INOCULATION
The first clue that proteins other than PrP might transmit disorder from cell to cell in the brain came from pathological studies of patients who had received striatal transplants of dopaminergic neurons. It was possible to find α-synuclein inclusions in cells that were at most approximately 15 years old, suggesting that aggregates may have moved from host neurons to the transplanted neurons [41,42]. Subsequent studies indicated that transneuronal movement of aggregates occurs in vivo [19,43,44]. Further, multiple studies have indicated that it is possible to produce disorder in experimental animals through inoculation with brain lysates that contain protein aggregates or purified protein. Typically, recipient mice are ‘primed’ to acquire disorder through overexpression of the pathogenic protein. For example, injection of β-amyloid mouse models with brain extracts from human Alzheimer’s disease cadavers produced variable patterns of disorder depending on both the host and the source of the agent. This prion-like ‘strain’ phenomenon depends on β-amyloid within the extracts [45]. Injection of transgenic mouse brain extract containing tau aggregates induces tau disorder at the site of injection, which is blocked by immunodepletion of tau [46]. Subsequent investigations further demonstrated acceleration of aggregation and pathological phenotype in human mutant α-synuclein transgenic mice after intracerebral injection of brain extracts derived from aged mice of the same line, whereas no phenotype was observed when brain extracts were derived from young, healthy transgenic mice [47].
The ‘protein-only’ hypothesis predicts that aggregated material alone should be sufficient to produce fibrillar disorder in vivo. There is now evidence of recombinant PrP-induced disease [48,49]. And two studies demonstrated that recombinant and synthetic α-synuclein and β-amyloid aggregates are sufficient to induce disorder [50▪▪,51▪▪]. Luk et al. [50▪▪] injected recombinant α-synuclein1–120 fibrils into the neocortex and striatum of young mice expressing human α-synuclein with the familial A53T mutation. Within 30 days after injection, an age at which uninjected mice harbor no disorder, mice receiving fibrils displayed significant thioflavin S-positive synuclein pathology around the site of injection. By 90 days, the pathology extended through the forebrain. In related work, Stohr et al. [51▪▪] used a luciferase reporter under the control of the glial fibrillary acidic protein (Gfap) promoter to monitor astrocytosis as a surrogate for cerebral β-amyloid deposition. Injection of synthetic wild-type Aβ(1–40) or mutant Aβ(S26C)2 fibrils into the right cerebral hemisphere of 2-month-old mice bigenic for the amyloid precursor protein Swedish double mutation and Gfap-luc accelerated β-amyloid disorder relative to age-matched noninoculated controls.
MOUSE STUDIES: RESTRICTED EXPRESSION
The requirement for prion-like spread in the progression of neurodegeneration remains uncertain, although recent studies support this idea. Two groups recently used a mouse model in which a mutant form of the tau protein (P301L) is expressed selectively in neurons of the medial entorhinal cortex [52▪▪,53▪▪]. Mice under 12 months of age produced misfolded tau within this region. By 24 months, however, tau accumulated in neurons downstream in the synaptic circuit, first in dentate gyrus granule cells, followed by the CA fields of the hippocampus. This work implied that misfolded tau can transfer from neuron to neuron in a hierarchical and network dependent manner however, it remains uncertain to what extent tau protein expression was truly restricted. Liu et al. [52▪▪] isolated dentate gyrus granule cells to quantify human tau mRNA levels. In aged transgenic mice, human tau mRNA was detected, suggesting tau gene expression in regions downstream of the entorhinal cortex. de Calignon et al. [53▪▪] found evidence of tau expression in downstream regions, and thus it is not possible to determine to what extent tau aggregates transferred across synapses versus between cell bodies. As it is clear from cell culture experiments that synapses are not required for cell–cell propagation, a central aspect of aggregate propagation is due to flux of aggregates in and out of cells. In the right setting (i.e., at a synapse) this may enable transneuronal spread, but this can also account for local progression of disorder.
CONCLUSION
It is helpful to understand propagation of protein disorder in the context of normal cell physiology that goes awry. Cellular mechanisms have evolved to adapt to frequent protein misfolding and aggregation events and to the presence of intracellular fibrillar species. These include quality control machinery, such as protein chaperones and degradation mechanisms. Multiple studies now strongly suggest that a flux of protein aggregates occurs in and out of cells. Thus, cell mechanisms seem to exist for release of aggregated material into the extracellular space. Whether this constitutes a protective mechanism, which appears likely, or is simply a byproduct of some other processing event remains to be determined. Conversely, cells also retain an ability to efficiently take up protein aggregates from the extracellular space through endocytic mechanisms. Thus, phenomena referred to as ‘propagation’ may represent an aberrant process whereby fibrillar aggregates that would ordinarily be degraded escape quality control, are released to enter neighboring cells, and amplify the misfolded state through templated conformational change. The cellular mechanisms that underlie uptake and release of aggregates are not yet well defined but do not appear to be restricted to neurons. In keeping with this, progressive protein aggregation disorder is not restricted to the central nervous system and is observed in diverse tissues such as the pancreas (diabetes) and skeletal muscle (myopathies). We propose that transneuronal propagation is probably best understood in the context of aggregate flux, rather than as a process that specifically requires functional synapses. In the brain, it is easy to account for local spread of disorder by aggregate flux in and out of cell bodies, whereas network-mediated spread may simply reflect aggregate release at synapses (by the same or different mechanism), with uptake by the nearest cell – another neuron. As we define the molecular mechanisms that govern aggregate flux and transcellular propagation of aggregation, we will learn much about cellular protein quality control but also about new opportunities to intervene in disease.
KEY POINTS.
Essential similarities exist between prion diseases and more common neurodegenerative diseases, based on biophysical studies, cell biology, and inoculation experiments in animals.
Templated conformational change is a biophysical property of prion replication that extends to other proteins associated with neurodegenerative diseases.
Fibrillar protein aggregates are taken into cells via endocytic mechanisms wherein they escape the vesicular compartment to directly contact intracellular protein.
Protein aggregates are released from cells freely into the extracellular space.
Protein aggregate flux in and out of cells can explain local spread of disorder and may also explain the role of neural networks in the progression of disease.
Acknowledgements
None.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1.Prusiner SB. Some speculations about prions, amyloid, and Alzheimer’s disease. N Engl J Med. 1984;310:661–663. doi: 10.1056/NEJM198403083101021. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner SB. Shattuck lecture: neurodegenerative diseases and prions. N Engl J Med. 2001;344:1516–1526. doi: 10.1056/NEJM200105173442006. [DOI] [PubMed] [Google Scholar]
- 3.Baker HF, Ridley RM, Duchen LW, et al. Induction of beta (A4)-amyloid in primates by injection of Alzheimer’s disease brain homogenate. Comparison with transmission of spongiform encephalopathy. Mol Neurobiol. 1994;8:25–39. doi: 10.1007/BF02778005. [DOI] [PubMed] [Google Scholar]
- 4.Ridley RM, Baker HF, Windle CP, Cummings RM. Very long term studies of the seeding of beta-amyloidosis in primates. J Neural Transm. 2006;113:1243–1251. doi: 10.1007/s00702-005-0385-2. [DOI] [PubMed] [Google Scholar]
- 5.Goudsmit J, Morrow CH, Asher DM, et al. Evidence for and against the transmissibility of Alzheimer disease. Neurology. 1980;30:945. doi: 10.1212/wnl.30.9.945. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa Y, Kaneko K, Watanabe S, Yamanaka K, Nukina N. A seeding reaction recapitulates intracellular formation of Sarkosyl-insoluble transactivation response element (TAR) DNA-binding protein-43 inclusions. J Biol Chem. 2011;286:18664–18672. doi: 10.1074/jbc.M111.231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 8.Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 278–284. [DOI] [PubMed] [Google Scholar]
- 9.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer Disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 10.Telling GC, Parchi P, DeArmond SJ, et al. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science. 1996;274:2079–2082. doi: 10.1126/science.274.5295.2079. [DOI] [PubMed] [Google Scholar]
- 11.Safar J, Wille H, Itri V, et al. Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med. 1998;4:1157–1165. doi: 10.1038/2654. [DOI] [PubMed] [Google Scholar]
- 12.Legname G, Nguyen HO, Peretz D, et al. Continuum of prion protein structures enciphers a multitude of prion isolate-specified phenotypes. Proc Natl Acad Sci USA. 2006;103:19105–19110. doi: 10.1073/pnas.0608970103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frost B, Ollesch J, Wille H, Diamond MI. Conformational diversity of wild-type tau fibrils specified by templated conformation change. J Biol Chem. 2009;284:3546–3551. doi: 10.1074/jbc.M805627200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petkova AT, Leapman RD, Guo Z, et al. Self-propagating, molecular-level polymorphism in Alzheimer’s beta-amyloid fibrils. Science. 2005;307:262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- 15.Yonetani M, Nonaka T, Masuda M, et al. Conversion of wild-type alphasynuclein into mutant-type fibrils and its propagation in the presence of A30P mutant. J Biol Chem. 2009;284:7940–7950. doi: 10.1074/jbc.M807482200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–12852. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kfoury N, Holmes BB, Jiang H, et al. Trans-cellular propagation of tau aggregation by fibrillar species. J Biol Chem. 2012;287:19440–19451. doi: 10.1074/jbc.M112.346072. These authors used a cell-based fluorescence resonance energy transfer assay to monitor intracellular and transcellular tau aggregation. This approach established that transcellular propagation of aggregated tau amplifies the misfolded state. The study also revealed that tau aggregates are directly released into the extracellular space in which they can be sequestered by an a-tau antibody that blocks cell uptake.
- 18.Lee HJ, Suk JE, Bae EJ, et al. Assembly-dependent endocytosis and clearance of extracellular alpha-synuclein. Int J Biochem Cell Biol. 2008;40:1835–1849. doi: 10.1016/j.biocel.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Desplats P, Lee HJ, Bae EJ, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volpicelli-Daley Laura A, Luk Kelvin C, Patel Tapan P, et al. Exogenous a-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren PH, Lauckner JE, Kachirskaia I, et al. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11:219–225. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trevino RS, Lauckner JE, Souriges Y, et al. Fibrillar structure and charge determine the interaction of polyglutamine protein aggregates with the cell surface. J Biol Chem. 2012;24:29722–29728. doi: 10.1074/jbc.M112.372474. The work details the first evidence of a cell surface structure mediating the binding and internalization of polyglutamine fibrils. Although the structure’s identity remains unknown, it is a proteinaceous, low affinity, and nonspecific receptor.
- 23.Horonchik L, Tzaban S, Ben-Zaken O, et al. Heparan sulfate is a cellular receptor for purified infectious prions. J Biol Chem. 2005;280:17062–17067. doi: 10.1074/jbc.M500122200. [DOI] [PubMed] [Google Scholar]
- 24.Gauczynski S, Nikles D, El-Gogo S, et al. The 37-kDa/67-kDa laminin receptor acts as a receptor for infectious prions and is inhibited by polysulfated glycanes. J Infect Dis. 2006;194:702–709. doi: 10.1086/505914. [DOI] [PubMed] [Google Scholar]
- 25.Kanekiyo T, Zhang J, Liu Q, et al. Heparan sulphate proteoglycan and the lowdensity lipoprotein receptor-related protein 1 constitute major pathways for neuronal amyloid-{beta} uptake. J Neurosci. 2011;31:1644–1651. doi: 10.1523/JNEUROSCI.5491-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Münch C, O’Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc Natl Acad Sci U S A. 2011;108:3548–3553. doi: 10.1073/pnas.1017275108. This study provides the first evidence that SOD-1 aggregates can amplify the misfolded state via transcellular propagation. The authors demonstrated that SOD-1 aggregates enter cells via macropinocytosis prior to seeding the aggregation of cytosolic SOD-1. Further, the induced mutant SOD-1 aggregation was persistent and heritable for up to 30 days in the absence of the progenitor SOD-1 seed.
- 27.Meier O, Boucke K, Hammer SV, et al. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J Cell Biol. 2002;158:1119–1131. doi: 10.1083/jcb.200112067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blumenthal R, Seth P, Willingham MC, Pastan I. pH-dependent lysis of liposomes by adenovirus. Biochemistry. 1986;25:2231–2237. doi: 10.1021/bi00356a057. [DOI] [PubMed] [Google Scholar]
- 29.Chandran K, Farsetta DL, Nibert ML. Strategy for nonenveloped virus entry: a hydrophobic conformer of the reovirus membrane penetration protein micro 1 mediates membrane disruption. J Virol. 2002;76:9920–9933. doi: 10.1128/JVI.76.19.9920-9933.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang ST, Zaitseva E, Chernomordik LV, Melikov K. Cell-penetrating peptide induces leaky fusion of liposomes containing late endosome-specific anionic lipid. Biophys J. 2010;99:2525–2533. doi: 10.1016/j.bpj.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Santa-Maria I, Varghese M, Ksiezak-Reding H, et al. Paired helical filaments from Alzheimer’s disease brain induce intracellular accumulation of tau in aggresomes. J Biol Chem. 2012;287:20522–20533. doi: 10.1074/jbc.M111.323279. This study offers the first account of the fate of internalized tau fibrils. PHFs derived from Alzheimer’s disease patients were shown to induce endogenous tau mis-folding that localized to aggresomes. It is currently unclear whether the aggresome is the site of seeded aggregation.
- 32.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamada K, Cirrito JR, Stewart FR, et al. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J Neurosci. 2011;31:13110–13117. doi: 10.1523/JNEUROSCI.2569-11.2011. This study used microdialysis to measure monomeric ISF tau levels in the hippocampus of living mice. This technique allowed for the detection of ISF tau in nontransgenic mice, demonstrating that tau is secreted in the absence of neurodegeneration. Additionally, this study provides evidence that extracellular monomeric tau is in steady state equilibrium with tau fibrils.
- 34.Lee S, Kim W, Li Z, Hall GF. Accumulation of vesicle-associated human tau in distal dendrites drives degeneration and tau secretion in an in situ cellular tauopathy model. Int J Alzheimer’s Dis. 2012;2012:16. doi: 10.1155/2012/172837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saman S, Kim W, Raya M, et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid (CSF) in early Alzheimer’s disease. J Biol Chem. 2012;287:3842–3849. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon D, Garcia-Garcia E, Royo F, et al. Proteostasis of tau. Tau over-expression results in its secretion via membrane vesicles. FEBS Lett. 2012;586:47–54. doi: 10.1016/j.febslet.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 37.Plouffe V, Mohamed NV, Rivest-McGraw J, et al. Hyperphosphorylation and cleavage at D421 enhance tau secretion. PLoS One. 2012;7:e36873. doi: 10.1371/journal.pone.0036873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chai X, Dage JL, Citron M. Constitutive secretion of tau protein by an unconventional mechanism. Neurobiol Dis. 2012;48:356–366. doi: 10.1016/j.nbd.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Saman S, Kim W, Raya M, et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem. 2012;287:3842–3849. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emmanouilidou E, Melachroinou K, Roumeliotis T, et al. Cell-produced {alpha}-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kordower JH, Chu Y, Hauser RA, et al. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. NatMed. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 42.Li JY, Englund E, Holton JL, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 43.Hansen C, Angot E, Bergstrom AL, et al. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Angot E, Steiner JA, Lema Tome CM, et al. Alpha-synuclein cell-to-cell transfer and seeding in grafted dopaminergic neurons in Vivo. PLoS One. 2012;7:e39465. doi: 10.1371/journal.pone.0039465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer-Luehmann M, Coomaraswamy J, Bolmont T, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–1784. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 46.Clavaguera F, Bolmont T, Crowther RA, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–913. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mougenot AL, Nicot S, Bencsik A, et al. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol Aging. 2012;33:2225–2228. doi: 10.1016/j.neurobiolaging.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 48.Legname G, Baskakov IV, Nguyen HO, et al. Synthetic mammalian prions. Science. 2004;305:673–676. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- 49.Makarava N, Kovacs GG, Bocharova O, et al. Recombinant prion protein induces a new transmissible prion disease in wild-type animals. Acta Neuropathol. 2010;119:177–187. doi: 10.1007/s00401-009-0633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Luk KC, Kehm VM, Zhang B, et al. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alphasynucleinopathy in mice. J Exp Med. 2012;209:975–986. doi: 10.1084/jem.20112457. See [51▪▪]
- 51. Stohr J, Watts JC, Mensinger ZL, et al. Purified and synthetic Alzheimer’s amyloid beta (Abeta) prions. Proc Natl Acad Sci USA. 2012;109:11025–11030. doi: 10.1073/pnas.1206555109. This study and [50▪▪] each revealed that injections of recombinant a-synuclein and synthetic b-amyloid fibrils are sufficient to induce disorder in the brains of Parkinson’s and Alzheimer’s disease mouse models, respectively, supporting a prion hypothesis for the noninfectious neurodegenerative diseases.
- 52. Liu L, Drouet V, Wu JW, et al. Trans-synaptic spread of tau pathology in vivo. PLoS One. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. See [53▪▪ ]
- 53. de Calignon A, Polydoro M, Suárez-Calvet M, et al. Propagation of tau pathology in a model of early Alzheimer’s disease. Neuron. 2012;73:685–697. doi: 10.1016/j.neuron.2011.11.033. This study and [52▪▪ ] each employed a mouse model with entorhinal cortex-restricted human mutant tau expression. In an age-dependent fashion, aggregated tau accumulated in regions synaptically connected and downstream to the entorhinal cortex, suggesting a prion-like spread of aggregated tau through neural networks.