Abstract
Background
Accurate assignment of gestational age at time of fetal death is important for research and clinical practice. An algorithm to estimate gestational age (GA) at fetal death was developed and evaluated.
Methods
The algorithm developed by the Stillbirth Collaborative Research Network (SCRN) incorporated clinical and postmortem data. The SCRN conducted a population-based case-control study of women with stillbirths and live births from 2006 to 2008 in five geographic catchment areas. Rules were developed to estimate a due date, identify an interval during which death likely occurred, and estimate GA at the time of fetal death. Reliability of using fetal foot length to estimate GA at death was assessed.
Results
The due date estimated for 620 singleton stillbirths studied was considered clinically reliable for 87%. Only 25.2% of stillbirths were documented alive within two days before diagnosis and 47.6% within one week of diagnosis. The algorithm-derived estimate of GA at time of fetal death was 1 or more weeks earlier than the GA at delivery for 43.5% of stillbirths. GA estimated from fetal foot length agreed with GA by algorithm within two weeks for 75% within a subset of well-dated stillbirths.
Conclusions
Precise assignment of GA at death, defined as reliable dating criteria and a short interval (≤1 week) during which fetal death was known to have occurred, was possible in 46.6% of cases. Fetal foot length is a relatively accurate measure of GA at death and should be collected in all stillbirth cases.
Investigation of the factors leading to stillbirth, defined as fetal death ≥ 20 weeks of gestation, should employ the best possible assessment of gestational age (GA) at the time of fetal death. Precise estimation of GA at fetal death requires accuracy of two key pieces of information: the timing of fetal death and the estimated due date (EDD). Unfortunately, precise data are often lacking, particularly regarding timing of fetal death. Some investigators have equated timing of fetal death with the time of delivery1 or have estimated timing of death from pathologic findings in the fetus or placenta2–4. Because there may be a prolonged period between fetal death and delivery, the estimated GA at time of fetal death may be overestimated, particularly when the date of delivery is used as the basis to determine the date of death. The accuracy of vital statistics data and clinicopathologic correlations are impacted when gestational age information is unreliable5,6.
We describe a set of rules (the “algorithm”) developed by the Stillbirth Collaborative Research Network (SCRN) to estimate gestational age at fetal death in cases of singleton stillbirth, incorporating clinical and pathologic data. We report the results obtained by applying the algorithm and evaluate the performance of a key component using a well-dated subset of cases in which both estimated due date and timing of death were known with precision.
METHODS
The Eunice Kennedy Shriver National Institute of Child Health and Human Development SCRN was formed to evaluate the scope and causes of stillbirth. The SCRN conducted a population-based case-control study of women with stillbirths and live births, enrolled at delivery between March 2006 and September 2008, who were residents of five geographic catchment areas defined by state and county boundaries. The study was conducted at 59 hospitals associated with the five clinical sites selected to ensure access to at least 90% of the stillbirths and live births among residents of the catchment areas. An attempt was made to enroll all stillbirths and a representative sample of live births. This study was reviewed and approved by the Institutional Review Boards at each of the clinical recruiting sites and at the data coordinating center.
The study design and methods have been described in detail elsewhere7. At the time of ascertainment and after consent was obtained, the clinically estimated GA at the diagnosis of death (for stillbirths) or GA at delivery (for live births) was recorded in completed weeks by study personnel (“clinical GA”). Women with a fetal loss were screened if the clinical gestational age was ≥18 weeks, but were subsequently excluded from enrollment if there was evidence that clearly estimated the gestational age at <20 weeks. Maternal and neonatal information was collected from multiple sources: maternal interview during the delivery hospitalization, medical chart abstraction, placental pathology examination, and biospecimen collection. A standardized postmortem examination was conducted for stillbirths in consenting cases8. Information collected from interview and chart abstraction included maternal demographics, obstetric history, prenatal ultrasound results, presenting complaints and diagnoses from prenatal and delivery hospitalizations, sex of the fetus, and birth weight.
The SCRN investigators were interested in developing a consistent approach for estimating GA at death for stillbirths and GA at delivery for live births. A set of rules (the “algorithm”) was developed to estimate GA at death for singleton stillbirths (N=620) and is the focus of this paper. Estimation of GA at delivery for live births is described in Appendix A along with the rules used to resolve cases where the algorithm resulted in a different EDD for infants (stillbirths or live births) from a multiple gestation.
Clinical data, postmortem data and, in some cases, the clinical GA were considered in estimating GA at death for singleton stillbirths. The algorithm was organized in three major steps: (1) calculation of an EDD using information from chart abstraction; (2) determination of a date interval during which death was likely to have occurred using chart abstraction data (“timing of death [TOD] interval); (3) estimation of GA at death and date of death, giving consideration to clinical reliability of the EDD and length of the TOD interval. In certain cases, information from postmortem examination (lack of maceration, foot length measurement) were utilized in executing the algorithm and calculations of gestational age estimates were retained in decimal form (e.g. 32 weeks and 2 days was retained as 32 and 2/7 = 32.2857…). When gestational age obtained from chart abstraction was recorded in the SCRN database as completed weeks only, the gestational age was entered in the algorithm as completed weeks plus 3 days (as day 3 is the middle of the completed week) and expressed in decimal form corresponding to completed weeks + 3/7. Calculation formulas are provided in Appendix B. GA estimates were truncated to completed weeks for final presentation of results.
Step 1—Estimating the due date
The estimated due date (EDD) was assigned using the last menstrual period (LMP) date derived from chart abstraction and the earliest dated obstetric ultrasound where the baby was alive, if available. The LMP date was recorded as “certain” or “uncertain” according to whether the chart indicated the woman was certain of the date. For each ultrasound, the estimated gestational age from the ultrasound report was recorded in completed weeks by the clinical site in addition to fetal measurements if available. In preparation for applying the algorithm, gestational age at the time of ultrasound (GAU/S) in completed weeks and days was computed using either crown rump length (CRL)9 or a composite of head circumference, femur length, biparietal diameter, and abdominal circumference10 depending on the parameters reported. If all measurements were available, CRL was used for fetuses <14 weeks gestational age and the other four measurements were used for fetuses ≥14 weeks of gestation. In cases where no ultrasound entered in the SCRN database included actual fetal measurements, but only a gestational age in completed weeks, GAU/S was computed from the earliest such report where the fetus was alive as the recorded ultrasound GA + 3/7.
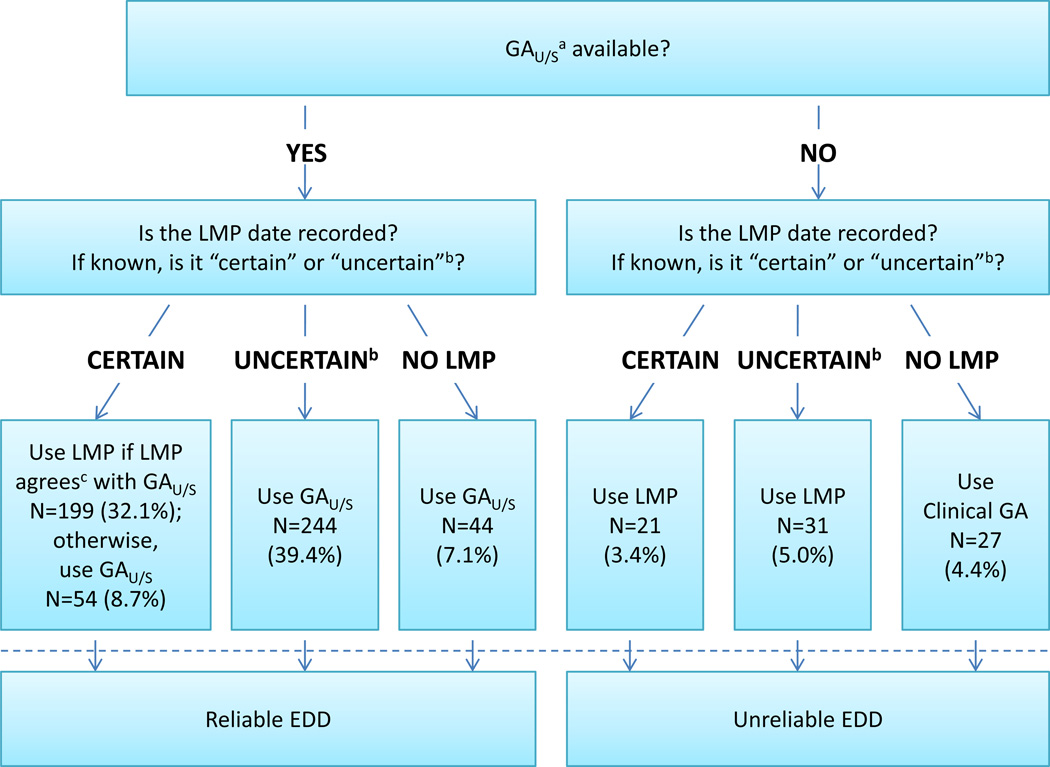
In order to assign the EDD, we incorporated the LMP date, the clinical reliability of the LMP date (certain/uncertain), and the GAU/S (Figure 1):
If both a dated ultrasound report, where the fetus was alive and a LMP date were available, the clinical reliability of the LMP date was considered. If the LMP date was “certain”, the gestational age at the time of the ultrasound was computed using the LMP date and compared to GAU/S. When the two estimates agreed within pre-determined limits (Table 1), the LMP date was used to compute the EDD; when they did not agree, GAU/S and the ultrasound date were used. If the LMP date was “uncertain” or certainty was not recorded, the EDD was estimated using the ultrasound date and GAU/S.
If a dated ultrasound report where the fetus was alive was available but no LMP date was recorded, then GAU/S and the ultrasound date were used to compute the EDD.
If there was no dated ultrasound report where the fetus was alive but an LMP date was available then the LMP date was used to compute the EDD.
If there was neither an LMP date nor a dated ultrasound report where the fetus was alive, EDD was estimated using the delivery date and the clinical GA at delivery recorded by the clinical site (plus 3/7, since the clinical GA was captured in completed weeks only).
FIGURE 1. Combining Lmp and Ultrasound Data to Determine Estimated Due Date.
This figure depicts the first step in the algorithm which is to determine the best estimated due date using clinical history and imaging findings. The first three rows (above the dashed line) show the determination of the EDD and apply equally to live births as well as stillbirths. Numbers and percentages are based on the 620 stillbirth cases. As indicated below the dashed line, the EDD was considered clinically reliable for purposes of determining GA at death if there was a dating ultrasound available. After application of the algorithm, the EDD was revised by case review (see text) for 13 cases with EDD originally determined as follows: by ultrasound that disagreed with certain LMP (4 cases); by ultrasound where the LMP was uncertain or certainty was not recorded (7 cases); by ultrasound when the LMP was not recorded (1 case); and by uncertain LMP when no ultrasound from a living fetus was available (1 case). EDD determined by case review was considered reliable. See Appendix B for formulas for calculating the EDD.
a GAU/S was derived from the earliest dated obstetric ultrasound when the baby was alive.
b “Uncertain” included cases in which certainty was not recorded.
c See text and Table 1 for details.
Abbreviations: LMP = Last menstrual period, U/S = Ultrasound, EDD = Estimated due date, GA = Gestational age, GAU/S = Gestational age at the date of the dating ultrasound examination
Table 1.
Ultrasound criteria for verifying EDD based on “certain” LMP19
| GALMP at time of ultrasound | EDD based on “certain” LMP is verified if the absolute difference between GALMP and GAU/S is: a |
|---|---|
| ≤19-6/7 weeks | ≤7.5 days |
| 20-0/7 to 29-6/7 weeks | ≤14.5 days |
| ≥30-0/7 weeks | ≤21.5 days |
U/S = ultrasound
EDD = estimated due date
LMP = last menstrual period
GA = gestational age
Calculated GALMP and GAU/S were retained in weeks and days with days represented in decimal form. To better match expected determination of agreement in a clinical setting, 0.5 days was added to the allowable differences recommended in the reference to allow for the potentially more precise differences calculated here.
Finally, the EDD assigned by the algorithm was considered reliable for the purpose of estimating gestational age at fetal death if estimated by ultrasound, or by “certain” LMP that agreed with ultrasound. The EDD was considered unreliable if estimated by LMP without ultrasound or by delivery date and the clinical GA.
Step 2—Determining the timing of death (TOD) interval for stillbirths
The date on which the fetus was last documented alive (“date last alive”, DLA) and the date the fetus was first diagnosed as dead (“date of diagnosis”, DoDx) were derived from chart abstraction based on information from prenatal care visits, hospitalizations, and ultrasound examinations. These included documentation of fetal heart tones by Doppler or sonogram. If no date of diagnosis was documented from chart abstraction, the delivery date was used as the date of diagnosis. A date last alive could not be determined for mothers who did not consent to chart abstraction or who had limited or no prenatal visits. In these cases the date last (known) alive was set to EDD minus 280 days (i.e. the back-calculated LMP). The DLA and the DoDx provided the lower and upper limits for the timing of death interval for each stillborn fetus. A corresponding gestational age range, [GADLA, GADoDx], when fetal death may have occurred was estimated using the EDD and the timing of death interval dates.
Individual cases were then reviewed by the SCRN clinical site investigator if the estimated gestational age at the DoDx differed by more than 2 weeks from the clinical GA recorded by the site. When this review identified transcription errors in medical charting or chart abstraction, the database was corrected and the algorithm re-applied. For discrepancies that remained, the EDD could be set by the clinical site investigator, overriding the EDD by algorithm if deemed valid based on clinical documentation. EDDs re-assigned by case review were considered reliable for the purpose of estimating GA at death.
Step 3—Estimating GA at fetal death
The algorithm estimated the GA at fetal death and the date of death by incorporating several factors: length of the TOD interval and the corresponding GA range, reliability of the EDD (Figure 1), and basic information from postmortem examination, when available, including presence or lack of fetal maceration4,8 and foot length (FtL) measurement. GA by foot length was calculated using a formula derived from Maroun and Graem (see Appendix B).11
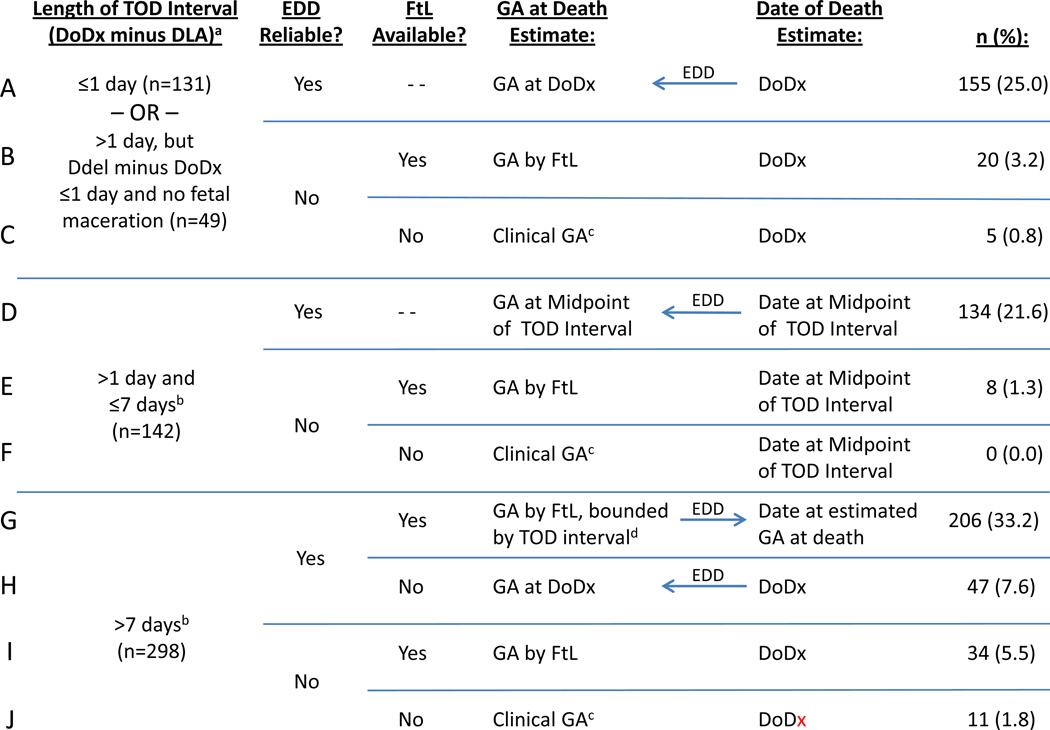
We first considered cases in which a) the length of the timing of death interval was ≤1 day, or b) the interval between date of diagnosis and delivery of the fetus was ≤1 day and fetal maceration was absent (maceration Grade 0, as evidenced by complete lack of desquamation) (Figure 2, rows A–C). These cases were considered to have the most precise information regarding the date of death, the estimate of which we took as the date of diagnosis identified by the algorithm. If the EDD was reliable, the GA at fetal death was estimated as the GA at date of diagnosis, calculated using the EDD (Figure 2, row A). If the EDD was unreliable, GA at death was estimated without using the estimated date of death. Instead, GA at death was estimated using foot length (row B), or using clinical GA when foot length information was not available (row C).
FIGURE 2. Estimating Gestational Age at Fetal Death.
This figure depicts the rules for determining the timing of fetal death, based on the length of the timing of death interval; the days between DoDx and delivery and presence/absence of maceration; the clinical reliability of the EDD, and the availability of the foot length measurement. Arrows show the direction of the computation of either the GA at Death estimate or the Date of Death estimate; where no arrow is shown, the two estimates were derived separately. Percentages are based on the 620 singleton stillbirths. See Appendix B for formulas for calculating the estimates. In the first box, there were 49 cases for which the TOD interval was >1 day but the days between the date of diagnosis of fetal death and the date of delivery were ≤1 and there was no fetal maceration; of these 27 had TOD interval >7 days.
a Date of diagnosis of fetal death minus the date last documented alive.
b Excluding cases with both {Ddel minus DoDx ≤1 day} and no fetal maceration, which are contained in the first box.
c The clinical GA at the date of diagnosis of fetal death, as reported by the clinical site. Because this value was recorded in completed weeks in our chart abstraction, we used the clinical GA in completed weeks + 3/7 for calculations, which assigned the middle day (day 3) of days 0 through 6 for the completed week. [The date of diagnosis as determined by the site was not recorded.]
d If GA by foot length fell outside the GA interval for TOD, the GA at death was estimated by the gestational age at the TOD interval boundary that was closest to the GA by foot length.
Abbreviations: GA = Gestational age, TOD = Timing of death, EDD = Estimated due date, FtL = Foot length, DLA = Date last documented alive, DoDx = Date of diagnosis of fetal death, DDel = Date of delivery
For the remaining women, estimation of GA at fetal death proceeded in a hierarchical fashion depending on the length of the timing of death interval and which clinical dating criteria applied. If the timing of death interval was >1 day and ≤7 days, the interval midpoint date was used as the date of death with GA at death estimated as the gestational age at the midpoint date when the EDD was reliable (Figure 2, row D), GA by foot length when the EDD was unreliable (row E), or clinical GA (in completed weeks, + 3/7) if the EDD was unreliable and foot length was not available (row F). If the timing of death interval was >7 days, foot length was considered in all cases where it was available. When the EDD was reliable and foot length available (row G), GA by foot length was used as the GA at death if it fell within the GA range associated with the timing of death interval; if it did not, the gestational age at the end of the timing of death interval closest to GA by foot length was used. If the EDD was reliable but the foot length was not available (row H), the date of diagnosis was taken as the most reasonable estimate of the date of death, and the reliable EDD was used to calculate the GA at date of diagnosis as the estimated GA at death. When the EDD was considered unreliable, the date of diagnosis was again taken as the estimate of the date of death, but the estimated GA at death was separately calculated as the GA by foot length with no adjustment for values outside the GA range associated with the timing of death interval (row I), or by the clinical GA (completed weeks +3/7) when foot length was not available (row J).
Reliability of foot length
We identified a well-dated subset of the stillbirth cases with both clinically reliable gestational dating criteria and a short timing of death interval, and who had a foot length measurement recorded. These cases met the following criteria: 1) TOD interval ≤7 days and 2) reliable EDD estimated by ultrasound on or before 20 6/7 weeks or by LMP that agreed with ultrasound ≤20 6/7 weeks. By the rules of the algorithm, GA by foot length was not needed to estimate GA at death in this subset. Thus, the GA at death estimated by the algorithm was compared to the GA by foot length in order to assess the validity of using foot length alone in the algorithm to estimate GA at death (figure 2, rows B, E, I).
Statistical Analysis
Summary statistics were used to describe the results. Statistical significance for comparisons of proportions between independent groups (e.g. stillbirths <24 vs. ≥24 weeks GA) was determined by the chi-square test and for comparisons between proportions in the same group by the McNemar test (e.g. percent of all stillbirths classified as <24 weeks GA by clinical GA vs. by the algorithm). The hypothesis that there were equal numbers of differences in each direction (positive and negative) for {GA estimated by the algorithm minus GA estimated by the foot length} was tested using the sign test.
RESULTS
The SCRN identified 953 eligible women with stillbirths for participation in the study. Of these, 126 were not approached, 164 were approached but refused to participate, and 663 (70%) were enrolled. These 620 singleton pregnancies are the subject of this analysis. Of these, 27 (4.4%) had neither a recorded LMP nor an ultrasound report where the fetus was alive. In 41 cases (6.6%) the medical record contained no documentation of a living fetus.
Estimating the Due Date
The algorithm-derived EDD was considered reliable in 87% (541/620) of the singleton stillbirths (Figure 1). Ultrasound results were used to revise the EDD in 54 cases (8.7% overall; 21% of 253 with reliable EDD and “certain” LMP). Among the 342 cases with EDD determined by ultrasound, 126 (37%) were derived from CRL measurements, 160 (47%) from combined head, abdomen, and femur measurements, and 56 (16%) from a gestational age assessed by ultrasound but recorded without the actual fetal measurements. The algorithm-derived EDD was overridden based on the clinical site investigators’ best judgment for 13 (2.1%) cases.
Estimating the Timing of Death Interval
Only 25.2% of the fetuses were documented to be alive within two days of the diagnosis of stillbirth, and less than half (47.6%) were documented as alive in the week preceding the stillbirth diagnosis (Table 2). Including the 41 cases in which there was no documentation of a living fetus at any point, there was no objective documentation that the fetus was alive within four weeks before the stillbirth diagnosis in 17.7% of cases (Table 2).
Table 2.
Length of TOD Interval for Stillbirth Cases
| Days between DLA and DoDx | N (%) | Cumulative % |
|---|---|---|
| 0 | 79 (12.7) | 12.7 |
| 1–2 | 77 (12.4) | 25.2 |
| 3–7 | 139 (22.4) | 47.6 |
| 8–14 | 108 (17.4) | 65.0 |
| 15–21 | 52 (8.4) | 73.4 |
| 22–28 | 55 (8.9) | 82.3 |
| >28 | 69 (11.1) | 93.4 |
| No DLA documented | 41 (6.6) | 100.0 |
TOD = Timing of death
DLA = (Algorithm-derived) date last documented alive
DoDx = (Algorithm-derived) date of diagnosis of fetal death
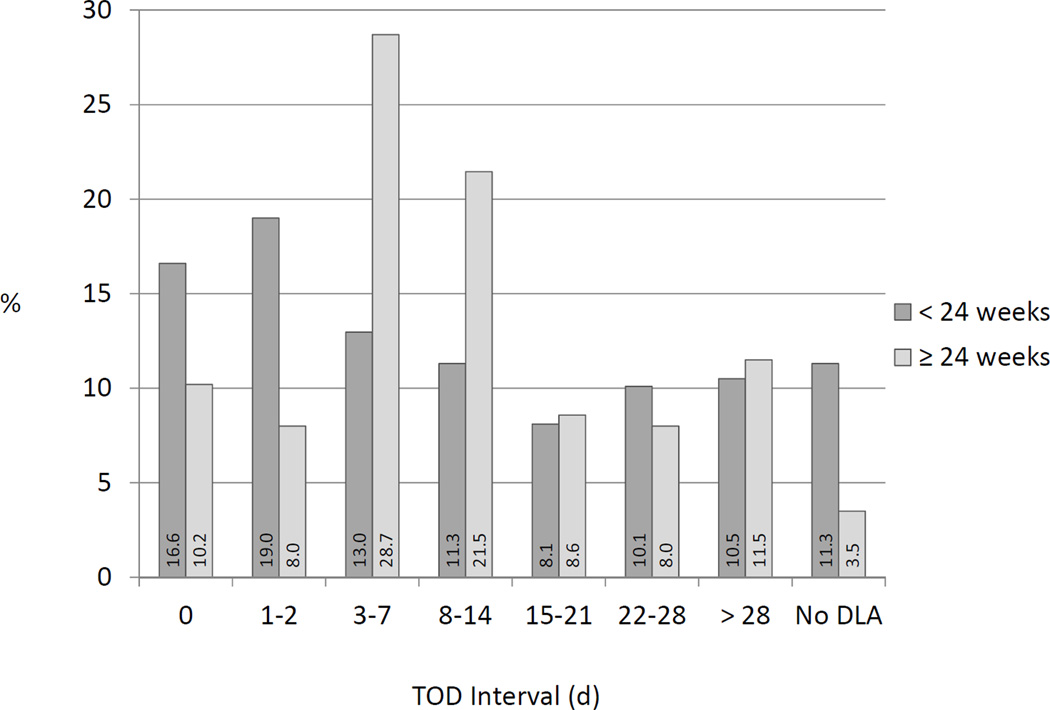
The TOD interval was ≤2 days in 35.6% of stillbirth cases with fetal death estimated as <24 weeks’ gestation by the algorithm compared to 18.2% of stillbirths with fetal death estimated as ≥24 weeks’ gestation (p<0.001) (Figure 3). About one fourth (24.3%) of stillbirths occurring <24 weeks’ gestation had TOD intervals 3–14 days in length, compared to half (50.1%) of later stillbirths. Additionally, 11.3% of the cases <24 weeks’ gestation had no documentation of a living fetus, compared to only 3.5% of the later stillbirths.
FIGURE 3. Distribution of Timing of Death Interval for Stillbirths <24 Weeks’ Gestation Versus ≥24 Weeks’ Gestation.
This figure demonstrates the distribution of the length of the TOD interval in stillbirth cases with age at fetal death estimated as <24 weeks’ gestation by the algorithm compared to those with death ≥24 weeks. Numbers at the base of each bar show the percentages represented by bar height. Abbreviation: TOD = Timing of Death
Estimating the GA at Death
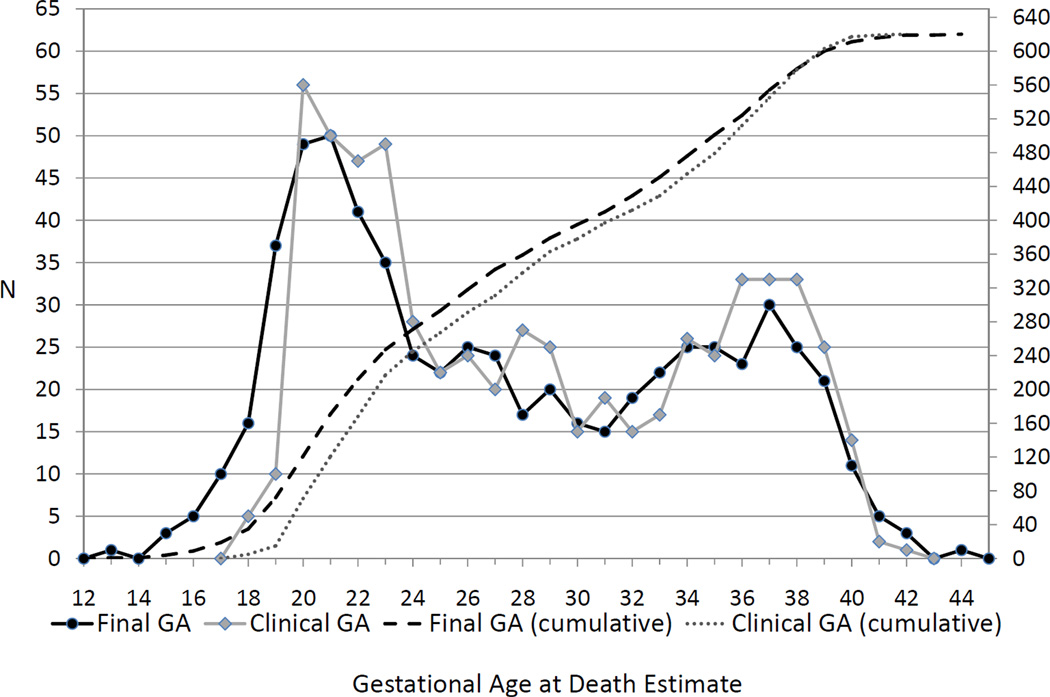
Figure 2 shows the number of cases for each method used to estimate the GA at death. The frequency and cumulative frequency distributions of the algorithm-assigned GA at death and the clinical GA for these singleton stillbirths are shown in Figure 4. The cumulative distributions indicate that the percentiles of the estimated GA are smaller than those of the clinical GA estimate up to 38 weeks gestation. Compared to the clinical GA captured from the chart abstraction data, application of the algorithm resulted in more stillbirth cases being assigned a GA at death of < 24 weeks (39.8% vs. 35.0%, p<0.001) and < 20 weeks (11.6% vs. 2.4%, p<0.001).
FIGURE 4. Frequency and Cumulative Frequency Distributions in Completed Weeks for Gestational Age at Death Estimates by the Scrn Algorithm and by the Clinical Gestational Age for Singleton Stillbirths.
This figure compares the distributions of the GA at fetal death estimated by the algorithm (“Final GA”) and estimated by the clinical gestational age at diagnosis of death reported by the clinical site (“Clinical GA”). The left axis shows the number of stillbirths with each gestational age for the frequency curves. The right axis shows the total number of stillbirths with gestational age at a given week or earlier for the cumulative distribution curves. Abbreviation: GA = gestational age
The final algorithm-assigned GA at fetal death was 1 or more weeks less than the gestational age at the algorithm-derived date of diagnosis of fetal death (DoDx) for 36.3% of stillbirth cases (Table 3). Similarly, the GA at fetal death was ≥1 week less than the gestational age at delivery for 43.5% of stillbirth cases. Cases with absolute differences of 3 or more weeks included larger proportions of women with no insurance (public or private), with no prenatal care in the first or second trimester, who were multiparous, and with algorithm-estimated GA at death <24 weeks than those with differences ≤2 weeks.
Table 3.
Comparison of Final (Algorithm-Derived) Gestational Age at Fetal Death versus Gestational Age at the Algorithm-Derived Date of Diagnosis of Fetal Deatha and Gestational Age at the Date of Deliverya
| Final GA compared with: | ||||
|---|---|---|---|---|
| GA at DoDx (by EDD) | GA at Date of Delivery (by EDD) | |||
| Difference (completed weeks)b |
N (%) | Cumulative % | N (%) | Cumulative % |
| ≤ −5 | 25 (4.0) | 4.0 | 29 (4.7) | 4.7 |
| −4 | 28 (4.5) | 8.5 | 30 (4.8) | 9.5 |
| −3 | 32 (5.2) | 13.7 | 34 (5.5) | 15.0 |
| −2 | 46 (7.4) | 21.1 | 51 (8.2) | 23.2 |
| −1 | 94 (15.2) | 36.3 | 126 (20.3) | 43.5 |
| 0 | 379 (61.1) | 97.4 | 334 (53.9) | 97.4 |
| 1 | 1 (0.2) | 97.6 | 2 (0.3) | 97.7 |
| 2 | 7 (1.1) | 98.7 | 6 (1.0) | 98.7 |
| 3 | 2 (0.3) | 99.0 | 2 (0.3) | 99.0 |
| 4 | 4 (0.6) | 99.7 | 4 (0.6) | 99.7 |
| ≥5 | 2 (0.3) | 100.0 | 2 (0.3) | 100.0 |
Calculated using the EDD.
Final (algorithm-derived) GA at fetal death in completed weeks minus: the gestational age in completed weeks at the algorithm-derived date of diagnosis of fetal death (DoDx) calculated using the estimated due date, and the gestational age in completed weeks at the date of delivery calculated using the estimated due date, respectively. The algorithm-derived GA at fetal death can sometimes exceed the estimated gestational age at the DoDx (or even at delivery). This can only occur when the EDD is considered unreliable, in which case the algorithm estimates the GA at fetal death independently of the DoDx (Figure 2, rows B, C, E, F, I, and J).
GA = Gestational age
DoDx = (Algorithm-derived) date of fetal death
EDD = estimated due date
Use of Foot Length to Determine GA at Death
Recorded foot length was available for 516 of the 620 stillborn fetuses (83.2%), and this proportion did not differ depending on estimated GA at death (<24 vs. ≥24 weeks: 83.4% vs. 83.1%, p=0.92). In 268 cases (43.2% of all stillbirths), foot length data were incorporated according to the algorithm in estimating GA at death (Figure 2, rows B, E, G, and I). Among cases with reliable EDD, foot length did not enter into determination of GA at death for those with a short timing of death interval (≤7 days) or with ≤1 day between date of diagnosis and delivery with no maceration (rows A and D, n=289). However, among the 253 cases with reliable EDD but a TOD interval >7 days (rows G and H), foot length was available in 81.4% (row G) and was used to estimate GA at death. Of those cases without a reliable EDD (n=78), foot length was used to estimate the GA at death in the 62 (79.5%) for which it was available; otherwise, the clinical GA was used.
A subset of 220 cases was well-dated, with a TOD interval ≤7 days and reliable dating criteria that included an ultrasound ≤20 6/7 weeks. This subset included larger proportions of women who were white, had prenatal care, had commercial health insurance or Veteran’s Administration benefits, and were nulliparous. The well-dated subset with available foot length included 191 (30.8%) of the singleton stillbirths. The difference between the GA at death calculated by the algorithm and the GA calculated by foot length alone is shown in Table 4. Calculation of GA by foot length showed no tendency for either overestimation or underestimation of the GA at death compared to the algorithm (p=0.74). For 75.4% of cases, the absolute difference between GA by foot length and the GA at death estimated by the algorithm was within two weeks. The remaining 47 cases differed by at least three weeks (range 3–8 weeks). In 8/47 (17.0%), fetal growth restriction had been identified prenatally. Four cases (8.5%) had abnormal karyotype on prenatal or postmortem testing, and an additional three cases (6.4%) had significant fetal anomalies with normal karyotype. Four babies (8.5%) weighed >4000 g at birth (range 4225 g – 4765 g), and GA by foot length overestimated the GA at death by algorithm in all four of these cases. In addition, the 47 cases included a larger proportion of white mothers and stillbirths with algorithm-estimated GA at death ≥24 weeks compared to the 144 cases with absolute difference within two weeks.
Table 4.
Comparison of the Algorithm-Derived Gestational Age at Fetal Death versus Gestational Age by Foot Length (FtL) in a Subset of 191 Singleton Stillbirths with Good Datinga
| Difference (completed weeks)b | N (%) | Cumulative % |
|---|---|---|
| ≤ −4 | 9 (4.7) | 4.7 |
| −3 | 10 (5.2) | 9.9 |
| −2 | 23 (12.0) | 22.0 |
| −1 | 33 (17.3) | 39.3 |
| 0 | 46 (24.1) | 63.4 |
| 1 | 27 (14.1) | 77.5 |
| 2 | 15 (7.9) | 85.3 |
| 3 | 11 (5.8) | 91.1 |
| ≥4 | 17 (8.9) | 100.0 |
Stillbirths with timing of death interval ≤7 days and reliable estimated due date by ultrasound at ≤20 6/7 weeks gestational age or by LMP that agreed with ultrasound at ≤20 6/7 for whom foot length was available.
Algorithm-derived gestational age at fetal death in completed weeks minus gestational age by foot length in completed weeks.
COMMENTS
The algorithm that is described to estimate GA at death in cases of stillbirth incorporates readily available clinical data and simple examination of the infant after delivery. Optimal determination of the GA at fetal death is important for the accuracy of vital statistics, for identifying epidemiologic patterns and risk factors for stillbirth at various stages of gestation, and for correctly identifying growth abnormalities. In our study, precise assignment of GA at death was possible in just under half of our cases (46.6%), considering those with both clinically reliable dating criteria and a short interval (≤1 week) in which the fetal death could have occurred (Figure 2, rows A and D). Given the geographic and racial diversity of our catchment areas, this is likely representative of general clinical practice in the United States.
Use of fetal foot length provided important and valid information with which GA at death could be estimated in cases with unreliable dating criteria, and we recommend that foot length be measured and recorded in all stillbirth cases. We demonstrated, using a well-dated subset of the stillbirths with both clinically reliable dating criteria, including ultrasound ≤20 6/7 weeks, and a timing of death interval ≤1 week, that foot length measurements provide accurate estimation of the GA at the time of fetal death (± 2 weeks) in 75% of cases. Similarly, other investigators have validated the correlation between fetal foot length and gestational age, whether measured prenatally by ultrasound14–16, or in postmortem examinations4,11. This is an important finding for two reasons. First, more than half the cases of stillbirth in this population-based study lacked precision of one or both of the key pieces of information needed to pinpoint the GA at death: good dating and short interval between the time the fetus was last documented alive and the time of stillbirth diagnosis. Second, fetal foot length can be measured easily at the time of delivery of the stillborn infant, and its conversion to GA requires no specialized expertise or equipment. Thus, even when families decline a comprehensive postmortem examination, this critical information can be ascertained.
Compared to the clinical GA, use of the algorithm to determine GA at death resulted in more cases falling within the <24 week (previable) and <20 weeks gestational age categories. This finding has implications for vital statistics reporting, as well as for determining the contribution of abnormal fetal growth to the etiology of stillbirth.
Over- or underestimation of GA at death renders interpretation of birth weight percentiles inaccurate, potentially distorting the role of fetal growth restriction or accelerated growth in the pathogenesis of fetal death. For example, a high rate of fetal growth restriction was found in a cohort of antepartum stillbirths ≥24 weeks’ gestation when GA at death was assumed to have occurred two days prior to stillbirth diagnosis or delivery.1 Our data suggest that almost 40% of deaths in stillbirths occurred at least a week before diagnosis, and over 40% occurred at least a week before delivery. Therefore, the contribution of fetal growth restriction to stillbirth might be less than previously reported. There is a paucity of information regarding the natural history of spontaneous labor following fetal death, but one retrospective study of antepartum stillbirths of a least 1000 g birth weight demonstrated that 10% of cases were retained for more than two weeks, and 7% for more than three weeks. Indeed, spontaneous labor occurred in eight cases after fetuses had been retained for 39–90 days after absence of fetal heart sounds.17
Use of the algorithm to estimate GA at death is likely to be less accurate for women with little prenatal care prior to stillbirth. We found that cases with the greatest differences between the estimated GA at death and GA at date of diagnosis included larger proportions of women with no insurance, with no prenatal care in the first or second trimester, and with stillbirth at <24 weeks’ gestation. These factors may affect reliability of dating criteria as well as estimates of the TOD interval. Where precise dating and timing of death are not possible, reliance on foot length to estimate GA at death may also contribute to under- or overestimation of GA in some cases, with consequences for determining fetal growth. In our subset of well-dated cases, GA estimated by foot length was ≥3 weeks earlier than the algorithm-derived estimate for 15% and ≥3 weeks later for 10% of cases.
Reporting of fetal death is fraught with the potential for miscommunication and misinterpretation of clinical data regarding gestational age and timing of death. Healthcare providers and hospital personnel who are responsible for providing this information generally do not have access to guidelines written in plain language or training in accurate collection of data and completion of reporting forms. For example, the American College of Obstetricians and Gynecologists’ Practice Bulletin regarding evaluation and management of stillbirth makes no mention of how to assign a gestational age at death for the stillborn fetus.12 The United States Standard Report of Fetal Death13 developed by the National Vital Statistics System of the National Center for Health Statistics asks for “Obstetric Estimate of Gestation at Delivery”, but limited guidance is provided for how this is to be determined. Relying on individual interpretation of proper procedures for completing standardized reporting forms can result in incomplete or erroneous data. Moreover, an improved estimate of the gestational age at fetal death would be a desirable addition to vital statistics reporting.
The algorithm described here can be easily applied in day-to-day clinical practice. First, every effort should be made to corroborate or revise historical pregnancy dating criteria (e.g. the LMP) with evidence from prior ultrasound examinations performed while the fetus was alive, and from as early in gestation as possible. Second, the date of the most recent objective evidence of fetal life should be documented and compared to the date of diagnosis of the stillbirth to determine the range of dates during which the stillbirth occurred. Presence or absence of maceration can be assessed by visual examination at the time of delivery. For timing of death intervals ≤7 days with reliable dating criteria, or when there is no maceration and the interval between diagnosis of fetal death and delivery is ≤1 calendar day, the GA at death is pinpointed with reasonable accuracy. For longer timing of death intervals and/or unreliable dating criteria, we recommend incorporating fetal foot length into determining the estimated GA at death. Exceptions may include cases with known aneuploidy or significant fetal anomalies, as well as those where delayed fetal growth had been identified by ultrasound while the fetus was alive. Foot length, while the best available fetal measure for gestational age determination, is affected by lack of or excessive fetal growth (18). Corroborating pathologic data with the known clinical scenario of each stillbirth is important. For example, several stillborn fetuses in our well-dated subset with the largest discrepancy between foot length and known gestational age at time of death were found to have severe IUGR by sonogram preceding fetal death.
There is no gold standard for estimating the gestational age of fetal death. Estimation must employ common-sense application of currently available knowledge and tools. Using our algorithm, imprecise timing of fetal death or unreliable dating criteria prevented precise determination of the gestational age of fetal death for more than half the cases of stillbirth. Nonetheless, our results suggest that the accuracy and uniformity of the information collected can be improved through the use of this algorithm.
ACKNOWLEDGEMENTS
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through the Cooperative Agreement mechanism (U01-HD045954, U10-HD045953, U10-HD045925, U10-HD045952, U10-HD045955, U10- HD045944).
APPENDIX A
-
Algorithm for estimating gestational age (GA) at delivery for live births
Step 1: The estimated due date (EDD) is determined as described for singleton stillbirths.
Step 2: Consider the reliability of the EDD.- If the EDD is considered reliable [estimated by ultrasound, last menstrual period (LMP) date that agrees with ultrasound, or by case review] then the EDD and the delivery date are used to calculate the estimated GA at delivery.
- If the EDD is not considered reliable [estimated by LMP date with no ultrasound available or by using the delivery date and the clinical GA at delivery recorded at screening for the study to estimate the due date] then the clinical GA at delivery from screening (+ 3/7, because it was recorded in the SCRN database as completed weeks) is used as the estimated GA at delivery.
-
Multiples with different EDD
In cases where the algorithm results in non-matching EDD for twins or triplets, the following rules are applied to set the final EDD. (For the SCRN, these are generally cases where small variations in ultrasound measurements between multiples results in different estimated due dates.)-
If ≤7 days between EDD of the multiples, thenIf the EDD is by “case review” for one infant, use the EDD for that infant for the other(s) in the set. All are subsequently labeled EDD by case review.If GA by ultrasound was used to compute the EDD for all infants in the set, take the average of the due dates and use that as the final EDD for each infant. All are labeled EDD by ultrasound.
-
If >7 days between EDD of the multiples, thenIf the EDD for one infant is by LMP that agrees with ultrasound GA, use the EDD for that infant for the other(s) in the set. All are labeled EDD by LMP that agrees with ultrasound.If GA by ultrasound was used to compute the EDD for all infants in the set,
- If one infant was a live birth, and one was a stillbirth, use the EDD for the live birth as the EDD for the stillbirth.
- If all infants were live births, take the average of the due dates and use that as the final EDD for each infant.
- All are labeled EDD by ultrasound.
-
APPENDIX B
This appendix gives formulas used in executing the algorithm.
-
GAU/S – Gestational age (GA) on the date of ultrasound (U/S), estimated by ultrasound:
- Crown rump length (CRL) in centimeters [Hadlock, 1992]9:
- GAU/S = exp [1.684969 + (0.315646 *CRL) − (0.049306 *CRL*CRL) + (0.004057*CRL*CRL*CRL) − (0.000120456*CRL*CRL*CRL*CRL) ]
- Head circumference (HC), Femur length (FL), Biparietal diameter (BPD), and Abdominal circumference (AC) in centimeters [Hadlock, 1984]10:
- GAU/S = 10.85 + 0.060(HC)(FL) + 0.6700(BPD) + 0.1680(AC)
- Estimate from an ultrasound report (recorded in the SCRN database as completed weeks):
- GAU/S = completed weeks of gestational age from ultrasound report + 3/7
In clinical practice, one would use the estimated gestational age in weeks and days from the earliest ultrasound report while the fetus was alive, and express the estimate in decimal form for further calculations.
- GALMP – Gestational age on the date of ultrasound, estimated by the last menstrual period (LMP) date:
- GALMP = (U/S date − LMP date) / 7
- Estimating the due date (EDD) from:
- LMP date:
- EDD = LMP date + 280
- U/S date and GAU/S:
- EDD = U/S date + (40 − GAU/S) × 7
-
Delivery date and GACLIN(completed weeks):
- EDD = Delivery date + [40 − (GACLIN(completed weeks) + 3/7)] × 7
In clinical practice, if both ultrasound data and an LMP date were unavailable, one would use the clinical estimate of gestational age in weeks and days (expressed as decimal weeks) on the clinical date of diagnosis of the fetal death (the latter was not recorded in the SCRN database) and estimate the EDD as:- EDD = clinical date of diagnosis of fetal death + [40 − GACLIN] × 7
- Range on gestational age by timing of death interval—date last alive (DLA) to date of diagnosis of fetal death (DoDx):
- GADLA = [280 − (EDD − date last documented alive)] / 7
- GADoDx = [280 − (EDD − date of stillbirth diagnosis)] / 7
-
GAFTL – Gestational age at fetal death, estimated by foot length (FtL)
Maroun and Graem11 provided norms for fetal foot length at delivery for stillbirths based on a quadratic function of completed weeks of gestation, but did not publish their regression equation. Their model can be formulated, using exact coefficients provided by the authors (personal communication), as- foot length (cm) = a(GAcompleted weeks)2 + b(GAcompleted weeks) + c
Taking the inverse by the quadratic formula, we have:- GAFtL(inverse) = (−b + [b2 − 4a{c − foot length (cm)}]1/2) / 2a
This is a fractional quantity, but is based on a formula derived using completed weeks of gestation. Since the number of completed weeks understates by 3 days the average gestational age in completed days for a completed week, the inverse estimate needs to be adjusted upward by 3 days. Thus, we define- GAFtL = GAFtL (inverse) + 3/7.
-
GAFtL,bounded – Gestational age at fetal death by foot length (FtL), bounded by the GA range corresponding to the timing of death interval:
In cases where the length of the timing of death interval is >7 days and the EDD is clinically reliable (Figure 2, row G), the gestational age range corresponding to the timing of death interval is used to bound the estimated GA at fetal death, i.e., if GAFtL falls outside the GA interval for timing of death, the gestational age at fetal death is defined as GADLA or GADoDx, whichever is closest to GAFtL. Thus,GAFtL,bounded = GADoDx where GAFtL ≥ GADoDx = GAFtL where GADLA < GAFtL < GADoDx = GADLA where GAFtL ≤ GADLA
In these cases, the estimated date of fetal death is back-calculated from GAFtL,bounded:- Date of death = EDD − [ (40 − GAFtL,bounded) × 7 ]
Footnotes
The members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Stillbirth Collaborative Research Network (SCRN) include: University of Texas Health Science Center at San Antonio — Donald J. Dudley, Deborah Conway, Josefine Heim-Hall, Karen Aufdemorte, Angela Rodriguez; University of Utah School of Medicine and Intermountain Health Care — Robert M. Silver, Michael W. Varner, Kristi Nelson; Emory University School of Medicine and the Rollins School of Public Health — Carol J. Rowland Hogue, Barbara J. Stoll, Janice Daniels Tinsley, Bahig Shehata, Carlos Abramowsky; Brown University — Donald Coustan, Halit Pinar, Marshall Carpenter, Susan Kubaska; University of Texas Medical Branch at Galveston: George R. Saade, Radek Bukowski, Jennifer Lee Rollins, Hal Hawkins, Elena Sbrana; RTI International — Corette B. Parker, Matthew A. Koch, Vanessa R. Thorsten, Holly Franklin, Pinliang Chen; Pregnancy and Perinatology Branch, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health — Marian Willinger, Uma M. Reddy; Drexel University School of Medicine — Robert L. Goldenberg.
REFERENCES
- 1.Gardosi J, Mul T, Mongelli M, Fagan D. Analysis of birthweight and gestational age in antepartum stillbirths. British Journal of Obstetrics and Gynaecology. 1998;105:524–530. doi: 10.1111/j.1471-0528.1998.tb10153.x. [DOI] [PubMed] [Google Scholar]
- 2.Genest DR, Williams MA, Greene MF. Estimating the time of death in stillborn fetuses: I. Histologic evaluation of fetal organs; an autopsy study of 150 stillborns. Obstetrics and Gynecology. 1992;80:575–584. [PubMed] [Google Scholar]
- 3.Genest DR. Estimating the time of death in stillborn fetuses: II. Histologic evaluation of the placenta; a study of 71 stillborns. Obstetrics and Gynecology. 1992;80:585–592. [PubMed] [Google Scholar]
- 4.Genest DR, Singer DB. Estimating the time of death in stillborn fetuses: III. External fetal examination; a study of 86 stillborns. Obstetrics and Gynecology. 1992;80:593–600. [PubMed] [Google Scholar]
- 5.Alexander GR, Petersen DJ, Powell-Griner E, Tompkins ME. A comparison of gestational age reporting methods based on physician estimate and date of last normal menses from fetal death reports. American Journal of Public Health. 1989;79:600–602. doi: 10.2105/ajph.79.5.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaudino JA, Jr, Blackmore-Prince C, Yip R, Rochat RW. Quality assessment of fetal death records in Georgia: a method for improvement. American Journal of Public Health. 1997;87:1323–1327. doi: 10.2105/ajph.87.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker CB, Carol JRH, Matthew AK, Willinger M, Reddy UM, Thorsten VR. Stillbirth Collaborative Research Network: design, methods and recruitment experience. Paediatrics and Perinatal Epidemiology. 2011;25:425–435. doi: 10.1111/j.1365-3016.2011.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinar H, Koch MA, Hawkins H, Heim-Hall J, Abramowsky CR, Thorsten VR, Carpenter MW, Zhou HH, Reddy UM. The Stillbirth Collaborative Research Network Postmortem Examination Protocol. American Journal of Perinatology. 2011;29:793–802. doi: 10.1055/s-0031-1284228. 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadlock FP, Shah YP, Kanon DJ, Lindsey JV. Fetal crown-rump length: reevaluation of relation to menstrual age (5–18 weeks) with high-resolution real-time US. Radiology. 1992;182:501–505. doi: 10.1148/radiology.182.2.1732970. [DOI] [PubMed] [Google Scholar]
- 10.Hadlock FP, Deter RL, Harrist RB, Park SK. Estimating fetal age: computer-assisted analysis of multiple fetal growth parameters. Radiology. 1984;152:497–501. doi: 10.1148/radiology.152.2.6739822. [DOI] [PubMed] [Google Scholar]
- 11.Maroun LL, Graem N. Autopsy standards of body parameters and fresh organ weights in nonmacerated and macerated human fetuses. Pediatric and Developmental Pathology. 2005;8:204–217. doi: 10.1007/s10024-004-7084-0. [DOI] [PubMed] [Google Scholar]
- 12.American College of Obstetricians and Gynecologists Practice Bulletin #102. Management of Stillbirth. Obstet Gynecol. 2009;113:748–761. doi: 10.1097/AOG.0b013e31819e9ee2. [DOI] [PubMed] [Google Scholar]
- 13. [Accessed 3/5/12];US Standard Certificate of Fetal Death, November 2003 Revision. Available at: http://www.cdc.gov/nchs/data/dvs/FDEATH11-03finalACC.pdf.
- 14.Mercer BM, Sklar S, Shariatmadar A, Gillieson MS, D’Alton ME. Fetal foot length as a predictor of gestational age. Obstetrics and Gynecology. 1987;156:350–355. doi: 10.1016/0002-9378(87)90282-1. [DOI] [PubMed] [Google Scholar]
- 15.Platt LD, Medearis AL, DeVore GR, Horenstein JM, Carlson DE, Brar HS. Fetal foot length: relationship to menstrual age and fetal measurements in the second trimester. Obstetrics and Gynecology. 1988;71:526–531. [PubMed] [Google Scholar]
- 16.Goldstein I, Reece EA, Hobbins JC. Sonographic appearance of the fetal 446 heel ossification centers and foot length measurements provide independent markers for gestational age estimation. Obstetrics and Gynecology. 1988;159:923–926. doi: 10.1016/s0002-9378(88)80172-8. [DOI] [PubMed] [Google Scholar]
- 17.Tricomi V, Kohl SG. Fetal death in utero. American Journal of Obstetrics and Gynecology. 1957;74:1092–1097. doi: 10.1016/0002-9378(57)90162-x. [DOI] [PubMed] [Google Scholar]
- 18.Meirowitz NB, Ananth CV, Smulian JC, McLean DA, Guzman ER, Vintzileos AM. Foot length in fetuses with abnormal growth. Journal of Ultrasound in Medicine. 2000;19:201–205. doi: 10.7863/jum.2000.19.3.201. [DOI] [PubMed] [Google Scholar]
- 19.American College of Obstetricians and Gynecologists Practice Bulletin #55. Management of postterm pregnancy. Obstet Gynecol. 2004;104:639–646. doi: 10.1097/00006250-200409000-00052. [DOI] [PubMed] [Google Scholar]