Abstract
Cell fate reprogramming makes possible the generation of new cell types from healthy adult cells to replace those lost or damaged in disease. Additionally, reprogramming patient cells into specific cell types allows for drug screening and the development of new therapeutic tools. Generation of new neurons is of particular interest because of the potential to treat diseases of the nervous system, such as neurodegenerative disorders and spinal cord injuries, with cell replacement. Recent advances in cell fate reprogramming have led to the development of novel methods for the direct conversion of fibroblasts into neurons and neural stem cells (NSCs). This review will highlight the advantages of these new methods over neuronal induction from embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), as well as outline the limitations and the potential for future applications.
Keywords: Cell fate reprogramming, neurogenesis, fibroblasts, embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), induced neurons (iNs), induced neural stem cells (iNSCs)
Introduction
Early attempts to generate functional neurons required the reprogramming of embryonic stem cells (ESCs) using media containing specific growth factors [1–5]. This process is time-consuming and difficult, but nevertheless demonstrates the tremendous promise of cell replacement therapies using ESCs. However, considerable ethical and political controversy continues to surround ESC research, which has stymied progress in the field and led researchers to look for other ways to approach the needs of generating new neurons [6, 7].
A major breakthrough in the field came in 2006 when the Yamanaka lab generated induced pluripotent stem cells (iPSCs) from mouse and human fibroblasts using a cocktail of transcription factors, consisting of Oct3/4, Sox2, Klf4 and c-Myc, termed OSKM [8, 9]. These iPSCs can be induced to differentiate into any cell type. Several groups have used various methods to promote the conversion of fibroblasts harvested from patients with various neurological disorders into iPSCs and subsequently functional neurons [10–13]. Since the first generation of iPSCs, somatic cell fate reprogramming has emerged as a field with growing potential, both in basic research and clinical work. However, these efforts are often limited by the low efficiency of iPSC conversion, as well as the risk of tumorigenesis, which is inherent when working with oncogenes and rapidly proliferating multipotent or pluripotent cells.
Recent work emerging in stem cell research offers a method that may avoid many of the ethical and clinical issues that have hindered previous techniques. Direct somatic cell fate reprogramming, sometimes referred to as induced trans-differentiation, involves the direct conversion from one differentiated cell type to another distinct cell type without a reversion to iPSCs or use of oncogenes. In the last few years, this novel technique has been shown to be a rapid and efficient method of generating functional induced neurons (termed iNs) and induced neural stem cells (iNSCs). These recent studies suggest the potential power of this technique to directly and efficiently convert fully differentiated cells into mature iNs while avoiding oncogene use, tumorigenic pluripotent or multipotent stem cell stages and ethical quandaries due to the origin of the tissue.
Starting cell types for generating neurons
Thus far, fibroblasts have been the starting point of choice for cell fate reprogramming to generate neurons, but the selection is not limited to fibroblasts. Several other cell types, including somatic cells such as hepatocytes as well as germ cells, have been successfully reprogrammed into functional iNs, demonstrating that interlineage trans-differentiation is possible [14, 15]. Nevertheless, fibroblasts remain the preferred cell type due to their relative availability. Fibroblasts can easily be obtained from patients through minimally invasive methods, making the generation of patient-specific cells relatively simple.
A second important factor that must be considered in cell fate reprogramming is the origin of the cell lineages. Fibroblasts differentiate from mesenchymal progenitor cells, some of which are derived from neural crest lineages. Neural crest cells originate in the ectoderm on the dorsal tip of the early embryonic neural tube. From there, they progress through an epithelial-mesenchymal transition and pervasive migration, ultimately differentiating into an array of tissues throughout the body. Consequently, fibroblasts share a neuro-ectodermal lineage with neuronal cells, unlike, for example, hepatocytes, which are derived from the endoderm. Moreover, fibroblast cultures are likely heterogeneous in cell types and often contain neural crest-derived stem cells [16]. These cultures may contain multipotent stem cells with the capacity to differentiate into neurons, due in part to their shared lineage. Therefore, easy access and lineage features make fibroblasts the favorite cell type for reprogramming to neurons.
Basic mechanism of iPSC generation
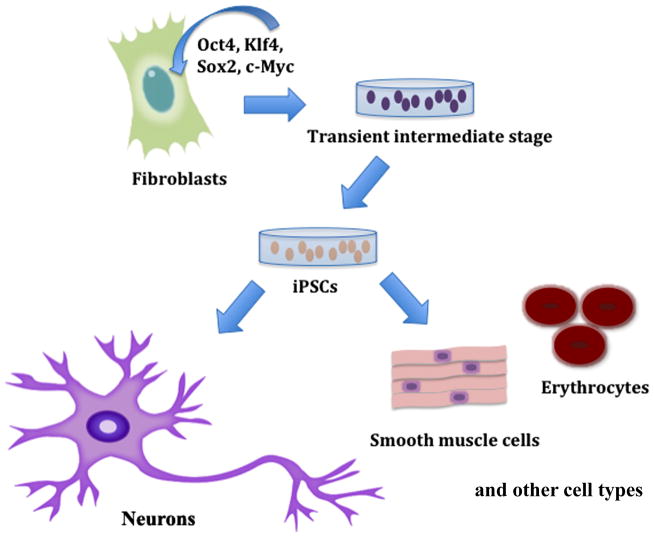
The function of the original OSKM transcription factor cocktail has been extensively studied to better understand how these factors are able to induce self-renewal and pluripotency in terminally differentiated cells (Fig. 1).
Figure 1.
Generation of neuronal cells via reprogramming of induced pluripotent stem cells (iPSCs). Fibroblast conversion to iPSCs is mediated by the OSKM cocktail. Neurons, and other cell types such as smooth muscle cells and erythrocytes, can be generated from IPSCs in different growth conditions. OSKM: Oct3/4, Sox2, Klf4, c-Myc.
Oct3/4, also called Pou5f1, regulates the self-renewal capacity of undifferentiated ESCs. This tightly regulated transcription factor may also play a similar role in blocking differentiation in adult stem cells, although this remains an area of debate [17–22]. Like Oct3/4, Sox2 is a regulator of self-renewal in stem cells [23–26]. Sox2 together with Oct4 promote pluripotency by driving expression of the transcription factor nanog, which regulates the self-renewal properties of ESCs [27, 28]. Klf4 is a positive regulator of cell proliferation and survival. Highly expressed in ESCs, Klf4 also acts as a tumor suppressor [29–33]. The oncogenic transcription factor c-Myc, a downstream target of the MAPK/ERK signaling pathway, functions as an activator of cell proliferation and a repressor of differentiation [34–37]. Although more detailed biochemical work is necessary to address the molecular mechanisms of reprogramming by these four transcription factors, it appears that the conjugate action of OSKM is sufficient for converting fibroblasts into iPSCs (Fig. 1).
Advantages and limitations of iPSCs
iPSCs offer several advantages over ESCs. First, use of iPSCs avoids the controversy that has surrounded ESC research because it does not require the destruction of an early stage embryo. Second, iPSCs can be derived from an individual patient’s fibroblasts and thus exhibit the unique genetic identity of that patient. This is important from a research perspective, because human iPSCs allow for the study of specific genetic abnormalities that can lead to neurological diseases, rather than attempting to replicate and study disease progression in animal models [12, 13, 38, 39]. For example, several groups have identified the cellular phenotypes of the rare neurodevelopmental disorder, Rett Syndrome, through study of neurons derived from iPSCs isolated from patients with Rett Syndrome [39, 40]. The disease has previously been shown to result from loss of function mutations in the gene that encodes methyl-CpG-binding protein 2 (MeCP2). Marchetto et al., described the morphological characteristics of these diseased neurons, such as a reduction in dendritic density and soma size and defects in synapse formation, in neurons derived from patient iPSCs. They also observed that synaptic defects could be partially rescued with treatments of IGF-1 [39]. This provides direct evidence that has previously only been observed in neurons of mouse models but not from humans. Furthermore, iPSC-derived neurons also have the potential to replace neurons that are lost or damaged in disorders of the nervous system. For example, motor and dopaminergic neurons have been derived from the iPSCs of patients with amyotrophic lateral sclerosis (ALS) and Parkinson’s disease (PD), respectively [10, 41]. Dimos et al., found that iPSCs generated from ALS patients appear similar to ESCs in gene expression, and in self-renewal and pluripotent capacity [10]. Devine et al., found that dopamine neurons differentiated from the PD patient-derived iPSCs express twice the α-synuclein protein as neurons from healthy individuals, making it an ideal system for studying the development of PD at the cellular level as well as drug screening [41]. The neurons derived from diseased patients are similar to iPSC-derived neurons from healthy individuals. However, whether the iPSC-derived neurons retain epigenetic memory from the diseased donor tissue remains to be determined before they can be considered suitable for transplantation in patients.
While these results are promising, there are still many limitations of this technique that make it difficult to translate into clinical application. Abnormalities, at the genetic and epigenetic level, that arise in iPSCs are a major hurdle that must be overcome before iPSC-derived neurons can be widely used in a clinical setting. These aberrations include genetic damage, such as copy number variations, abnormal karyotypes, including chromosomal translocations and duplications, and point mutations, which accumulate in cultured iPSCs [42, 43]. Presence of epigenetic factors that may contribute to disease susceptibility and progression in patient-derived iPSCs is another area of concern. For example, iPSCs may carry epigenetic memory from their starting tissue. Epigenetic errors that have been observed in iPSCs include aberrant histone modifications and DNA methylation defects. Recent studies that analyze extensive genome-wide genetic and epigenetic profiling across iPSC cell lines aim to better elucidate the changes at the genome level, which occur in de-differentiated cells [44, 45].
Furthermore, the use of the oncogene c-Myc in the OSKM cocktail, while helping to induce stem cell qualities from fibroblasts, increases the risk of tumor formation, thus limiting the potential for clinical use of iPSC-derived neurons. Researchers have attempted to address this problem by using growth factors or chemical cocktails to guide re-differentiation. Numerous molecules have been shown to enhance fibroblast de-differentiation and replace the oncogenic components of the reprogramming cocktail [46–50]. These include growth factors, such as fibroblast growth factors (FGFs), the cytokine leukemia inhibitory factor (LIF) and cell signaling molecules such as Wnts [48, 51–53]. Chemicals, such as histone deacetylase (HDAC) inhibitors and DNA methyltransferase inhibitors, have also been used by several groups to enhance fibroblast reprogramming [54, 55]. The promising technological development of such chemicals provides great hope that patient-specific iNs can be generated and implanted without unnecessary tumor risk to the patient by using oncogenes in the reprogramming.
Basic mechanisms of direct fibroblast to neuron conversion
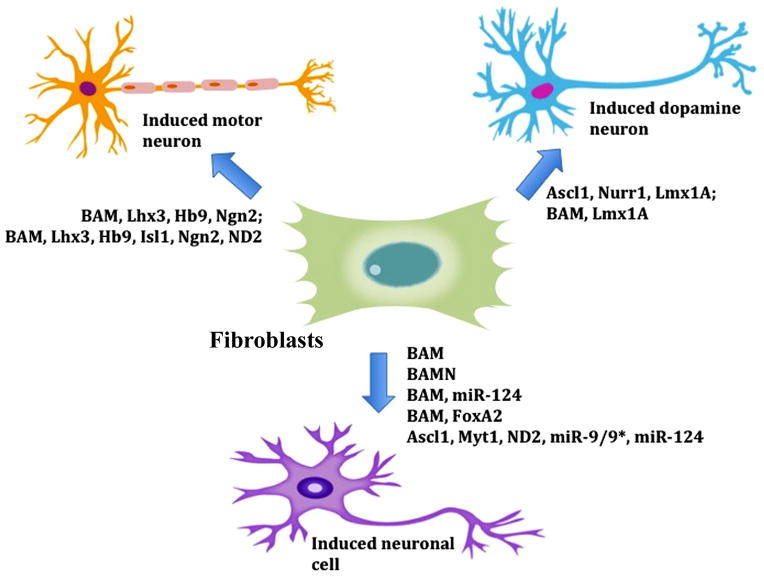
The next step forward in iN generation is direct generation of neurons from fibroblasts. This process involves inducing increased expression levels of particular reprogramming factors in fibroblasts, thereby mediating a cell fate conversion process that entirely avoids the iPS cell phase. Successful attempts have induced trans-differentiation with neurogenic transcription factors such as Brn2, in combination with microRNAs, and chemicals, including HDAC inhibitors, all of which mediate and enhance the reprogramming process (Fig. 2).
Figure 2.
Direct generation of induced neurons (iNs) from fibroblasts. Fibroblasts are directly converted into iNs, bypassing intermediate and pluripotent cell stages. A variety of factors can mediate and enhance the conversion process to distinct neurons. BAM: Brn2, Ascl1, Myt11.
Neurogenic transcription factors
In the last few years, several groups have generated iNs from fibroblasts using combinations of neurogenic factors (Fig. 2). Although the mechanism by which these factors promote induced trans-differentiation is poorly understood, it appears that the transcription factors are capable of reprogramming embryonic and adult fibroblasts to stable, functioning iNs. Several groups independently used neurogenic cocktails containing combinations of Brn2/Pou3f2, Ascl1/MASH1 and Myt1l (called the BAM cocktail) in addition to other neurogenic factors to directly convert fibroblasts to iNs [56–61].
Brn2 is expressed specifically in the neuro-ectodermal cell lineage [62–64]. Ascl1/Mash1 is important for certain neural lineages, for example autonomic neuronal precursors, during early development [65, 66]. Myt1l appears to play a role in neuronal development and differentiation [62–64, 67, 68]. This BAM cocktail was initially considered to be the minimum components required to generate functional iNs. More recent evidence suggests that some of these factors are dispensable and that the reprograming process can be enhanced and the efficiency improved by other factors, such as neurogenic differentiation 1/2 (NeuroD1/2) [57]. Pang et al., found that the addition of NeuroD1 to the cocktail, termed BAMN, can induce reprogramming of both fetal and postnatal human fibroblasts to neurons (hiNs) with similar efficiencies of 2–4% [57]. Using the BAM cocktail, Pfisterer et al., demonstrated a successful reprogramming of embryonic and adult human fibroblasts to hiNs with efficiencies of 16% and 4%, respectively [58]. They observed that the successful conversion of human fetal fibroblasts to immature hiNs required 20 days of transgene expression.
Dopaminergic factors
Several groups have directly reprogrammed fibroblasts to induced dopamine neurons (iDA) by overexpressing dopamine neuron lineage-specific factors that act during brain development, including genes involved in midbrain dopamine neuron development. Pfisterer et al., found that two such genes, Lmx1a and FoxA2, optimize generation of human iDAs when added to the BAM cocktail [61] (Fig. 2). These iDAs express genes characteristic of normal midbrain DAs. With the use of doxycycline-inducible lentiviral vectors, Caiazzo et al., overexpressed varying combinations of 11 dopamine neuron-inducing factors in mouse embryonic fibroblasts (MEFs) from TH-GFP transgenic mice [69]. The authors found that the combination of Mash1/Nurr1 (Nr4a2)/Lmx1a induces the highest efficiency of reprogrammed (GFP-positive) cells at 18%. The majority (85%) of the induced cells are double positive for the neuronal marker, Tuj1, and tyrosine hydrolase (TH), a dopamine cell marker [69]. Expression of the reprogramming factors is only necessary for 6 days for successful conversion. This group also demonstrated that the same combination of factors can efficiently reprogram human embryonic fibroblasts (HEFs) to Tuj1+/TH+ neuronal cells. Additionally, the cocktail is successfully used to convert the fibroblasts of Parkinson’s disease patients and healthy adult donors to iNs and iDAs [69]. The resulting reprogrammed cells express multiple midbrain DA markers and are functional through electrophysiological analyses.
Motor neuron-specific factors
Using a cocktail of BAM and different combinations of seven known motor neuron specific factors, Son et al., determined the most effective combinations (Lhx3, Hb9, Isl1, Ngn2 and Lhx3, Hb9, Isl1, Ngn2, NeuroD1) to efficiently convert embryonic fibroblasts to functional induced motor neurons (iMNs) [59] (Fig. 2). This combination of factors yields a conversion efficiency of 5%–10% functional mouse iMNs. The cocktail was also applied, with the addition of NeuroD1, to HEFs, inducing the generation of functional cholinergic iMNs in under 2 weeks.
microRNAs
Several microRNAs (miRNAs) have been shown to be required for rapid cell proliferation and cell-cycle progression in embryonic and neural stem cells, including the miR-290–295 cluster in mice, the miR-371–373 cluster in humans, and miR-302/367, miR-9/9* and miR-124 in both species [70–76]. Yoo et al., demonstrated that the miRNAs miR-9/9* and miR-124 are critical in the regulation of neuronal differentiation [60]. The forced expression of miR-9/9* and miR-124, along with Ascl1, Myt1 and NeuroD2 can efficiently reprogram human fibroblasts to iNs (Fig. 2). The efficiency of the conversion is significantly increased by the addition of the neurogenic transcription factor NeuroD2, at ~10% compared to ~5% in the absence of NeuroD2. Interestingly, they also found that the transcription factor ASCL1/MASH1 is not necessary for the induced trans-differentiation of fibroblasts to iNs [60]. miR-124 and miR-9/9* appear to mediate neuronal differentiation synergistically. In fact, these miRNAs promote neuronal differentiation and are highly expressed in postmitotic neurons [72, 77, 78]. The addition of these miRNAs to the reprogramming cocktail induces spontaneous synaptic activity in iNs [60]. Ambasudhan et al., found that a combination of miR-124 and two neurogenic transcription factors Brn2 and Myt1l can directly reprogram both postnatal and adult human fibroblasts to functional iNs with comparable conversion efficiencies [56]. These iNs exhibit normal neuronal morphology, gene expression, spontaneous action potential firing and functional synapse formation. Interestingly, this cocktail primarily generates GABAergic and glutamatergic iNs, while yielding few dopamine or serotonin iNs.
Characterization of iNs
An essential question is whether iNs converted directly from fibroblasts are really functional neurons. Thus, characterization of iNs is critical for attempts to apply these cells clinically. Morphologically, iNs are observed for characteristics of normal neuronal morphology such as axonal and dendritic projections. Genetically, iNs are determined if they exhibit gene expression normally observed in a mature neuron. iNs should express Tuj1 and NeuN, as well as genes specific to a particular neuronal subtype while also demonstrating the loss of genes specific to the original cell-type. Caiazzo et al., found that reprogramming with their dopamine-specific neurogenic gene cocktail largely eliminates the expression of genes characteristic of fibroblasts as well as other closely related neuronal subtypes, such as serotonergic and adrenergic genes [69].
Functionally, iNs are analyzed by the capacity for synaptic transmission as well as electrophysiological properties. Potential for synaptic transmission is determined by both visualizing the expression of proteins such as synapsin and synaptotagmin, in addition to synaptic vesicle fusion and release of neurotransmitters [79, 80]. Electrophysiological analysis determines if the iNs are capable of spontaneous action potential firing, necessary for functional characterization of mature iNs. BAMN directed iNs generate spontaneous action potentials and are capable of forming synapses [57]. Caiazzo et al., observed neuronal electrophysiological properties and dopamine uptake in iDAs [69]. Son et al., found that iMNs also exhibit neuronal electrophysiological and synaptic properties [59]. Moreover, these iMNs are successfully grafted in vivo, with results similar to those seen using embryo-derived motor neurons.
Another important consideration in cell fate reprogramming is the stability of induction. Stably induced neurons should exhibit the functional neuronal characteristics described above in the absence of the initial inducing factors. Caiazzo et al., found that the number of functional iDAs remains unchanged up to 24 days after doxycycline withdrawal and deactivation of the reprogramming factor cocktail [69]. Likewise, Yoo et al., observed stable neuronal identity three weeks after removal of the reprogramming cocktail [60]. These results suggest that once fibroblasts are reprogrammed into iNs, these iNs maintain neuronal properties even after withdrawal of inducing factors. However, further analysis is required to ensure that expression of the reprogramming factors is in fact eliminated after doxycycline withdrawal, as residual expression is sometimes observed in this system.
Direct generation of induced neural stem cells from fibroblasts
One method of generating neuronal cells from fibroblasts without the use of iPSCs is to first generate neural stem cells (NSCs), which can give rise to neurons, astrocytes or oligodendrocytes. Recently, several groups directly converted fibroblasts into induced NSCs (iNSCs), effectively bypassing the pluripotent stage [81] (Fig. 3). Thier et al., used a reprograming cocktail similar to OSKM, but with restricted expression of Oct4. By infecting MEFs with a retrovirus constitutively expressing SKM and a doxycycline-inducible lentiviral vector expressing Oct4 only for up to 5 days, they observed neurosphere-like colonies 11 days after infection. Neurosphere-like colonies with what appear to be axonal processes were observed in the iNSCs cultures 25 days after infection. After 31 passages the iNSCs showed expression of neural stem cell and neuronal precursor-specific markers Nestin and Pax6. Most of the iNSC-derived neurons are GABAergic [82]. Han et al., used two reprogramming cocktails, both containing Brn4/Pou3f4, Sox2, Klf4, c-Myc (referred to as 4 factors or 4F) with one also including the transcription factor E47/Tcf3 (5F) [83]. The iNSC cultures can be stably maintained for more than 130 passages. After 4 to 5 weeks of expression of the reprogramming factors, iNSC clusters that express NSC-specific markers, such as Olig2 and SSEA1, were observed. The reprogramming cocktail lacking E47 expression yielded similar results but with a lower efficiency. Most of the iNSCs neurons are GABAergic or glutamatergic with a small percentage of cholinergic neurons. Interestingly, some of the 5F iNSC-derived neurons also express TH [83]. In both studies, the iNSCs express synaptic proteins and appear to be capable of forming synapses.
Figure 3.
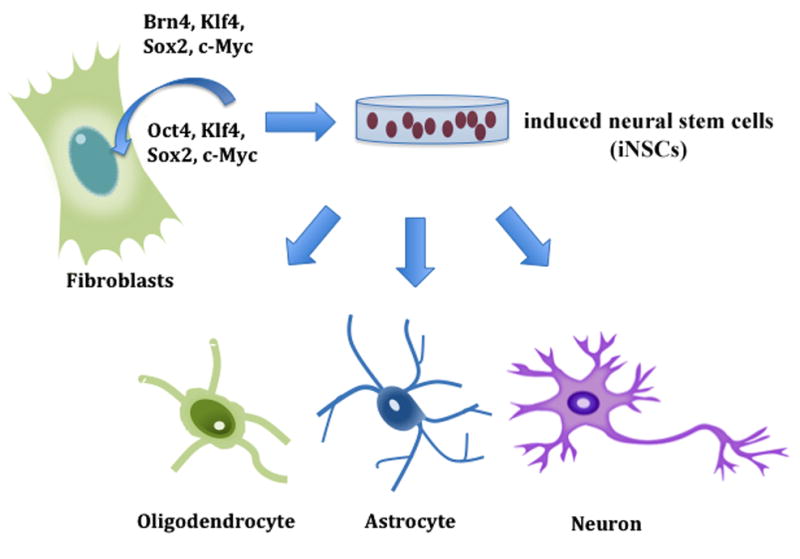
Direct generation of induced neural stem cells (iNSCs) from fibroblasts. Fibroblasts are directly converted into multipotent iNSCs, which can subsequently be differentiated into neurons, oligodendrocytes and astrocytes.
Both groups used a combination of reprogramming factors and neural-specific transcription factors to generate iNSC lines that are similar in morphology and gene expression profiles to control NSC lines. The iNSCs can differentiate into neurons and glia with efficiencies similar to control NSC lines, indicating the multipotency of the iNSCs. The iNSC-derived neurons are functional as demonstrated by electrophysiology analysis. Finally, Thier et al., and Han et al., tested the ability of iNSCs to integrate and differentiate in vivo by transplanting them into the neonatal rat brain and the subventricular zone of the adult mouse brain, respectively. Both groups observed that grafted iNSCs are able to survive and differentiate in vivo [82, 83].
Although this method is promising, it does have several limitations. First, the expression profile of the iNSC-derived neural cells suggests that they might retain some epigenetic memory from the donor tissue. However, some reprogramming does occur at the epigenetic level, as evidenced by the similarities in methylation of Nestin in control and iNSC-derived neurons, when compared to fibroblasts. Second, Han et al., observed expression of the transgenes Sox2, Klf4 and Brn2 in the 5F-derived population [83]. Finally, the efficiency in differentiation of oligodendrocytes appears to be low. This is also observed in neural progenitor induction from fibroblasts temporally treated with the OSKM cocktail and subsequently with neural reprogramming medium [84].
Advantages of direct neuronal induction
iPSCs are a great leap forward, giving us the theoretical ability to generate patient-specific cells for developing tools for clinical treatment, for instance generating genetically matched dopamine neurons from patients with Parkinson’s disease [41]. The process, however, has not been particularly conducive to the clinic. The low efficiency of iPSC generation has limited the clinical application of iPSCs. In many cases, the reprogramming efficiencies have been as low as 0.1%. The recent emerging methods for direct conversion of fibroblasts to iNs have yielded far higher efficiencies using mouse (2–18%) and human (2–10%) fibroblasts (Table 1). Induced trans-differentiation is also a more rapid process than differentiation from stem cells, taking as little as 6–8 days to generate mature iNs from fibroblasts, compared to 2–6 weeks required by stem cell reprogramming. Additionally, non-coding RNAs and small molecules can enhance the reprogramming process and improve efficiency.
Table 1.
Summary of direct conversion of fibroblasts to induced neurons (iNs), neural stem cells (iNSCs) and neural progenitor cells (iNPCs).
| Cell type | Reprogramming factors | Efficiency | Time (days) | Reference |
|---|---|---|---|---|
| iN | BAM | 1.8–7.7% | 8–20 | [61] |
| hiN | BAM, miR-124 | 4–8% | 14–21 | [56] |
| hiN, iDA, hiDA | Ascl1, Nurr1, Lmx1A | 5–10%, 3–6% | 6–16 | [69] |
| hiN | BAM, NeuroD1 | 2–4% | 14–34 | [57] |
| hiN, hiDA | BAM, FoxA2, Lmx1A | 4–16%; ~10% | 20–24 | [58] |
| iMN | BAM, Lhx3, Hb9, Ngn2; BAM, Lhx3, Hb9, Isl1, Ngn2, NeuroD1 | 5–10% | 7–14 | [59] |
| hiN | Ascl1, Myt1, NeuroD2, miR-9/9*, miR-124 | ~5–10% | 28 | [60] |
| iNSC, iNPC | Oct4, Sox2, Klf4, c-Myc | N/A* | 13–25 | [82, 84] |
| iNSC | Brn4, Sox2, Klf4, c-Myc, E47/Tcf3 | N/A* | 28–35 | [83] |
BAM: Brn2, Ascl1, Myt1l
Efficiency represents percentage of neural cells that were generated from fibroblast-derived iNSC and iNPCs.
A distinct advantage of direct neuronal induction is avoiding the potential for tumor growth inherent in generating neurons, or indeed any other cell, from iPSCs. Induced multipotent and pluripotent cells can form after transplantation, and retroviral vector-mediated transgene delivery used in the iPSCs conversion studies can integrate into the host genome and activate oncogenes [85]. The original cocktail of transcription factors used to differentiate iPSCs contains the oncogene c-Myc, which can promote tumorigenesis [86–89]. Takahashi et al., found that in mouse iPS clones, approximately 20% of the mice derived from iPSCs develop tumors [9]. Direct cell fate reprogramming bypasses the tumorigenic pluripotent stem cell stage, and avoids the use of c-Myc, thus greatly reducing the risk of tumor formation after transplantation. Moreover, the issue of retroviral integration presents a different hurdle. However, emerging nonviral methods of transgene delivery may help to eliminate this particular obstacle.
Limitations and disadvantages
Low efficiency is not uncommon in cell fate reprogramming and the efficiency of direct reprogramming of fibroblasts to iNs is much higher than admittedly low efficiencies observed in generation of neurons from iPSCs. However, Yoo et al., observed that this efficiency gain is slightly less in generation of iNs from adult human fibroblasts [60]. Maturation of the iNs is also delayed in these cells. In general, conversion of human fibroblasts to hiNs was less efficient than mouse fibroblast conversion.
In several important characteristics, iNs more closely resemble neurons than fibroblasts. Specifically, in genetic profiling they express crucial neuronal factors and signaling molecules, synaptic proteins, neurotransmitter synthesis proteins and receptors, as well as axonal guidance molecules. However, the question still remains of whether iNs are truly functional neurons applicable in a clinical setting. Expression of fibroblast-specific genes is reduced, but is still detected in iN cell populations [11, 69]. The extent and effect of the expression of these uncharacteristic genes in iNs has yet to be evaluated. Similarly, it is still unknown whether iNs are abnormal in their expression of other key neuronal genes and whether they will perform as expected in the host tissue.
The retroviral delivery of neurogenic transcription factors can cause potentially damaging integration into the host genome. In fact, iPS clones contain multiple retroviral integrations for each factor used. Moreover, a recent study showed that a significant number of mutations in the genome can be induced during cell reprogramming. Genes that function in the regulation of cell cycling and growth are often sites of retroviral integration. This propensity for integration makes certain retroviral vectors particularly genotoxic. Lentiviral vectors, such as those used by Caiazzo et al., to generate iDAs [69], are less likely than other retroviral vectors to integrate into the host genome in proto-oncogenes or other cancer-associated hot spots, but the risk remains [85, 91]. Recently, a highly efficient, nonviral system of transgene delivery called ePiggyBac has been shown to successfully differentiate human ESCs into neuronal precursors and neurons [92]. This system is more efficient than retroviral-mediated differentiation as well as previous PiggyBac transgenes. The method is also reversible, allowing for removal of the ePiggyBac transgenes without mutations in the human genome. Another advantage of the ePiggyBac system is its ability to integrate large inserts into the genome. Non-viral transgene delivery systems like ePiggyBac eliminate the risk for genotoxicity that results from use of retroviral vectors. This system has the potential to mediate safer and more efficient direct trans-differentiation of fibroblasts into induced neurons and neural stem cells.
Perspectives
One promising approach for the next wave of technology is synthetic messenger RNAs. Synthetic mRNAs offer the possibility to reprogram cells without the risk of genomic integration present with the use of retroviral vectors. Warren et al., described a method of highly efficient cell fate reprogramming by administering synthetic mRNA to several somatic cell types, including embryonic and adult fibroblasts. They are able to reprogram these somatic cells to pluripotent cells at efficiencies greater than 2% [93]. The use of synthetic mRNA to mediate reprogramming is an exciting and promising prospect.
The addition or replacement of individual transcription factors with small molecules, such as the HDAC inhibitor valproic acid, in a reprogramming cocktail has been shown to improve efficiency [46–49, 54]. Notably, valproic acid, when administered with the miR-302/367 cluster, is required for the reprogramming of mouse cells. Anokye-Danso et al., demonstrated that expression of the miR302/367 cluster with the addition of valproic acid-mediated suppression of HDAC2 is sufficient to promote efficient fibroblast de-differentiation [70]. The application of small molecules like valproic acid to induce the trans-differentiation of fibroblasts to iNs can enhance the conversion process and increase efficiency.
Novel methods for cell fate reprogramming such as synthetic mRNAs, small molecules and a non-integrating transgene delivery system, all of which eliminate the risk of genomic integration, should be further developed for use in a clinical setting. The implications of these novel technologies offer considerable promise for the future of cell replacement therapies to treat neurological disorders.
Acknowledgments
This work was supported by New York State Department of Health, C026878 (A. P.), the Ellison Medical Foundation (T. S.), an award from the Hirschl/Weill-Caulier Trust (T. S.) and an R01-MH083680 grant from the NIH/NIMH (T. S.).
References
- 1.Kawasaki H, et al. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28(1):31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- 2.Lee SH, et al. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18(6):675–9. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- 3.Okabe S, et al. Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cells in vitro. Mech Dev. 1996;59(1):89–102. doi: 10.1016/0925-4773(96)00572-2. [DOI] [PubMed] [Google Scholar]
- 4.Reubinoff BE, et al. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18(4):399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 5.Thomson JA, V, Marshall S, Trojanowski JQ. Neural differentiation of rhesus embryonic stem cells. APMIS. 1998;106(1):149–56. doi: 10.1111/j.1699-0463.1998.tb01330.x. discussion 156–7. [DOI] [PubMed] [Google Scholar]
- 6.Cohen CB. Ethical and policy issues surrounding the donation of cryopreserved and fresh embryos for human embryonic stem cell research. Stem Cell Rev. 2009;5(2):116–22. doi: 10.1007/s12015-009-9060-6. [DOI] [PubMed] [Google Scholar]
- 7.Jung JU, et al. The roles of glycosphingolipids in the proliferation and neural differentiation of mouse embryonic stem cells. Exp Mol Med. 2009;41(12):935–45. doi: 10.3858/emm.2009.41.12.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Dimos JT, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321(5893):1218–21. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 11.Jin J, et al. Analysis of differential proteomes of induced pluripotent stem cells by protein-based reprogramming of fibroblasts. J Proteome Res. 2011;10(3):977–89. doi: 10.1021/pr100624f. [DOI] [PubMed] [Google Scholar]
- 12.Park IH, et al. Disease-specific induced pluripotent stem cells. Cell. 2008;134(5):877–86. doi: 10.1016/j.cell.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiang L, et al. Directed conversion of Alzheimer’s disease patient skin fibroblasts into functional neurons. Cell. 2011;146(3):359–71. doi: 10.1016/j.cell.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Marro S, et al. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell. 2011;9(4):374–82. doi: 10.1016/j.stem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tursun B, et al. Direct conversion of C. elegans germ cells into specific neuron types. Science. 2011;331(6015):304–8. doi: 10.1126/science.1199082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayreuther K, et al. Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Proc Natl Acad Sci U S A. 1988;85(14):5112–6. doi: 10.1073/pnas.85.14.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hochedlinger K, et al. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121(3):465–77. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Lengner CJ, et al. Oct4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell. 2007;1(4):403–15. doi: 10.1016/j.stem.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lengner CJ, Welstead GG, Jaenisch R. The pluripotency regulator Oct4: a role in somatic stem cells? Cell Cycle. 2008;7(6):725–8. doi: 10.4161/cc.7.6.5573. [DOI] [PubMed] [Google Scholar]
- 20.Looijenga LH, et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res. 2003;63(9):2244–50. [PubMed] [Google Scholar]
- 21.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24(4):372–6. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 22.Zangrossi S, et al. Oct-4 expression in adult human differentiated cells challenges its role as a pure stem cell marker. Stem Cells. 2007;25(7):1675–80. doi: 10.1634/stemcells.2006-0611. [DOI] [PubMed] [Google Scholar]
- 23.Avilion AA, et al. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17(1):126–40. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyer LA, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122(6):947–56. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chew JL, et al. Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol. 2005;25(14):6031–46. doi: 10.1128/MCB.25.14.6031-6046.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masui S, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9(6):625–35. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- 27.Loh YH, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38(4):431–40. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 28.Rodda DJ, et al. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280(26):24731–7. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, et al. Transcriptional profiling of Kruppel-like factor 4 reveals a function in cell cycle regulation and epithelial differentiation. J Mol Biol. 2003;326(3):665–77. doi: 10.1016/S0022-2836(02)01449-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rowland BD, Bernards R, Peeper DS. The KLF4 tumour suppressor is a transcriptional repressor of p53 that acts as a context-dependent oncogene. Nat Cell Biol. 2005;7(11):1074–82. doi: 10.1038/ncb1314. [DOI] [PubMed] [Google Scholar]
- 31.Wei D, et al. Kruppel-like factor 4 induces p27Kip1 expression in and suppresses the growth and metastasis of human pancreatic cancer cells. Cancer Res. 2008;68(12):4631–9. doi: 10.1158/0008-5472.CAN-07-5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang WT, Zheng PS. Kruppel-like factor 4 function as a tumor suppressor in cervical carcinoma. Cancer. 2011 doi: 10.1002/cncr.26698. [DOI] [PubMed] [Google Scholar]
- 33.Yoon HS, Chen X, Yang VW. Kruppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage. J Biol Chem. 2003;278(4):2101–5. doi: 10.1074/jbc.M211027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bouchard C, Staller P, Eilers M. Control of cell proliferation by Myc. Trends Cell Biol. 1998;8(5):202–6. doi: 10.1016/s0962-8924(98)01251-3. [DOI] [PubMed] [Google Scholar]
- 35.Facchini LM, Penn LZ. The molecular role of Myc in growth and transformation: recent discoveries lead to new insights. FASEB J. 1998;12(9):633–51. [PubMed] [Google Scholar]
- 36.Leone G, et al. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature. 1997;387(6631):422–6. doi: 10.1038/387422a0. [DOI] [PubMed] [Google Scholar]
- 37.Marcu KB, Bossone SA, Patel AJ. myc function and regulation. Annu Rev Biochem. 1992;61:809–60. doi: 10.1146/annurev.bi.61.070192.004113. [DOI] [PubMed] [Google Scholar]
- 38.Urbach A, et al. Differential modeling of fragile X syndrome by human embryonic stem cells and induced pluripotent stem cells. Cell Stem Cell. 2010;6(5):407–11. doi: 10.1016/j.stem.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchetto MC, et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143(4):527–39. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheung AY, et al. Isolation of MECP2-null Rett Syndrome patient hiPS cells and isogenic controls through X-chromosome inactivation. Hum Mol Genet. 2011;20(11):2103–15. doi: 10.1093/hmg/ddr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devine MJ, et al. Parkinson’s disease induced pluripotent stem cells with triplication of the alpha-synuclein locus. Nat Commun. 2011;2:440. doi: 10.1038/ncomms1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gore A, et al. Somatic coding mutations in human induced pluripotent stem cells. Nature. 2011;471(7336):63–7. doi: 10.1038/nature09805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hussein SM, Nagy K, Nagy A. Human induced pluripotent stem cells: the past, present, and future. Clin Pharmacol Ther. 2011;89(5):741–5. doi: 10.1038/clpt.2011.37. [DOI] [PubMed] [Google Scholar]
- 44.Bock C, et al. Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell. 2011;144(3):439–52. doi: 10.1016/j.cell.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boulting GL, et al. A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol. 2011;29(3):279–86. doi: 10.1038/nbt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen S, et al. Self-renewal of embryonic stem cells by a small molecule. Proc Natl Acad Sci U S A. 2006;103(46):17266–71. doi: 10.1073/pnas.0608156103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen S, et al. Dedifferentiation of lineage-committed cells by a small molecule. J Am Chem Soc. 2004;126(2):410–1. doi: 10.1021/ja037390k. [DOI] [PubMed] [Google Scholar]
- 48.Li W, et al. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4(1):16–9. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Xu Y, et al. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci U S A. 2010;107(18):8129–34. doi: 10.1073/pnas.1002024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 51.Maherali N, Hochedlinger K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;3(6):595–605. doi: 10.1016/j.stem.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 52.Marson A, et al. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3(2):132–5. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ying QL, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453(7194):519–23. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huangfu D, et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26(7):795–7. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shi Y, et al. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;2(6):525–8. doi: 10.1016/j.stem.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 56.Ambasudhan R, et al. Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell. 2011;9(2):113–8. doi: 10.1016/j.stem.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pang ZP, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476(7359):220–3. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pfisterer U, et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A. 2011;108(25):10343–8. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Son EY, et al. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9(3):205–18. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoo AS, et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476(7359):228–31. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vierbuchen T, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–41. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Catena R, et al. Conserved POU binding DNA sites in the Sox2 upstream enhancer regulate gene expression in embryonic and neural stem cells. J Biol Chem. 2004;279(40):41846–57. doi: 10.1074/jbc.M405514200. [DOI] [PubMed] [Google Scholar]
- 63.Jin Z, et al. Different transcription factors regulate nestin gene expression during P19 cell neural differentiation and central nervous system development. J Biol Chem. 2009;284(12):8160–73. doi: 10.1074/jbc.M805632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sugitani Y, et al. Brn-1 and Brn-2 share crucial roles in the production and positioning of mouse neocortical neurons. Genes Dev. 2002;16(14):1760–5. doi: 10.1101/gad.978002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lo L, Sommer L, Anderson DJ. MASH1 maintains competence for BMP2-induced neuronal differentiation in post-migratory neural crest cells. Curr Biol. 1997;7(6):440–50. doi: 10.1016/s0960-9822(06)00191-6. [DOI] [PubMed] [Google Scholar]
- 66.Parras CM, et al. Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 2002;16(3):324–38. doi: 10.1101/gad.940902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim JG, et al. Myelin transcription factor 1 (Myt1) of the oligodendrocyte lineage, along with a closely related CCHC zinc finger, is expressed in developing neurons in the mammalian central nervous system. J Neurosci Res. 1997;50(2):272–90. doi: 10.1002/(SICI)1097-4547(19971015)50:2<272::AID-JNR16>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 68.Nielsen JA, et al. Myelin transcription factor 1 (Myt1) modulates the proliferation and differentiation of oligodendrocyte lineage cells. Mol Cell Neurosci. 2004;25(1):111–23. doi: 10.1016/j.mcn.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 69.Caiazzo M, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476(7359):224–7. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 70.Anokye-Danso F, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8(4):376–88. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delaloy C, et al. MicroRNA-9 coordinates proliferation and migration of human embryonic stem cell-derived neural progenitors. Cell Stem Cell. 2010;6(4):323–35. doi: 10.1016/j.stem.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Makeyev EV, et al. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007;27(3):435–48. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyoshi N, et al. Reprogramming of mouse and human cells to pluripotency using mature microRNAs. Cell Stem Cell. 2011;8(6):633–8. doi: 10.1016/j.stem.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 74.Subramanyam D, et al. Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat Biotechnol. 2011;29(5):443–8. doi: 10.1038/nbt.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Visvanathan J, et al. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 2007;21(7):744–9. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao C, et al. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol. 2009;16(4):365–71. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krichevsky AM, et al. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells. 2006;24(4):857–64. doi: 10.1634/stemcells.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Papagiannakopoulos T, Kosik KS. MicroRNA-124: micromanager of neurogenesis. Cell Stem Cell. 2009;4(5):375–6. doi: 10.1016/j.stem.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 79.Rosahl TW, et al. Short-term synaptic plasticity is altered in mice lacking synapsin I. Cell. 1993;75(4):661–70. doi: 10.1016/0092-8674(93)90487-b. [DOI] [PubMed] [Google Scholar]
- 80.Schiavo G, et al. Binding of the synaptic vesicle v-SNARE, synaptotagmin, to the plasma membrane t-SNARE, SNAP-25, can explain docked vesicles at neurotoxin-treated synapses. Proc Natl Acad Sci U S A. 1997;94(3):997–1001. doi: 10.1073/pnas.94.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou Q, Tripathi P. How to remake a fibroblast into a neural stem cell. Cell Stem Cell. 2012;10(4):347–48. doi: 10.1016/j.stem.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 82.Thier M, et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell. 2012;10(4):473–79. doi: 10.1016/j.stem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 83.Han DW, et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10(4):465–72. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 84.Kim J, et al. Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci. 2011;108(19):7838–7843. doi: 10.1073/pnas.1103113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cattoglio C, et al. Hot spots of retroviral integration in human CD34+ hematopoietic cells. Blood. 2007;110(6):1770–8. doi: 10.1182/blood-2007-01-068759. [DOI] [PubMed] [Google Scholar]
- 86.Adams JM, et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318(6046):533–8. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- 87.Harris AW, et al. The E mu-myc transgenic mouse. A model for high-incidence spontaneous lymphoma and leukemia of early B cells. J Exp Med. 1988;167(2):353–71. doi: 10.1084/jem.167.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leder A, et al. Consequences of widespread deregulation of the c-myc gene in transgenic mice: multiple neoplasms and normal development. Cell. 1986;45(4):485–95. doi: 10.1016/0092-8674(86)90280-1. [DOI] [PubMed] [Google Scholar]
- 89.Morgenbesser SD, DePinho RA. Use of transgenic mice to study myc family gene function in normal mammalian development and in cancer. Semin Cancer Biol. 1994;5(1):21–36. [PubMed] [Google Scholar]
- 90.Barrilleaux B, Knoepfler PS. Inducing iPSCs to escape the dish. Cell Stem Cell. 2011;9(2):103–11. doi: 10.1016/j.stem.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Montini E, et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24(6):687–96. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- 92.Lacoste A, Berenshteyn F, Brivanlou AH. An efficient and reversible transposable system for gene delivery and lineage-specific differentiation in human embryonic stem cells. Cell Stem Cell. 2009;5(3):332–42. doi: 10.1016/j.stem.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 93.Warren L, et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell. 2010;7(5):618–30. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]