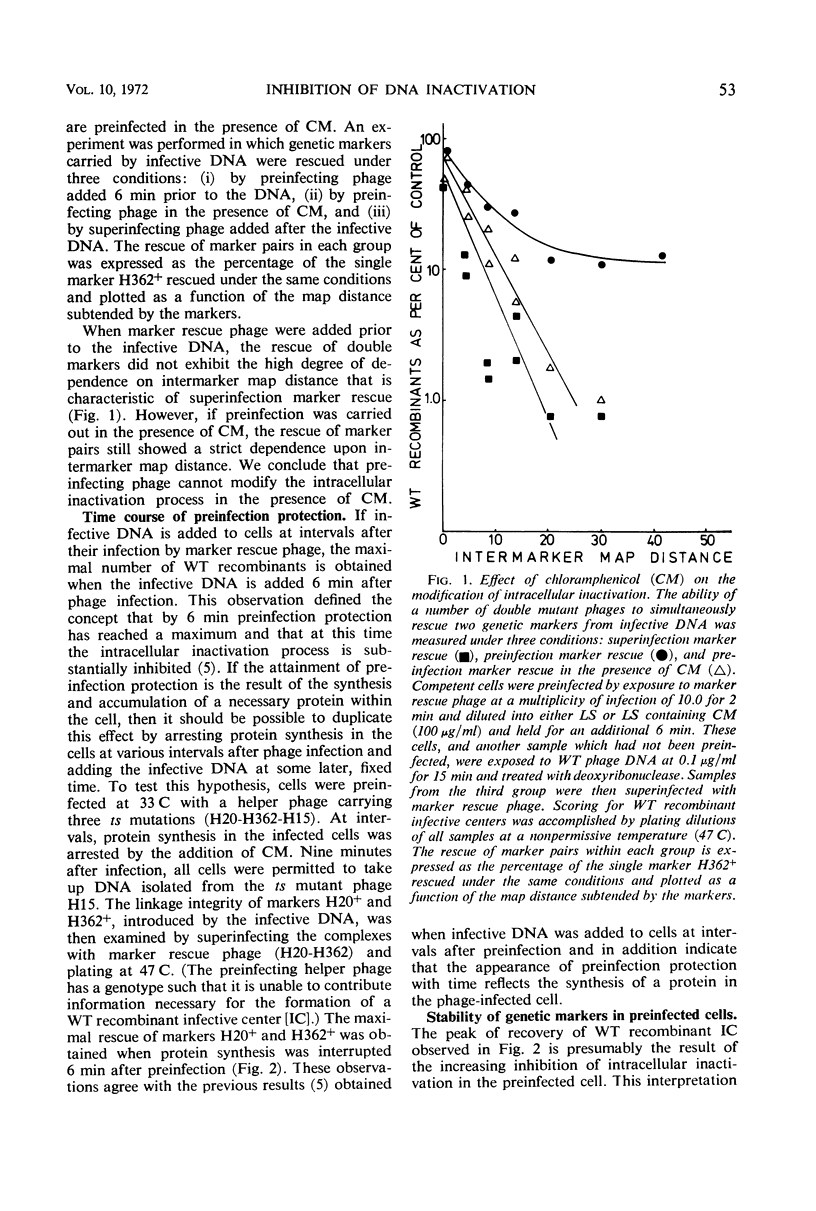
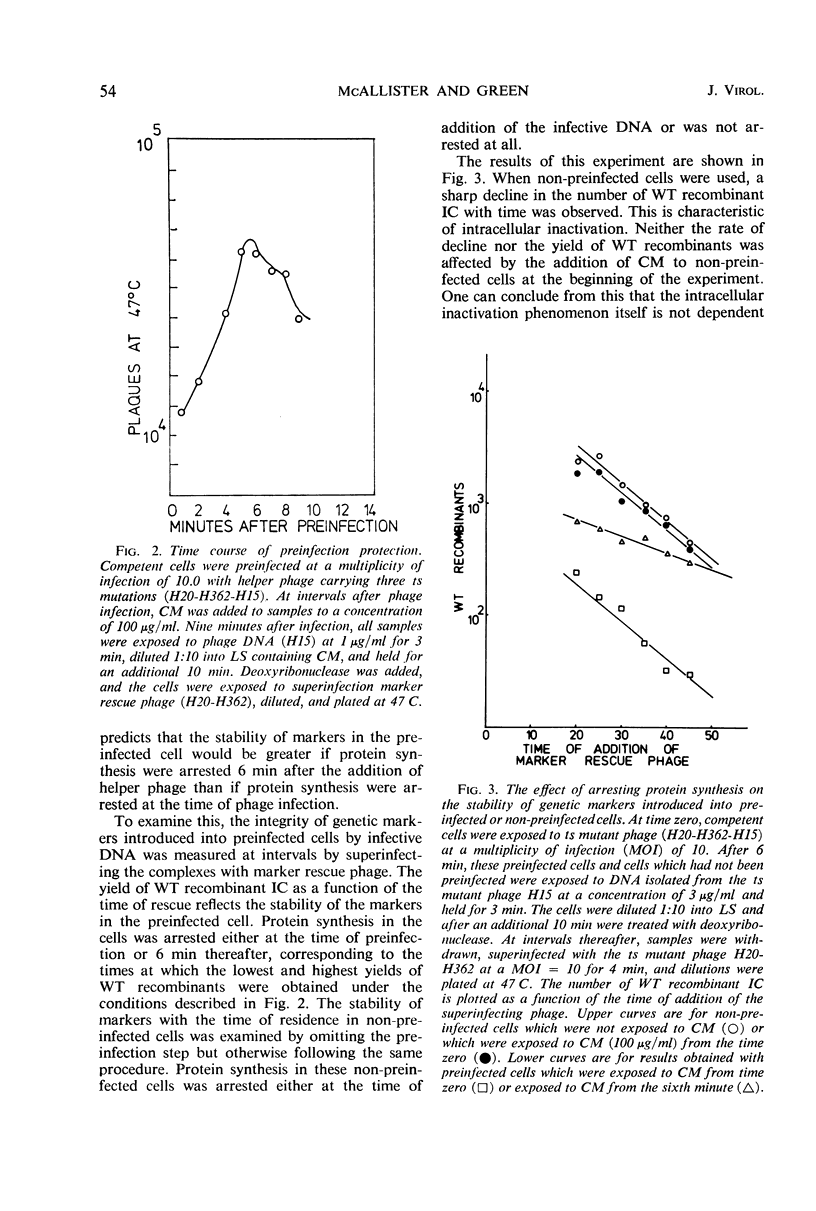
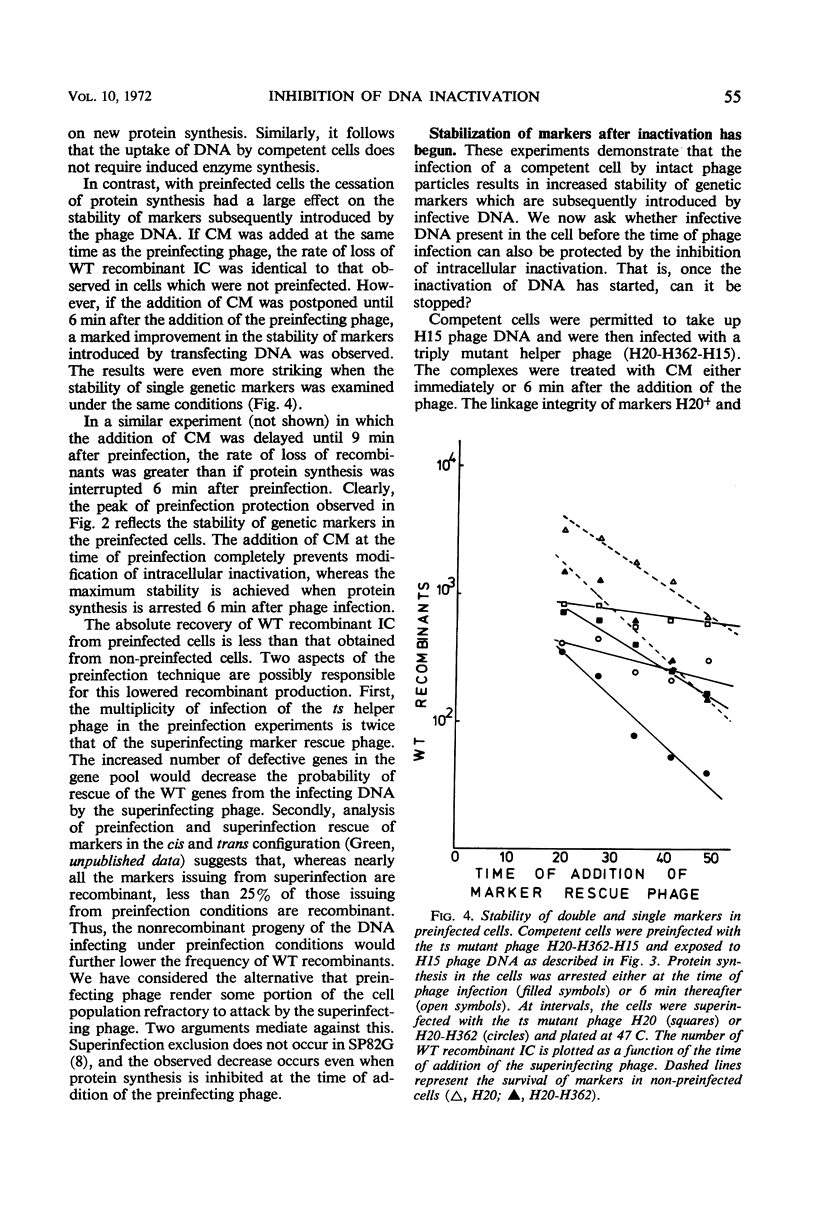
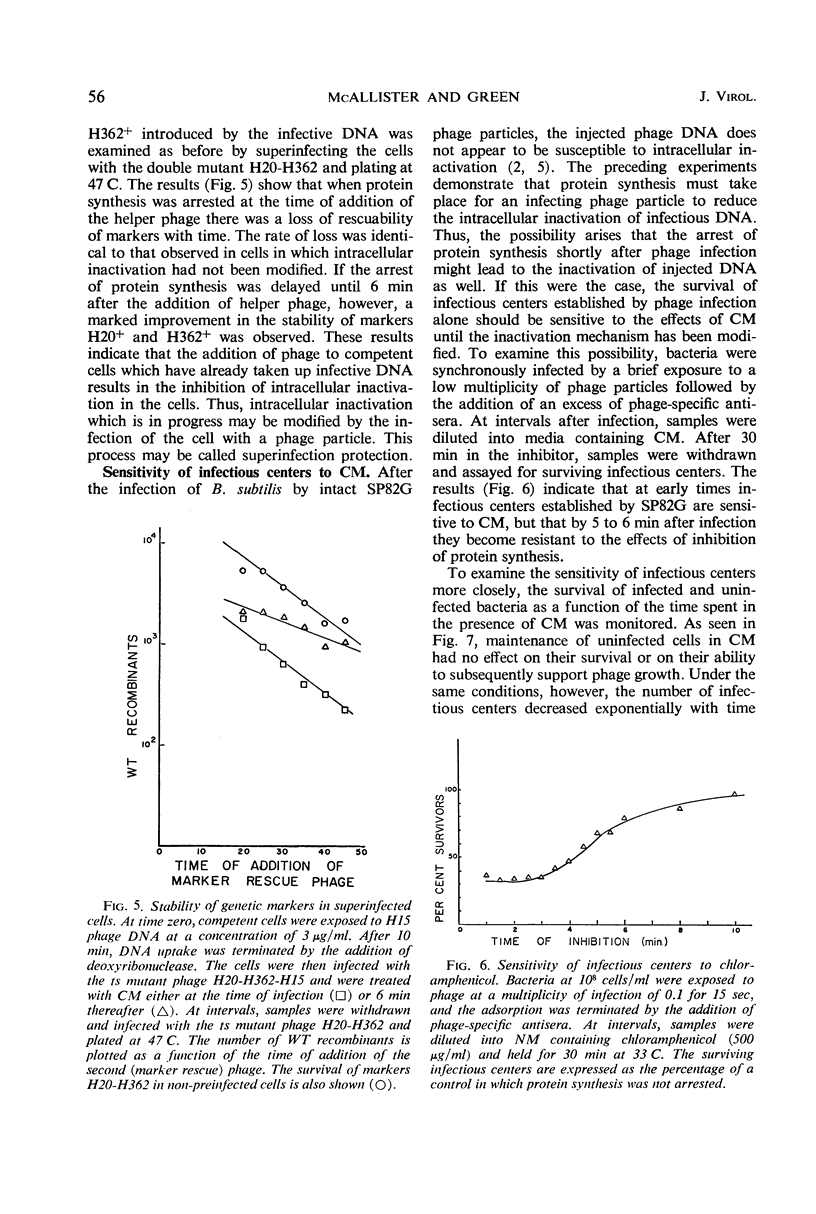
Abstract
The stability of SP82G bacteriophage deoxyribonucleic acid (DNA) after its uptake by competent Bacillus subtilis was examined by determining the ability of superinfecting phage particles to rescue genetic markers carried by the infective DNA. These experiments show that a DNA inactivation process within the cell is inhibited after infection of the cell by intact phage particles. The inhibition is maximally expressed 6 min after phage infection and is completely prevented by the addition of chloramphenicol at the time of infection. The protective effect of this function extends even to infective DNA which was present in the cell before the addition of intact phage. Continued protein synthesis does not appear to be a requirement for the maintenance of the inhibition. In an analogous situation, if infectious centers resulting from singly infecting phage particles are exposed to chloramphenicol shortly after the time of infection, an exponential decrease in the survival of infectious centers with time held in chloramphenicol is observed. If the addition of chloramphenicol is delayed until 6 min after infection, the infectious centers are resistant to chloramphenicol. The sensitivity of infectious centers treated with chloramphenicol at early times after infection is strongly dependent upon the multiplicity of infection and is consistent with a model of multiplicity reactivation. These results indicate that injected DNA is also susceptible to the intracellular inactivation process and suggest that the inhibition of this system is necessary for the successful establishment of an infectious center.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Epstein H. T., Mahler I. Mechanisms of enhancement of SP82 transfection. J Virol. 1968 Jul;2(7):710–715. doi: 10.1128/jvi.2.7.710-715.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H. T. Transfection enhancement by ultraviolet irradiation. Biochem Biophys Res Commun. 1967 Apr 20;27(2):258–262. doi: 10.1016/s0006-291x(67)80071-8. [DOI] [PubMed] [Google Scholar]
- FOELDES J., TRAUTNER T. A. INFECTIOUS DNA FROM A NEWLY ISOLATED B. SUBTILIS PHAGE. Z Vererbungsl. 1964 Apr 10;95:57–65. doi: 10.1007/BF00898184. [DOI] [PubMed] [Google Scholar]
- GREEN D. M. INFECTIVITY OF DNA ISOLATED FROM BACILLUS SUBTILIS BACTERIOPHAGE, SP82. J Mol Biol. 1964 Dec;10:438–451. doi: 10.1016/s0022-2836(64)80065-6. [DOI] [PubMed] [Google Scholar]
- Javor G. T., Tomasz A. An autoradiographic study of genetic transformation. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1216–1222. doi: 10.1073/pnas.60.4.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan E. A genetic study of temperature-sensitive mutants of the subtilis phage SP82. Virology. 1966 Dec;30(4):650–660. doi: 10.1016/0042-6822(66)90170-x. [DOI] [PubMed] [Google Scholar]
- Kahan E. Early and late gene function in bacteriophage SP82. Virology. 1971 Dec;46(3):634–637. doi: 10.1016/0042-6822(71)90066-3. [DOI] [PubMed] [Google Scholar]
- LEVINTHAL C., KEYNAN A., HIGA A. Messenger RNA turnover and protein synthesis in B. subtilis inhibited by actinomycin D. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1631–1638. doi: 10.1073/pnas.48.9.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- McAllister W. T. Bacteriophage infection: which end of the SP82G genome goes in first? J Virol. 1970 Feb;5(2):194–198. doi: 10.1128/jvi.5.2.194-198.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- NESTER E. W., LEDERBERG J. Linkage of genetic units of Bacillus subtilis in DNA transformation. Proc Natl Acad Sci U S A. 1961 Jan 15;47:52–55. doi: 10.1073/pnas.47.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKUBO S., STRAUSS B., STODOLSKY M. THE POSSIBLE ROLE OF RECOMBINATION IN THE INFECTION OF COMPETENT BACILLUS SUBTILIS BY BACTERIOPHAGE DEOXYRIBONUCLEIC ACID. Virology. 1964 Dec;24:552–562. doi: 10.1016/0042-6822(64)90207-7. [DOI] [PubMed] [Google Scholar]
- REILLY B. E., SPIZIZEN J. BACTERIOPHAGE DEOXYRIBONUCLEATE INFECTION OF COMPETENT BACILLUS SUBTILIS. J Bacteriol. 1965 Mar;89:782–790. doi: 10.1128/jb.89.3.782-790.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMIG W. R. Infection of Bacillus subtilis with phenol-extracted bacteriophages. Virology. 1962 Apr;16:452–459. doi: 10.1016/0042-6822(62)90226-x. [DOI] [PubMed] [Google Scholar]
- Riva S., Polsinelli M., Falaschi A. A new phage of Bacillus subtilis with infectious DNA having separable strands. J Mol Biol. 1968 Jul 28;35(2):347–356. doi: 10.1016/s0022-2836(68)80029-4. [DOI] [PubMed] [Google Scholar]
- SPIZIZEN J. Genetic activity of deoxyribonucleic acid in the reconstitution of biosynthetic pathways. Fed Proc. 1959 Dec;18:957–965. [PubMed] [Google Scholar]
- Schell J. The mechanism of restriction of bacteriophage lambda in Escherichia coli strains: demonstration of an in vivo requirement for S-adenosylmethionine. Virology. 1969 Sep;39(1):66–73. doi: 10.1016/0042-6822(69)90348-1. [DOI] [PubMed] [Google Scholar]
- Somma S., Polsinelli M. Quantitive autoradiographic study of competence and deoxyribonucleic acid incorporation in Bacillus subtilis. J Bacteriol. 1970 Mar;101(3):851–855. doi: 10.1128/jb.101.3.851-855.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz H. C., Trautner T. A. The role of recombination in transfection of B. subtilis. Mol Gen Genet. 1971;113(2):174–190. doi: 10.1007/BF00333191. [DOI] [PubMed] [Google Scholar]
- Swift R. L., Wiberg J. S. Bacteriophage T4 inhibits colicin E2-induced degradation of Escherichia coli deoxyribonucleic acid. I. Protein synthesis-dependent inhibition. J Virol. 1971 Sep;8(3):303–310. doi: 10.1128/jvi.8.3.303-310.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner D., Oishi M. The effect of bacteriophage T4 infection on an ATP-dependent deoxyribonuclease in Escherichia coli. Biochim Biophys Acta. 1971 Feb 11;228(3):767–769. doi: 10.1016/0005-2787(71)90747-7. [DOI] [PubMed] [Google Scholar]



