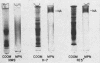Abstract
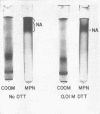
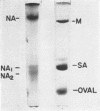
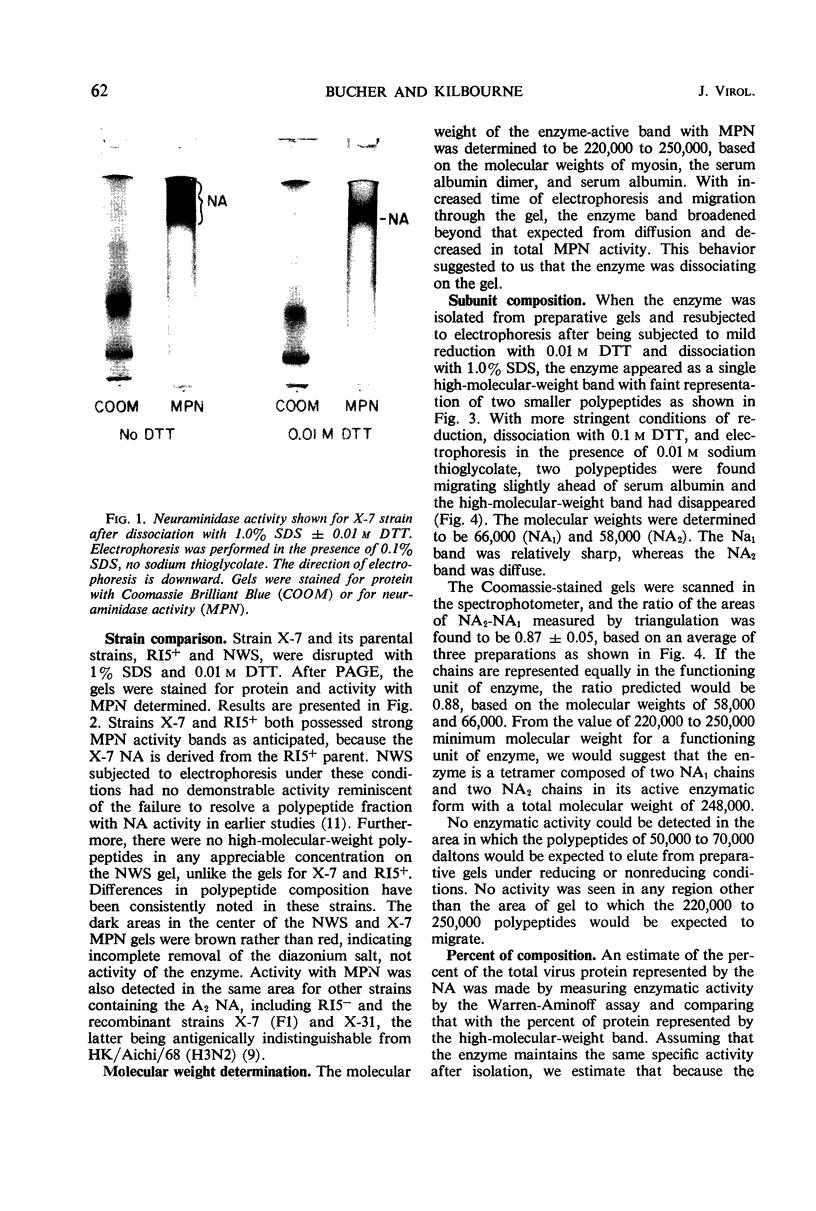
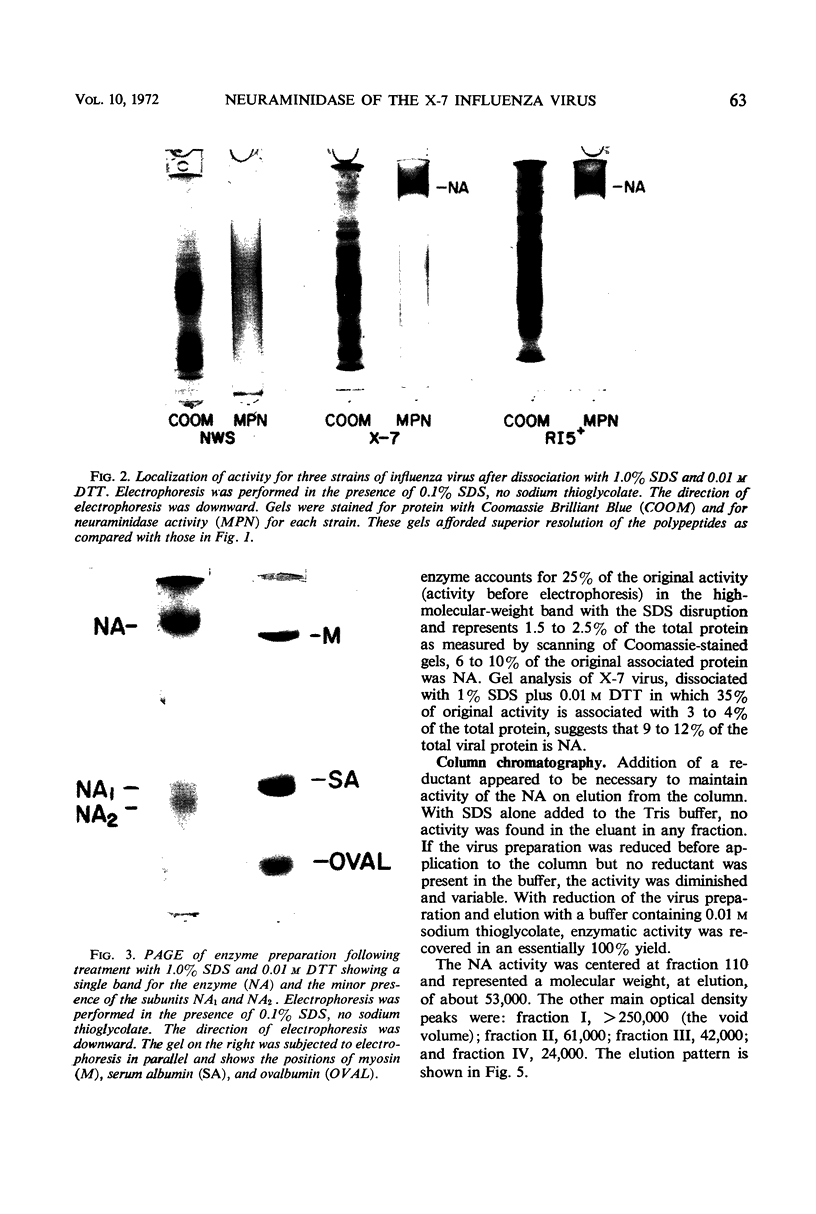
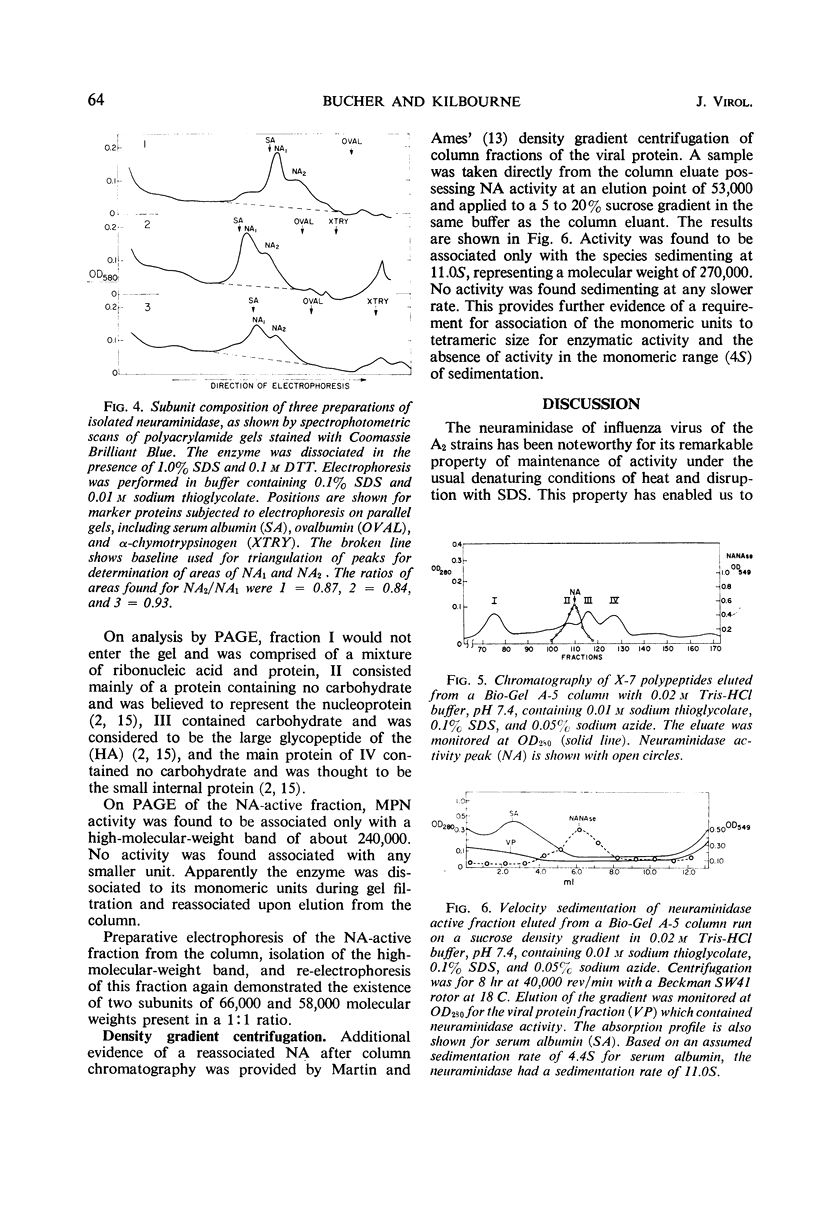
Neuraminidase activity of influenza virus was directly seen on sodium dodecyl sulfate polyacrylamide gels with the aid of the synthetic substrate, methoxyphenol neuraminic acid. Neuraminidase (NA) appeared as a high-molecular-weight fraction with a size in the range of 220,000 to 250,000 daltons. Isolation of this fraction from the X-7 strain of influenza virus, dissociation with sodium dodecyl sulfate, and reduction showed the presence of two polypeptides of 66,000 (NA1) and 58,000 (NA2) molecular weights in equimolar concentration. We postulate that the minimum active unit for the viral A2 neuraminidase is a tetramer composed of two NA1 and two NA2 subunits.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fish W. W., Reynolds J. A., Tanford C. Gel chromatography of proteins in denaturing solvents. Comparison between sodium dodecyl sulfate and guanidine hydrochloride as denaturants. J Biol Chem. 1970 Oct 10;245(19):5166–5168. [PubMed] [Google Scholar]
- Haslam E. A., Hampson A. W., Radiskevics I., White D. O. The polypeptides of influenza virus. 3. Identification of the hemagglutinin, neuraminidase and nucleocapsid proteins. Virology. 1970 Nov;42(3):566–575. doi: 10.1016/0042-6822(70)90303-x. [DOI] [PubMed] [Google Scholar]
- Kendal A. P., Eckert E. A. The preparation and properties of 14 C-carboxamidomethylated subunits from A 2 -1957 influenza neuraminidase. Biochim Biophys Acta. 1972 Feb 28;258(2):484–495. doi: 10.1016/0005-2744(72)90240-9. [DOI] [PubMed] [Google Scholar]
- Kilbourne E. D., Schulman J. L., Schild G. C., Schloer G., Swanson J., Bucher D. Related studies of a recombinant influenza-virus vaccine. I. Derivation and characterization of virus and vaccine. J Infect Dis. 1971 Nov;124(5):449–462. doi: 10.1093/infdis/124.5.449. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laver W. G., Kilbourne E. D. Identification in a recombinant influenza virus of structural proteins derived from both parents. Virology. 1966 Nov;30(3):493–501. doi: 10.1016/0042-6822(66)90125-5. [DOI] [PubMed] [Google Scholar]
- Laver W. G. Separation of two polypeptide chains from the hemagglutinin subunit of influenza virus. Virology. 1971 Jul;45(1):275–288. doi: 10.1016/0042-6822(71)90134-6. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Palese P., Bodo G., Tuppy H. Quantitative determination of neuraminidase-active foci in cell monolayer cultures infected with influenza or newcastle disease virus. J Virol. 1970 Oct;6(4):556–558. doi: 10.1128/jvi.6.4.556-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze I. T. The structure of influenza virus. I. The polypeptides of the virion. Virology. 1970 Dec;42(4):890–904. doi: 10.1016/0042-6822(70)90338-7. [DOI] [PubMed] [Google Scholar]
- Tuppy H., Palese P. A chromogenic substrate for the investigation of neuraminidases. FEBS Lett. 1969 Apr;3(1):72–75. doi: 10.1016/0014-5793(69)80100-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Webster R. G. Estimation of the molecular weights of the polypeptide chains from the isolated hemagglutinin and neuraminidase subunits of influenza viruses. Virology. 1970 Mar;40(3):643–654. doi: 10.1016/0042-6822(70)90209-6. [DOI] [PubMed] [Google Scholar]