Abstract
Chagas disease is caused by Trypanosoma cruzi, a protozoan parasite. Chagas disease remains a serious health problem in large parts of Mexico and Central and South America, where it is a major cause of morbidity and mortality. This disease is being increasingly recognized in non-endemic regions due to immigration. Heart disease develops in 10–30% of infected individuals. It is increasingly clear that parasite- and host-derived bioactive lipids potently modulate disease progression. Many of the changes that occur during acute and chronic Chagas disease can be accounted for by the effects of arachidonic acid (AA)-derived lipids such as leukotrienes, lipoxins, H(P)ETEs, prostaglandins (PGs) and thromboxane. During the course of infection with T. cruzi, changes in circulating levels of AA metabolites are observed. Antagonism of PG synthesis with cyclooxygenase (COX) inhibitors has both beneficial and adverse effects. Treatment with COX inhibitors during acute infection may result in increased parasite load and mortality. However, treatment instituted during chronic infection may be beneficial with no increase in mortality and substantial improvement with cardiac function. Recently, T. cruzi infection of mice deficient in AA biosynthetic enzymes for various pathways has yielded more insightful data than pharmacological inhibition and has highlighted the potential deleterious effects of inhibitors due to “off-target” actions. Using COX-1 null mice, it was observed that parasite biosynthesis is dependent upon host metabolism, that the majority of TXA2 liberated during T. cruzi infection is derived from the parasite and that this molecule may act as a quorum sensor to control parasite growth/differentiation. Thus, eicosanoids present during acute infection may act as immunomodulators aiding the transition to, and maintenance of, the chronic stage of the disease. It is also likely that the same mediators that initially function to ensure host survival may later contribute to cardiovascular damage. Collectively, the eicosanoids represent a new series of targets for therapy in Chagas disease with defined potential therapeutic windows in which to apply these agents for greatest effect. A deeper understanding of the mechanism of action of non-steroidal anti-inflammatory drugs may provide clues to the differences between host responses in acute and chronic T. cruzi infection.
1.1. INTRODUCTION
In Latin America, millions of people are at risk for infection with the parasite Trypanosoma cruzi the causative agent of Chagas disease. Clinically, T. cruzi (Tc) infection causes acute myocarditis followed by chronic cardiomyopathy and vasculopathy in humans and experimental models. There are three stages of infection: acute, intermediate and chronic. Acute myocarditis is characterized by an intense inflammatory response typified by upregulation of inflammatory mediators such as cytokines, chemokines, nitric oxide (NO) and endothelin-1 (Huang et al., 1999a, b; Machado et al., 2008; Machado and Aliberti, 2009; Petkova et al., 2000, 2001; Tanowitz et al., 2005). As the acute infection wanes, individuals may remain asymptomatic, but 10–30% of infected individuals may ultimately develop chronic cardiomyopathy (Tanowitz et al., 2009). The manifestations of the chronic disease include dilated cardiomyopathy with congestive heart failure, conduction abnormalities and thromboembolic events (Tanowitz et al., 1992, 2009). Parasite persistence is central to the aetiology of the cardiomyopathy and is aggravated by microvascular spasm with focal ischaemia and autoimmune mechanisms (Factor et al., 1985; Petkova et al., 2000, 2001; Tanowitz et al., 1996). As early as the 1990s, it was suggested that eicosanoids may play a significant role in the vasospasmand platelet aggregation that characterize Chagas disease (Tanowitz et al., 1990).
1.2. EICOSANOID SYNTHESIS IN VERTEBRATES
Eicosanoids are a family of lipid mediators that participate in a wide range of biological activities including vascular tone, inflammation, ischaemia and tissue homeostasis (Haeggstrom et al., 2010). In mammals, the biosynthetic pathways for these important biological mediators are dependent upon liberation of arachidonic acid (AA) from the inner leaflet of the plasma membrane. The biosynthetic pathways for eicosanoid biosynthesis are well described in vertebrates and are outlined below.
1.2.1. AA metabolism
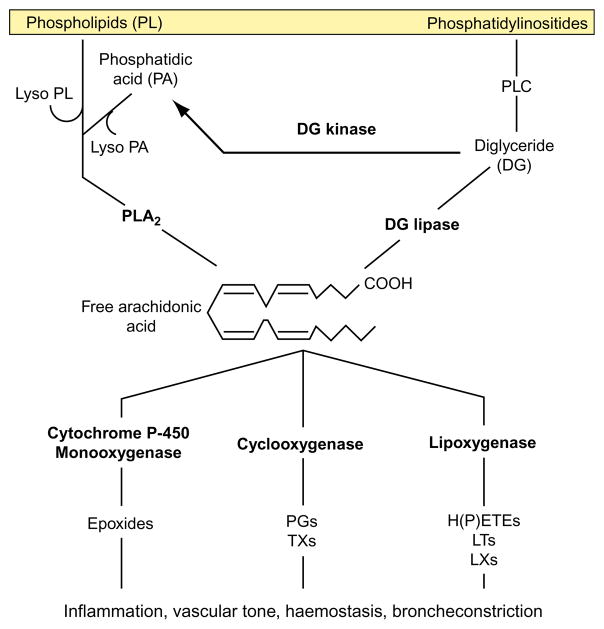
AA is a 20-carbon polyunsaturated fatty acid derived from linoleic acid. Once synthesized, AA is stored as a part of glycerophospholipids that compose the lipid bilayer of the plasma membrane and can be released via the action of phospholipases A2, C and D (PLA2, PLC and PLD, respectively; Fig. 1.1). AA can be reincorporated into cellular lipids via reacylation and recombination with lysophospholipid. AA is metabolized predominantly by the following three independent metabolic pathways:
FIGURE 1.1.
Production and use of free arachidonic acid from various intracellular sources.
The cyclooxygenase (COX) pathway: producing prostaglandins (PGs) and thromboxane A2 (TXA2).
The lipoxygenase (LO) pathway: producing leukotrienes (LTs), lipoxins (LXs), hydroxyeicosatetraenoic acids (HETE) and hydroperoxyeicosatetraenoic acids (HPETE).
Cytochrome P-450 monooxygenase pathway: producing epoxides and hydroxyeicosatetraenoic acids.
In addition to its role in eicosanoid synthesis, AA itself is capable of regulating cellular responses. AA controls the activity of PLA2 and PLC via a negative feedback mechanism (Sumida et al., 1993), triggers mobilization of intracellular calciumstores in a manner similar to that of inositol 1,4,5-phosphate (Chow and Jondal, 1990) and activates the classical isoforms of protein kinase C (PKC) (Naor, 1991). Activation of PKC by fatty acids may form a positive feedback loop to enhance fatty acid liberation through amplification of PLA2 activity (Sumida et al., 1993). AA also suppresses tumour necrosis factor (TNF)-α, interleukin (IL)-1α and lipopolysaccharide (LPS)-induced activation of endothelial cells (Stuhlmeier et al., 1996), indicating that AA may negatively regulate endothelial cell activation.
1.2.2. The LO pathway of AA metabolism
There are four LO enzymes, 5-, 8-, 12- and 15-LO, that metabolize AA by oxygenation of a single carbon resulting in the formation of a variety of compounds with diverse biological activities (Sumida et al., 1993). The nomenclature of the LO enzymes is derived from the position of the hydroperoxy group in the product of that enzyme (Chiang et al., 2006). For example, 5-LO converts AA into a hydroperoxide by insertion of molecular oxygen at position 5 of AA backbone (Brock et al., 1995). In humans, the primary products of AA metabolism by LO are 5-, 12- and 15- HPETE which, by the action of peroxidases, yield their hydroxy derivatives (HETE). The 5- and 12-LO enzymes have a ubiquitous distribution, while 15-LO is confined to eosinophils (Ivanov et al., 2010). The 5-LO in neutrophils is translocated from the cytoplasm to the cell membrane in the presence of raised intracellular calcium (Brock et al., 1995). 5-LO with the help of 5-LO-activating protein (FLAP) converts AA to 5-HPETE, which spontaneously reduces 5-HETE. 5-LO again acts on 5-HETE to convert it to Leukotriene (LTA4) (Chiang et al., 2006). The biosynthetic pathways involved in Lipoxin (LXA4) formation are complex, involve the actions of at least two independent LOs and can occur through transcellular cascades, particularly those involving platelets and leukocytes (Chiang et al., 2006). However, the activity of 5-LO seems to be a common step in LXA4 synthesis (Serhan et al., 1984). LXA4 is secreted by neutrophils and inhibited the activating effects of LTB4 on platelets (Serhan et al., 1984); however, a growing list of anti-inflammatory/pro-resolving effects have been associated with LXA4 resulting in the suggestion that they act as “braking signals” in inflammation (Maderna and Godson, 2009). These properties include limiting leukocyte trafficking and preventing endothelial cell activation at the inflammatory site, stimulation of phagocytosis of apoptotic cells by macrophages and are potential anti-fibrotic mediators (Aliberti, 2005; Baker et al., 2009; Maderna and Godson, 2009; Ryan and Godson, 2010).
LTs were first discovered in leukocytes but are now known to be synthesized in many other cells and tissues including neutrophils, monocytes, mast cells, macrophages, keratinocytes as well as in lung, brain, spleen and heart. LTs are so named because it contains three conjugated double bonds (trienes). LTA4 is the central metabolite from which other LTs are derived. Cells expressing LTA4 hydrolase (neutrophils and monocytes) convert it to LTB4, while the cells that express LTC4 synthase convert LTA4 by the addition of tripeptide glutathione to LTC4. Subsequent removal of the glutamic acid from the glutathione leads to the formation of LTD4 with further degradation (by removal of the glycine residue) leading to the formation of LTE4. LTC4, D4 and E4 contain a cysteine residue in its structure; therefore, they are often referred to as cysteinyl leukotrienes (cys-LTs). LTB4, 5-HETE and LTC4 are subsequently secreted from the cell (Lam et al., 1990).
1.2.3. The cytochrome P-450 pathway of AA metabolism
AA can also be metabolized by the cytochrome P-450 monooxygenases. This pathway oxidizes AA to 19-hydroxy and 19-oxoeicosatetraenoic acids (omega-1 oxidation) and 20-hydroxyeicosatetraenoic and eicosatetraen-1,20-dioic acids (omega-oxidation). Hepatic and renal P-450 monooxygenases also produce a series of epoxides that are further converted to diols. Moreover, omega-1 and omega-oxidation occur in conjunction, and then trihydroxy-AA derivatives, or lipoxins, are formed. The cytochrome P-450 monooxygenases are not only responsible for eicosanoid synthesis but also for catabolism and have been shown to inactivate several eicosanoids including PGE2 and LTB4. Thus, cytochrome P-450 monooxygenases, in addition to PG 15-hydroxydehydrogenase, provide a secondary pathway to catalyze the oxidation and inactivation of these important bioactive lipids.
1.2.4. The COX pathway of AA metabolism
AA is hydrolyzed by the COX enzymes to PGH2 (Rouzer and Marnett, 2008). PGH2 is the central substrate for prostaglandin synthesis and is further metabolized by specific terminal synthases to generate PGs and TXA2 (Santovito et al., 2009). The relevance of these enzymes and the bioactive lipids they produce are not well understood in parasitic disease.
1.2.4.1. The COX family of enzymes
Enzymes in the COX family are structurally and enzymatically similar but have mechanistically different pathophysiological roles. There are two COX isoenzymes (COX-1 and -2) in humans, sharing approximately 61% sequence homology (based on amino acid sequences) with the active sites highly conserved. The two human COX enzymes share 61% sequence homology (based on amino acid sequences) with highly conserved active sites. COX-1 is constitutively expressed and mediates gastric mucus production, platelet activation and vascular tone, while COX-2 is inducible and functions in inflammation, cancer and tissue damage (Haeggstrom et al., 2010; Rouzer and Marnett, 2008).
COX-1 is a 576-amino acid polypeptide (MW 66.2 kDa) that is constitutively and ubiquitously expressed by many tissues, including gastric mucosa, endothelial cells and platelets (Yokoyama and Tanabe, 1989). COX-1 has a half-life of 10 min (Wu et al., 1988), indicating constant synthesis is required to keep the PG/TX synthesis operative. This short half-life is probably a reflection of the high degree of instability of the COX-1 mRNA. It has been hypothesized that inactivation of an apoprotein moiety in the enzyme by free radicals formed during normal enzyme function may be one of the reasons for its instability (Egan et al., 1976; Ham et al., 1979). Indeed, inhibition of protein synthesis prevents the release of prostacyclin (PGI2), a major protective factor for endothelial cells. COX-2 is a 604-amino acid polypeptide (70 kDa) (Hla and Neilson, 1992) and is an inducible form of COX (with induction varying between 10- and 80-fold). Its activity has been reported to account for 40–60% of PG synthesis in some tissues (Karim et al., 1996).
COX-1 and COX-2 are highly segregated in their intracellular compartmentalization prompting speculation that each isozyme is restricted to a certain pool(s) of substrates and that COX-2 may not exist just to augment the activity of COX-1 in times of physiological stress where enhanced PG synthesis is required. Karim et al. (1996) found no evidence for the hypothesis that COX-1 and -2 have separate synthetic pathways. Indeed, the Vmax and Km of both enzymes for arachidonate are nearly identical (Meade et al., 1993a,b). However, since COX-1 is constitutively expressed and COX-2 is inducible, there arises a physiological division in the function of these two enzymes with COX-1 mediating the effect of prostanoids on normal cellular responses and COX-2 mediating pathological responses in processes such as inflammation.
The COX isoforms use two sequential reactions to generate PGH2. The COX reaction produces oxygenation and cyclization of a pentane ring in AA leading to the formation of the unstable metabolite PGG2. The peroxidase reaction then catalyses the reduction of PGG2 and the endoperoxide PGH2 is formed (Miyamoto et al., 1976; Ohki et al., 1979). The residues most important for the function of COX-1 and -2 are the two heme-binding sites in exons 7 (His295) and 8 (His374), the active site tyrosine in exon 8 (Tyr371) and the aspirin (ASA) acetylation site in exon 10 (Ser506) (DeWitt et al., 1990; Shimokawa and Smith, 1991; Shimokawa et al., 1990; Smith et al., 1991). COX-1 contains four potential asparagine-linked glycosylation sites at residues 67, 103, 143 and 409 and contains an epidermal growth factor (EGF) homology domain (residues 33–71) which is encoded by the whole of exon 2 (Toh, 1989; Yokoyama and Tanabe, 1989). These sites are well conserved in COX-2; however, the active site in COX-2 varies by a substitution of a valine to an isoleucine residue at position 509. This substitution appears to be solely responsible for the ability of some classes of inhibitors to preferentially bind and inactivate COX-2 over COX-1. The C-terminus of COX-2 also contains an 18-amino acid sequence, not present in COX-1, giving rise to an additional N-linked glycosylation site (Hla and Neilson, 1992).
1.2.4.2. Synthesis of PGs and thromboxane
PGH2 is the central substrate for the synthesis of all PGs and TXA2 (Haeggstrom et al., 2010; Rouzer and Marnett, 2008). PGI2 is derived from PGH2 by the action of PGI2 synthase, a cytochrome P-450-like membrane resident enzyme (Moncada and Vane, 1979). PGI2 spontaneously hydrolyzes to form the inactive metabolite 6-keto PGF1α (t1/2 = 3 min). PGF2α, PGD2 and PGE2 are derived from PGH2 by their respective synthases. PGF2α is also formed by the degradation of PGE2 via the action of the enzymes PG 15-hydroxydehydrogenase, cytochrome P-450 monooxygenase or PGE9-ketoreductase (Hecker and Ullrich, 1989). TXA2 is derived from PGH2 by the action of TXA2 synthase, a cytochrome P-450-like heme-thiolate enzyme, which adds an oxan: oxetane ring to the structure of PGH2, producing equimolar quantities of hydroxyheptadecatrienoic acid and malondialdehyde in the process. TXA2 (t1/2 = 30 s) spontaneously hydrolyzes to a stable but biologically inactive hemiacetal form, TXB2 (Hecker and Ullrich, 1989). The determining factor in the formation of prostanoid/TXA2 synthesis is the presence of the necessary enzymes and not necessarily their regulation, which leads to tissue-specific expression patterns for many of these products, for example, TXA2 is synthesized mainly by platelets and macrophages, while PGI2 is synthesized predominantly by vascular smooth muscle and endothelial cells (Hecker and Ullrich, 1989).
1.2.4.3. The biological responses to eicosanoids are mediated by cell surface receptors
Prostaglandins and LTs both act through G-protein-coupled receptors located on the plasma membrane of multiple cell types. Four LT receptors are reported, B leukotriene receptor 1 and 2 (BLT1 and BLT2) and cys-LT receptor 1 and 2 (Cyslt1 and Cyslt2). Prostaglandin receptors are similarly named after the prostanoid that serves as the respective ligand, “D-, F-, E- and I-type” prostanoid receptor (DP, FP, EP and IP). TXA2 receptor is known as TXA2 prostanoid receptor (TP). LXA4 is thought to bind to two types of receptor: (a) a surface seven-transmembrane G-protein-coupled receptor, ALX/FPR2 (Brink et al., 2003; Chiang et al., 2006; Devchand et al., 2003) and (b) a cytosolic nuclear ligand-activated transcription factor, the aryl hydrocarbon receptor (AhR) (Schaldach et al., 1999). LXA4–AhR translocates to the nucleus and binds with aryl hydrocarbon receptor nuclear translocator, which interacts with regulatory regions of dioxin-responsive genes. This leads to the activation of a series of genes to facilitate biotransformations and elimination of toxic substances. The binding of ligands (e.g. LXA4) to AhR results in the formation of an active transcription factor that binds to DNA domains— dioxin-responsive elements—that activate the expression of a panel of specific genes.
The receptors signal via heterotrimeric G-proteins to manifest their biological effects that include modulation of immunity, vascular permeability, tissue fibrosis, mucus secretion and vascular tone (Beller et al., 2004; Hui et al., 2004; Tager and Luster, 2003).
1.3. LIPID METABOLISM AND EICOSANOID BIOSYNTHETIC PATHWAYS IN TRYPANOSOMA CRUZI
Kinetoplasts have specialized, adaptable biosynthetic pathways for lipids that reflect the extreme environments they must endure during their life cycle, each with its own unique requirements for lipid synthesis. Prokaryote biosynthetic pathways synthesize fatty acids using a type I or type II synthase (Cox, 1982; Lee et al., 2006). Unlike mammalian cells, T. cruzi and Trypanosoma brucei utilize elongases for nearly all fatty acid synthesis (Lee et al., 2006; Livore et al., 2007). Four novel elongase genes have been identified in T. brucei and five in T. cruzi (Lee et al., 2006; Livore et al., 2007). Of the elongases identified, ELO1 extends C4 to C10, ELO2 extends C10 to C14, ELO3 elongates C14 to C18 and other elongases extend beyond C18 (such as AAX69821 from T. brucei). In all cases, a preference for n6 polyunsaturated fatty acids was observed (Lee et al., 2006). Thus, fatty acid synthesis would appear modular to reflect the requirement for, in the case of T. brucei, mostly stearate (C18) in the insect vector and myristate (C14) in a mammalian host. As such, enzyme expression is highly regulated. For example, ELO3 (that would prevent accumulation of myristate) is downregulated in blood-borne parasite, and elevated concentrations of exogenous fatty acids upregulate the entire pathway, along with T. cruzi metacyclogenesis, allowing the needs of the parasite to be highly adaptable to the surrounding environment (Lee et al., 2006; Wainszelbaum et al., 2003). However, unlike Leishmania major, which has a complete pathway for polyunsaturated fatty acid biosynthesis, trypanosomes contain only Delta5 elongases and Delta4 desaturases (Livore et al., 2007) which allow them to use eicosapentaenoic acid and AA, a precursor that is relatively abundant in the host, for C22 polyunsaturated fatty acid synthesis but have otherwise incomplete synthetic pathways for polyunsaturated fatty acid synthesis.
Eicosanoid synthesis begins with liberation of AA from membrane phospholipids via the activity of PLs that cleave the Sn-1 acyl chain. PLA1- and PLA2-like activities have been reported in T. cruzi and T. brucei (Belaunzaran et al., 2007; Opperdoes and van Roy, 1982; Sage et al., 1981; Shuaibu et al., 2001). In all cases, activity was membrane associated and Ca2+ dependent; however, activity was greatly enhanced in the infective life stages of T. cruzi (trypomastigote and amastigote). Moreover, the infective life stages release a PLA1-like activity with increased secretion coinciding with metacyclogenesis (Belaunzaran et al., 2007). An additional non-PLA-dependent pathway, using sequential deacylation of diacyl glycerophospholipids, has also identified (Ridgley and Ruben, 2001). The outcome of these activities is the liberation of lipid-based second messengers from both the parasite (membrane bound form PLA1) and the host (secreted form). Liberation of AA in such a fashion results in activation of a plasma membrane localized Ca2+ channel and mobilization of intracellular Ca2+ stores in T. cruzi and T. brucei (Catisti et al., 2000; Eintracht et al., 1998). Responses were specific to AA as shorter-chain lipids were without effect. Moreover, the liberation of diacylglycerol and lysophosphatidylcholine from host membranes activates kinases cascades that may be critical in parasite–host cell interactions that precede invasion.
PGF2α is the dominant eicosanoid species produced in Leishmania and T. brucei, along with smaller quantities of PGE2 and PGD2 (Kabututu et al., 2003; Kubata et al., 2000; Opperdoes and van Roy, 1982). Importantly, T. cruzi preferentially synthesizes TXA2 (Ashton et al., 2007) with smaller amounts of PGF2α, and no significant levels of PGD2 produced. Eicosanoid synthesis relies on a series of terminal synthases, each specific to its own species of lipid produced. Surprisingly, few homologues of the mammalian eicosanoid biosynthetic enzymes have been identified and characterized in kinetoplasts. PGF2α synthases have been identified only in Old World Leishmania spp. and absent in New World Leishmania spp. In T. cruzi, PGF2α synthase is similar to yeast old yellow enzyme (TcOYE) and T. brucei (TbPGFS) (Kabututu et al., 2003; Kubata et al., 2000, 2002). The primary sequence of TbPGFS and TcOYE is distinct from their mammalian counterparts (Kubata et al., 2000, 2002), and the enzymatic activity is resistant to pharmacological agents (ASA or indomethacin) that inhibit mammalian enzymes indicating that the active sites are also topographically or structurally different (Kabututu et al., 2003). The crystal structures of TcOYE (Sugiyama et al., 2007) and TbPGFS (Kilunga et al., 2005; Okano et al., 2002) have recently been solved. Both form barrel-like structures with a central hydrophobic core, but TcOYE functions as a dimer (Sugiyama et al., 2007; Yamaguchi et al., 2011) which is more analogous to its mammalian homologue.
Both TcOYE and TbPGFS function in drug resistance. In T. cruzi, TcOYE is essential for drug resistance (Kubata et al., 2002) and TcOYE levels were recently found to be sixfold different between benznidazole-sensitive and-resistant strains of T. cruzi (Murta et al., 2006). T. cruzi possesses four copies of TcOYE demonstrating the importance of this enzyme to parasite well-being. While differential expression of PGF2α synthases has been reported in other studies (Andrade et al., 2008; Dost et al., 2004), they have failed to corroborate the relationship between PGF2α synthase expression and drug resistance. A reason for this may be the presence of additional NADPH oxidoreductases from the cytochrome P-450 family in T. cruzi (Portal et al., 2008). Three new enzymes in this class were recently identified and displayed a role in drug resistance as well as predicted roles in fatty acid/eicosanoid synthesis (Portal et al., 2008). The enzymes possess conserved binding domains for FMN, FAD and NADPH and are strongly inhibited by diphenyleneiodonium, a classical flavoenzyme inhibitor. It is perhaps this last property that distinguishes these new enzymes from TcOYE and TbPGFS, as they appear more like their mammalian counterparts. However, the function of these enzymes is largely undetermined.
The biological responses of T. cruzi to eicosanoids are likely to be highly unconventional in nature. Neither orthologues of heterotrimeric G proteins nor heptahelical G-protein-coupled receptors have been annotated in the T. cruzi genome. Therefore, it is possible that the production of these lipid mediators is exclusively to manipulate host responses to infection and ensure parasite survival/transmission.
1.4. ENDOGENOUS REGULATION OF EICOSANOIDS DURING EXPERIMENTAL CHAGAS DISEASE
It is now appreciated that the release of eicosanoids during infection with T. cruzi regulates host responses and controls disease progression (Ashton et al., 2007). The role of these bioactive lipids in acute and chronic Chagas disease is largely unexplored. However, recent studies (see previous section) have demonstrated that trypanosomes are capable of AA metabolism. Thus, the interpretation of the importance of these bioactive lipids to disease pathogenesis is potentially further complicated by whether the host or the parasite is the primary source of synthesis (Kabututu et al., 2003; Kubata et al., 2000, 2002). Despite this uncertainty, it is clear that eicosanoids play essential and potent roles in the pathogenesis of experimental Chagas disease. Essential fatty acid deficiency (including AA) results in up to 63% reduction in peripheral parasitaemia and more than twice the usual survival rate during acute disease (Santos et al., 1992). Moreover, rates of eicosanoid synthesis are higher in resistant versus susceptible strains of mice (Cardoni and Antunez, 2004). Eicosanoids released by T. cruzi may contribute to parasite differentiation, phagocytosis (Freire-de-Lima et al., 2000) and host survival (Sterin-Borda et al., 1996) by acting as immunomodulators to aid transition and maintenance of the chronic phase of the disease. In support of this concept, CD11b+ myeloid cells from infected mice have been shown to secrete an unidentified PG that mediates the loss of immature B-cell populations by apoptosis. This compromises host defence and favours chronic disease (Zuniga et al., 2005).
1.4.1. Acute infection
Several species of eicosanoids have been implicated in both acute and chronic Chagas disease. The question that remains is which AA derivatives are most important for disease pathogenesis? Plasma from infected mice displays increased levels of PGF2α, PGI2, TXA2 and PGE2 (Cardoni and Antunez, 2004; Pinge-Filho et al., 1999; Tanowitz et al., 1990) compared to uninfected mice from 10 days post-infection onwards. We have previously determined that the main PGs derived from T. cruzi are TXA2 and PGF2α (Ashton et al., 2007), indicating that host is the likely source of the elevated PGI2 and PGE2. PGE2 release is likely from activated macrophages and CD8+ T-cells (Oliveira et al., 2010; Sterin- Borda et al., 1996). No specific role has been delineated for the elevated PGI2 and PGF2α observed in plasma from bona fide or experimental Chagas disease. Minimal work on PGF2α indicates that PGF2α levels in the TXA2 synthase null and wild-type mice were similar, indicating this PG was likely not involved with the augmentation of parasitaemia observed in the COX-1 null and ASA-treated mice or in the regulation of mortality (Mukherjee et al., 2011). This leaves the potential role of PGF2α in Chagas disease largely unexplored. However, the significant amounts of PGF2α produced by T. cruzi and the fact that all members of the kinetoplast family have an identifiable PGF2α synthases indicate that this eicosanoid is of significant value to the parasite.
Most studies suggest the primary role of PGs/eicosanoids is to manipulate the host response and enhance the likelihood of transition to the chronic state (Kristensson et al., 2010; Pinge-Filho et al., 1999; Sterin-Borda et al., 1996). During acute infection, PGE2 has been shown to modulate the virulence of the T. cruzi strain. A non-lethal strain (K98) provoked elevated circulating PGE2, while lethal strains (RA or K98-2) did not (Celentano et al., 1995). Inhibition of COX activity (and therefore PGE2 release) increased mortality in K98-strain-infected mice, but PGE2 infusion did not attenuate the virulence of the RA strain. The effects of PGE2 may stem from a number of sources. PGE2 release from monocytes drives Th1 immunity which has greater effect at controlling parasitaemia (Oliveira et al., 2010). In addition, PGE2 is essential to the suppression of TNF-α release and lymphoproliferation by the host during acute infection in both patients and mice (Borges et al., 1998; de Barros-Mazon et al., 2004; Pinge-Filho et al., 1999). Inhibition of PGE2 synthesis reduces number of parasite nests, inflammatory infiltrates and cardiac fibrosis during acute disease (Abdalla et al., 2008) all of which likely aids the transition to chronic disease (Sterin-Borda et al., 1996).
Prostaglandin release from the parasite, primarily TXA2, also appears to aid survival of the acute infection and transition to the chronic state. Preventing host response to parasite-derived TXA2 augmented death and parasitaemia (Ashton et al., 2007). Platelets exert a direct anti-trypanosomal activity (Momi et al., 2000), and over the course of disease, there is a generalized thrombocytopenia characterized by increased platelet adherence and aggregation that likely limits the anti-parasitic action of these cells (Tanowitz et al., 1990). TXA2 may regulate vasospasm, thrombosis, vascular permeability and endothelial cell dysfunction during acute disease; however, TXA2 also displays immunosuppressive properties with the wild-type mice displaying minimal pathology, but TXA2 receptor null mice exhibiting pronounced inflammation in the myocardium with an almost threefold increased in parasite load in cardiac tissue. There is also evidence that TXA2 signalling by the host acts as a potential quorum-sensing mechanism for T. cruzi and regulates amastigote proliferation to prevent overwhelming the host during acute infection (Ashton et al., 2007). It is clear that TXA2 plays a prominent role in acute T. cruzi infection; however, the previous belief that TXA2 manifests as a host response to infection, and not directly from the parasite, suggests the role of T. cruzi-derived mediators have been undervalued in disease pathogenesis.
In fact, quorum sensing may involve a variety of eicosanoids. These short-lived auto/paracrine messengers are well suited to this role due to quick inactivation and need for constant synthesis. Quorum sensing in T. brucei involves release of PGD2 which slows proliferation largely through the induction of apoptosis (Figarella et al., 2005). However, PGD2 is metabolised to J-series PGs (PGJ2 and 12Δ-PGJ2) in the presence of albumin, and these subsequent “metabolic” products are more potent than PGD2 in regulating survival in T. brucei (Figarella et al., 2005, 2006). Similar effects are also likely in T. cruzi, despite the fact that this parasite produces no discernable PGD2. Administration of 15Δ-PGJ2 during the acute stage of T. cruzi infection reduced the density of amastigote nests in cardiac muscle (Rodrigues et al., 2010). However, 15Δ-PGJ2 also displays immunosuppressive properties and reduced inflammation at the site of infection and peripheral leukopenia via increased levels of IL-10 release which clouds the interpretation of the results as to whether this molecule may act as a quorum sensor during Chagas disease.
During the acute inflammatory phase of the T. cruzi infection, high-level expression of proinflammatory cytokines and other mediators is prevalent. The inflammatory cytokines and lipid mediators are essential for host survival during acute infection (Borges et al., 2009; Hideko Tatakihara et al., 2008). 5-LO has received the most attention in inflammation research involved in T. cruzi infection due to its involvement in LT synthesis. LTs are known to participate actively in the control of infections by protozoa, as demonstrated in several studies (Henderson and Chi, 1998; Machado et al., 2005). LTB4 and LTC4 enhance association with and uptake of T. cruzi by monocytes by stimulation of phagocytosis (Wirth and Kierszenbaum, 1985a,b). Conversely, since LTC4 treatment increased association with non-phagocytic cells, LTC4 may facilitate parasite invasion, not uptake, as a part of the enhanced parasite clearance. However, the role of these molecules in host resistance and induction of myocarditis during infection remains unclear. LTB4 promotes recruitment of inflammatory cells (Tager et al., 2003); however, unlike LTB4, cys-LTs (LTC4) do not induce leukocyte migration into inflamed tissue but increase vascular permeability and subsequent oedema (Dahlen et al., 1981). Moreover, they have also been described as detrimental factors to heart contractility (Gorelik et al., 1992). Thus, the absence of 5-LO seems to prevent the harmful effects of these mediators on heart contractile function. Many of the effects of LTC4 are mediated by guanylate cyclase/NOinhibition. NO is an important mediator of parasite killing in experimental T. cruzi infection (Vespa et al., 1994) and is potently regulated by LTs.
While some lipid mediators drive acute inflammation, other endogenous mediators counter-balance these proinflammatory events. In addition to LTs, lipoxins (LXAs) play a pro-resolving role in inflammatory reactions (Maderna and Godson, 2009; Ryan and Godson, 2010; Stables and Gilroy, 2010). To date, the role of LXAs in myocardial inflammation and modulation of the immune response during T. cruzi infection remains unresolved, but the action of LXAs in the regulation of Toxoplasma gondii infection allows us to extrapolate the potential roles that this eicosanoid may play during T. cruzi infection. The T. gondii model demonstrates that challenge with parasite triggers endogenous LXA4 release that down-modulates dendritic cell activation in vivo and in vitro (Aliberti et al., 2002a). T. gondii infection in 5-LO null mice resulted in more extensive tissue pathology, mainly due to lack of LXA4 production, as treatment with LXAs analogs restored the resistance to tissue pathology with no mortality associated with uncontrolled proinflammatory responses, in a similar manner as for wild-type mice (Aliberti et al., 2002b). Moreover, there is evidence that the AhR mediates the bioactions of LXAs during T. gondii infection. This receptor is a ligand-activated transcription factor that regulates many of the biologic actions of LXAs, including increasing the expression of suppressor of cytokine signalling 2 (SOCS-2) (Machado et al., 2006). Ongoing work in our laboratories on inflammation during T. cruzi infection has revealed that SOCS-2 is important in the modulation of inflammation during this infection (Mukherjee et al., 2011).
Thus, it appears that the eicosanoids present during acute infection largely act as immunomodulators that aid in the transition to and maintenance of the chronic phase of the disease (Sterin-Borda et al., 1996). It is unclear whether T. cruzi generates PGs as a defence against host immune system or whether it hijacks the host PG metabolic pathway in its favour. To this end, further studies using null mice missing biosynthetic enzymes or receptors are required to fully elucidate the role of the identified PGs in Chagas disease.
1.4.2. Chronic infection
In contrast to acute infection, where plasma levels of multiple prostanoids are elevated, only increased levels of TXA2 are observed in chronic disease (>180 days post-infection) (Cardoni and Antunez, 2004). In chronic disease, the effects of TXA2 largely promote tissue damage especially in the heart, where it may exacerbate myocyte apoptosis and enhance progression to dilated cardiomyopathy and heart failure, a major cause of death in patients with this disease. In support of this hypothesis, treatment of mice with chronic T. cruzi infection with ASA may result in improvement of cardiac function which likely results from negating the detrimental effects of TXA2 on myocyte contractility, platelet function and vascular tone. In addition to the maelstrom of changes that TXA2 mediates during acute infection, the secretion of TXA2 would prevent the initiation of an adaptive immune response by the host (Kabashima et al., 2003), enabling maintenance of the chronic phase of the disease. Finally, the role for TXA2 in chronic disease is made more complicated by its control of parasite proliferation. We have suggested that parasite-derived TXA2 is a possible quorum sensor for the parasite (Ashton et al., 2007); however, parasite-derived TXA2 release is insufficient to suppress peripheral parasitaemia in chronic disease. This indicates a need for host-derived TXA2 for control of the severity of the chronic disease.
Despite the fact that TXA2 is the chief PG detected in plasma, other eicosanoids may play a significant role during chronic disease. T-lymphocytes from patients with chronic Chagas disease affect cardiac function and remodelling in a rat model (de Bracco et al., 1984). Similarly, lymphocytes derived from acute and chronic infection of mice display negative and positive inotrophism, respectively (Gorelik et al., 1992). LO products (primarily LTC4) released from the lymphocytes were shown to positively affect cardiac function, while COX products (principally PGE2) exerted a depressor inotropic action. Thus, eicosanoid release during chronic disease appears to be more focused on damage to the host than during the acute phase of infection.
1.4.3. Insect vectors
While several groups have investigated the impact of eicosanoids on mammalian hosts, little has been done to determine their potential role in insect vectors. Prostaglandin biosynthesis and release occur in all three life stages of T. cruzi (Ashton et al., 2007; Kabututu et al., 2003). While it is clear that in the trypomastigote and amastigote forms of the parasite, multiple functions exist for bioactive lipids, almost nothing is known about the role of these mediators in the epimastigote stage. Insects fed on blood treated with inhibitors to COX, LO and PLA2 enhance parasitaemia and mortality due to parasite challenge with T. rangeli (Garcia et al., 2004), leading to a hypothesis that parasite-derived PGs suppress immunity and permit the chronic habitation of the vector (Azambuja and Garcia, 2005; Azambuja et al., 2005). Eicosanoids such as TXA2 may aid in the colonization of the gut by producing mucosal injury (Walt et al., 1987) and increase the potential spread of the parasite through increasing gut motility (Schultheiss and Diener, 1999). The survival of T. cruzi in the gut of its insect vector is largely dependent upon nutritional status (Azambuja and Garcia, 2005; Azambuja et al., 2005). Thus, the same scavenging mechanism that operates to provide lipid precursors in the mammalian host (see next section for a discussion of these mechanisms) may also apply to the insect vector.
1.5. LESSONS FROM PHARMACOLOGICAL MANIPULATION AND FROM NULL MICE
1.5.1. Pharmacological intervention
Given the increasing interest in the role of eicosanoids in T. cruzi infection, it is not unexpected that there should be interest in pharmacological manipulation of eicosanoid biosynthesis in the pathogenesis and clinical management of this infection. Previous studies have attempted to document the role of eicosanoids in early infection using pharmacological intervention with mixed results (Celentano et al., 1995; Freire-de-Lima et al., 2000; Hideko Tatakihara et al., 2008; Michelin et al., 2005; Mukherjee et al., 2011; Pinge-Filho et al., 1999). Pharmacological antagonists selective for COX-1 (ASA), COX-2 (celecoxib) or both (indomethacin) increase mortality and parasitaemia (both peripheral blood counts and cardiac parasite nests) regardless of mouse or parasite strain used (Celentano et al., 1995; Hideko Tatakihara et al., 2008; Mukherjee et al., 2011; Pinge-Filho et al., 1999; Sterin-Borda et al., 1996). Moreover, administration of non-steroidal anti-inflammatory drugs (NSAIDs)may enhance-mortality in patients (Celentano et al., 1995; Sterin-Borda et al., 1996). Conversely, others have found inhibition of PG synthesis/release ablates parasitaemia and extend survival in mice infected with T. cruzi (Abdalla et al., 2008; Freire-de-Lima et al., 2000; Michelin et al., 2005; Paiva et al., 2007). This was usually associated with a decrease in the circulating levels of inflammatory cytokines (such as TNF-α, IFN-γ and IL-10) (Michelin et al., 2005). These outcomes are not unexpected with the use of such general inhibitors and highlight the need for specific receptor blockers and terminal synthase antagonists to be applied to identifying the PGs most relevant to the pathogenesis of Chagas disease.
The dichotomy of the effects seen with NSAIDs in acute disease might result from the different combination of agents, mice and parasite strains previously employed. The expression of both COX isoforms remains unchanged during infection, and there is no increase in COX-2 levels in COX-1 null mice by immunoblotting (S. Mukherjee, unpublished data). While the role of COX-2 in T. cruzi infection is largely undefined, both COX-1 and COX-2 appear to play different roles during acute infection. Inhibition of COX-2 (celecoxib), but not COX-1 (ASA), prevented the thrombocytopenia and leukopenia associated with acute infection and increased reticulocyte counts in response to infection (Hideko Tatakihara et al., 2008). Inhibition of COX-1 and -2 reciprocally regulates NO release from M1 and M2 macrophages which may correlate with resistance to infection. Consistent with this observation, COX-2-derived PGs mediate most of the immunosuppressive effects during the initial phase of T. cruzi infection (Michelin et al., 2005). This may result from the observations that PGI2 and PGE2 are more closely linked to COX-2 metabolism, while COX-1 is responsible for TXA2 synthesis (Parente and Perretti, 2003; Smith et al., 1997). In addition, timing of onset of treatment is important; that is, administration of ASA early in disease, 5 days post-infection increased parasitaemia and mortality (Mukherjee et al., 2011). This observation suggests that caution should be exercised when employing COX inhibitors for controlling fever and pain in the setting of acute Chagas disease. Conversely, use of ASA during chronic disease had no effect on mortality or parasitaemia but improved cardiac function, suggesting the same COX-1 products that mediate host survival during the acute disease are likely to contribute to the progression of cardiac damage and heart failure in the chronic stage.
The selectivity of the NSAIDs used may also determine whether parasite or host production of PGs is the primary target of the treatment regimen used. Many of the biosynthetic enzymes in trypanosomes appear to be unaffected by inhibitors of their mammalian counterparts (Kabututu et al., 2003). Conversely, indomethacin-amides were recently shown to have anti-T. cruzi activity (Konkle et al., 2009). Although these compounds were tested for inhibition of steroid biosynthesis in T. cruzi, they are uniquely specific to COX-2 inhibition in mammals. Thus, a logical hypothesis is that free fatty acid, eicosanoid and sterol biosynthesis may be linked in T. cruzi through the use of enzymes whose biosynthetic capabilities allow them to participate in more than one pathway.
LTs are necessary for control of parasitaemia and survival in the acute T. cruzi infection (Borges et al., 2009) due to modulation of NO and cytokine release. The treatment of T. cruzi-infected mice with a BLT1 receptor antagonist or the 5-LO inhibitor nordihydroguaiaretic acid was accompanied by increased parasitaemia and tissue parasitism but not lethality (Talvani et al., 2002). NO is a major effector molecule of trypanocidal activity in mice (Malvezi et al., 2004; Petray et al., 1994; Vespa et al., 1994) and is also a significant target for LTs in vivo. Therefore, the enhanced parasitaemia is likely due to a reduction in NO activity with 5-LO/BLT1 antagonism; however, studies have shown that elevated NO production occurred in the absence of 5-LO activity suggesting that mechanisms independent of LTs may be operative. Indeed, 5-LO was recently demonstrated to modulate the severity of myocardial inflammation during T. cruzi infection likely through the same mechanism (Pavanelli et al., 2010). The impairment of LT synthesis clearly resulted in increased parasite persistence most likely due to a combination of low leukocyte infiltration and NO production during acute disease (Borges et al., 2009).
Aside from studies suggesting that LT production is necessary for efficient effector mechanisms during T. cruzi infection, a growing body of the literature indicates that other bioactive lipids, such as the chemoattractant platelet-activating factor (1-o-alkyl-2-acetyl-sn-glyceryl-3-phosphorocholine) (PAF), may also activate NO-dependent trypanocidal activity in macrophages. PAF is a non-AA-derived membrane phospholipid mediator with widely recognized proinflammatory activities. PAF is produced by and exerts its biological actions on a variety of mononuclear cells and is implicated in several systemic inflammatory disorders (Braquet et al., 1987; Chao and Olson, 1993; Chignard et al., 1979; Im et al., 1996, 1997; Ishii et al., 1998). In vitro, PAF induced inhibition of parasite growth via NO secretion by T. cruzi-infected macrophages. Addition of a PAF antagonist, WEB 2170, inhibited both NO biosynthesis and trypanocidal activity which appear to be dependent on TNF-α production. Treatment of T. cruzi-infected mice with PAF antagonist (WEB 2170) promoted higher parasitaemia and earlier mortality, when compared with controls, suggesting that PAF may help coordinate mechanisms of resistance to T. cruzi infection. PAF also plays a role in acute myocarditis in T. cruzi-infected mice (Chandrasekar et al., 1998). PAF secretion during infection likely acts as a chemoattractant for several leukocyte populations resulting in inflammatory cell infiltration and production of proinflammatory cytokines that bring about damage in this tissue. Exacerbation of parasitaemia and mortality with PAF receptor blockade suggests that these molecules might regulate effector responses to the parasite.
In addition to these effects on the host, PAF may regulate T. cruzi differentiation and growth (Rodrigues et al., 1996). PAF failed to significantly alter T. cruzi growth; however, parasite growth in the presence of PAF was significantly more differentiated than in its absence. Blockade of the PAF receptor abrogated the PAF effect on cell differentiation. T. cruzi produce a PAF-like lipid with all the biological properties of mammalian- PAF (Tc-PAF) (Gomes et al., 2006). This Tc-PAF augments infection of host cells by T. cruzi and triggers the differentiation of epimastigotes into metacyclic trypomastigotes (Gomes et al., 2006). These effects were abrogated by WEB 2086, and antibodies against murine PAF receptor labelled epimastigotes, suggesting that T. cruzi expresses an orthologue of the PAF receptor. Moreover, these data suggest that T. cruzi contains the components of an autocrine PAF-like ligand–receptor system that modulates cell differentiation towards the infectious stage (Gomes et al., 2006). It remains to be seen if T. cruzi infection in vivo can modulate host response by secreting a PAF-like mediator.
1.5.2. Phenotypes of transgenic/knockout mice
It appears that multiple eicosanoids contribute significantly to the pathogenesis of Chagas disease. Yet little is known of the role played by each. The pharmacological approaches used to date have been in general to decipher the role of individual eicosanoids; for instance, a COX-2 inhibitor would decrease PGI2 and PGE2 production without identifying which species might be causal. Further, pharmacological inhibition of one pathway (COX) may shunt the precursor AA into other metabolic pathways (such as LOX) along with increased synthesis of downstream metabolites. Thus, it becomes difficult to identify the bioactive lipid(s) responsible for the action of these generalized inhibitors. A cornucopia of transgenic and knockout mouse lines has been generated in the biosynthetic pathway and receptors for eicosanoids. Little work has been done using these models to identify specific roles for individual eicosanoid species; however, the work that has been performed has yielded surprising results.
The inconsistency over the nature of COX inhibitors as modulators of Chagas disease was recently resolved with the first use of COX-1 null mice to model the changes in the course of experimental T. cruzi infection (Mukherjee et al., 2011). This report confirmed that PGs derived from COX-1-mediated biosynthesis of the host contribute to the suppression of parasite proliferation, but not mortality in acute disease. Like in the COX-1 null mice, ASA ablated the release of PGF2α and TXA2 in response to T. cruzi infection. However, infection of COX-1 null mice only mimicked the effects of ASA on parasitaemia, indicating control of parasite proliferation, but not mortality, was due to regulation of PGs.
The mechanism for the enhanced mortality with NSAID treatment during acute disease may lie with “off-target” effects of these agents (Claria and Serhan, 1995). Aside from preventing PG synthesis, ASA-mediated acetylation of COX-2 induces the synthesis of aspirin-triggered lipoxin (ATL, or 15-epi-LXA4) (Serhan et al., 1984). ATL has the same activity but is more metabolically stable than LXA4. Multiple studies by several groups have shown that ATL regulates cytokine production and release (Maderna and Godson, 2009; Ryan and Godson, 2010), induces SOCS-2 and suppresses TNF receptor-associated factor-6 (TRAF-6) to silence cytokine signalling (Machado et al., 2006). Importantly, Machado et al. (2006) demonstrated that ASA-treated SOCS-2 null mice given LPS by the intra-peritoneal route could not inhibit neutrophil migration and TNF-α. It is therefore possible that mortality may have more to do with modulation of the impending cytokine storm during acute disease than actual PG production. Thus, the effects of ASA in T. cruzi infection may be via dual mechanisms that operate during different phases of disease.
Pharmacological inhibition may not distinguish between the potential for the host and parasite to function as the source of eicosanoid synthesis during disease. Importantly, the differences between pharmacological inhibition of COX-1 and the COX-1 null mice indicated that there was an unrecognized and essential host–parasite interdependence that dictates the biosynthetic activity of the parasite. The basis for this relationship appears to be the requirement for host-derived PGH2 for PG synthesis throughout infection. A reduction in PGF2α release in COX-1 null, but not TXA2 synthase null, mice was observed. As TXA2 synthase null mice have normal COX activity, the data indicate that COX activity in the host likely provides precursormolecules required for the biosynthetic pathways of this parasite. This “scavenging” hypothesis is confirmed by the inability of the parasite (the primary source of TXA2 during infection) to sustain TXA2 release in the COX-1 null mice. Moreover, the fatty acid synthesis pathways in trypanosomes are defective regarding synthesis of polyunsaturated lipids (Livore et al., 2007) which makes scavenging precursors from the host not just energetically favourable but a requirement. Secretion of PLA-like activity into the host cell from intracellular amastigotes (Belaunzaran et al., 2007) would liberate AA from the inner layer of the host cytoplasmic membrane. This variation on transcellular synthesis would ensure a constant supply of precursors for parasite biosynthetic pathways.
If the parasite were scavenging precursors from the host, then they would only need the terminal synthases to produce bioactive lipids. The fatty acid biosynthetic pathways in trypanosomes are poorly defined, and little homology is reported between the mammalian biosynthetic enzymes and their trypanosomal homologues (Kubata et al., 2000). The PGF2a synthase identified in the parasite is more similar to yeast old yellow enzyme than to mammalian enzyme (Kubata et al., 2002), however, the recent report of anti-parasitic activity of indomethacin-amide derivatives indicates that the active site of some parasite enzymes, if not their primary sequences, is sufficiently homologous to their mammalian counterparts (Konkle et al., 2009). Interestingly, no enzyme other than COX has been identified as being sensitive to indomethacin. However, it remains to be determined whether the target gene (TcCYP51) of these indomethacin-amide derivatives is an integral component of the eicosanoid biosynthetic pathway of T. cruzi (Konkle et al., 2009). These data highlight, for the first time, an interdependence of the parasite on hostmetabolism for prostanoid biosynthesis, which would never have been identified using inhibitor/pharmacological studies alone, and reveal a deeper understanding of host–parasite relationships with potential new avenue for therapeutic options.
The interdependence between host and parasite for endogenous precursors begs the question whether the host or parasite is the primary source of the lipid mediators regulating the pathogenesis of disease. TXB2 levels are elevated in mice infected with T. cruzi (Ashton et al., 2007; Mukherjee et al., 2011; Tanowitz et al., 1990) and levels were maintained in acute infection in the TXA2 synthase null mice (Ashton et al., 2007). These experiments identified TXA2 as a parasite-derived molecule that modulates survival and disease progression. However, the primary source of TXA2 had always been assumed to manifest through a host response to infection, such as inflammation and platelet activation. These data dispelled this myth and firmly indicated that parasite-derived eicosanoids are primary modulators of disease. Further, the use of TXA2 receptor (TP) null mice revealed a second interdependence in the host–parasite relationship. The feedback from the TP on host cells initiates a signalling cascade that controls parasite growth and permits parasite replication at a rate that enables continued survival of the host. Conversely, T. cruzi-derived TXA2 elicits a robust response from host cells that appears to be largely anti-inflammatory. TP null mice had an increased mortality, and their coronary artery endothelial cells had a higher intracellular parasitism and degree of dysfunction compared with wild-type mice that displayed minimal pathology. These data suggest that parasite-derived TXA2 is sufficient to stimulate host TP to ensure normal disease progression, parasitaemia and host survival. The combination of these effects allows for a balance between the needs of the parasite (proliferation and survival through evading the host immune response) and the host (to survive the initial infection and largely limit collateral damage to organs during acute infection). Thus, the proposed interdependence of the parasite on host precursors and host on parasite-derived TXA2 for controlled disease pathogenesis is unique as it inter-twines host biochemistry and parasite biology.
Recently, the regulatory role of endogenous LTs and lipoxins in experimental Chagas disease was determined in mice with targeted deletion of the 5-LO gene. The deficiency of 5-LO during T. cruzi infection enhances peripheral parasite load and the number of myocardial parasite nests compared to wild-type mice. Despite these observations, infected 5-LO null mice controlled parasite burden and survived acute infection. Similar results were obtained in mice treated with MK886, an inhibitor of LT biosynthesis. These studies show that the endogenous LT production is an important regulator of iNOS expression in the heart, which in turn appears to help control parasite burden during early phase of infection in mice. The high levels of NO observed in T. cruzi-infected wild-type mice may impact the efficiency of immune response, allowing the proliferation and persistence of parasite in the tissue. The lower NO production observed in infected 5-LO null mice could be sufficient to control parasite replication while avoiding extensive myocardial damage. Indeed, it was recently demonstrated that 5-LO products are responsible for oxidative stress in erythrocytes during this infection (Borges et al., 2009). Thus, the lipid mediators derived from 5-LO metabolism, especially LTB4, may induce inflammatory damage into parasitized cardiac tissues as a consequence of controlling initial parasite load.
5-LO null mice displayed reduced leukocyte migration to the myocardium with increased circulating IL-6 and IL-10 (Chandrasekar et al., 1996; Saavedra et al., 1999; Truyens et al., 1994) and decreased levels of TNF-α, IFN-γ and NOS in the hearts of 5-LO null compared to WT mice (Pavanelli et al.); however, the functional significance of these changes in vivo is not clear. The diminished migration of CD4+ and CD8+ T cells into the myocardium of 5-LO null mice is likely a product of diminished cytokine/chemokine production combined with lower NO release. 5-LO null mice present reduced indices of myocardial fibrosis at a late stage of acute infection, which is a clear consequence of decreased tissue destruction due to reduced leukocyte infiltration. In addition, the reduction in release of fibrosis promoting cytokines, such as IL-4, TNF-α and TGF-β (Piguet et al., 1989; Rossi, 1998), in the 5-LO null mice during infection would also ameliorate the cardiac damage during infection. Further investigations focused on the chronic heart pathology should demonstrate if absence of 5-LO could lead to reduce matrix remodelling and perhaps reduce chronic morbidity. Thus, all the above factors indicate that host 5-LO and its enzymatic products are essential for controlling T. cruzi replication and pathogenesis of disease during T. cruzi infection. These data highlight the insights and profound understanding about the pathogenesis of Chagas disease that can be gained from the use of knockout mouse models and the opportunities that yet exist to further define the roles on individual eicosanoids on the pathogenesis of Chagas disease.
Acknowledgments
This work was supported by grants from the United States National Institutes of Health (H. B. T. [AI-76248]), the National Health and Medical Research Council of Australia (A. W. A. [512154]) and a Scientist Development Grant from the American Heart Association (S. M. [0735252N]). This work was also supported by Career Development Awards from the National Health and Medical Research Council of Australia (A. W. A. [402847]), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (F. S. M. [576200/2008-5; 473670/2008-9]) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) (F. S. M. [14916]).
ABBREVIATIONS
- AA
arachidonic acid
- AhR
aryl hydrocarbon receptor
- ALX
lipoxin receptor
- ARNT
aryl hydrocarbon receptor nuclear translocator
- ASA
aspirin
- ATL
aspirin-triggered lipoxin
- BLT1/2
B leukotriene receptor 1 or 2
- CD
cell differentiation antigen
- COX
cyclooxygenase
- Cyslt1/2
cysteinyl leukotriene receptor 1 or 2
- cys-LTs
cysteinyl leukotrienes
- FLAP
5-LO activating protein
- FPRL-1
fMLP phagocyte receptor with low affinity
- H(P)ETE
hydro(peroxy)eicosatetraenoic acid
- IFN
interferon
- IL
interleukin
- LO
lipoxygenase
- LT
leukotriene
- LX
lipoxin
- lyso PA
lysophospholipid
- NDGA
nordihydroguaiaretic acid
- NO
nitric oxide
- NOS
nitric oxide synthase
- NSAID
non-steroidal anti-inflammatory drug
- PAF
platelet-activating factor
- PG
prostaglandin
- PKC
protein kinase C
- PL(A/C/D)
phospholipase A/C/D
- PMA
phorbol myristal acetate
- SOCS-2
suppressor of cytokine signalling 2
- Tb
T. brucei
- TbPGFS
T. brucei PGF2α synthase
- Tc
T. cruzi
- TcOYE
old yellow enzyme
- TGF-β
transforming growth factor β
- TNF-α
tumour necrosis factor-α
- TP, DP, FP, EP, IP
T-, D-, F-, E- and I-type prostanoid receptors
- TRAF
TNF receptor-associated factor
- TXA2
thromboxane A2
References
- Abdalla GK, Faria GE, Silva KT, Castro EC, Reis MA, Michelin MA. Trypanosoma cruzi: the role of PGE2 in immune response during the acute phase of experimental infection. Exp Parasitol. 2008;118:514–521. doi: 10.1016/j.exppara.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Aliberti J. Host persistence: exploitation of anti-inflammatory pathways by Toxoplasma gondii. Nat Rev Immunol. 2005;5:162–170. doi: 10.1038/nri1547. [DOI] [PubMed] [Google Scholar]
- Aliberti J, Serhan C, Sher A. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J Exp Med. 2002a;196:1253–1262. doi: 10.1084/jem.20021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliberti J, Hieny S, Reis e Sousa C, Serhan CN, Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nat Immunol. 2002b;3:76–82. doi: 10.1038/ni745. [DOI] [PubMed] [Google Scholar]
- Andrade HM, Murta SM, Chapeaurouge A, Perales J, Nirde P, Romanha AJ. Proteomic analysis of Trypanosoma cruzi resistance to Benznidazole. J Proteome Res. 2008;7:2357–2367. doi: 10.1021/pr700659m. [DOI] [PubMed] [Google Scholar]
- Ashton AW, Mukherjee S, Nagajyothi FN, Huang H, Braunstein VL, Desruisseaux MS, et al. Thromboxane A2 is a key regulator of pathogenesis during Trypanosoma cruzi infection. J Exp Med. 2007;204:929–940. doi: 10.1084/jem.20062432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azambuja P, Garcia ES. Trypanosoma rangeli interactions within the vector Rhodnius prolixus: a mini review. Mem Inst Oswaldo Cruz. 2005;100:567–572. doi: 10.1590/s0074-02762005000500019. [DOI] [PubMed] [Google Scholar]
- Azambuja P, Ratcliffe NA, Garcia ES. Towards an understanding of the interactions of Trypanosoma cruzi and Trypanosoma rangeli within the reduviid insect host Rhodnius prolixus. An Acad Bras Cienc. 2005;77:397–404. doi: 10.1590/s0001-37652005000300004. [DOI] [PubMed] [Google Scholar]
- Baker N, O’Meara SJ, Scannell M, Maderna P, Godson C. Lipoxin A4: anti-inflammatory and anti-angiogenic impact on endothelial cells. J Immunol. 2009;182:3819–3826. doi: 10.4049/jimmunol.0803175. [DOI] [PubMed] [Google Scholar]
- Belaunzaran ML, Wainszelbaum MJ, Lammel EM, Gimenez G, Aloise MM, Florin-Christensen J, et al. Phospholipase A1 from Trypanosoma cruzi infective stages generates lipid messengers that activate host cell protein kinase C. Parasitology. 2007;134:491–502. doi: 10.1017/S0031182006001740. [DOI] [PubMed] [Google Scholar]
- Beller TC, Friend DS, Maekawa A, Lam BK, Austen KF, Kanaoka Y. Cysteinyl leukotriene 1 receptor controls the severity of chronic pulmonary inflammation and fibrosis. Proc Natl Acad Sci USA. 2004;101:3047–3052. doi: 10.1073/pnas.0400235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges MM, Kloetzel JK, Andrade HF, Jr, Tadokoro CE, Pinge-Filho P, Abrahamsohn I. Prostaglandin and nitric oxide regulate TNF-α production during Trypanosoma cruzi infection. Immunol Lett. 1998;63:1–8. doi: 10.1016/s0165-2478(98)00034-0. [DOI] [PubMed] [Google Scholar]
- Borges CL, Cecchini R, Tatakihara VL, Malvezi AD, Yamada-Ogatta SF, Rizzo LV, et al. 5-Lipoxygenase plays a role in the control of parasite burden and contributes to oxidative damage of erythrocytes in murine Chagas’ disease. Immunol Lett. 2009;123:38–45. doi: 10.1016/j.imlet.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Braquet P, Touqui L, Shen TY, Vargaftig BB. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987;39:97–145. [PubMed] [Google Scholar]
- Brink C, Dahlen SE, Drazen J, Evans JF, Hay DW, Nicosia S, et al. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol Rev. 2003;55:195–227. doi: 10.1124/pr.55.1.8. [DOI] [PubMed] [Google Scholar]
- Brock TG, McNish RW, Peters-Golden M. Translocation and leukotriene synthetic capacity of nuclear 5-lipoxygenase in rat basophilic leukemia cells and alveolar macrophages. J Biol Chem. 1995;270:21652–21658. doi: 10.1074/jbc.270.37.21652. [DOI] [PubMed] [Google Scholar]
- Cardoni RL, Antunez MI. Circulating levels of cyclooxygenase metabolites in experimental Trypanosoma cruzi infections. Mediators Inflamm. 2004;13:235–240. doi: 10.1080/09637480400003022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catisti R, Uyemura SA, Docampo R, Vercesi AE. Calcium mobilization by arachidonic acid in trypanosomatids. Mol Biochem Parasitol. 2000;105:261–271. doi: 10.1016/s0166-6851(99)00186-3. [DOI] [PubMed] [Google Scholar]
- Celentano AM, Gorelik G, Solana ME, Sterin-Borda L, Borda E, Gonzalez Cappa SM. PGE2 involvement in experimental infection with Trypanosoma cruzi subpopulations. Prostaglandins. 1995;49:141–153. doi: 10.1016/0090-6980(95)00002-r. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Melby PC, Troyer DA, Freeman GL. Induction of proinflammatory cytokine expression in experimental acute Chagasic cardiomyopathy. Biochem Biophys Res Commun. 1996;223:365–371. doi: 10.1006/bbrc.1996.0900. [DOI] [PubMed] [Google Scholar]
- Chandrasekar B, Melby PC, Troyer DA, Colston JT, Freeman GL. Temporal expression of pro-inflammatory cytokines and inducible nitric oxide synthase in experimental acute Chagasic cardiomyopathy. Am J Pathol. 1998;152:925–934. [PMC free article] [PubMed] [Google Scholar]
- Chao W, Olson MS. Platelet-activating factor: receptors and signal transduction. Biochem J. 1993;292 (Pt. 3):617–629. doi: 10.1042/bj2920617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- Chignard M, Le Couedic JP, Tence M, Vargaftig BB, Benveniste J. The role of platelet-activating factor in platelet aggregation. Nature. 1979;279:799–800. doi: 10.1038/279799a0. [DOI] [PubMed] [Google Scholar]
- Chow SC, Jondal M. Polyunsaturated free fatty acids stimulate an increase in cytosolic Ca2+ by mobilizing the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool in T cells through a mechanism-independent of phosphoinositide turnover. J Biol Chem. 1990;265:902–907. [PubMed] [Google Scholar]
- Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci USA. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox FE. Trypanosoma cruzi: signals for transformation. Nature. 1982;300:685. doi: 10.1038/300685a0. [DOI] [PubMed] [Google Scholar]
- Dahlen SE, Bjork J, Hedqvist P, Arfors KE, Hammarstrom S, Lindgren JA, et al. Leukotrienes promote plasma leakage and leukocyte adhesion in postcapillary venules: in vivo effects with relevance to the acute inflammatory response. Proc Natl Acad Sci USA. 1981;78:3887–3891. doi: 10.1073/pnas.78.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Barros-Mazon S, Guariento ME, da Silva CA, Coffman RL, Abrahamsohn IA. Differential regulation of lymphoproliferative responses to Trypanosoma cruzi antigen in patients with the cardiac or indeterminate form of Chagas disease. Clin Immunol. 2004;111:137–145. doi: 10.1016/j.clim.2004.01.002. [DOI] [PubMed] [Google Scholar]
- de Bracco MM, Sterin-Borda L, Fink S, Finiasz M, Borda E. Stimulatory effect of lymphocytes from Chagas’ patients on spontaneously beating rat atria. Clin Exp Immunol. 1984;55:405–412. [PMC free article] [PubMed] [Google Scholar]
- Devchand PR, Arita M, Hong S, Bannenberg G, Moussignac RL, Gronert K, et al. Human ALX receptor regulates neutrophil recruitment in transgenic mice: roles in inflammation and host defense. FASEB J. 2003;17:652–659. doi: 10.1096/fj.02-0770com. [DOI] [PubMed] [Google Scholar]
- DeWitt DL, el-Harith EA, Kraemer SA, Andrews MJ, Yao EF, Armstrong RL, et al. The aspirin and heme-binding sites of ovine and murine prostaglandin endoperoxide synthases. J Biol Chem. 1990;265:5192–5198. [PubMed] [Google Scholar]
- Dost CK, Saraiva J, Monesi N, Zentgraf U, Engels W, Albuquerque S. Six Trypanosoma cruzi strains characterized by specific gene expression patterns. Parasitol Res. 2004;94:134–140. doi: 10.1007/s00436-004-1188-3. [DOI] [PubMed] [Google Scholar]
- Egan RW, Paxton J, Kuehl FA., Jr Mechanism for irreversible self-deactivation of prostaglandin synthetase. J Biol Chem. 1976;251:7329–7335. [PubMed] [Google Scholar]
- Eintracht J, Maathai R, Mellors A, Ruben L. Calcium entry in Trypanosoma brucei is regulated by phospholipase A2 and arachidonic acid. Biochem J. 1998;336 (Pt. 3):659–666. doi: 10.1042/bj3360659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor SM, Cho S, Wittner M, Tanowitz H. Abnormalities of the coronary micro-circulation in acute murine Chagas’ disease. Am J Trop Med Hyg. 1985;34:246–253. doi: 10.4269/ajtmh.1985.34.246. [DOI] [PubMed] [Google Scholar]
- Figarella K, Rawer M, Uzcategui NL, Kubata BK, Lauber K, Madeo F, et al. Prostaglandin D2 induces programmed cell death in Trypanosoma brucei bloodstream form. Cell Death Differ. 2005;12:335–346. doi: 10.1038/sj.cdd.4401564. [DOI] [PubMed] [Google Scholar]
- Figarella K, Uzcategui NL, Beck A, Schoenfeld C, Kubata BK, Lang F, et al. Prostaglandin-induced programmed cell death in Trypanosoma brucei involves oxidative stress. Cell Death Differ. 2006;13:1802–1814. doi: 10.1038/sj.cdd.4401862. [DOI] [PubMed] [Google Scholar]
- Freire-de-Lima CG, Nascimento DO, Soares MB, Bozza PT, Castro-Faria-Neto HC, de Mello FG, et al. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- Garcia ES, Machado EM, Azambuja P. Effects of eicosanoid biosynthesis inhibitors on the prophenoloxidase-activating system and microaggregation reactions in the hemolymph of Rhodnius prolixus infected with Trypanosoma rangeli. J Insect Physiol. 2004;50:157–165. doi: 10.1016/j.jinsphys.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Gomes MT, Monteiro RQ, Grillo LA, Leite-Lopes F, Stroeder H, Ferreira-Pereira A, et al. Platelet-activating factor-like activity isolated from Trypanosoma cruzi. Int J Parasitol. 2006;36:165–173. doi: 10.1016/j.ijpara.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Gorelik G, Borda E, Postan M, Gonzalez Cappa S, Sterin-Borda L. T lymphocytes from T. cruzi-infected mice alter heart contractility: participation of arachidonic acid metabolites. J Mol Cell Cardiol. 1992;24:9–20. doi: 10.1016/0022-2828(92)91155-x. [DOI] [PubMed] [Google Scholar]
- Haeggstrom JZ, Rinaldo-Matthis A, Wheelock CE, Wetterholm A. Advances in eicosanoid research, novel therapeutic implications. Biochem Biophys Res Commun. 2010;396:135–139. doi: 10.1016/j.bbrc.2010.03.140. [DOI] [PubMed] [Google Scholar]
- Ham EA, Egan RW, Soderman DD, Gale PH, Kuehl FA., Jr Peroxidase-dependent deactivation of prostacyclin synthetase. J Biol Chem. 1979;254:2191–2194. [PubMed] [Google Scholar]
- Hecker M, Ullrich V. On the mechanism of prostacyclin and thromboxane A2 biosynthesis. J Biol Chem. 1989;264:141–150. [PubMed] [Google Scholar]
- Henderson WR, Jr, Chi EY. The importance of leukotrienes in mast cell-mediated Toxoplasma gondii cytotoxicity. J Infect Dis. 1998;177:1437–1443. doi: 10.1086/517833. [DOI] [PubMed] [Google Scholar]
- Hideko Tatakihara VL, Cecchini R, Borges CL, Malvezi AD, Graca-de Souza VK, Yamada-Ogatta SF, et al. Effects of cyclooxygenase inhibitors on parasite burden, anemia and oxidative stress in murine Trypanosoma cruzi infection. FEMS Immunol Med Microbiol. 2008;52:47–58. doi: 10.1111/j.1574-695X.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci USA. 1992;89:7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Calderon TM, Berman JW, Braunstein VL, Weiss LM, Wittner M, et al. Infection of endothelial cells with Trypanosoma cruzi activates NF-kappaB and induces vascular adhesion molecule expression. Infect Immun. 1999a;67:5434–5440. doi: 10.1128/iai.67.10.5434-5440.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Chan J, Wittner M, Jelicks LA, Morris SA, Factor SM, et al. Expression of cardiac cytokines and inducible form of nitric oxide synthase (NOS2) in Trypanosoma cruzi-infected mice. J Mol Cell Cardiol. 1999b;1:75–88. doi: 10.1006/jmcc.1998.0848. [DOI] [PubMed] [Google Scholar]
- Hui Y, Cheng Y, Smalera I, Jian W, Goldhahn L, Fitzgerald GA, et al. Directed vascular expression of human cysteinyl leukotriene 2 receptor modulates endothelial permeability and systemic blood pressure. Circulation. 2004;110:3360–3366. doi: 10.1161/01.CIR.0000147775.50954.AA. [DOI] [PubMed] [Google Scholar]
- Im SY, Ko HM, Kim JW, Lee HK, Ha TY, Lee HB, et al. Augmentation of tumor metastasis by platelet-activating factor. Cancer Res. 1996;56:2662–2665. [PubMed] [Google Scholar]
- Im SY, Choi JH, Ko HM, Han SJ, Chun SB, Lee HK, et al. A protective role of platelet-activating factor in murine candidiasis. Infect Immun. 1997;65:1321–1326. doi: 10.1128/iai.65.4.1321-1326.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Kuwaki T, Nagase T, Maki K, Tashiro F, Sunaga S, et al. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998;187:1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I, Heydeck D, Hofheinz K, Roffeis J, O’Donnell VB, Kuhn H, et al. Molecular enzymology of lipoxygenases. Arch Biochem Biophys. 2010;503:161–174. doi: 10.1016/j.abb.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Kabashima K, Murata T, Tanaka H, Matsuoka T, Sakata D, Yoshida N, et al. Thromboxane A2 modulates interaction of dendritic cells and T cells and regulates acquired immunity. Nat Immunol. 2003;4:694–701. doi: 10.1038/ni943. [DOI] [PubMed] [Google Scholar]
- Kabututu Z, Martin SK, Nozaki T, Kawazu S, Okada T, Munday CJ, et al. Prostaglandin production from arachidonic acid and evidence for a 9,11-endoperoxide prostaglandin H2 reductase in Leishmania. Int J Parasitol. 2003;33:221–228. doi: 10.1016/s0020-7519(02)00254-0. [DOI] [PubMed] [Google Scholar]
- Karim S, Habib A, Levy-Toledano S, Maclouf J. Cyclooxygenase-1 and -2 of endothelial cells utilize exogenous or endogenous arachidonic acid for transcellular production of thromboxane. J Biol Chem. 1996;271:12042–12048. doi: 10.1074/jbc.271.20.12042. [DOI] [PubMed] [Google Scholar]
- Kilunga KB, Inoue T, Okano Y, Kabututu Z, Martin SK, Lazarus M, et al. Structural and mutational analysis of Trypanosoma brucei prostaglandin H2 reductase provides insight into the catalytic mechanism of aldo-ketoreductases. J Biol Chem. 2005;280:26371–26382. doi: 10.1074/jbc.M413884200. [DOI] [PubMed] [Google Scholar]
- Konkle ME, Hargrove TY, Kleshchenko YY, von Kries JP, Ridenour W, Uddin MJ, et al. Indomethacin amides as a novel molecular scaffold for targeting Trypanosoma cruzi sterol 14alpha-demethylase. J Med Chem. 2009;52:2846–2853. doi: 10.1021/jm801643b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensson K, Nygard M, Bertini G, Bentivoglio M. African trypanosome infections of the nervous system: parasite entry and effects on sleep and synaptic functions. Prog Neurobiol. 2010;91:152–171. doi: 10.1016/j.pneurobio.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Kubata BK, Duszenko M, Kabututu Z, Rawer M, Szallies A, Fujimori K, et al. Identification of a novel prostaglandin F2α synthase in Trypanosoma brucei. J Exp Med. 2000;192:1327–1338. doi: 10.1084/jem.192.9.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubata BK, Kabututu Z, Nozaki T, Munday CJ, Fukuzumi S, Ohkubo K, et al. A key role for old yellow enzyme in the metabolism of drugs by Trypanosoma cruzi. J Exp Med. 2002;196:1241–1251. doi: 10.1084/jem.20020885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam BK, Gagnon L, Austen KF, Soberman RJ. The mechanism of leukotriene B4 export from human polymorphonuclear leukocytes. J Biol Chem. 1990;265:13438–13441. [PubMed] [Google Scholar]
- Lee SH, Stephens JL, Paul KS, Englund PT. Fatty acid synthesis by elongases in trypanosomes. Cell. 2006;126:691–699. doi: 10.1016/j.cell.2006.06.045. [DOI] [PubMed] [Google Scholar]
- Livore VI, Tripodi KE, Uttaro AD. Elongation of polyunsaturated fatty acids in trypanosomatids. FEBS J. 2007;274:264–274. doi: 10.1111/j.1742-4658.2006.05581.x. [DOI] [PubMed] [Google Scholar]
- Machado ER, Ueta MT, Lourenco EV, Anibal FF, Sorgi CA, Soares EG, et al. Leukotrienes play a role in the control of parasite burden in murine strongyloidiasis. J Immunol. 2005;175:3892–3899. doi: 10.4049/jimmunol.175.6.3892. [DOI] [PubMed] [Google Scholar]
- Machado FS, Aliberti J. Lipoxins as an immune-escape mechanism. Adv Exp Med Biol. 2009;666:78–87. doi: 10.1007/978-1-4419-1601-3_6. [DOI] [PubMed] [Google Scholar]
- Machado FS, Johndrow JE, Esper L, Dias A, Bafica A, Serhan CN, et al. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat Med. 2006;12:330–334. doi: 10.1038/nm1355. [DOI] [PubMed] [Google Scholar]
- Machado FS, Souto JT, Rossi MA, Esper L, Tanowitz HB, Aliberti J, et al. Nitric oxide synthase-2 modulates chemokine production by Trypanosoma cruzi-infected cardiac myocytes. Microbes Infect. 2008;10:1558–1566. doi: 10.1016/j.micinf.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maderna P, Godson C. Lipoxins: resolutionary road. Br J Pharmacol. 2009;158:947–959. doi: 10.1111/j.1476-5381.2009.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvezi AD, Cecchini R, de Souza F, Tadokoro CE, Rizzo LV, Pinge-Filho P. Involvement of nitric oxide (NO) and TNF-α in the oxidative stress associated with anemia in experimental Trypanosoma cruzi infection. FEMS Immunol Med Microbiol. 2004;41:69–77. doi: 10.1016/j.femsim.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993a;268:6610–6614. [PubMed] [Google Scholar]
- Meade EA, Smith WL, DeWitt DL. Expression of the murine prostaglandin (PGH) synthase-1 and PGH synthase-2 isozymes in cos-1 cells. J Lipid Mediat. 1993b;6:119–129. [PubMed] [Google Scholar]
- Michelin MA, Silva JS, Cunha FQ. Inducible cyclooxygenase released prostaglandin mediates immunosuppression in acute phase of experimental Trypanosoma cruzi infection. Exp Parasitol. 2005;111:71–79. doi: 10.1016/j.exppara.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Ogino N, Yamamoto S, Hayaishi O. Purification of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem. 1976;251:2629–2636. [PubMed] [Google Scholar]
- Momi S, Perito S, Mezzasoma AM, Bistoni F, Gresele P. Involvement of platelets in experimental mouse trypanosomiasis: evidence of mouse platelet cytotoxicity against Trypanosoma equiperdum. Exp Parasitol. 2000;95:136–143. doi: 10.1006/expr.2000.4517. [DOI] [PubMed] [Google Scholar]
- Moncada S, Vane JR. The role of prostacyclin in vascular tissue. Fed Proc. 1979;38:66–71. [PubMed] [Google Scholar]
- Mukherjee S, Machado FS, Huang H, Oz HS, Jelicks LA, Prado CM, et al. Aspirin treatment of mice infected with Trypanosoma cruzi and implications for the pathogenesis of Chagas disease. PLoS one. 2011;6(2):e16959. doi: 10.1371/journal.pone.0016959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murta SM, Krieger MA, Montenegro LR, Campos FF, Probst CM, Avila AR, et al. Deletion of copies of the gene encoding old yellow enzyme (TcOYE), a NAD(P)H flavin oxidoreductase, associates with in vitro-induced benznidazole resistance in Trypanosoma cruzi. Mol Biochem Parasitol. 2006;146:151–162. doi: 10.1016/j.molbiopara.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Naor Z. Is arachidonic acid a second messenger in signal transduction? Mol Cell Endocrinol. 1991;80:C181–C186. doi: 10.1016/0303-7207(91)90135-f. [DOI] [PubMed] [Google Scholar]
- Ohki S, Ogino N, Yamamoto S, Hayaishi O. Prostaglandin hydroperoxidase, an integral part of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J Biol Chem. 1979;254:829–836. [PubMed] [Google Scholar]
- Okano Y, Inoue T, Kubata BK, Kabututu Z, Urade Y, Matsumura H, et al. Crystallization and preliminary X-ray crystallographic studies of Trypanosoma brucei prostaglandin F2α synthase. J Biochem. 2002;132:859–861. doi: 10.1093/oxfordjournals.jbchem.a003298. [DOI] [PubMed] [Google Scholar]
- Oliveira LG, Kuehn CC, Santos CD, Toldo MP, do Prado JC., Jr Enhanced protection by melatonin and meloxicam combination in experimental infection by Trypanosoma cruzi. Parasite Immunol. 2010;32:245–251. doi: 10.1111/j.1365-3024.2009.01185.x. [DOI] [PubMed] [Google Scholar]
- Opperdoes FR, van Roy J. The phospholipases of Trypanosoma brucei bloodstream forms and cultured procyclics. Mol Biochem Parasitol. 1982;5:309–319. doi: 10.1016/0166-6851(82)90038-x. [DOI] [PubMed] [Google Scholar]
- Paiva CN, Arras RH, Lessa LP, Gibaldi D, Alves L, Metz CN, et al. Unraveling the lethal synergism between Trypanosoma cruzi infection and LPS: a role for increased macrophage reactivity. Eur J Immunol. 2007;37:1355–1364. doi: 10.1002/eji.200636705. [DOI] [PubMed] [Google Scholar]
- Parente L, Perretti M. Advances in the pathophysiology of constitutive and inducible cyclooxygenases: two enzymes in the spotlight. Biochem Pharmacol. 2003;65:153–159. doi: 10.1016/s0006-2952(02)01422-3. [DOI] [PubMed] [Google Scholar]
- Pavanelli WR, Gutierrez FR, Mariano FS, Prado CM, Ferreira BR, Teixeira MM, et al. 5-Lipoxygenase is a key determinant of acute myocardial inflammation and mortality during Trypanosoma cruzi infection. Microbes Infect. 2010;(8–9):587–597. doi: 10.1016/j.micinf.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Petkova SB, Tanowitz HB, Magazine HI, Factor SM, Chan J, Pestell RG, et al. Myocardial expression of endothelin-1 in murine Trypanosoma cruzi infection. Cardiovasc Pathol. 2000;9:257–265. doi: 10.1016/s1054-8807(00)00045-4. [DOI] [PubMed] [Google Scholar]
- Petkova SB, Huang H, Factor SM, Pestell RG, Bouzahzah B, Jelicks LA, et al. The role of endothelin in the pathogenesis of Chagas’ disease. Int J Parasitol. 2001;31:499–511. doi: 10.1016/s0020-7519(01)00168-0. [DOI] [PubMed] [Google Scholar]
- Petray P, Rottenberg ME, Grinstein S, Orn A. Release of nitric oxide during the experimental infection with Trypanosoma cruzi. Parasite Immunol. 1994;16:193–199. doi: 10.1111/j.1365-3024.1994.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Piguet PF, Collart MA, Grau GE, Kapanci Y, Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med. 1989;170:655–663. doi: 10.1084/jem.170.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinge-Filho P, Tadokoro CE, Abrahamsohn IA. Prostaglandins mediate suppression of lymphocyte proliferation and cytokine synthesis in acute Trypanosoma cruzi infection. Cell Immunol. 1999;193:90–98. doi: 10.1006/cimm.1999.1463. [DOI] [PubMed] [Google Scholar]
- Portal P, Villamil SF, Alonso GD, De Vas MG, Flawia MM, Torres HN, et al. Multiple NADPH-cytochrome P450 reductases from Trypanosoma cruzi suggested role on drug resistance. Mol Biochem Parasitol. 2008;160:42–51. doi: 10.1016/j.molbiopara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Ridgley EL, Ruben L. Phospholipase from Trypanosoma brucei releases arachidonic acid by sequential sn-1, sn-2 deacylation of phospholipids. Mol Biochem Parasitol. 2001;114:29–40. doi: 10.1016/s0166-6851(01)00234-1. [DOI] [PubMed] [Google Scholar]
- Rodrigues CO, Dutra PM, Souto-Padron T, Cordeiro RS, Lopes AH. Effect of platelet-activating factor on cell differentiation of Trypanosoma cruzi. Biochem Biophys Res Commun. 1996;223:735–740. doi: 10.1006/bbrc.1996.0965. [DOI] [PubMed] [Google Scholar]
- Rodrigues WF, Miguel CB, Chica JE, Napimoga MH. 15d-PGJ2 modulates acute immune responses to Trypanosoma cruzi infection. Mem Inst Oswaldo Cruz. 2010;105:137–143. doi: 10.1590/s0074-02762010000200005. [DOI] [PubMed] [Google Scholar]
- Rossi MA. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens. 1998;16:1031–1041. doi: 10.1097/00004872-199816070-00018. [DOI] [PubMed] [Google Scholar]
- Rouzer CA, Marnett LJ. Non-redundant functions of cyclooxygenases: oxygenation of endocannabinoids. J Biol Chem. 2008;283:8065–8069. doi: 10.1074/jbc.R800005200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan A, Godson C. Lipoxins: regulators of resolution. Curr Opin Pharmacol. 2010;10:166–172. doi: 10.1016/j.coph.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Saavedra E, Herrera M, Gao W, Uemura H, Pereira MA. The Trypanosoma cruzi trans-sialidase, through its COOH-terminal tandem repeat, upregulates interleukin 6 secretion in normal human intestinal microvascular endothelial cells and peripheral blood mononuclear cells. J Exp Med. 1999;190:1825–1836. doi: 10.1084/jem.190.12.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage L, Hambrey PN, Werchola GM, Mellors A, Tizard IR. Lysophospholipase 1 in Trypanosoma brucei. Tropenmed Parasitol. 1981;32:215–220. [PubMed] [Google Scholar]
- Santos CF, Silva ME, Nicoli JR, Crocco-Afonso LC, Santos JE, Bambirra EA, et al. Effect of an essential fatty acid deficient diet on experimental infection with Trypanosoma cruzi in germfree and conventional mice. Braz J Med Biol Res. 1992;25:795–803. [PubMed] [Google Scholar]
- Santovito D, Mezzetti A, Cipollone F. Cyclooxygenase and prostaglandin synthases: roles in plaque stability and instability in humans. Curr Opin Lipidol. 2009;20:402–408. doi: 10.1097/MOL.0b013e32832fa22c. [DOI] [PubMed] [Google Scholar]
- Schaldach CM, Riby J, Bjeldanes LF. Lipoxin A4: a new class of ligand for the Ah receptor. Biochemistry. 1999;38:7594–7600. doi: 10.1021/bi982861e. [DOI] [PubMed] [Google Scholar]
- Schultheiss G, Diener M. Inhibition of spontaneous smooth muscle contractions in rat and rabbit intestine by blockers of the thromboxane A2 pathway. Zentralbl Veterinarmed A. 1999;46:123–131. doi: 10.1046/j.1439-0442.1999.00200.x. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hamberg M, Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci USA. 1984;81:5335–5339. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa T, Smith WL. Essential histidines of prostaglandin endoperoxide synthase. His309 is involved in heme binding. J Biol Chem. 1991;266:6168–6173. [PubMed] [Google Scholar]
- Shimokawa T, Kulmacz RJ, DeWitt DL, Smith WL. Tyrosine 385 of prostaglandin endoperoxide synthase is required for cyclooxygenase catalysis. J Biol Chem. 1990;265:20073–20076. [PubMed] [Google Scholar]
- Shuaibu MN, Kanbara H, Yanagi T, Ameh DA, Bonire JJ, Nok AJ. Phospholipase A2 from Trypanosoma brucei gambiense and Trypanosoma brucei brucei: inhibition by organotins. J Enzyme Inhib. 2001;16:433–441. doi: 10.1080/14756360109162392. [DOI] [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Shimokawa T. The aspirin and heme binding sites of PGG/H synthase. Adv Prostaglandin Thromboxane Leukot Res. 1991;21A:77–80. [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Arakawa T, Spencer AG, Thuresson ED, Song I. Independent prostanoid biosynthetic systems associated with prostaglandin endoperoxide synthases-1 and -2. Thromb Haemost. 1997;78:627–630. [PubMed] [Google Scholar]
- Stables MJ, Gilroy DW. Old and new generation lipid mediators in acute inflammation and resolution. Prog Lipid Res. 2010;50:35–51. doi: 10.1016/j.plipres.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Sterin-Borda L, Gorelik G, Goren N, Cappa SG, Celentano AM, Borda E. Lymphocyte muscarinic cholinergic activity and PGE2 involvement in experimental Trypanosoma cruzi infection. Clin Immunol Immunopathol. 1996;81:122–128. doi: 10.1006/clin.1996.0167. [DOI] [PubMed] [Google Scholar]
- Stuhlmeier KM, Tarn C, Csizmadia V, Bach FH. Selective suppression of endothelial cell activation by arachidonic acid. Eur J Immunol. 1996;26:1417–1423. doi: 10.1002/eji.1830260703. [DOI] [PubMed] [Google Scholar]
- Sugiyama S, Tokuoka K, Uchiyama N, Okamoto N, Okano Y, Matsumura H, et al. Preparation, crystallization and preliminary crystallographic analysis of old yellow enzyme from Trypanosoma cruzi. Acta Crystallogr F Struct Biol Cryst Commun. 2007;63:896–898. doi: 10.1107/S1744309107044879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumida C, Graber R, Nunez E. Role of fatty acids in signal transduction: modulators and messengers. Prostaglandins Leukot Essent Fatty Acids. 1993;48:117–122. doi: 10.1016/0952-3278(93)90019-s. [DOI] [PubMed] [Google Scholar]
- Tager AM, Luster AD. BLT1 and BLT2: the leukotriene B4 receptors. Prostaglandins Leukot Essent Fatty Acids. 2003;69:123–134. doi: 10.1016/s0952-3278(03)00073-5. [DOI] [PubMed] [Google Scholar]
- Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, Friedrich EB, et al. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol. 2003;4:982–990. doi: 10.1038/ni970. [DOI] [PubMed] [Google Scholar]
- Talvani A, Machado FS, Santana GC, Klein A, Barcelos L, Silva JS, et al. Leukotriene B4 induces nitric oxide synthesis in Trypanosoma cruzi-infected murine macrophages and mediates resistance to infection. Infect Immun. 2002;70:4247–4253. doi: 10.1128/IAI.70.8.4247-4253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanowitz HB, Burns ER, Sinha AK, Kahn NN, Morris SA, Factor SM, et al. Enhanced platelet adherence and aggregation in Chagas’ disease: a potential pathogenic mechanism for cardiomyopathy. Am J Trop Med Hyg. 1990;43:274–281. doi: 10.4269/ajtmh.1990.43.274. [DOI] [PubMed] [Google Scholar]
- Tanowitz HB, Kirchhoff LV, Simon D, Morris SA, Weiss LM, Wittner M. Chagas’ disease. Clin Microbiol Rev. 1992;5:400–419. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanowitz HB, Kaul DK, Chen B, Morris SA, Factor SM, Weiss LM, et al. Compromised microcirculation in acute murine Trypanosoma cruzi infection. J Parasitol. 1996;82:124–130. [PubMed] [Google Scholar]
- Tanowitz HB, Huang H, Jelicks LA, Chandra M, Loredo ML, Weiss LM, et al. Role of endothelin 1 in the pathogenesis of chronic chagasic heart disease. Infect Immun. 2005;73:2496–2503. doi: 10.1128/IAI.73.4.2496-2503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanowitz HB, Machado FS, Jelicks LA, Shirani J, de Carvalho AC, Spray DC, et al. Perspectives on Trypanosoma cruzi-induced heart disease (Chagas disease) Prog Cardiovasc Dis. 2009;51:524–539. doi: 10.1016/j.pcad.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh H. Prostaglandin endoperoxide synthase contains an EGF-like domain. FEBS Lett. 1989;258:317–319. doi: 10.1016/0014-5793(89)81683-7. [DOI] [PubMed] [Google Scholar]
- Truyens C, Angelo-Barrios A, Torrico F, Van Damme J, Heremans H, Carlier Y. Interleukin-6 (IL-6) production in mice infected with Trypanosoma cruzi: effect of its paradoxical increase by anti-IL-6 monoclonal antibody treatment on infection and acute-phase and humoral immune responses. Infect Immun. 1994;62:692–696. doi: 10.1128/iai.62.2.692-696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa GN, Cunha FQ, Silva JS. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect Immun. 1994;62:5177–5182. doi: 10.1128/iai.62.11.5177-5182.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainszelbaum MJ, Belaunzaran ML, Lammel EM, Florin-Christensen M, Florin- Christensen J, Isola EL. Free fatty acids induce cell differentiation to infective forms in Trypanosoma cruzi. Biochem J. 2003;375:705–712. doi: 10.1042/BJ20021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walt RP, Kemp RT, Filipowicz B, Davies JG, Bhaskar NK, Hawkey CJ. Gastric mucosal protection with selective inhibition of thromboxane synthesis. Gut. 1987;28:541–544. doi: 10.1136/gut.28.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth JJ, Kierszenbaum F. Effects of leukotriene C4 on macrophage association with and intracellular fate of Trypanosoma cruzi. Mol Biochem Parasitol. 1985a;15:1–10. doi: 10.1016/0166-6851(85)90024-6. [DOI] [PubMed] [Google Scholar]
- Wirth JJ, Kierszenbaum F. Stimulatory effects of leukotriene B4 on macrophage association with and intracellular destruction of Trypanosoma cruzi. J Immunol. 1985b;134:1989–1993. [PubMed] [Google Scholar]
- Wu KK, Hatzakis H, Lo SS, Seong DC, Sanduja SK, Tai HH. Stimulation of de novo synthesis of prostaglandin G/H synthase in human endothelial cells by phorbol ester. J Biol Chem. 1988;263:19043–19047. [PubMed] [Google Scholar]
- Yamaguchi K, Okamoto N, Tokuoka K, Sugiyama S, Uchiyama N, Matsumura H, et al. Structure of the inhibitor complex of old yellow enzyme from Trypanosoma cruzi. J Synchrotron Radiat. 2011;18:66–69. doi: 10.1107/S0909049510033595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama C, Tanabe T. Cloning of human gene encoding prostaglandin endoperoxide synthase and primary structure of the enzyme. Biochem Biophys Res Commun. 1989;165:888–894. doi: 10.1016/s0006-291x(89)80049-x. [DOI] [PubMed] [Google Scholar]
- Zuniga E, Acosta-Rodriguez E, Merino MC, Montes C, Gruppi A. Depletion of immature B cells during Trypanosoma cruzi infection: involvement of myeloid cells and the cyclooxygenase pathway. Eur J Immunol. 2005;35:1849–1858. doi: 10.1002/eji.200526005. [DOI] [PubMed] [Google Scholar]