Abstract
Background
The potential consequences of asthma in childhood and young adulthood on lung structure in older adults have not been studied in a large, population-based cohort.
Objective
The authors hypothesized that a history of asthma onset in childhood (age 18 or before) or young adulthood (age 19 to 45) was associated with altered lung structure on computed tomography (CT) in later life.
Methods
The Multi-Ethnic Study of Atherosclerosis Lung Study recruited 3,965 participants and assessed asthma history using standardized questionnaires, spirometry following guidelines, and segmental airway dimensions and percent low attenuation areas on CT scans.
Results
Asthma with onset in childhood and young adulthood was associated with large decrements in the forced expiratory volume in one second among participants with a mean age of 66 years (−365 ml and −343 ml, respectively; P<0.001). Asthma with onset in childhood and young adulthood was associated with increased mean airway wall thickness standardized to an internal perimeter of 10 mm (Pi10) (0.1 mm, P<0.001 for both), predominantly from narrower segmental airway lumens (−0.39 mm and −0.34 mm, respectively; P<0.001). Asthma with onset in childhood and young adulthood also was associated with a greater percentage of low attenuation areas (1.69% and 4.30%, respectively; P<0.001). Findings were similar among never smokers except that differential percentage of low attenuation areas in child-onset asthma was not seen in them.
Conclusion
Asthma with onset in childhood or young adulthood, was associated with reduced lung function, narrower airways and, among asthmatics who smoked, greater percentage of low attenuation areas in later life.
MeSH terms: airway remodeling, airway structure, asthma, emphysema, epidemiology
INTRODUCTION
Asthma prevalence exceeds ten percent in the developed world, and is increasing rapidly in the developing world (1). Asthma is thought to contribute to low lung function in later life (2, 3), which predicts mortality (4) and defines chronic obstructive pulmonary disease (COPD) (5), now the third-leading cause of death in the United States (6).
The majority of children with asthma experience clinical remission and are symptom-free by early adulthood (7). Nonetheless, those with remitted child-onset asthma have basement membrane thickening on endobronchial biopsy and relatively few have completely normal lung function at age 18–25 years old (8). The European Community Respiratory Health Survey found that a history of childhood asthma, among other factors, was associated with a larger decline in lung function and increased risk of COPD later in life, and the Burden of Obstructive Lung Disease group similarly found that childhood respiratory disease was a risk factor for COPD among never smokers (9, 10).
Case-control studies suggest that asthma may contribute to airway wall thickening and irreversible airflow limitation (11–20). Early and repeated mechanical stress to alveoli from persistent asthma may lead to alveolar rupture, strain on adjacent alveolar walls and alveolar enlargement (21) with consequences in later life. The relationship of child-onset asthma to lung function in later life is poorly defined since the longest prospective cohort study of child- onset asthma has follow-up to a mean age of 42 years (2) and no such studies have evaluated lung structure. We therefore examined asthma with onset in childhood and young adulthood in a cross-sectional analysis of a population-based cohort of older adults. We hypothesized that a history of asthma onset in childhood (age 18 or before) or young adulthood (age 19 to 45) would be associated with altered lung structure on computed tomography (CT) in later life. For completeness, we also report results for participants with asthma onset after age 45 years.
METHODS
Multi-Ethnic Study of Atherosclerosis
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multicenter cohort study of subclinical cardiovascular disease in whites, African Americans, Hispanics, and Asians (22). Between 2000 and 2002, MESA recruited 6814 men and women 45 to 84 years of age from Forsyth County, North Carolina; New York City; Baltimore; St. Paul, Minnesota; Chicago; and Los Angeles. Exclusion criteria were clinical cardiovascular disease, weight exceeding 136 kg (300 lb), pregnancy, and impediment to long-term participation. Written informed consent was obtained from all participants. The protocols were approved by the institutional review boards of all collaborating institutions and by the National Heart, Lung, and Blood Institute.
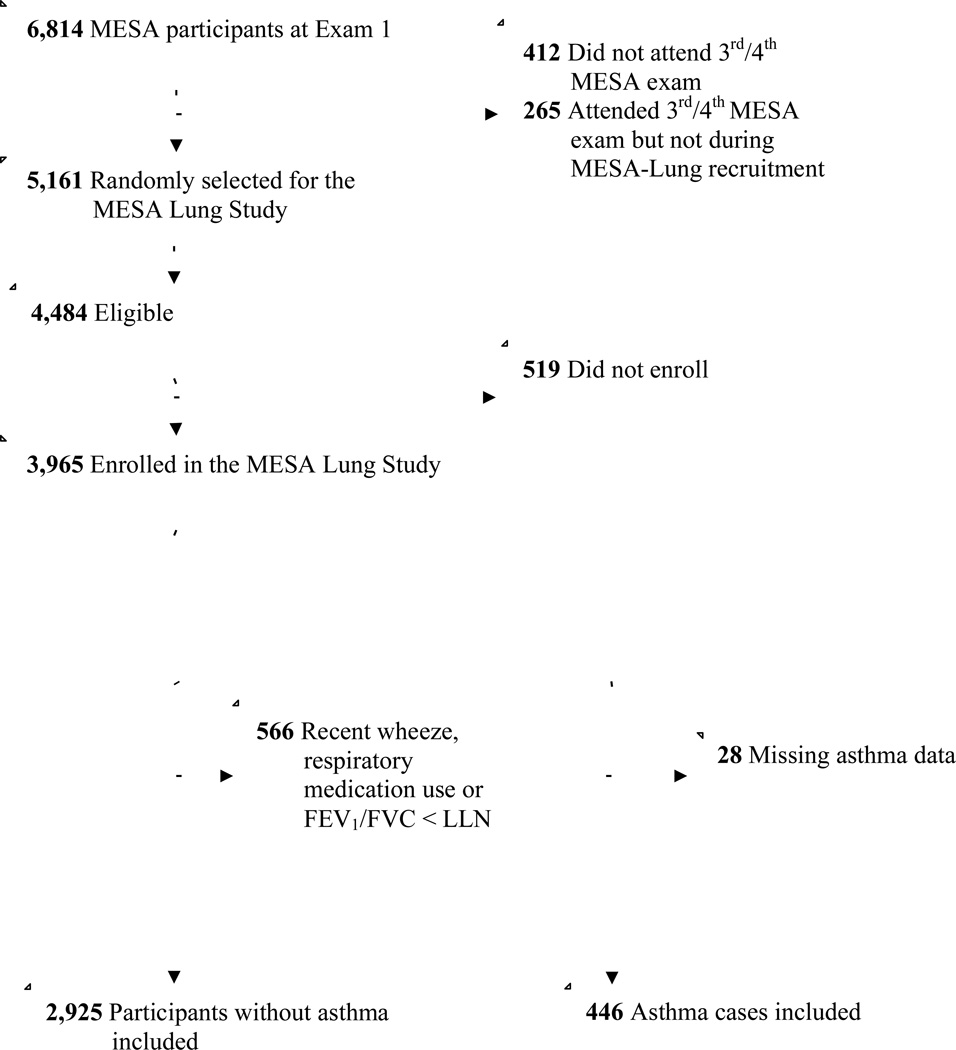
The MESA Lung Study enrolled MESA participants who underwent an examination between 2004 and 2006 (Figure 1). Characteristics of MESA participants in the MESA Lung Study compared to not in the MESA Lung Study are reported in the Online Repository (Table E1); non-participants in the MESA Lung Study were somewhat older and more likely to be white or African-American but the prevalence of asthma was similar in both groups.
Figure 1. The Study Sample.
Asthma
Asthma and respiratory history was ascertained by self report using standard questionnaire items (23). Participants were asked, “Have you ever had asthma?” If so, participants were asked at what age they “developed first asthma symptoms?” and at what age a “doctor first diagnosed asthma.” Ninety-nine percent of participants who reported asthma also reported a physician-diagnosis of asthma. Child-onset asthma was defined as report of first symptoms or diagnosis of asthma at or before age 18 years and young adult-onset asthma as onset of symptoms or diagnosis between age 19 and 45 years.
Remission of asthma was defined as “10-year (or more) period without asthma symptoms.” Participants who reported a remission were asked about “age at first recurrence of asthma symptoms.” Recurrence was defined as participants with a history of remission who subsequently reported use of respiratory medication at the time of the visit, or wheezing in the prior twelve months. Participants without a remission were defined as having persistent asthma.
Participants without asthma
Participants were classified as being without asthma if they reported no history of asthma, had no current respiratory medication use, no wheezing in the last twelve months, and an FEV1/FVC ≥ lower limit of normal.
Spirometry
Spirometry was conducted in 2004–2006 in accordance with the American Thoracic Society/European Respiratory Society guidelines (24) (See Online Repository for details).
CT Measures
CT scans were acquired as previously described and as detailed in the Online Repository (25).
Airway Dimensions
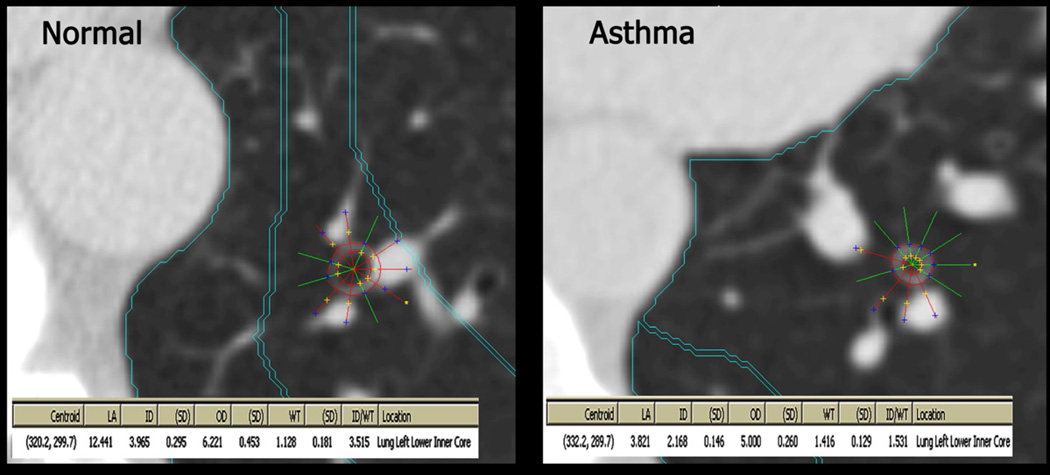
Airways were sampled by CT imaging perpendicular to the airway long axis (i.e., the airway lumen was approximately circular). They were measured in two dimensions using a modified full-width-half-maximum principle to identify the outer and inner airway wall borders (Figure 2) (26). Pi10 in the lower lobes was calculated following the standard method. Individual regression plots were created for each participant, plotting the square root of the wall area against the corresponding internal perimeter for each measured airway belonging to that participant (mean = nine airways per participant). As is standard, airways with an internal perimeter ≤ 6 mm were excluded given the technical limitations of the CT scanners. The resulting regression line was used to calculate the standardized measure of airway wall thickness for a hypothetical airway with an internal perimeter of 10mm (Pi10) for each participant (27).
Figure 2. Airway measurements on CT Scan.
Upon locating an airway perpendicular to the plane, a centroid was placed in the airway, from which the Pulmonary Analysis Software Suite generated rays and outer airway diameters. If a ray extended into adjacent tissue the analyst would exclude it and the PASS system would regenerate the inner and outer diameter to conform to the shape of the airway. The remaining rays were averaged to calculate wall thickness and lumen diameter.
Since asthma may be related to narrowed airway lumens or thickened airway walls, (11) and standard CT measures of airway wall thickness such as Pi10 combine wall thickness with luminal diameter (by regressing airway wall thickness on inner perimeter, or taking the ratio of the two), we assessed airway lumen diameter and airway wall thickness separately in a single segmental, lower lobe (LB10 or RB10) airway for each participant. Thus, for purposes of this study, the terms ‘airway wall thickness’ and ‘airway lumen diameter’ refer to the dimensions of a single airway for each participant, while ‘Pi10’ is a measure derived from both the wall thicknesses and lumen diameters of multiple airways per participant. All analyses of individual airway dimensions (lumen, wall thickness) are adjusted for body size.
Percentage of lung low attenuation areas (%LAA)
%LAA (also known as percent emphysema) was defined as the percentage of the total lung voxels with attenuation < –910 Hounsfield units (HU) (28). %LAA reflects emphysematous and hyperinflated lung, in addition to distal airway lumens (29).
Additional details regarding scanner types, slice thickness and reconstruction kernels, Pi10 calculations, individual airway measurements and %LAA can be found in the Online Repository.
Covariates
Age, gender, race/ethnicity, educational attainment and family history were self-reported. Assessment of other covariates has been previously described, including anthropometry (30), current smoking, pack-years and pipe-years (31), environmental tobacco smoke (ETS) and occupational exposures (23) and particulate matter (32).
Statistical Analysis
Participants with asthma were categorized according to onset of asthma. Linear regression models regressed measures of lung function and structure on asthma categories. Initial regression models adjusted for age, sex, height, height2 and race/ethnicity. Additional adjustment was performed for the following potential confounders: smoking status, pack- years, cotinine, pipe-years, household ETS, education, body mass index, waist circumference, occupational exposures, family history of emphysema and particulate matter exposure. Models for CT measures also were adjusted for CT scanner type and tube current in milliamperes as precision variables. Differences in lung function and structure across categories of asthma were tested with the −2 log-likelihood test of nested models. In order to account for the multiple comparisons (four measures of lung function and structure), statistical significance was defined post-hoc as a Bonferroni-corrected, two-tailed P<0.0125 for this test. If a statistically significant difference were detected for a given endpoint using this strict criteria, within-category comparisons were considered significant at a P<0.05. Analyses were performed using SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
The mean age of the 3,371 participants was 65 years at the time of spirometry, 49% were male, and the race/ethic distribution was 34% white, 25% African-American, 23% Hispanic, and 18% Chinese-American. Fifty percent of the cohort never smoked cigarettes, 42% were former smokers and 8% currently smoked.
Of the 3,371 participants who met inclusion criteria, 446 had asthma and 2,925 did not. Two hundred seventeen participants reported asthma onset in childhood and 102 as young adults.
Participants with young adult-onset asthma were more likely to be female, have shorter height and report ETS exposure (Table I). Smoking history did not vary appreciably across categories of asthma.
Table I.
Characteristics of Participants Stratified by Asthma Age of Onset
| No Asthma* |
Child Onset Asthma ≤18 years† |
Young Adult Onset Asthma 19 to 45 years‡ |
Late Adult Onset Asthma > 45 years§ |
|
|---|---|---|---|---|
| N=3371 | 2925 | 217 | 102 | 127 |
| Age, mean (SD), years | 65 (10) | 63 (10) | 62 (10) | 68 (9) |
| Female gender — % | 50 | 48 | 79 | 67 |
| Race/ethnicity — % | ||||
| White | 34 | 38 | 38 | 40 |
| Chinese-American | 19 | 11 | 6 | 10 |
| African American | 25 | 31 | 29 | 21 |
| Hispanic | 23 | 20 | 28 | 29 |
| Education, years — % | ||||
| < 12 | 17 | 11 | 19 | 17 |
| 12 | 18 | 10 | 12 | 20 |
| > 12 | 65 | 79 | 69 | 63 |
| Asthma age-of-onset, median (IQR) | n/a | 8 (4–13) | 35 (30–40) | 57 (50–64) |
| Age at remission, median (IQR) | n/a | 17 (12–25) | 40 (33–45) | 57 (50–67) |
| Taking asthma medications, — %¶ | 0 | 29 | 47 | 60 |
| Wheezing in last 12 months, — % | 0 | 35 | 48 | 54 |
| Current asthma, — % | 0 | 52% | 55% | 91% |
| Hay fever – % | 29 | 62 | 55 | 48 |
| Height, mean (SD), cm | 166 (10) | 167 (10) | 163 (8) | 163 (10) |
| Weight, mean (SD), lbs | 169 (37) | 182 (42) | 178 (39) | 175 (37) |
| Waist circumference, mean (SD), cm | 96 (13) | 100 (16) | 101 (16) | 103 (15) |
| Body mass index, mean (SD), kg/m2 | 28 (5) | 29 (6) | 31 (7) | 30 (6) |
| Cigarette smoking status, — % | ||||
| Never | 51 | 50 | 50 | 40 |
| Past | 42 | 39 | 41 | 47 |
| Current | 8 | 11 | 9 | 13 |
| Cigarette pack years, median (IQR)# | 13 (3–30) | 16 (5–35) | 12 (6–23) | 24 (6–44) |
| Pipe-years, median (IQR)** | 21 (9–70) | 48 (25–120) | 9 (8–25) | 18 (4–30) |
| Urine cotinine, median (IQR) ng/ml | 7 (7–11) | 7 (7–19) | 7 (7–22) | 7 (7–12) |
| Household ETS, — % | 69 | 70 | 77 | 67 |
| Occupational exposures to dust — % | 34 | 46 | 45 | 39 |
| Family history of emphysema, — % | 3 | 7 | 6 | 10 |
| CT scanner type, — % | ||||
| Multi-detector CT | 39 | 41 | 35 | 39 |
| Electron-beam CT | 61 | 59 | 65 | 61 |
| Particulate Matter < 10 µm, mean % (SD) | 16 (7) | 15 (7) | 17 (7) | 15 (8) |
| FEV1, mean % predicted (SD) | 98 (16) | 83 (18) | 83 (21) | 82 (23) |
| FVC, mean % predicted (SD) | 97 (15) | 90 (16) | 90 (18) | 90 (19) |
| FEV1/FVC % (SD) | 77 (7) | 71 (10) | 72 (11) | 69 (13) |
Abbreviations: SD, standard deviation; IQR, inter-quartile range; CT, computed tomography; ETS, environmental tobacco smoke; FEV1, forced expiratory volume in one second; FVC, forced vital capacity
No asthma = no history of asthma, no current respiratory medication use, no wheezing in the last twelve months, and an FEV1/FVC ≥ lower limit of normal.
Child onset asthma = report of first asthma symptoms or diagnosis before age 19 years
Young adult onset asthma = report of first asthma symptoms or diagnosis between ages 19 to 45 years
Late adult onset asthma = report of first asthma symptoms or diagnosis between after age 45 years
Inhaled corticosteroids, β-agonists, leukotriene inhibitors
Among ever smokers
Among pipe smokers
Lung Function
As expected, participants with asthma onset at any age, including in childhood or young adulthood, had significantly lower lung function in later life compared to participants without asthma (P< 0.001) (Table II).
Table II.
Asthma age of onset, lung structure and function
| ≤ 18 years n=217 |
19 to 45 years n=102 |
> 45 years n=127 |
P-value | |
|---|---|---|---|---|
| FEV1 (ml) |
−365 (−419 to −310) *** |
−343 (−420 to −266) *** |
−359 (−428 to −289) *** |
< 0.001 |
| Single airway lumen diameter (mm) |
−0.39 (−0.58 to −0.2) *** |
−0.34 (−0.61 to −0.07) * |
−0.52 (−0.76 to −0.28) *** |
< 0.001 |
| Single airway wall thickness (mm) |
0.01 (−0.01 to 0.04) |
0.02 (−0.02 to 0.05) |
−0.02 (−0.06 to 0.009) |
0.06 |
| % LAA (%) |
1.69 (0.09 to 3.29) * |
4.30 (2.00 to 6.59) *** |
3.52 (1.45 to 5.58) *** |
< 0.001 |
Results represent the mean difference compared to participants without asthma from linear regression models adjusted for age, sex, height, height2, race/ethnicity, smoking status, pack-years, pipe-years, urine cotinine, household ETS, education, body mass index, waist circumference, occupational exposures, particulate matter exposure. Lung structure models (lumen diameter, wall thickness and %LAA) were additionally adjusted for tube current in milliamperes and CT scanner type. The %LAA model was additionally adjusted for family history of emphysema. P-values represent the −2 log likelihood test for overall significance among asthma age of onset subgroups vs. participants without asthma.
Where significant overall, subgroups were tested individually, with p < 0.001 = *** and p < 0.05 = *.
Lung Structure
Participants with asthma had an increased Pi10 compared to participants without asthma (multivariate mean difference was 0.10 mm [95%CI: 0.06, 0.13]; P<0.001 for those with asthma onset in childhood, 0.09 mm [95%CI: 0.04, 0.13]; P<0.001 for those with asthma onset in young adulthood, and 0.05 mm [95%CI: 0.006, 0.09]; P<0.001 for those with onset after age 45 years).
Associations for Pi10, however, were predominantly due to lumen rather than wall size (Table II). Participants with asthma had narrower segmental airway lumens than participants without asthma (P< 0.001). In contrast to findings for airway lumens, segmental airway wall thickness was not significantly increased in asthma compared to participants without asthma (P=0.06).
Percent Low Attenuation Areas
Participants with asthma had greater %LAA than participants without asthma (P<0.001), whether with onset in childhood, young adulthood or after age 45 years (Table II).
Never Smokers
Findings were generally similar among the 50% of the cohort that never smoked cigarettes (Table III). Decrements in lung function related to asthma with onset in childhood or young adulthood were large, and findings for the lumen were statistically significant. The only major difference compared to the whole cohort was that %LAA was not increased in never smokers with child-onset asthma. As in the whole cohort, Pi10 was increased within each category of asthma (P<0.001).
Table III.
Asthma age of onset, lung structure and lung function in never smokers
| ≤ 18 years n=107 |
19 to 45 years n=51 |
> 45 years n=51 |
P-value | |
|---|---|---|---|---|
| FEV1 (ml) |
−293 (−364 to −221) *** |
−237 (−338 to −137) *** |
−307 (−407 to −207) *** |
< 0.001 |
| Single airway lumen diameter (mm) |
−0.35 (−0.62 to −0.08) * |
−0.28 (−0.64 to 0.08) |
−0.77 (−1.14 to −0.40) *** |
< 0.001 |
| Single airway wall thickness (mm) |
0.01 (−0.02 to 0.05) |
0.02 (−0.02 to 0.07) |
−0.03 (−0.08 to 0.02) |
0.09 |
| % LAA (%) |
−0.68 (−2.83 to 1.47) |
3.52 (0.46 to 6.58) * |
3.44 (0.391 to 6.50) * |
< 0.001 |
Results represent the mean difference compared to participants without asthma from linear regression models adjusted for age, sex, height, height2, race/ethnicity, smoking status, pack-years, pipe-years, urine cotinine, household ETS, education, body mass index, waist circumference, occupational exposures, particulate matter exposure. Lung structure models (lumen diameter, wall thickness and %LAA) were additionally adjusted for tube current in milliamperes and CT scanner type. The %LAA model was additionally adjusted for family history of emphysema.
P < 0.001 = *** and P < 0.05 = *.
Exploratory Analysis of Duration, Remission, Recurrence or Persistence of Asthma
Each year of duration of asthma was associated with narrower airway lumens (mean difference −0.01 mm [95%CI: −0.02 to −0.003];P = 0.003), thicker airway walls (mean difference 0.001 mm [95%CI: 0.0003 to 0.002];P = 0.01) and increased %LAA (mean difference 0.15% [95%CI 0.11 to 0.20]; P < 0.001) in fully adjusted models. Even participants with remitted asthma had evidence of narrow airway lumens and large decrements in lung function; participants with persistent asthma had evidence of increased %LAA (Table E2).
Lung Structure and Lung Function
In fully adjusted models, each increased ml of FEV1 was associated with wider airway lumens (mean difference 0.03mm (95%CI 0.03 to 0.04); p < 0.001), was not associated with a change in airway wall thickness (mean difference 0.0005mm (95%CI −0.0003 to 0.00014; p = 0.2), and was associated with reduced %LAA (mean difference −0.4% (95%CI −0.5 to −0.4);p < 0.001).
Sensitivity Analyses
Results were similar when inclusion criteria for the control group were relaxed to include any participant without a history of asthma even if they had lung function below the lower limit of normal, respiratory medication use or wheezing in the last twelve months. When age of onset was varied, findings were generally similar (Online Repository Tables E3 and E4). When the threshold for %LAA was decreased to −950 HU results were similar (multivariate mean difference was 0.7% [95%CI: 0.2, 1.3] for those with asthma onset in childhood, 1.4% [95%CI: 0.7, 2.2] for those with asthma onset in young adulthood, and 1.3% [95%CI: 0.6, 1.9] for those with onset after age 45 years). Results were similar in analyses stratified by scanner type (Tables E5a and E5b). Results adjusted for medication use are reported in Table E6 and for those with recurrent disease in Table E7. Results also were similar after adjusting for recent upper respiratory infection (Table E8), and stratified by current vs. former asthma (Table E9).
DISCUSSION
A history of asthma, particularly in childhood and young adulthood, was associated both with large decrements in lung function in later life and narrower segmental airways in this large, population-based cohort study. In addition, asthma onset in young adulthood was associated with increased %LAA.
The mean decrement in FEV1 in later life among participants with asthma with onset in childhood and young adulthood was larger than that among participants with a history of cigarette smoking (−365 ml for child onset asthma and −343 ml for young adult onset asthma vs. −82 mL for participants who smoked) (31) in this multi-ethnic, predominantly urban US cohort. This finding confirms that asthma in childhood and young adulthood is a major correlate of lung function later in life.
Our findings for lung function are consistent with prior cross-sectional studies of self- reported asthma in older adults (3); these studies, however, did not report asthma age-of-onset and so it is unclear if they assessed early or late-onset asthma, or mis-classified COPD (33).
A novel finding of this study is that mean segmental airway lumen diameter was narrower among participants with asthma onset in childhood and young adulthood compared to participants without asthma in this general population sample. Though there is controversy about the optimal approach to measuring airways on CT (34), Nakano et al. showed that the relative change in smaller airways may be greater than what can be observed on CT in the larger airways, which would suggest associations of greater magnitude in airways smaller than measured in the present study (35). Furthermore, proximal airway changes may lead to regional ventilatory defects (36). This finding suggests either that participants with asthma had narrower airways throughout their lives that predisposed them to asthma or that asthma also contributed to narrow airways, either through airway remodeling (12) or because of bronchoconstriction and increased airway smooth muscle tone (37). We speculate that dysynapsis, or a relatively smaller airway size compared to lung volume, may explain these consistent findings for lung function and airway lumens in asthma.
Multiple prior studies have observed evidence of airway wall thickening on CT scan in patients with asthma (11, 14–20). However, most of these used the Pi10 and other metrics of airway wall thickness such as airway wall area percent that combine lumen with airway wall thickness and therefore it is unclear if their results were driven by smaller airway lumens or thicker airway walls. In addition, most of these studies were case-control studies in which the cases had fairly severe asthma. For example Aysola et al.(11) found that patients with severe asthma had increased wall thickness percent and wall area percent compared to those with mild to moderate asthma or to those without asthma, but no difference between those with mild to moderate asthma vs. those without asthma. We did see an association between asthma and increased Pi10, but not between asthma and wall thickness. Our validated, reproducible approach to the measurement of segmental airway wall lumen diameter and thickness was similar to that utilized by Nakano et al (38), and suggests that in participants with asthma, composite measures such as Pi10 are driven more by narrowing of the airway lumens than by thickening of the airway walls. Alternatively, it may be due to the generally milder asthma phenotype found in this population based study.
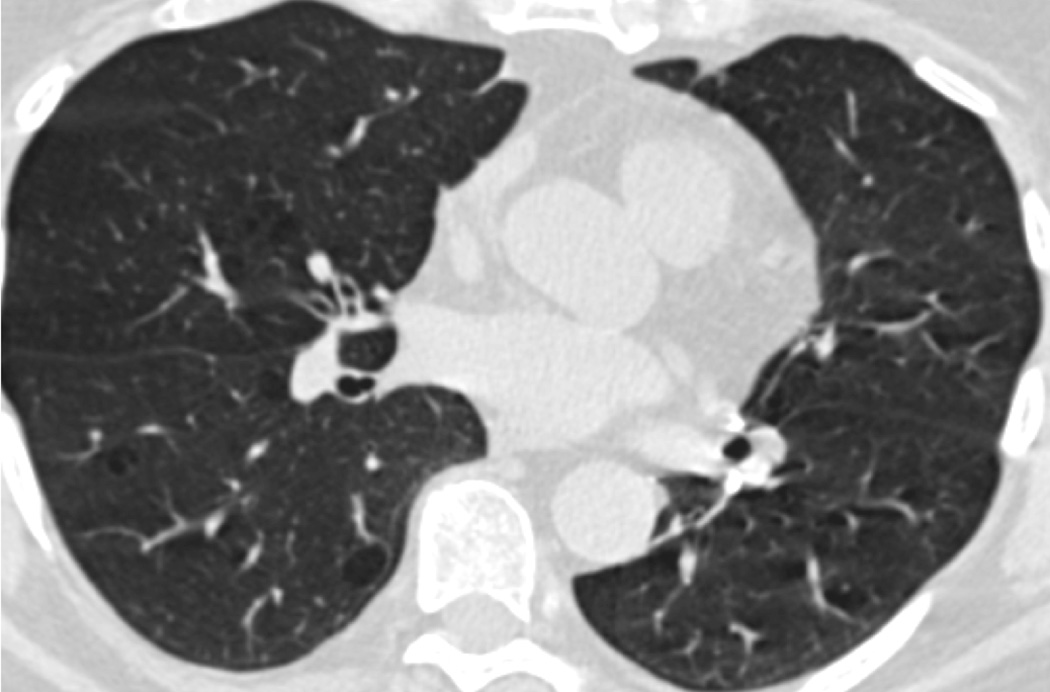
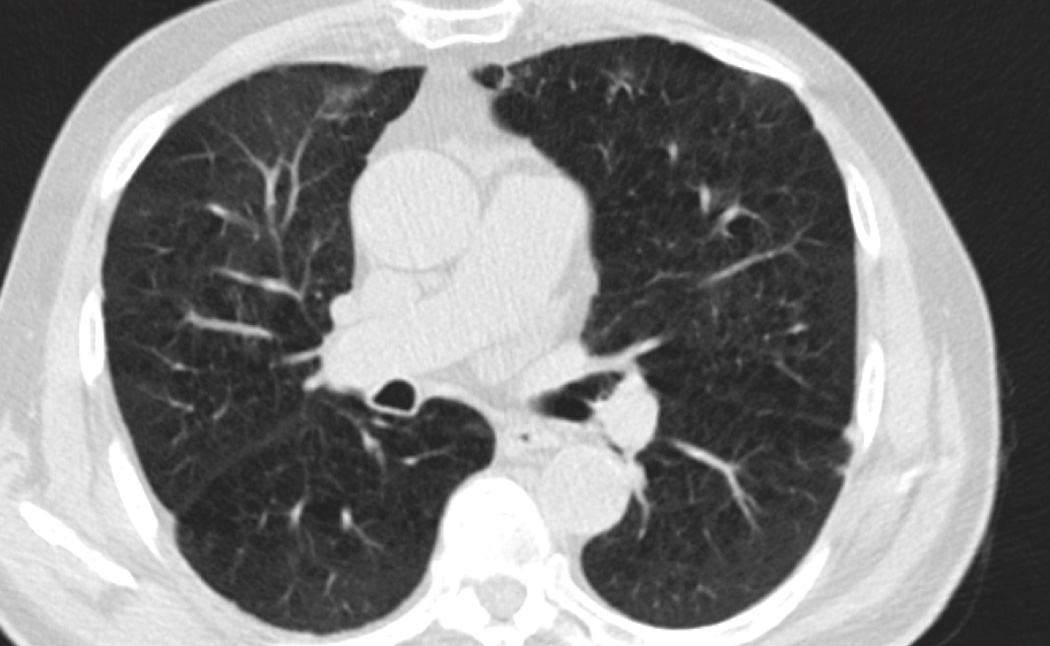
Asthma onset in young adulthood was associated with increased %LAA, consistent with prior small case-control studies (39, 40). Asthma is a known risk factor for COPD (41). This risk is due in part to airway remodeling (42); however, these results suggest that damage to lung parenchyma might also contribute. The mechanical hypothesis of emphysema posits that early and repeated injury leads to alveolar rupture, strain on adjacent alveoli, and propagation of emphysematous sac enlargement (21). Conversely, Gelb et al. posit a “pseudophysiologic emphysema” due to loss of lung elastic recoil (43). Exacerbations in older smokers accelerate progression of percent emphysema/%LAA (44). It is possible that these results are due to either chronic air trapping in stable asthma or acute hyperinflation during an exacerbation. Indeed, Gelb et al. found evidence of “pseudophysiologic emphysema,” with reduced diffusing capacity and marked hyperinflation but minimal evidence of emphysema on autopsy in a study of ten participants with severe small airways disease (43). However, participants in our study were drawn from a population-based sample and thus would, on average, have had milder disease, and were scanned when clinically stable. Furthermore, results were similar after controlling for recent upper respiratory infection. Further, visual assessment of CT scans from participants with persistent asthma demonstrated pulmonary emphysema among smokers and never-smokers (Figure 4).
The present study has a number of limitations. Some degree of misclassification of the age of onset of asthma is likely in this cross-sectional study of adults given the retrospective ascertainment of asthma. However, the probability that misclassification of asthma was differential was minimized (although not ruled out) given the use of the cohort design and the subclinical outcome measures. Furthermore, our findings of large decrements in lung function among participants with self-reported asthma, including those with child onset asthma, are supportive, as are our findings of narrower airway lumens and large decrements in lung function even among participants with remitted asthma. Post-bronchodilator spirometry was not conducted in this large population-based study, but mortality risk related to lung function is based on pre-bronchodilator measures.
Lung structure was measured on the lung regions of gated cardiac CT scans. The cardiac scans did not allow assessment of upper lobes but cardiac-gating reduced the motion artifact typical in the left lower-lobe of full lung scans, allowing more precise measures in these lobes. Three-dimensional airway reconstructions were not possible for these scans; however we validated the location of the airways against full-lung, three-dimensional scans. Variations in slice thickness and scanner resolution by scanner type may have affected airway measurement accuracy. However, this variability existed across all subgroups and scanner type was accounted for within the statistical models. Beam hardening may contribute to low attenuation around the pulmonary vessels, but would have been non-differential with respect to asthma subgroups. Expiratory CT scans were not obtained, and thus some of the %LAA may be due to air trapping.
Cross-sectional studies may have selection bias, but this is unlikely to explain the results since the study was population-based and participants were not selected by asthma status. Although MESA is population based, patients with subclinical cardiovascular disease were excluded; this is unlikely to bias results for asthma because asthma is not related to subclinical cardiovascular disease.
In conclusion, a history of asthma in childhood and young adulthood, in addition to later adulthood, was associated with significant alterations in both lung function and lung structure in later life. All categories of asthma were associated with narrower segmental airways whereas asthma onset between ages 19 and 45 years was associated with increased %LAA.
Figure 3. Increased %LAA in Participants with Persistent Asthma.
- Never-smoker
- Smoker
Key Messages.
Asthma with onset in childhood and young adulthood was associated with narrower segmental airway lumens.
Participants with asthma who smoked had reduced lung attenuation compared to participants without asthma.
ACKNOWLEDGEMENTS
This manuscript has been reviewed by the MESA Investigators for scientific content and consistency of data interpretation with previous MESA publications. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. Dr. Donohue had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was supported by National Institutes of Health (grants R01 HL077612, R01 HL075476, RC1 HL100543, and N01-HC95159-169). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- %LAA
percent low attenuation areas
- COPD
chronic obstructive pulmonary disease
- CT
computed tomography
- ETS
environmental tobacco smoke
- FEV1
forced expiratory volume in one second
- FVC
forced vital capacity
- HU
Hounsfield units
- ICC
intraclass correlation coefficient
- LLN
lower limit of normal
- MESA
Multi-Ethnic Study of Atherosclerosis
- Pi10
wall thickness for a hypothetical airway with an internal perimeter of 10 mm
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions: Dr. Donohue performed the statistical analysis and drafted the manuscript. Drs. Hoffman, Guo, Jacobs and Enright and Ms. Baumhauer provided data collection and critical revisions. Drs. Ahmed and Lovasi provided assistance with statistical analyses and critical revisions. Dr. Barr provided funding, data collection and critical revisions.
REFERENCES
- 1.Braman SS. The global burden of asthma. Chest. 2006;130(1 Suppl):4S–12S. doi: 10.1378/chest.130.1_suppl.4S. Epub 2006/07/15. [DOI] [PubMed] [Google Scholar]
- 2.Phelan PD, Robertson CF, Olinsky A. The Melbourne Asthma Study: 1964–1999. The Journal of allergy and clinical immunology. 2002;109(2):189–194. doi: 10.1067/mai.2002.120951. Epub 2002/02/14. [DOI] [PubMed] [Google Scholar]
- 3.Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339(17):1194–1200. doi: 10.1056/NEJM199810223391703. Epub 1998/10/22. [DOI] [PubMed] [Google Scholar]
- 4.Lee HM, Le H, Lee BT, Lopez VA, Wong ND. Forced vital capacity paired with Framingham Risk Score for prediction of all-cause mortality. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2010;36(5):1002–1006. doi: 10.1183/09031936.00042410. Epub 2010/06/22. [DOI] [PubMed] [Google Scholar]
- 5.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American journal of respiratory and critical care medicine. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. Epub 2007/05/18. [DOI] [PubMed] [Google Scholar]
- 6.Minino AM, Xu J. National Vital Statistics Reports: Deaths: Preliminary Data for 20082010. 2010 Dec 16; Available from: http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_02.pdf. [PubMed]
- 7.Grol MH, Gerritsen J, Postma DS. Asthma: from childhood to adulthood. Allergy. 1996;51(12):855–869. doi: 10.1111/j.1398-9995.1996.tb04485.x. Epub 1996/12/01. [DOI] [PubMed] [Google Scholar]
- 8.van den Toorn LM, Overbeek SE, de Jongste JC, Leman K, Hoogsteden HC, Prins JB. Airway inflammation is present during clinical remission of atopic asthma. American journal of respiratory and critical care medicine. 2001;164(11):2107–2113. doi: 10.1164/ajrccm.164.11.2006165. Epub 2001/12/12. [DOI] [PubMed] [Google Scholar]
- 9.Svanes C, Sunyer J, Plana E, Dharmage S, Heinrich J, Jarvis D, et al. Early life origins of chronic obstructive pulmonary disease. Thorax. 2010;65(1):14–20. doi: 10.1136/thx.2008.112136. Epub 2009/09/05. [DOI] [PubMed] [Google Scholar]
- 10.Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, Nizankowska-Mogilnicka E, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139(4):752–763. doi: 10.1378/chest.10-1253. Epub 2010/10/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aysola RS, Hoffman EA, Gierada D, Wenzel S, Cook-Granroth J, Tarsi J, et al. Airway remodeling measured by multidetector CT is increased in severe asthma and correlates with pathology. Chest. 2008;134(6):1183–1191. doi: 10.1378/chest.07-2779. Epub 2008/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Blic J, Tillie-Leblond I, Emond S, Mahut B, Dang Duy TL, Scheinmann P. High-resolution computed tomography scan and airway remodeling in children with severe asthma. The Journal of allergy and clinical immunology. 2005;116(4):750–754. doi: 10.1016/j.jaci.2005.07.009. Epub 2005/10/08. [DOI] [PubMed] [Google Scholar]
- 13.Hoshino M, Matsuoka S, Handa H, Miyazawa T, Yagihashi K. Correlation between airflow limitation and airway dimensions assessed by multidetector CT in asthma. Respiratory medicine. 2010;104(6):794–800. doi: 10.1016/j.rmed.2009.12.005. Epub 2010/01/08. [DOI] [PubMed] [Google Scholar]
- 14.Awadh N, Muller NL, Park CS, Abboud RT, FitzGerald JM. Airway wall thickness in patients with near fatal asthma and control groups: assessment with high resolution computed tomographic scanning. Thorax. 1998;53(4):248–253. doi: 10.1136/thx.53.4.248. Epub 1998/09/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. American journal of respiratory and critical care medicine. 2003;167(10):1360–1368. doi: 10.1164/rccm.200209-1030OC. Epub 2003/01/18. [DOI] [PubMed] [Google Scholar]
- 16.Gono H, Fujimoto K, Kawakami S, Kubo K. Evaluation of airway wall thickness and air trapping by HRCT in asymptomatic asthma. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2003;22(6):965–971. doi: 10.1183/09031936.03.00085302. Epub 2003/12/19. [DOI] [PubMed] [Google Scholar]
- 17.Kasahara K, Shiba K, Ozawa T, Okuda K, Adachi M. Correlation between the bronchial subepithelial layer and whole airway wall thickness in patients with asthma. Thorax. 2002;57(3):242–246. doi: 10.1136/thorax.57.3.242. Epub 2002/02/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ketai L, Coutsias C, Williamson S, Coutsias V. Thin-section CT evidence of bronchial thickening in children with stable asthma: bronchoconstriction or airway remodeling? Academic radiology. 2001;8(3):257–264. doi: 10.1016/S1076-6332(03)80535-4. Epub 2001/03/16. [DOI] [PubMed] [Google Scholar]
- 19.Little SA, Sproule MW, Cowan MD, Macleod KJ, Robertson M, Love JG, et al. High resolution computed tomographic assessment of airway wall thickness in chronic asthma: reproducibility and relationship with lung function and severity. Thorax. 2002;57(3):247–253. doi: 10.1136/thorax.57.3.247. Epub 2002/02/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niimi A, Matsumoto H, Amitani R, Nakano Y, Mishima M, Minakuchi M, et al. Airway wall thickness in asthma assessed by computed tomography. Relation to clinical indices. American journal of respiratory and critical care medicine. 2000;162(4 Pt 1):1518–1523. doi: 10.1164/ajrccm.162.4.9909044. Epub 2000/10/13. [DOI] [PubMed] [Google Scholar]
- 21.Mishima M, Hirai T, Itoh H, Nakano Y, Sakai H, Muro S, et al. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(16):8829–8834. doi: 10.1073/pnas.96.16.8829. Epub 1999/08/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. Epub 2002/10/25. [DOI] [PubMed] [Google Scholar]
- 23.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118(6 Pt 2):1–120. Epub 1978/12/01. [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. Epub 2005/08/02. [DOI] [PubMed] [Google Scholar]
- 25.Donohue KM ea. Cigarette smoking and airway wall thickness on CT scan in a multi-ethnic cohort: The MESA Lung Study. Respiratory medicine. 2012 doi: 10.1016/j.rmed.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinhardt JMDSN, Hoffman EA. Accurate Measurement of Intra-thoracic Airways. IEEE transactions on medical imaging. 1998;16(6):820–827. doi: 10.1109/42.650878. [DOI] [PubMed] [Google Scholar]
- 27.Grydeland TB, Dirksen A, Coxson HO, Eagan TM, Thorsen E, Pillai SG, et al. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. American journal of respiratory and critical care medicine. 2010;181(4):353–359. doi: 10.1164/rccm.200907-1008OC. Epub 2009/11/21. [DOI] [PubMed] [Google Scholar]
- 28.Coxson HO, Mayo JR, Behzad H, Moore BJ, Verburgt LM, Staples CA, et al. Measurement of lung expansion with computed tomography and comparison with quantitative histology. J Appl Physiol. 1995;79(5):1525–1530. doi: 10.1152/jappl.1995.79.5.1525. Epub 1995/11/01. [DOI] [PubMed] [Google Scholar]
- 29.Mitsunobu F, Mifune T, Ashida K, Hosaki Y, Tsugeno H, Okamoto M, et al. Influence of age and disease severity on high resolution CT lung densitometry in asthma. Thorax. 2001;56(11):851–856. doi: 10.1136/thorax.56.11.851. Epub 2001/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MESA Manual of Operations. Field center and laboratory procedures. Seattle: University of Washington; 2008. [cited 2011 February 16]; Available from: http://www.mesa-nhlbi.org/manuals.aspx. [Google Scholar]
- 31.Rodriguez J, Jiang R, Johnson WC, MacKenzie BA, Smith LJ, Barr RG. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med. 2010;152(4):201–210. doi: 10.1059/0003-4819-152-4-201002160-00004. Epub 2010/02/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park SK, Auchincloss AH, O'Neill MS, Prineas R, Correa JC, Keeler J, et al. Particulate Air Pollution, Metabolic Syndrome and Heart Rate Variability: the Multi-Ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2010;118(10):1406–1411. doi: 10.1289/ehp.0901778. Epub 2010/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tinkelman DG, Price DB, Nordyke RJ, Halbert RJ. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. The Journal of asthma : official journal of the Association for the Care of Asthma. 2006;43(1):75–80. doi: 10.1080/02770900500448738. Epub 2006/02/02. [DOI] [PubMed] [Google Scholar]
- 34.Castro M, Fain SB, Hoffman EA, Gierada DS, Erzurum SC, Wenzel S. Lung imaging in asthmatic patients: the picture is clearer. The Journal of allergy and clinical immunology. 2011;128(3):467–478. doi: 10.1016/j.jaci.2011.04.051. Epub 2011/06/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, et al. The prediction of small airway dimensions using computed tomography. American journal of respiratory and critical care medicine. 2005;171(2):142–146. doi: 10.1164/rccm.200407-874OC. Epub 2004/11/02. [DOI] [PubMed] [Google Scholar]
- 36.de Lange EE, Altes TA, Patrie JT, Battiston JJ, Juersivich AP, Mugler JP, 3rd, et al. Changes in regional airflow obstruction over time in the lungs of patients with asthma: evaluation with 3He MR imaging. Radiology. 2009;250(2):567–575. doi: 10.1148/radiol.2502080188. Epub 2009/02/04. [DOI] [PubMed] [Google Scholar]
- 37.Brown RH, Scichilone N, Mudge B, Diemer FB, Permutt S, Togias A. High-resolution computed tomographic evaluation of airway distensibility and the effects of lung inflation on airway caliber in healthy subjects and individuals with asthma. American journal of respiratory and critical care medicine. 2001;163(4):994–1001. doi: 10.1164/ajrccm.163.4.2007119. Epub 2001/04/03. [DOI] [PubMed] [Google Scholar]
- 38.Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, et al. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. American journal of respiratory and critical care medicine. 2000;162(3 Pt 1):1102–1108. doi: 10.1164/ajrccm.162.3.9907120. Epub 2000/09/16. [DOI] [PubMed] [Google Scholar]
- 39.Ueda T, Niimi A, Matsumoto H, Takemura M, Hirai T, Yamaguchi M, et al. Role of small airways in asthma: investigation using high-resolution computed tomography. The Journal of allergy and clinical immunology. 2006;118(5):1019–1025. doi: 10.1016/j.jaci.2006.07.032. Epub 2006/11/08. [DOI] [PubMed] [Google Scholar]
- 40.Vignola AM, Paganin F, Capieu L, Scichilone N, Bellia M, Maakel L, et al. Airway remodelling assessed by sputum and high-resolution computed tomography in asthma and COPD. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2004;24(6):910–917. doi: 10.1183/09031936.04.00032603. Epub 2004/12/02. [DOI] [PubMed] [Google Scholar]
- 41.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9691):733–743. doi: 10.1016/S0140-6736(09)61303-9. Epub 2009/09/01. [DOI] [PubMed] [Google Scholar]
- 42.Silva GE, Sherrill DL, Guerra S, Barbee RA. Asthma as a risk factor for COPD in a longitudinal study. Chest. 2004;126(1):59–65. doi: 10.1378/chest.126.1.59. Epub 2004/07/14. [DOI] [PubMed] [Google Scholar]
- 43.Gelb AF, Zamel N. Unsuspected pseudophysiologic emphysema in chronic persistent asthma. American journal of respiratory and critical care medicine. 2000;162(5):1778–1782. doi: 10.1164/ajrccm.162.5.2001037. Epub 2000/11/09. [DOI] [PubMed] [Google Scholar]
- 44.Tanabe N, Muro S, Hirai T, Oguma T, Terada K, Marumo S, et al. Impact of exacerbations on emphysema progression in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2011;183(12):1653–1659. doi: 10.1164/rccm.201009-1535OC. Epub 2011/04/08. [DOI] [PubMed] [Google Scholar]
- 45.Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the multi-ethnic study of atherosclerosis (MESA) lung study. Chest. 2010;137(1):138–145. doi: 10.1378/chest.09-0919. Epub 2009/09/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. American journal of respiratory and critical care medicine. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. Epub 1999/01/05. [DOI] [PubMed] [Google Scholar]
- 47.Hoffman EA, Simon BA, McLennan G. State of the Art. A structural and functional assessment of the lung via multidetector-row computed tomography: phenotyping chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(6):519–532. doi: 10.1513/pats.200603-086MS. Epub 2006/08/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D'Souza N RJaHE. ASAP: Interactive Quantification of 2D Airway Geometry. SPIE Medical Imaging. 1996;2709:197–208. [Google Scholar]
- 49.Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, Detrano R, et al. Reproducibility and validity of lung density measures from cardiac CT Scans--The Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Academic radiology. 2009;16(6):689–99. doi: 10.1016/j.acra.2008.12.024. Epub 2009/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]