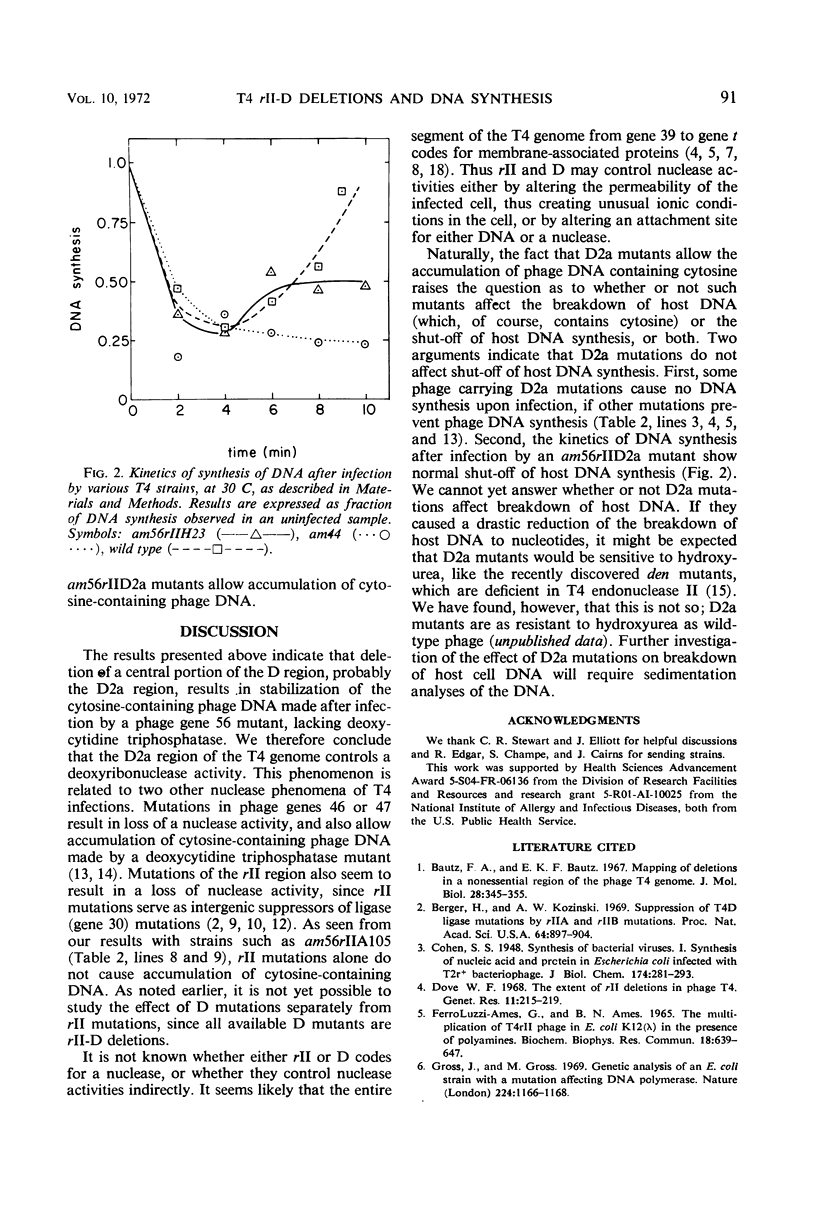
Abstract
When T-even phage infect Escherichia coli, synthesis of host deoxyribonucleic acid (DNA) rapidly ceases. If the phage carry a mutation in a gene essential for phage DNA synthesis, then the infected bacteria should make no DNA, either host DNA or phage DNA. However, we have found that infection with certain T4 gene 56 (deoxycytidine triphosphatase)-rII double mutants leads to substantial DNA synthesis. Only rII deletion mutations which extend into the middle third of the adjacent, nonessential D region lead to the anomalous DNA synthesis, when combined with a gene 56 mutation; the requirement probably is that the deletion extend into the D2a transcriptional unit identified by Sederoff et al. Genetic evidence indicates that the observed anomalous DNA synthesis is synthesis of phage DNA. We suggest that the D2a region controls, directly or indirectly, a nuclease involved in the breakdown of cytosine-containing DNA. In the absence of the D2a product, the cytosine-containing phage DNA made by the gene 56 mutant is stabilized.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bautz F. A., Bautz E. K. Mapping of deletions in a non-essential region of the phage T4 genome. J Mol Biol. 1967 Sep 14;28(2):345–355. doi: 10.1016/s0022-2836(67)80014-7. [DOI] [PubMed] [Google Scholar]
- Berger H., Kozinski A. W. Suppression of T4D ligase mutations by rIIa and rIIb mutations. Proc Natl Acad Sci U S A. 1969 Nov;64(3):897–904. doi: 10.1073/pnas.64.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove W. The extent of rII deletions in phage T4. Genet Res. 1968 Apr;11(2):215–219. doi: 10.1017/s001667230001140x. [DOI] [PubMed] [Google Scholar]
- Gross J., Gross M. Genetic analysis of an E. coli strain with a mutation affecting DNA polymerase. Nature. 1969 Dec 20;224(5225):1166–1168. doi: 10.1038/2241166a0. [DOI] [PubMed] [Google Scholar]
- Guttman B. S., Begley L. Evidence for a magnesium pump induced by bacteriophage T4. Virology. 1968 Dec;36(4):687–690. doi: 10.1016/0042-6822(68)90203-1. [DOI] [PubMed] [Google Scholar]
- Josslin R. The lysis mechanism of phage T4: mutants affecting lysis. Virology. 1970 Mar;40(3):719–726. doi: 10.1016/0042-6822(70)90216-3. [DOI] [PubMed] [Google Scholar]
- Karam J. D., Barker B. Properties of bacteriophage T4 mutants defective in gene 30 (deoxyribonucleic acid ligase) and the rII gene. J Virol. 1971 Feb;7(2):260–266. doi: 10.1128/jvi.7.2.260-266.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam J. D. DNA replication of phage T4 rII mutants without polynucleotide ligase (gene 30). Biochem Biophys Res Commun. 1969 Oct 22;37(3):416–422. doi: 10.1016/0006-291x(69)90931-0. [DOI] [PubMed] [Google Scholar]
- Kasai T., Bautz E. K. Regulation of gene-specific RNA synthesis in bacteriophage T4. J Mol Biol. 1969 May 14;41(3):401–417. doi: 10.1016/0022-2836(69)90284-8. [DOI] [PubMed] [Google Scholar]
- Krisch H. M., Shah D. B., Berger H. Replication and recombination in ligase-deficient rII bacteriophage T4D. J Virol. 1971 Apr;7(4):491–498. doi: 10.1128/jvi.7.4.491-498.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Biological effects of substituting cytosine for 5-hydroxymethylcytosine in the deoxyribonucleic acid of bacteriophage T4. J Virol. 1969 Oct;4(4):439–453. doi: 10.1128/jvi.4.4.439-453.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Degradation of cytosin-containing bacterial and bacteriophage DNA after infection of Escherichia coli B with bacteriophage T4D wild type and with mutants defective in genes 46, 47 and 56. J Mol Biol. 1968 Dec;38(3):395–411. doi: 10.1016/0022-2836(68)90394-x. [DOI] [PubMed] [Google Scholar]
- SILVER S. ACRIFLAVINE RESISTANCE: A BACTERIOPHAGE MUTATION AFFECTING THE UPTAKE OF DYE BY THE INFECTED BACTERIAL CELLS. Proc Natl Acad Sci U S A. 1965 Jan;53:24–30. doi: 10.1073/pnas.53.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski P. D., Warner H. R., Hercules K., Munro J. L., Mendelsohn S., Wiberg J. S. Mutants of bacteriophage T4 defective in the induction of T4 endonuclease II. J Biol Chem. 1971 May 25;246(10):3431–3433. [PubMed] [Google Scholar]
- Schmidt D. A., Mazaitis A. J., Kasai T., Bautz E. K. Involvement of a phage T4 sigma factor and an anti-terminator protein in the transcription of early T4 genes in vivo. Nature. 1970 Mar 14;225(5237):1012–1016. doi: 10.1038/2251012a0. [DOI] [PubMed] [Google Scholar]
- Sederoff R., Bolle A., Epstein R. H. A method for the detection of specific T4 messenger RNAs by hybridization competition. Virology. 1971 Aug;45(2):440–455. doi: 10.1016/0042-6822(71)90344-8. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]



