Abstract
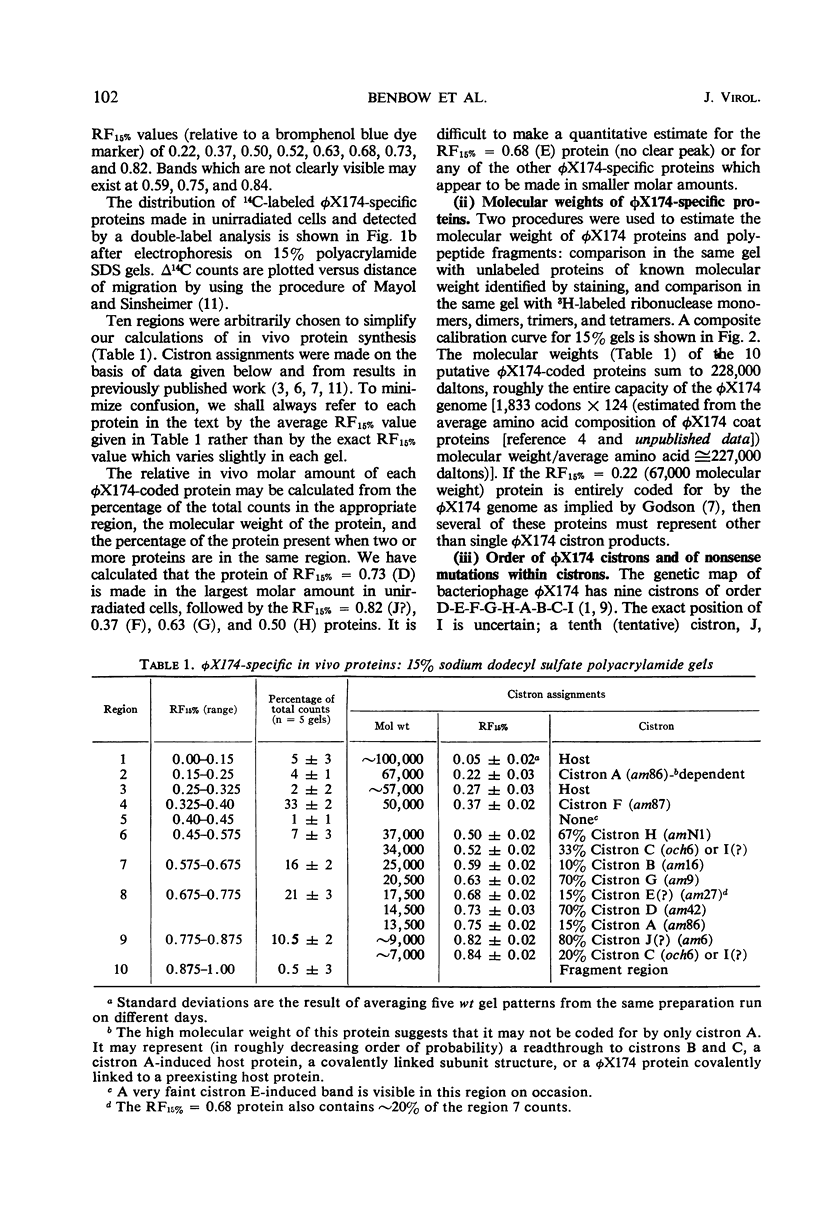
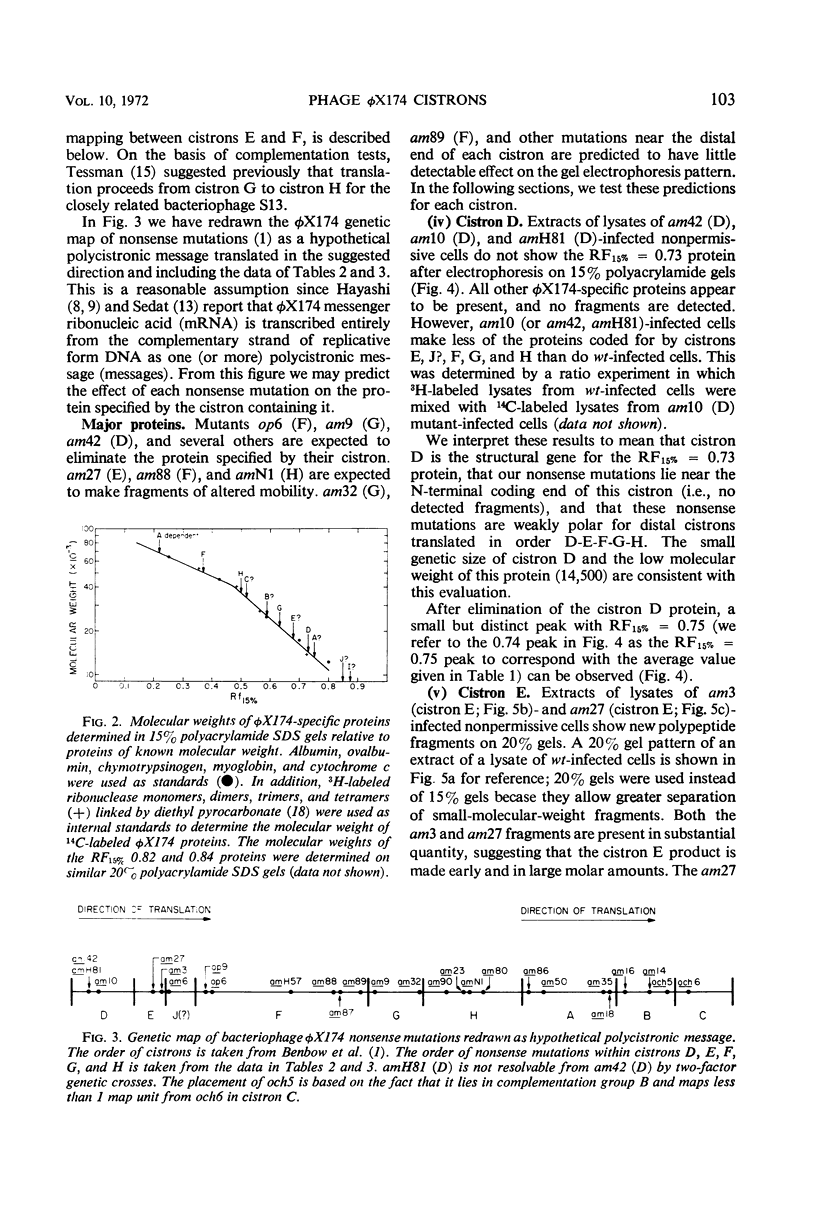
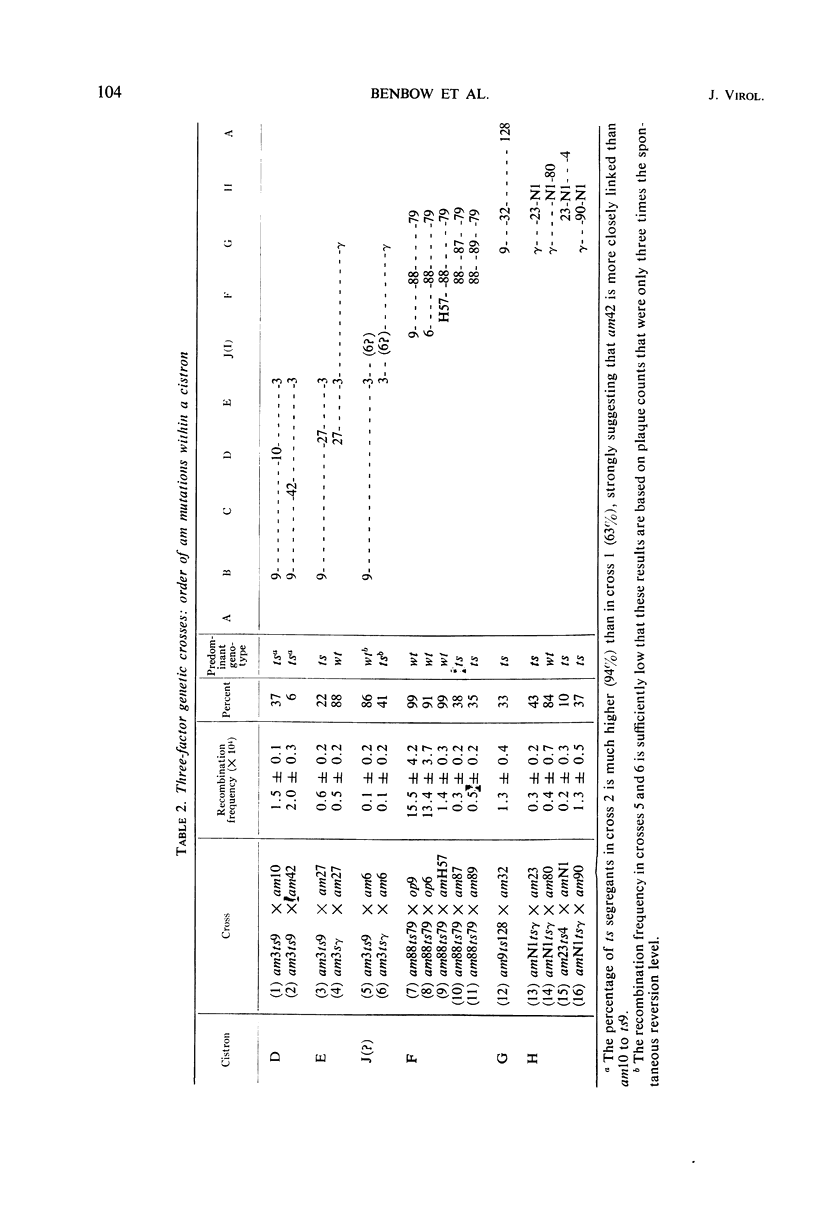
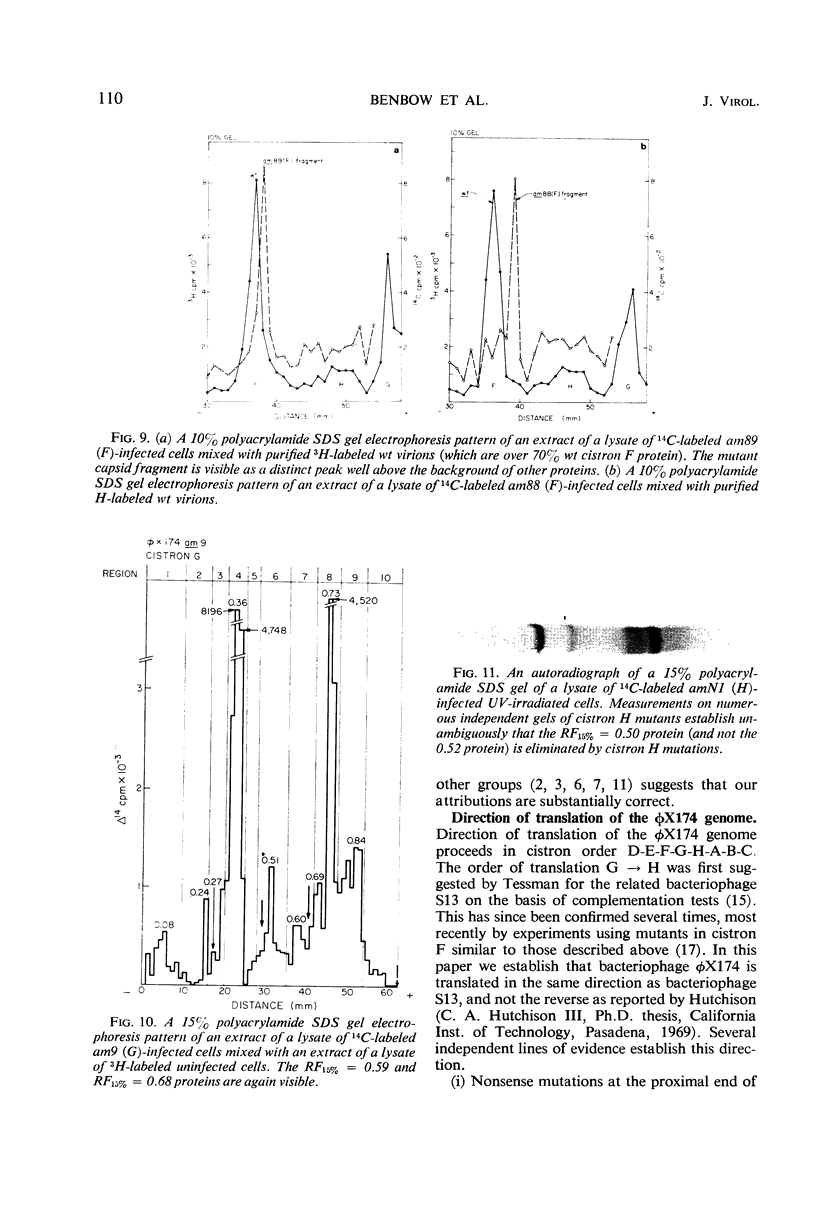
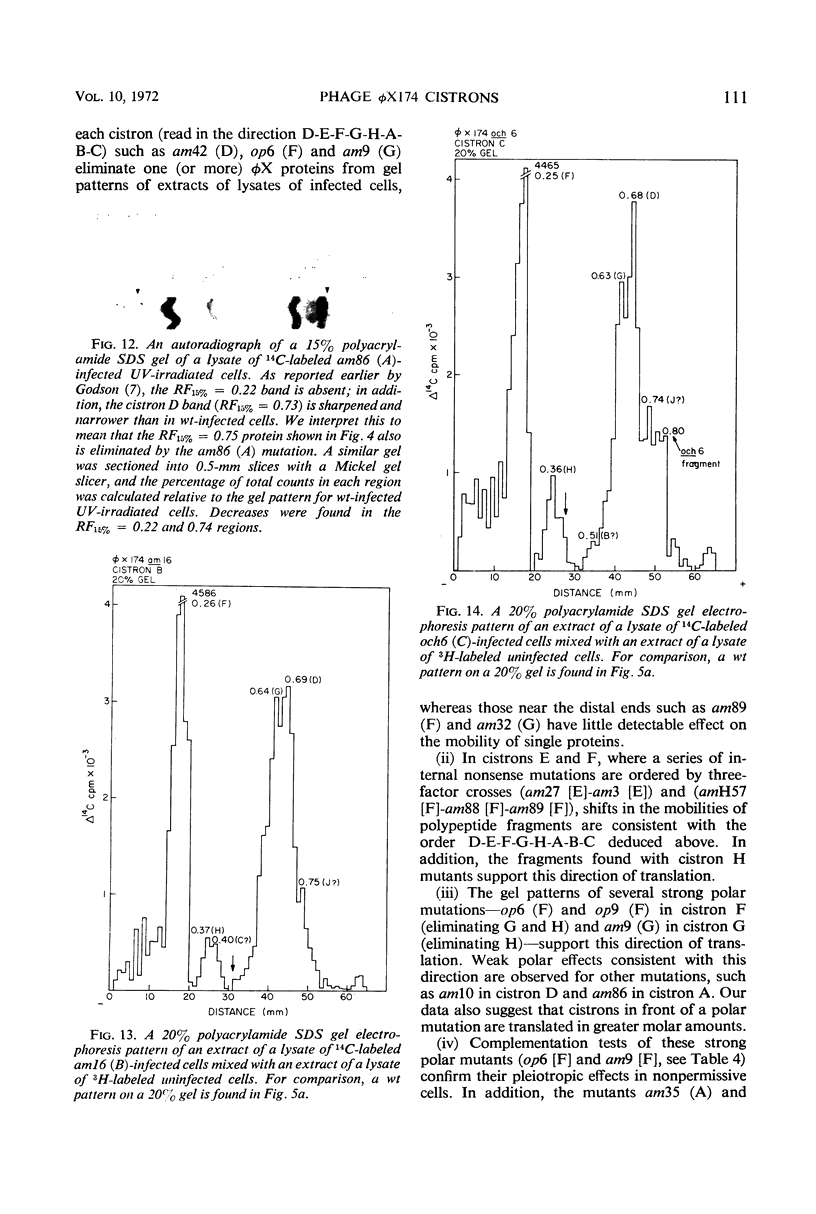
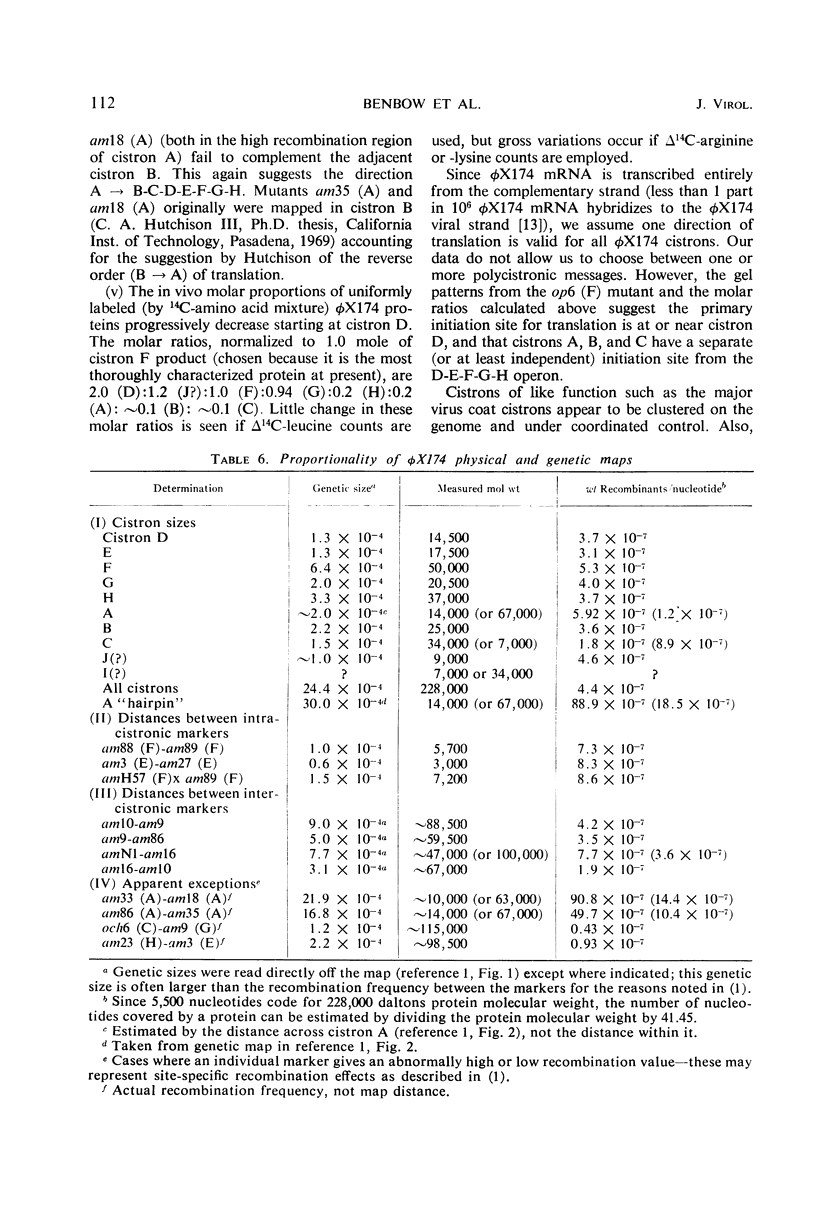
Translation of the bacteriophage φX174 genome follows cistron order D-E-F-G-H-A-B-C. To establish this, the position of a nonsense mutation on the genetic map was compared with the physical size (molecular weight) of the appropriate protein fragment generated in nonpermissive cells. Distances on the φX174 genetic map and distances on a physical map constructed from the molecular weights of φX174 proteins and protein fragments are proportional over most of the genome with the exception of the high recombination region within cistron A.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benbow R. M., Hutchison C. A., Fabricant J. D., Sinsheimer R. L. Genetic Map of Bacteriophage phiX174. J Virol. 1971 May;7(5):549–558. doi: 10.1128/jvi.7.5.549-558.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A. B., Denhardt D. T. Studies on phiX174 proteins. I. Phage-specific proteins synthesized after infection of Escherichia coli. J Mol Biol. 1969 Sep 28;44(3):377–386. doi: 10.1016/0022-2836(69)90367-2. [DOI] [PubMed] [Google Scholar]
- Burgess A. B. Studies on the proteins of phi X174. II. The protein composition of the phi X coat. Proc Natl Acad Sci U S A. 1969 Oct;64(2):613–617. doi: 10.1073/pnas.64.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgell M. H., Hutchison C. A., 3rd, Sinsheimer R. L. The process of infection with bacteriophage phi-X174. 28. Removal of the spike proteins from the phage capsid. J Mol Biol. 1969 Jun 28;42(3):547–557. doi: 10.1016/0022-2836(69)90242-3. [DOI] [PubMed] [Google Scholar]
- Funk F. D., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XXXV. Cistron 8. J Virol. 1970 Jul;6(1):12–19. doi: 10.1128/jvi.6.1.12-19.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand D. H., Hayashi M. Electrophoretic characterization of phiX174-specific proteins. J Mol Biol. 1969 Sep 28;44(3):501–516. doi: 10.1016/0022-2836(69)90376-3. [DOI] [PubMed] [Google Scholar]
- Godson G. N. Characterization and synthesis of phi X174 proteins in ultraviolet-irradiated and unirradiated cells. J Mol Biol. 1971 May 14;57(3):541–553. doi: 10.1016/0022-2836(71)90108-2. [DOI] [PubMed] [Google Scholar]
- HAYASHI M., HAYASHI M. N., SPIEGELMAN S. RESTRICTION OF IN VIVO GENETIC TRANSCRIPTION TO ONE OF THE COMPLEMENTARY STRANDS OF DNA. Proc Natl Acad Sci U S A. 1963 Oct;50:664–672. doi: 10.1073/pnas.50.4.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. Process of infection with bacteriophage phi-X174. XIV. Studies on macromolecular synthesis during infection with a lysis-defective mutant. J Mol Biol. 1967 Aug 28;28(1):87–94. doi: 10.1016/s0022-2836(67)80079-2. [DOI] [PubMed] [Google Scholar]
- Mayol R. F., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XXXVI. Measurement of virus-specific proteins during a normal cycle of infection. J Virol. 1970 Sep;6(3):310–319. doi: 10.1128/jvi.6.3.310-319.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C. Marker-specific effects in genetic recombination. J Mol Biol. 1970 Aug;51(3):633–655. doi: 10.1016/0022-2836(70)90013-6. [DOI] [PubMed] [Google Scholar]
- Sedat J., Lyon A., Sinsheimer R. L. Purification of Escherichia coli pulse-labeled RNA by benzoylated DEAE-cellulose chromatography. J Mol Biol. 1969 Sep 28;44(3):415–434. doi: 10.1016/0022-2836(69)90370-2. [DOI] [PubMed] [Google Scholar]
- Tessman I., Kumar S., Tessman E. S. Direction of translation in bacteriophage S13. Science. 1967 Oct 13;158(3798):267–268. doi: 10.1126/science.158.3798.267. [DOI] [PubMed] [Google Scholar]
- Vanderbilt A. S., Borrás M. T., Tessman E. S. Direction of translation in phage S13 as determined from the sizes of polypeptide fragments of nonsense mutants. Virology. 1971 Feb;43(2):352–355. doi: 10.1016/0042-6822(71)90307-2. [DOI] [PubMed] [Google Scholar]
- Wolf B., Lausarot P. M., Lesnaw J. A., Reichmann M. E. Preparation of polymeric protein markers and an investigation of their behavior in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1970 Jan 20;200(1):180–183. doi: 10.1016/0005-2795(70)90060-7. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C., CARLTON B. C., GUEST J. R., HELINSKI D. R., HENNING U. ON THE COLINEARITY OF GENE STRUCTURE AND PROTEIN STRUCTURE. Proc Natl Acad Sci U S A. 1964 Feb;51:266–272. doi: 10.1073/pnas.51.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipser D. Polar mutations and operon function. Nature. 1969 Jan 4;221(5175):21–25. doi: 10.1038/221021a0. [DOI] [PubMed] [Google Scholar]