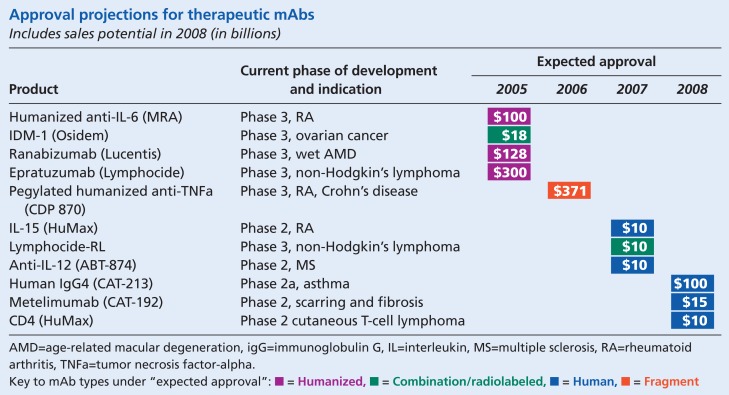
According to a recent report by the Tufts Center for the Study of Drug Development, the success rate in bringing monoclonal antibody (mAb) therapeutics through clinical trials and to market has improved significantly in recent years. Based on an analysis of past approval rates, Tufts projects that by 2008, the U.S. Food and Drug Administration is likely to approve 11 of the current mAb products in the pipeline.* In effect, these approvals would come close to doubling the number of mAbs now on the market.
According to the report, newer mAbs, which include humanized mAbs and human products, are of types that have had a much higher success rate in attaining FDA approval than original murine (mouse-derived) products. Historically, chimeric mAbs (such as the recently approved cetuximab [Erbitux]) have had the greatest success with FDA approval, with 26 percent of products in development hitting the market. The approval rate for murine products, by comparison, is 4.5 percent.
To date, the FDA has approved 17 mAbs. Data-monitor, a London-based company specializing in industry analysis, predicts a significant effect on the global market for mAb therapeutics once the additional products are approved, with the market expanding to $16.7 billion in 2008. By that time, oncologic products, such as cetuximab and bevacizumab (Avastin), both of which were approved last year, will represent the bulk of mAb sales — $7.2 billion, or 43 percent of the total mAb market.
Approval projections for therapeutic mAbs
Includes sales potential in 2008 (in billions)
SOURCES: PROTEIN THERAPEUTIC SUCCESS RATES INCREASE WITH BIOTECH ADVANCES, TUFTS CENTER FOR THE STUDY OF DRUG DEVELOPMENT, BOSTON, MARCH 2005, AND MONOCLONAL ANTIBODY THERAPIES: EVOLVING INTO A $30 BILLION MARKET, DATAMONITOR, LONDON, APRIL 2005
Footnotes
The Tufts report named 16 mAbs, three of which (cetuximab, bevacizumab, and natalizumab [Tysabri, later voluntarily withdrawn from the market by its manufacturers]) received FDA approval before the release of the report. The manufacturers of two other products cited in the report but not named above have indicated since that they are unlikely to proceed with development of those products (lerdelimumab [Trabio], for postsurgical use in glaucoma patients, and R1549, for ovarian cancer) after disappointing phase 3 trial results.