Abstract
Surgical ablation procedure can restore sinus rhythm (SR) in patients with atrial fibrillation (AF) undergoing cardiac surgery. However, it is not known whether it has any impact on clinical outcomes. There is a need for a randomized trial with long‐term follow‐up to study the outcome of surgical ablation in patients with coronary and/or valve disease and AF. Patients are prospectively enrolled and randomized either to group A (cardiac surgery with left atrial ablation) or group B (cardiac surgery alone). The primary efficacy outcome is the SR presence (without any AF episode) during a 24‐hour electrocardiogram after 1 year. The primary safety outcome is the combined end point of death, myocardial infarction, stroke, and renal failure at 30 days. Long‐term outcomes are a composite of total mortality, stroke, bleeding, and heart failure at 1 and 5 years. We finished the enrollment with a total of 224 patients from 3 centers in 2 countries in December 2011. Currently, the incomplete 1‐year data are available, and the patients who enrolled first will have their 5‐year visits shortly. PRAGUE‐12 is the largest study to be conducted so far comparing cardiac surgery with surgical ablation of AF to cardiac surgery without ablation in an unselected population of patients who are operated on for coronary and/or valve disease. Its long‐term results will lead to a better recognition of ablation's potential clinical benefits.
The PRAGUE‐12 trial is partially funded by the Charles University Research Projects MSM0021620817 and UNCE 204010/2012.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Introduction
Atrial Fibrillation
Atrial fibrillation (AF) is the most common cardiac arrhythmia affecting 1% to 2% of the general population.1 It is associated with increased morbidity and mortality.1, 2 Regardless of other known predictors of mortality, death rates are doubled with AF.3, 4 Approximately every fifth stroke is due to the AF,3 and patients with paroxysmal AF carry the same stroke risk as those with a permanent or persistent form.5 Patients with AF have a significantly poorer quality of life compared with the general population.6 The direct (medical and drugs) and indirect (work loss) annual costs associated with AF make it an extremely costly public health problem.7, 8 As its prevalence increases with age and with the presence of significant valve or ischemic heart disease, AF is also frequently present in patients who are scheduled for cardiac surgery.
Together with the imperfect results of current pharmacological and catheter ablation therapy, all of those facts today make surgical possibilities of AF treatment a much discussed and demanding topic.
Surgical Ablation Procedures
The first surgical AF ablation procedures in early 1980s (left atrial isolation procedure9 and the corridor procedure10) have not expanded widely. In 1987, Dr. James L. Cox introduced his Maze procedure,11, 12 and for more than 2 decades the Cox‐Maze procedure represented the standard for the surgical AF treatment. A success rate of 95% in restoring sinus rhythm (SR) that persisted 5 years after surgery has been described13, 14 as well as its significant effect on the reduction in the rate of cerebrovascular accidents and transient ischemic events.15, 16 However, this method has not expanded as its results would justify, mainly because it is a technically difficult and demanding procedure.
To simplify the surgery and make it feasible for an average surgeon, the incisions of the traditional cut‐and‐sew Cox‐Maze III procedure were replaced by linear lines of ablation. These lines can be created by using a variety of energy sources. A systematic review published in 2005 by Khargi and colleagues reports comparable efficacy rates for the cut‐and‐sew Maze III surgery and ablation procedures using alternative energy sources.17 However, the limitation of that review was that the rhythms were evaluated in the fairly short time of 6 months postprocedure. Despite all the facts, a large data registry study showed that <50% of patients received some type of concomitant AF ablation when undergoing cardiac surgery.18 We believe that this fact is strongly related to the lack of convincing results based on randomized studies with long‐term follow‐ups.
Previous Randomized Studies
Only a few randomized studies have been published to date, and they suffered from relatively small sample sizes involving various groupings of patients and from inconsistent published data relative to mid‐ and long‐term results.19, 20, 21, 22, 23, 24, 25, 26, 27 Most of these studies enrolled only patients scheduled for mitral valve surgery, thus the efficacy of surgical ablation in patients undergoing other types of surgery (eg, coronary artery bypass graft [CABG] or aortic valve replacement) is even less well established, with 1 study showing an increased rate of perioperative complications in the ablation group.23 Most studies were able to demonstrate safety of the ablation procedure and the SR restoration rates, but whether this had any impact (positive or negative)on major clinical events is not known. A recently published meta‐analysis of the above‐mentioned randomized controlled trials concluded that there is a need for large, multicenter, randomized trials to assess the long‐term efficacy and safety of surgical ablation procedures for the maintenance of the sinus rhythm.28
Thus, our study was designed to contribute to evaluating outcomes and determining the appropriate role for surgical ablation procedures in the management of AF.
Methods
Trial Design
The PRAGUE‐12 trial is a large, prospective, open, randomized, multicenter clinical trial assessing the outcome of cardiac surgery with left atrial ablation vs cardiac surgery alone (without ablation) in patients with coronary and/or valve disease and AF. The trial hypotheses assume that surgical ablation of the left atrium would result in (1) a higher incidence of the SR in the treated group, (2) nonincreasing periprocedural mortality and complications, and (3) possible improvement of the long‐term clinical outcomes.
The trial is approved by the institutional ethics committee of each participating center and is conducted in accordance with the Declaration of Helsinki. After obtaining written informed consent, patients are randomly assigned (by envelope method) to undergo surgery combined with left atrial ablation (A group) or surgery without left atrial ablation (B group).
Patient Selection
As the aim of our study was to assess the effect of ablation in a nonselected, typical, realistic population of patients undergoing CABG and/or valve surgery, no narrow selection criteria were used. The inclusion criteria were: indication for cardiac surgery (CABG, valve replacement or repair, others or combinations) and AF (paroxysmal, persistent, or longstanding persistent) documented at least twice in the previous 6 months before surgery, a signed informed consent, and an age >18 years. The only exclusion criterion was emergency surgery.
Surgical Procedure
Only experienced surgeons could participate in the study, residents were not allowed to participate as primary surgeons. The left atrial lesion set was chosen according to the preference of the cardiac surgery department, as it represents a standard ablation modification in this center. It was precisely defined and accepted by all participating centers. The lesion set is the same for all patients in group A and was intended to be performed prior to the valve or CABG procedure after placing the patient on cardiopulmonary bypass (CPB) and arresting the heart. It included pulmonary veins (PV) ablation (separately left‐sided and right‐sided PV pairs), left atrial appendage (LAA) surgical resection, and 3 other lesions, namely an interconnecting lesion between PV pairs, a connecting lesion from the PV to mitral annulus, and a lesion from the left upper pulmonary vein to the rim of the LAA (Figure 1).
Figure 1.
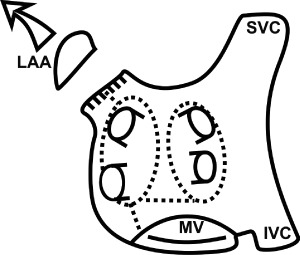
Schematic drawing of the left atrium with the ablation lesions (dotted lines). Abbreviations: IVC, inferior vena cava; LAA, left atrial appendage; MV, mitral valve; SVC, superior vena cava.
The energy source for creating lesions was chosen according to the preference of the lead surgeon and guidelines of the particular department. The lesion lines were not planned to be assessed for conduction block so as not to prolong the surgical procedure.
Treatment Strategy
Patient medication is maintained until the day of surgery except for anticoagulation or antiplatelet therapy, which should be either discontinued 5 days prior to surgery or switched to heparin. Postoperative care is identical for both groups. Unless contraindicated, all patients receive postoperatively on the day of surgery antiarrhythmic drugs (AADs); amiodarone as the first choice, or propafenone or sotalol as the second choice. All patients are put on warfarin with a target international normalized ratio of 2 to 2.5. Other medication, including β‐blockers, is adjusted routinely according to the patient's comorbidities. It is recommended to discontinue AADs 3 months after surgery if the patient appears to be AF free. Unless otherwise contraindicated, warfarin is recommended to be discontinued 6 months after surgery (ie, 3 month after discontinuation of AADs) if the patient remains in stable SR Direct current cardioversion is strongly recommended if AF is present at the 30‐day follow‐up. Nevertheless, the actual treatment strategy is left at the discretion of the treating cardiologists (according to patients' CHADS2 score and other characteristics).
Follow‐up
Cardiac rhythm is continuously monitored until discharge from the hospital. Postoperative follow‐ups are scheduled at 1, 3, and 6 months and 1 and 5 years after surgery. All of the follow‐ups are performed in the participating cardiology centers. The first 3 follow‐ups include clinical examination and electrocardiogram (ECG), and the 1‐ and 5‐year follow‐ups also include a 24‐hour Holter‐ECG and echocardiography. Data regarding current medications, recent complications, or hospitalizations are recorded at each follow‐up. Because the study is an open design, a blinded clinical events committee was not established. Nevertheless, the primary end point analysis will be blinded because the Holter‐ECGs will be performed and analyzed by arrhythmologists, who will not have detailed information about the patients.
Study Outcomes
The study end points were established in accordance with definitions and recommendations for the AF‐related trials previously published by American Heart Association/American College of Cardiology, Society of Thoracic Surgeons, European Heart Rhythm Association, and German Atrial Fibrillation Competence Network.3, 29, 30
The primary efficacy end point is the SR presence (without any AF episodes) during a 24‐hour Holter‐ECG 1 year after surgery. The primary safety end point is the occurrence of the composite of death, nonfatal myocardial infarction (MI), nonfatal stroke, or new onset renal failure (with the need for hemodialysis) at 30 days postsurgery. The long‐term secondary end point is the occurrence of the composite of death, nonfatal stroke, major bleeding, and hospitalization for heart failure over 1 and 5 years after surgery.
The assessment of perioperative data includes clamp time, CBP time, blood loss, overall time of surgery, and duration of hospitalization. The echocardiography parameters including the left ventricular ejection fraction and the size of left atrium were measured preoperatively, and are scheduled to be measured after 1 and 5 years after surgery. The long‐term need for anticoagulation and use of antiarrhythmic drugs, as well as the need for a pacemaker or implantable cardioverter‐defibrillator will be assessed.
Total mortality is selected to be used rather than cardiovascular mortality, as the exact cause of death is difficult to determine in patients who develop multiorgan failure. Stroke is defined as a new acute focal neurologic deficit, with symptoms lasting >24hours and confirmed by a neurologist. Renal failure is defined as an onset of a renal insufficiency, with a need for hemodialysis. Myocardial infarction is defined as the detection of rise and/or fall of cardiac biomarkers with at least 1 value above the 99th percentile of the upper reference limit together with at least 1 of the following: development of pathologic Q waves in the ECG, ECG changes indicative of new ischemia (new ST‐T changes or new left bundle branch block), and symptoms of ischemia. Bleeding is defined as any clinically overt sign of hemorrhage that requires diagnostic studies, hospitalization, or treatment by a healthcare professional. Heart failure is defined as any situation where signs and symptoms of heart failure require hospitalization.
Paroxysmal AF is defined as recurrent AF (≥2 episodes) that terminates spontaneously within 7 days or is terminated in ≤48 hours with electrical or pharmacologic cardioversion. Persistent AF is defined as continuous AF that is sustained beyond 7 days. Episodes of AF, in which a decision is made to electrically or pharmacologically cardiovert the patient after ≥48 hours of the AF but prior to 7 days, are also classified as persistent AF episodes. Longstanding persistent AF is defined as continuous AF of >12‐months duration.
Sample Size and Statistical Analysis
According to available publications, we assumed that the sinus rhythm restoration rate 1 year after surgery would be 70% in the ablation group and 30% in the control group. Study protocol required a powered evaluation of specific study subgroups, namely of patients with preoperative permanent AF and patients who had mitral valve surgery. A power analysis revealed that 37 patients per group or subgroup were required to assure at least 90% power for detecting the anticipated between‐groups differences in the SR prevalence at 1 year. It was estimated that the smallest subgroup of interest will represent approximately 40% of the sample, which leads to the requirement of 93 subjects per group. To compensate for the expected attrition rate due to dropouts and failure to obtain the primary end point information at the 1‐ and 5‐year evaluation, the figure was increased by 15%, yielding a sample size of 107 subjects per group. This will provide a 90% statistical power to demonstrate the difference between the study groups and the subgroups.
All analyses will be based on the intention‐to‐treat principle, which means that everyone randomized to a particular treatment arm is analyzed within the arm regardless of the treatment actually received. Continuous data will be presented as arithmetic mean and standard deviation for normally distributed variables, or as median and 25th to 75th percentile range for log‐normally distributed variables. Normality will be tested by the Shapiro‐Wilk test. Comparison of groups will be based on the Student 2‐sample t test and Mann‐Whitney test. Within‐subjects comparison at 2 time points will be done by paired t test. Categorical data will be given as absolute and relative frequencies (percentages). The differences in proportions between groups will be analyzed using the Fisher exact test and its generalization. Logistic regression model will be used to identify independent predictors of the endpoint occurrence and in particular of failure of restoring the SR At the 1‐year and 5‐year evaluations, methods of survival analysis will be used for modeling of the time to the first occurrence of any of the components of primary efficacy outcome and for group comparisons. These include Kaplan‐Meier estimators of survival curves, log‐rank test, and Cox proportional hazards regression model. The model will be used after checking of the proportionality assumption, and the treatment effect will be presented as the hazard ratio and the corresponding 95% confidence interval. Interval censoring will be used where necessary.
To investigate whether particular categories of patients might be more or less responsive to treatment, subgroups formed by the type of the preoperative AF and the type of surgery will be analyzed. The interaction term between each subgroup factor and the treatment group will be included in the respective logistic or Cox models.
Statistical software nQuery Advisor 5.0 (Statistical Solutions, Boston, MA) was used to determine the sample size requirements. All statistical tests will be treated as 2‐sided and evaluated at a significance level of 0.05.
Study Progress
A total of 224 patients were enrolled to study in 3 centers in the Czech Republic and Slovakia. Cryo‐energy was used to create the lesions in 96.6% of patients, and radiofrequency was used in 3.4%. Both study groups were comparable in all baseline characteristics, except for history of myocardial infarction and chronic renal disease, which were more frequent in group B (Table 1). Currently, the last patients are scheduled for their 1‐year visits, and the first patients enrolled will have their 5‐year visits shortly.
Table 1.
Baseline Characteristics
| Characteristics | A (With Ablation) (n = 117) | B (Without Ablation) (n = 107) |
|---|---|---|
| Demography | ||
| Age (y) | 69.9 ± 7.8 | 71.0 ± 7.9 |
| Female gender, n (%) | 50 (42.7) | 44 (41.2) |
| AF duration, mo | 15.0 (5.0–64.0) | 16.0 (5.0–60.0) |
| Type of AF, n (%) | ||
| Paroxysmal | 26 (22.2) | 33 (30.8) |
| Persistent | 30 (25.6) | 25 (23.4) |
| Permanent | 61 (52.1) | 49 (45.8) |
| Preoperative rhythm, n (%) | ||
| Sinus rhythm, n (%) | 24 (20.5) | 33 (30.8) |
| AF, n (%) | 91 (77.8) | 70 (65.4) |
| Paced rhythm | 1 (0.9) | 4 (3.7) |
| Atrial flutter (typical) | 1 (0.9) | 0 (0.0) |
| Preoperative cardioversion, n (%) | 18 (15.4) | 15 (14.0) |
| Preoperative catheter ablation, n (%) | 2 (1.7) | 2 (1.9) |
| Left atrial diameter (mm) | 48.7 ± 7.3 | 47.7 ± 7.1 |
| NYHA functional class, n (%) | ||
| I | 7 (6.0) | 16 (14.9) |
| II | 66 (56.4) | 51 (47.7) |
| III | 43 (36.7) | 37 (34.6) |
| IV | 1 (0.9) | 3 (2.8) |
| Mean NYHA functional class | 2.3 ± 0.6 | 2.3 ± 0.7 |
| Comorbidity, n (%) | ||
| Hypertension | 95 (81.2) | 86 (80.4) |
| Myocardial infarction | 23 (19.7) | 37 (34.6) |
| Stroke/TIA | 13 (11.1) | 15 (14.0) |
| Diabetes | 41 (35.0) | 40 (37.4) |
| Renal failure | 7 (6.0) | 18 (16.8) |
| Bleeding | 4 (3.4) | 6 (5.6) |
| Heart failure | 29 (24.8) | 34 (31.8) |
| Lung disease | 19 (16.2) | 19 (17.8) |
| Thyroid gland disease | 10 (8.5) | 17 (15.9) |
| Thrombosis | 5 (4.3) | 7 (6.5) |
| Pacemaker/ICD | 9 (7.7) | 15 (14.0) |
| Ejection fraction (%) | 52.6 ± 10.9 | 49.9 ± 12.5 |
| Logistic EuroSCORE | 5.8 (3.2–9.9) | 6.8 (4.0–11.6) |
Abbreviations: AF, atrial fibrillation; EuroSCORE, European System for Cardiac Operative Risk Evaluation; ICD, implantable cardioverter‐defibrillator; n, number of patients; NYHA, New York Heart Association; TIA, transitory ischemic attack.
Data are presented as mean ± standard deviation or median with 25th to 75th percentiles range in parentheses, unless otherwise stated.
Discussion
The aim of our trial was to assess the long‐term efficacy and safety of surgical ablation compared to pharmacological treatment in a nonselected, typical, realistic population of patients undergoing CABG and/or valve surgery. Therefore, no narrow selection criteria were used, and the only exclusion criteria were unwillingness to participate or emergency surgery. Our patients underwent a wide variety of surgical procedures. Together with a highly heterogeneous nonselected patients set, a comparison of 2 randomized groups (1 with and 1 without ablation) should clarify the real benefits of ablation in real clinical situations. Subanalysis will be done according to the types of preoperative AF and the types of surgery to show the different effects of ablation relative to different subgroups of patients.
As mentioned in the Introduction, there are many types of energy sources that are widely used for creating the lesions: cryo‐energy, radiofrequency, microwaves, laser, and high‐frequency ultrasound. In addition, different sets of lesions are used in different cardiac surgery departments, from simple pulmonary veins ablation to extensive biatrial lesion sets. Finding the best and most effective combination of energy source and lesion set for each patient is a very hot topic for discussion today. In our study, we decided to choose and define the lesion set (described in the Surgical Procedure part of the article), as it was the standard frequently used method in participating cardiac surgery centers. The energy source was left to each participating center; however, it was in almost 97% cryo‐energy, again as it represents the most frequently used method in those centers.
When preparing the study, we discussed at length a rhythm monitoring strategy. Some implantable device would, of course, be the best choice, but unfortunately there were strong objections from the ethics committee. Finally, because an important aim of our study was to have as much high‐level follow‐up completeness as possible, we decided to have repeated ECGs during the year and 1 single 24‐hour Holter‐ECG monitoring as the key rhythm evaluating method after 1 and 5 years. Despite the possible risk of missing some AF paroxysms, we preferred this patient‐friendly monitoring strategy in our study, because we felt that it gave us better compliance compared to the Holter‐ECGs that would be scheduled more frequently.
Conclusion
Objective evaluation of the benefits and possibilities of surgical ablation among the unselected cardiac surgery candidates with AF is difficult. Nevertheless, we consider studies in a prospective randomized fashion to be the source of the most objective data.
References
- 1. Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 2. Chugh S, Blackshear J, Shen W, et al. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37:371–378. [DOI] [PubMed] [Google Scholar]
- 3. Kirchhof P, Auricchio A, Bax J, et al. Outcome parameters for trials in atrial fibrillation: executive summary. Recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NETwork (AFNET) and the European Heart Rhythm Association (EHRA). Eur Heart J. 2007;28:2803–2817. [DOI] [PubMed] [Google Scholar]
- 4. Stewart S, Hart CL, Hole DJ, et al. A population‐based study of the longterm risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. [DOI] [PubMed] [Google Scholar]
- 5. Friberg L, Hammar N, Rosenqvist M. Stroke in paroxysmal atrial fibrillation: report from the Stockholm Cohort of Atrial Fibrillation. Eur Heart J. 2010;31:967–975. [DOI] [PubMed] [Google Scholar]
- 6. Thrall G, Lane D, Carroll D, et al. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006;119:448.e1–e19. [DOI] [PubMed] [Google Scholar]
- 7. Wu EQ, Birnbaum HG, Mareva M, et al. Economic burden and co‐morbidities of atrial fibrillation in a privately insured population. Curr Med Res Opin. 2005;21:1693–1699. [DOI] [PubMed] [Google Scholar]
- 8. Stewart S, Murphy NF, Walker A, et al. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart. 2004;90:286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams JM, Ungerleider RM, Lofland GK, et al. Left atrial isolation: new technique for the treatment of supraventricular arrhythmias. J Thorac Cardiovasc Surg. 1980;80:373. [PubMed] [Google Scholar]
- 10. Guiraudon GM, Campbell CS, Jones DL, et al. Combined sinoatrial node atrioventricular node isolation: a surgical alternative to His bundle ablation in patients with atrial fibrillation. Circulation. 1985;72(suppl 3):220. [Google Scholar]
- 11. Cox JL, Schuessler RB, D'Agostino HJ, et al. The surgical treatment of atrial fibrillation. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991;101:569–583. [PubMed] [Google Scholar]
- 12. Cox JL. The first Maze procedure. J Thorac Cardiovasc Surg. 2011;141:1093–1097. [DOI] [PubMed] [Google Scholar]
- 13. Damiano RJ Jr, Gaynor SL, Bailey M, et al. The long‐term outcome of patients with coronary disease and atrial fibrillation undergoing the Cox maze procedure. J Thorac Cardiovasc Surg. 2003;126:2016–2021. [DOI] [PubMed] [Google Scholar]
- 14. Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long‐term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–1827. [DOI] [PubMed] [Google Scholar]
- 15. Bando K, Kobayashi J, Sasako Y, et al. Effect of maze procedure in patients with atrial fibrillation undergoing valve replacement. J Heart Valve Dis. 2002;11:719–724. [PubMed] [Google Scholar]
- 16. Ad N, Cox JL. Stroke prevention as an indication for the Maze procedure in the treatment of atrial fibrillation. Semin Thorac Cardiovasc Surg. 2000;12:56–62. [DOI] [PubMed] [Google Scholar]
- 17. Khargi K, Hutten BA, Lemke B, et al. Surgical treatment of atrial fibrillation; a systematic review. Eur J Cardiothorac Surg. 2005;27:258–265. [DOI] [PubMed] [Google Scholar]
- 18. Gammie JS, Haddad M, Milford‐Beland S, et al. Atrial fibrillation correction surgery: lessons from the Society of Thoracic Surgeons National Cardiac Database. Ann Thorac Surg. 2008;85: 909–914. [DOI] [PubMed] [Google Scholar]
- 19. Abreu Filho CAC, Lisboa LAF, Dallan LAO, et al. Effectiveness of the Maze procedure using cooled‐tip radiofrequency ablation in patients with permanent atrial fibrillation and rheumatic mitral valve disease. Circulation. 2005;112(suppl 9):I20–I25. [DOI] [PubMed] [Google Scholar]
- 20. Deneke T, Khargi K, Grewe PH, et al. Efficacy of an additional MAZE procedure using cooled‐tip radiofrequency ablation in patients with chronic atrial fibrillation and mitral valve disease. A randomized, prospective trial. Eur Heart J. 2002;23:558–566. [DOI] [PubMed] [Google Scholar]
- 21. Doukas G, Samani NJ, Alexiou C, et al. Left atrial radiofrequency ablation during mitral valve surgery for continuous atrial fibrillation: a randomized controlled trial. JAMA. 2005;294: 2323–2329. [DOI] [PubMed] [Google Scholar]
- 22. Schuetz A, Schulze CJ, Sarvanakis KK, et al. Surgical treatment of permanent atrial fibrillation using microwave energy ablation: a prospective randomized clinical trial. Eur J Cardiothorac Surg. 2003;24:475–480. [DOI] [PubMed] [Google Scholar]
- 23. Blomstrom‐Lundqvist C, Johansson B, Berglin E, et al. A randomized double‐blind study of epicardial left atrial cryoablation for permanent atrial fibrillation in patients undergoing mitral valve surgery: the SWEDish Multicentre Atrial Fibrillation study (SWEDMAF). Eur Heart J. 2007;28:2902–2908. [DOI] [PubMed] [Google Scholar]
- 24. Chevalier P, Leizorovicz A, Maureira P, et al. Left atrial radiofrequency ablation during mitral valve surgery: A prospective randomized multicentre study (SAFIR). Arch Cardiovasc Dis. 2009;102:769–775. [DOI] [PubMed] [Google Scholar]
- 25. de Lima GG, Kalil RAK, Leiria TLL, et al. Randomized study of surgery for patients with permanent atrial fibrillation as a result of mitral valve disease. Ann Thorac Surg. 2004;77:2089. [DOI] [PubMed] [Google Scholar]
- 26. Von Oppell U, Masani N, O'Callaghan P, et al. Mitral valve surgery plus concomitant atrial fibrillation ablation is superior to mitral valve surgery alone with an intensive rhythm control strategy. Eur J Cardiothorac Surg. 2009;35:641–650. [DOI] [PubMed] [Google Scholar]
- 27. Albrecht A, Kalil RAK, Schuch L, et al. Randomized study of surgical isolation of the pulmonary veins for correction of permanent atrial fibrillation associated with mitral valve disease. J Thorac Cardiovasc Surg. 2009;138:454–459. [DOI] [PubMed] [Google Scholar]
- 28. Kong MH, Lopes RD, Piccini JP, et al. Surgical Maze procedure as a treatment for atrial fibrillation: a meta‐analysis of randomized controlled trials. Cardiovasc Ther. 2010;28:311–326. [DOI] [PubMed] [Google Scholar]
- 29. McNamara RL, Brass LM, Drozda JP Jr, et al. American College of Cardiology; American Heart Association. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with atrial fibrillation. Circulation. 2004;109:3223–3324. [DOI] [PubMed] [Google Scholar]
- 30. Shemin R, Cox J, Gillinov A, et al. Guidelines for reporting data and outcomes for the surgical treatment of atrial fibrillation. Ann Thorac Surg. 2007;83:1225–1230. [DOI] [PubMed] [Google Scholar]


