Abstract
Despite the well-accepted notion that early maternal influences persist beyond fetal life and may underlie many adult diseases, the risks imposed by the maternal environment on breast cancer development and underlying biological mechanisms remain poorly understood. In this study, we investigated whether early exposure to blueberry (BB) via maternal diet alters oncogene Wnt1-induced mammary tumorigenesis in offspring. Wnt1-transgenic female mice were exposed to maternal Casein (CAS, control) or blueberry-supplemented (CAS + 3%BB) diets throughout pregnancy and lactation. Offspring were weaned to CAS and mammary tumor development was followed until age 8 months. Tumor incidence and latency were similar for both groups; however, tumor weight at killing and tumor volume within 2 weeks of initial detection were lower (by 50 and 60%, respectively) in offspring of BB- versus control-fed dams. Dietary BB exposure beginning at weaning did not alter mammary tumor parameters. Tumors from maternal BB-exposed offspring showed higher tumor suppressor (Pten and Cdh1) and lower proproliferative (Ccnd1), anti-apoptotic (Bcl2) and proangiogenic (Figf, Flt1 and Ephb4) transcript levels, and displayed attenuated microvessel density. Expression of Pten and Cdh1 genes was also higher in mammary tissues of maternal BB-exposed offspring. Mammary tissues and tumors of maternal BB-exposed offspring showed increased chromatin-modifying enzyme Dnmt1 and Ezh2 transcript levels. Body weight, serum insulin and serum leptin/adiponectin ratio were lower for maternal BB-exposed than control tumor-bearing offspring. Tumor weights and serum insulin were positively correlated. Results suggest that dietary influences on the maternal environment contribute to key developmental programs in the mammary gland to modify breast cancer outcome in adult progeny.
Introduction
The concept that the risk of adult diseases is partially established in the womb (i.e. fetal origin of adult diseases) was initially advanced by Professor David Barker for coronary heart disease in the 1980s (1). Since this seminal (and highly provocative) report, many epidemiological studies have linked poor maternal health to subsequent detrimental health effects in adult offspring (2–4). Nevertheless, the mechanisms underlying these life-long linkages, by which limited exposure (less than a year) to a non-supportive maternal environment can affect susceptibility to many devastating adult diseases, such as diabetes and cancer, are essentially unknown (5).
Breast cancer is the second leading cause of cancer-related deaths and the most common malignancy among women (6). Although this complex disease exhibits prominent genetic underpinnings (7), there is growing acceptance that susceptibility to breast cancer may be partly predetermined in utero (8). Initial support for this concept came from findings of increased breast cancer risk in daughters of women who used the synthetic estrogen diethylstilbestrol during pregnancy to prevent miscarriage (9,10). Diet and nutrition are well-accepted modifiable risks for breast cancer. Maternal exposure to dietary components has been reported to elicit significant effects on mammary gland development and breast cancer outcome in offspring in rodent models. For example, female rat offspring exposed to a maternal high fat diet during gestation have higher incidence of and larger mammary tumors when administered the carcinogen 7,12-dimethylbenz[a]anthracene as young adults (11,12). A fat-enriched maternal diet similarly increased spontaneous mammary tumor formation in outbred mice (13) and in transgenic mice overexpressing the c-neu oncogene (14). Protein restriction during pregnancy and lactation resulted in early onset of mammary tumorigenesis (by 2-fold) in rat offspring after N-methyl-N-nitrosourea administration (15). Further, agouti mice exposed to soy isoflavone genistein early in life via maternal diet were more likely to adopt a ‘healthy’ pseudoagouti rather than the ‘disease-prone’ yellow agouti phenotype (16). Our own studies showed that in utero and lactational exposure to a soy protein diet increased the latency and decreased the multiplicity of N-methyl-N-nitrosourea–induced mammary tumors in rat offspring (17). These protective effects were partly mediated by increased expression of the tumor suppressor E-cadherin in the mammary gland prior to carcinogenic insult. Nonetheless, whereas pharmacological agents, carcinogens and other environmental endocrine disruptors (e.g. high estrogen concentrations) are known to disrupt the normal course of mammary gland development via their gene-altering activities (18), a clear understanding of how maternal nutritional history and specifically, how maternal consumption of diets readily available to the Western population may predispose to breast cancer risk in progeny upon fetal exposure, is yet to be achieved.
To further explore the role of maternal diet in breast cancer outcome of adult progeny following fetal/neonatal exposure and potential underlying mechanisms, we evaluated the effects of whole blueberry (BB) powder consumed by pregnant and lactating dams on mammary tumor incidence and progression in their offspring. Our study used MMTV-Wnt1-transgenic (Wnt1-Tg) mice that overexpress the oncogene Wnt1 in the mammary gland (19). Although mutations in Wnt components are rarely seen in women with breast cancer, with the exception of metastatic breast carcinoma (20), overexpression of Wnt1 leads to increased β-catenin nuclear pools, a feature of >50% of human breast carcinomas (21). In a previous study, we showed enhanced prepubertal mammary gland differentiation in female rat offspring of dams consuming BB diet (22), although mammary tumor parameters were not evaluated. Recently, dietary intake of whole BB powder was shown to elicit anti-tumor and anti-metastatic activity in nude mice when transplanted with MDA-MB-231 triple negative breast cancer cells (23). Further, studies in humans (24) and rodents (25) demonstrated that dietary BB supplementation inhibited obesity-associated insulin resistance, in part by decreasing inflammation. In this study, we tested the hypothesis that fetal and lactational exposure to maternal BB-supplemented diet will suppress mammary tumor incidence and/or progression in adult offspring through effects on critical oncogenic and metabolic (insulin) signaling pathways, and which may involve altered expression of key DNA-modifying enzymes.
Materials and methods
Animals and diets
Animal studies were performed in compliance with protocols approved by the University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee. Animals were housed in polycarbonate cages under conditions of 24ºC, 40% humidity and a 12h light, 12h dark cycle. Wnt1-Tg male mice [B6SJL-Tg(Wnt1)1Hev/J] were purchased from Jackson Laboratories (Bar Harbor, ME) and mated with wild-type (WT) females of the same strain to generate female Wnt1-Tg offspring. In Study 1, WT dams were randomly assigned to one of two diets prior to mating: (i) American Institute of Nutrition-93G-based pelleted diet containing casein (CAS) as the major protein source (Harlan, Indianapolis, IN) and (ii) CAS to which was added freeze-dried whole wild BB (Vaccinium angustifolium) powder (generous gift of the Wild Blueberry Association of North America, courtesy of Susan Davis) at 3% by weight of feed (designated BB). Supplementary Table 1, available at Carcinogenesis Online describes the preparation and processing of the composite BB powder, its average total anthocyanin content and relative anthocyanin concentrations. Diet with added BB powder was formulated to be isoenergetic and isonitrogenous (Supplementary Table 2, available at Carcinogenesis Online). Dams were maintained on the same diets throughout pregnancy and lactation. At weaning (PND21), female pups from both diet groups were weighed and genotyped for presence or absence of Wnt1-transgene following protocols suggested by the supplier (Jackson Laboratories). Dams fed either diet had comparable pregnancy weight gains, litter size and pup weaning weights (data not shown). A cohort of the WT and Wnt1-Tg female offspring from each diet group (n = 5–6 per group, per genotype) was euthanized at PND21 for analyses of mammary tissue parameters and gene expression (below). The remainder of the WT females were weaned to CAS (control) diet and at PND50, mammary tissues were collected for similar analyses (n = 5–6 per diet group per genotype). Corresponding Wnt1-Tg female offspring from the maternal diet groups (CAS: n = 33; BB: n = 28) were weaned to CAS and followed for mammary tumor development until age 8 months. In Study 2, WT dams mated to Wnt1-Tg males were fed CAS throughout pregnancy and lactation. Female Wnt1-Tg offspring were weaned to either CAS (n = 33; same group from Study 1) or BB-supplemented CAS (n = 22) diets and followed for mammary tumor development until age 8 months. Mice were provided food and water ad libitum and weight gains were documented weekly. All animals that did not develop tumors by 8 months of age were euthanized.
Mammary tissue collection and tumor analyses
Mammary gland pairs 2, 3 and 4 were harvested from PND21 (WT and Wnt1-Tg) and PND50 (WT) mice and used for analyses as follows. The left inguinal mammary gland (4) was fixed for whole-mount analyses, whereas the corresponding right gland (with lymph nodes removed) was immediately homogenized in Trizol (Invitrogen, Carlsbad, CA) for RNA analyses (below). The right-halves of mammary glands 2 and 3 were snap frozen at –80ºC for western blot analyses. Wnt1-Tg mice were palpated twice weekly from 4 weeks of age to monitor tumor formation. Tumors were measured using a caliper for length, width and height at initial detection and 2 weeks later were excised, weighed and re-measured. Tumor volume was calculated using the standard calculation for a sphere (4/3×3.14 × ab2) (26). Tumors were divided into three parts: one part was processed for immunohistochemistry and pathology, the second was extracted for RNA analyses and the third was stored at –80ºC for protein analyses.
Microvessel density analysis
Microvessel density was determined in tumors from Wnt1-Tg mice (n = 3–4 per diet group) as described previously (27). Microvessels were immunolabeled with rat anti-mouse CD34 (AbD-serotec, Kidlington, UK) at 1:25 dilution, followed by biotinylated rabbit anti-rat IgG at 1:100 dilution (Vector Laboratories, Burlingame, CA). Staining was visualized using VectaStain Elite ABC kit followed by Vector DAB substrate kit as per manufacturer’s instructions (Vector Laboratories). Sections were lightly stained with hematoxylin. Stained tissue sections were scanned by Aperio ScanScope (Aperio Technologies, Vista, CA) to capture high resolution digital images of the entire section. Vessels were counted in five random areas of tumor infiltration (1mm2/area) per tumor sample in a blinded fashion.
Whole-mount analyses, immunohistochemistry and tumor pathology
Whole mounts of fat pads were prepared as described (22) and evaluated under a dissecting microscope. The total numbers of terminal end buds (TEBs), located at the leading edge of the fat pad, were counted from whole mounts of the left mammary gland 4; four to five mice were used for each diet group. Branching density for each gland was quantified by counting the number of branched points within a box of fixed dimensions (22). Histopathology of tumors from hematoxylin–eosin stained paraffin sections (17) was determined by a residency-trained veterinary pathologist (L.J.H.) using accepted criteria established by an expert panel of surgical, veterinary and experimental pathologists (28). Immunostaining for cyclin D1 used anti-cyclin D1 antibody (1:250 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) following the manufacturer’s protocols. Sections of solid carcinoma tumors from four individual mice per diet group were analyzed. Negative controls were tumor sections processed in parallel, except with the omission of primary antibody.
Quantitative real-time PCR
Total RNA isolation, preparation of corresponding cDNAs and quantitative real-time PCR (QPCR) analyses followed previously described protocols (29). Primers (Supplementary Table 3, available at Carcinogenesis Online) were designed to span introns, using Primer Express software (Applied Biosystems, Foster City, CA) and were synthesized by Integrated DNA Technologies (Coralville, IA). The expression of each target mRNA was calibrated to a standard curve generated using pooled cDNAs and normalized to that of TATA-box binding protein (Tbp).
Western blot analyses
Whole cell lysates and nuclear extract proteins were prepared from frozen mammary tumors and subjected to immunoblotting as described previously (30). Primary antibodies used were anti-PTEN (1:1000; Cell Signaling, Danvers, MA), anti-cyclin D1 (1:400; Santa Cruz Biotechnology), anti-DNMT1 (1:1000; Abcam, Cambridge, MA), anti-EZH2 (1:1000; Cell Signaling) and anti-β-actin (1:2000; Sigma Chemical Co., St Louis, MO). The immunoreactive proteins were visualized with the Amersham ECL Plus kit (GE Healthcare Life Sciences, Piscataway, NJ). Digital images were captured using the GE Image Scanner III detection system and quantified using Quantity One software (Bio-Rad Laboratories, Hercules, CA).
Serum hormone assays
The levels of insulin and leptin in sera of Wnt1-Tg mice displaying solid carcinoma tumors 2 weeks post initial tumor detection (Study 1: n = 9 individual mice per diet group) were measured using the Milliplex MAP mouse serum adipokine kit (Millipore Corp., Billerica, MA), following the manufacturer’s instructions. For insulin and leptin assays, the sensitivity thresholds were 24.9 and 3.7 pg/ml, respectively, whereas intra- and interassay coefficients of variations were <4.5 and 10.3%, respectively. Serum insulin levels for mice in Study 2 (n = 8–9 individual mice per diet group) were measured using the Rat/Mouse Insulin ELISA kit (Millipore); sensitivity was 0.2ng/ml and intra- and interassay coefficients of variations were 5 and 11%, respectively. Serum adiponectin concentrations were measured using a mouse adiponectin ELISA kit (Millipore Corp.). Adiponectin assay sensitivity was 0.5ng/ml and intra- and interassay coefficients of variations were <6%. Serum IGF-1 levels were measured using the Mouse/Rat IGF-1 immunoassay kit (R&D Systems, Minneapolis, MN), with a sensitivity threshold of 1.6 pg/ml.
Data analyses
Data are presented as mean ± SEM and were compared by t-test or one-way analysis of variance, using SigmaStat version 3.5 Software (SPSS, Chicago, IL). Differences in tumor incidence between diet groups were determined by Fisher Exact test. P ≤ 0.05 was considered to be statistically significant.
Results
Dietary exposure to BB through maternal diet altered mammary parameters in offspring
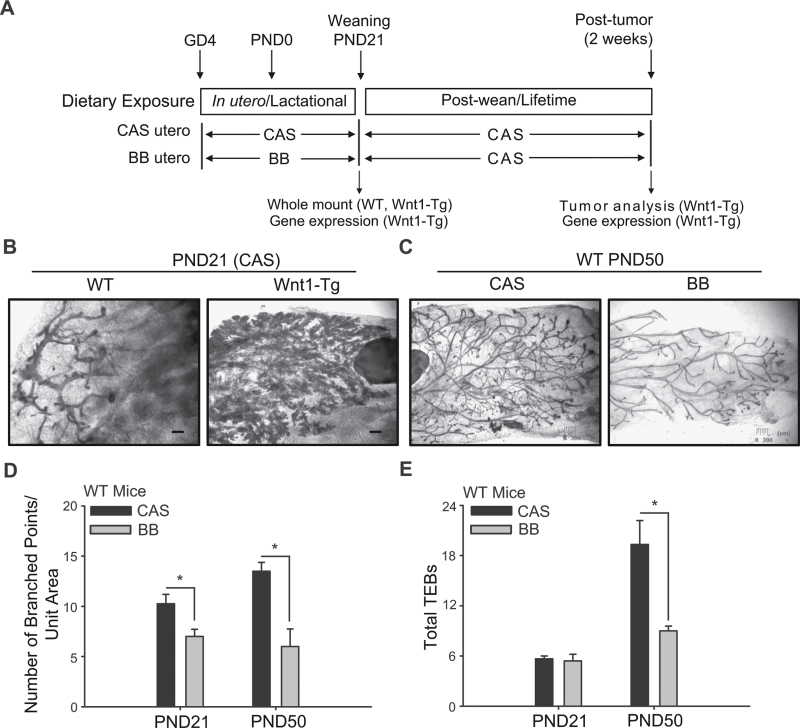
Whole mounts of fat pads were prepared from WT and Wnt1-Tg female offspring of dams that were fed control (CAS) or BB-supplemented (BB) diets during pregnancy and lactation (Figure 1A). At weaning (PND21), mammary glands from Wnt1-Tg offspring of CAS-fed dams displayed extensive ductal side-branching and highly developed lobuloalveoli structures (Figure 1B). In contrast, mammary glands from WT littermates (Figure 1B) showed rudimentary branching and distinct TEBs, with no detectable alveoli. The extensive mammary hyperplasia elicited by Wnt1 overexpression in PND21 Wnt1-Tg offspring precluded an accurate determination of differences in mammary branching and TEB numbers with diet. To determine whether maternal diet affected mammary gland development, whole mounts of fat pads from young adult (PND50) WT female offspring that were exposed to maternal CAS or BB diets were evaluated. Dietary BB-exposed PND50 WT offspring displayed mammary glands that had less side-branching than CAS-exposed counterparts (Figure 1C). The numbers of branched points (a measure of the ductal network) in mammary glands of BB offspring were lower than those of control offspring at both PND21 and PND50 (Figure 1D). The numbers of TEBs were comparable for mammary glands of offspring of the two diet groups at PND21 and were lower in mammary glands of BB-exposed offspring relative to those of control offspring at PND50 (Figure 1E).
Fig. 1.
In utero and lactational exposure to BB reduces ductal branching and TEB numbers in offspring. (A) Dietary regimen. Wild-type (WT) dams were mated with Wnt1-Tg males. Dams were fed control (CAS) diet or the BB-supplemented CAS diet from mating through pregnancy and lactation. Wnt1-Tg and WT female pups were weaned to CAS diet. Inguinal mammary gland (4) was collected for RNA (right MG) and whole mount (left MG) from weaning (PND21) pups. For the tumor study, Wnt1-Tg female offspring that were exposed via maternal diet to either CAS (n = 33) or BB (n = 28) were monitored weekly by palpation for tumor development. Two weeks post-tumor detection, serum was collected and tumors were harvested for RNA and protein analyses. (B) Whole-mount analysis revealed excessive branching and hyperplasia in mammary glands of Wnt1-Tg female pups at weaning (PND21), compared with WT mice, as previously reported (19). (C) Shown are the mammary glands of PND50 virgin WT female mice exposed to CAS or BB via maternal diet. (D) Ductal branching was quantified in PND21 and PND50 WT mice. (E) Quantification of mammary TEB in PND21 and PND50 WT mice exposed to maternal CAS or BB diets. Results are mean ± SEM; *P < 0.05 relative to CAS (n = 5 mice per diet, per PND). Bar, 300 µm. 3× magnification.
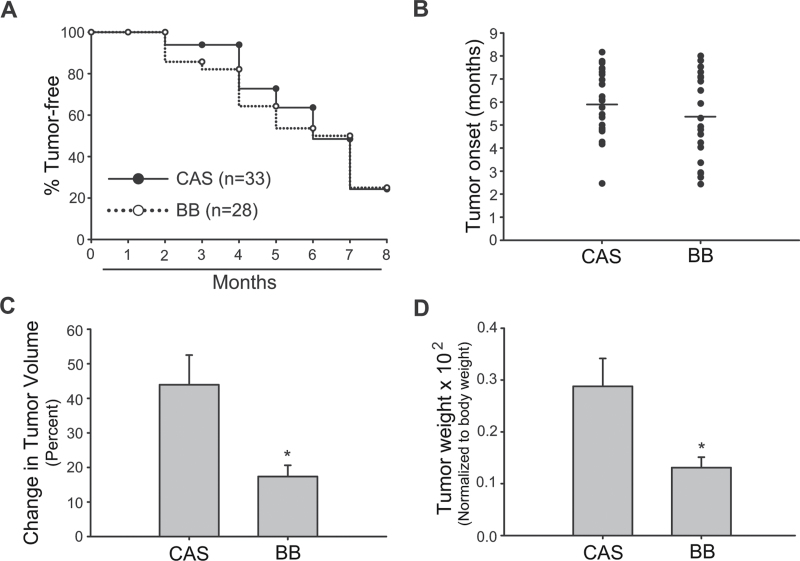
Dietary exposure in utero and at lactation to BB inhibited mammary tumor growth in offspring
Female Wnt1-Tg offspring of dams that were fed CAS or BB diets during pregnancy and lactation were followed for mammary tumor onset up to age 8 months. Exposure to maternal BB diet did not alter mammary tumor incidence in these females, as determined by Kaplan–Meier analysis (Figure 2A). The average age of tumor onset (5–6 months) was comparable (P = 0.265) between offspring of both diet groups (Figure 2B). However, the change in tumor volume, defined as the percentage change in tumor size from initial detection to tumor harvest at 2 weeks postdetection, and tumor weights (measured at tumor harvest and normalized to body weights) were lower for offspring of the BB-fed dams (Figure 2C and D). Histopathological analyses of the tumors showed predominance of solid carcinoma (28) (Supplementary Table 4, available at Carcinogenesis Online).
Fig. 2.
Maternal intake of BB suppresses tumor growth in Wnt1-Tg female offspring. Tumor incidence (A), onset (B), change in tumor volume (C) and tumor weight at killing (D) were compared for Wnt1-Tg female offspring of CAS- or BB-fed dams. Presence of tumors was monitored by weekly visual inspection and palpation from 1 to 8 months of age. The mean tumor onset was calculated as the age of initial tumor appearance. Tumor weight was normalized to body weight. Change in tumor volume was calculated as described in Materials and methods. Results are mean ± SEM; *P < 0.05 for BB relative to CAS.
Dietary BB exposure postweaning did not alter mammary tumor parameters
To determine if postweaning exposure to BB relative to the control diet, recapitulated the effects of early BB exposure through maternal diet, female Wnt1-Tg offspring of control (CAS) diet-fed dams were weaned (at PND21) to CAS or BB diets and monitored for tumors until age 8 months (Supplementary Figure 1A, available at Carcinogenesis Online). The incidence, onset, change in volume and weights of mammary tumors of Wnt1-Tg females fed BB diets did not differ from those of Wnt1-Tg females fed CAS (Supplementary Figure 1B–3E, available at Carcinogenesis Online). Diet did not affect the type of mammary tumors that developed (Supplementary Table 4, available at Carcinogenesis Online).
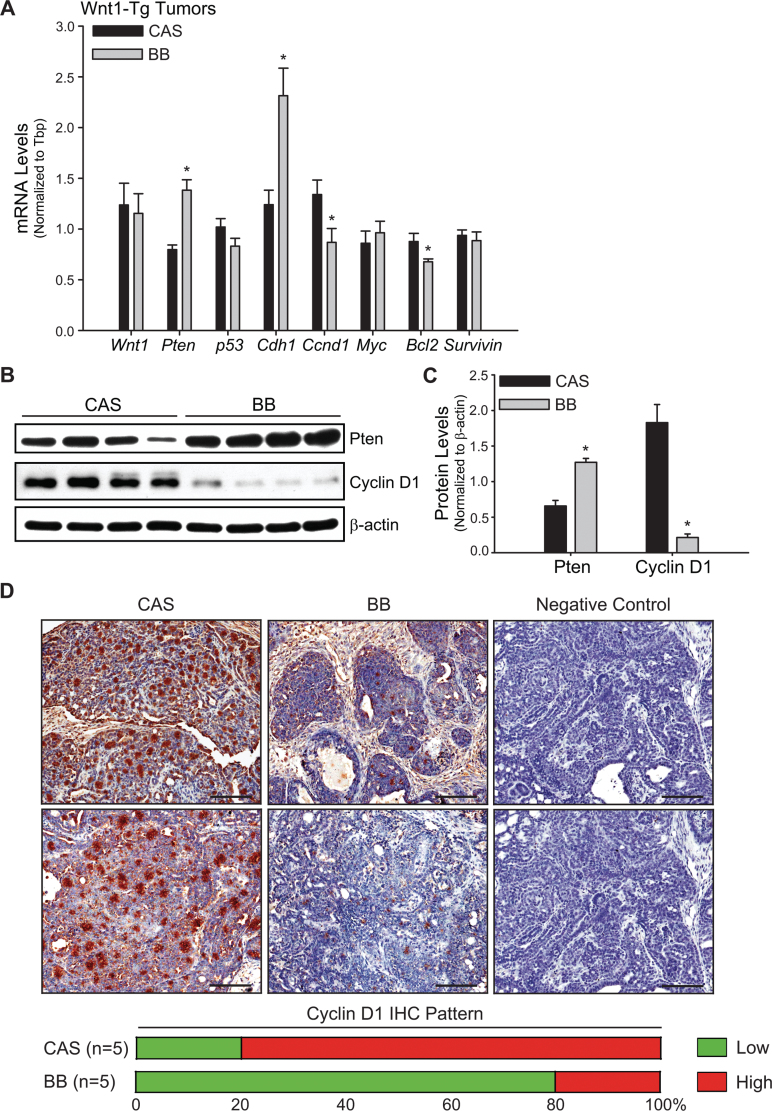
Gestational + lactational exposure to BB diet promoted tumor suppressor expression in mammary tumors
Solid carcinoma tumors from Wnt1-Tg offspring of dams that were fed CAS or BB diets (n = 9/diet group) were evaluated for expression of tumor suppressor (Pten; p53; cadherin 1, Cdh1), proproliferative (Cyclin D1, Ccnd1; c-Myc) and anti-apoptotic (Bcl2, survivin) genes by QPCR. Transcript levels of Pten and Cdh1 were increased, whereas those of Ccnd1 and Bcl2 were decreased, in tumors of maternal BB-exposed offspring (Figure 3A). Diet did not influence expression of Wnt1, p53, c-Myc and survivin in tumors. The changes in gene expression for Pten and Ccnd1 in mammary tumors with exposure to maternal BB diet were confirmed at the protein level by western (Figure 3B and C) and for Ccnd1, also by immunohistochemistry (Figure 3D). The majority (80%) of tumors analyzed in the BB group by immunohistochemistry had low to undetectable cyclin D1 protein (Figure 3D).
Fig. 3.
Tumors from Wnt1-Tg mice exposed to BB via maternal diet exhibit higher expression of anti-proliferative, proapoptotic and prodifferentiation markers. (A) Genes involved in cell proliferation, apoptosis, differentiation and tumor progression were evaluated by QPCR in tumors from Wnt1-Tg mice exposed to CAS or BB diets in utero and during lactation. Tbp was used as a normalizing control; *P < 0.05 relative to CAS (n = 9 per diet group). (B) Western blot analysis of PTEN and cyclin D1 proteins in CAS or BB tumors. Each lane (50 μg of total protein) represents an individual animal. (C) Immunoreactive bands (in B) were quantified by densitometry. Normalized values relative to β-actin are presented as histograms (*P < 0.05 relative to CAS). (D) Representative cyclin D1 immunostaining of CAS and BB tumors from five tumored mice (per diet group) is shown at ×200 magnification. Each panel for CAS and BB represents solid carcinoma tumor sections (Supplementary Table 4, available at Carcinogenesis Online) from an individual mouse. Negative control shows lack of immunostaining in the absence of primary antibody. Bar, 100 µm.
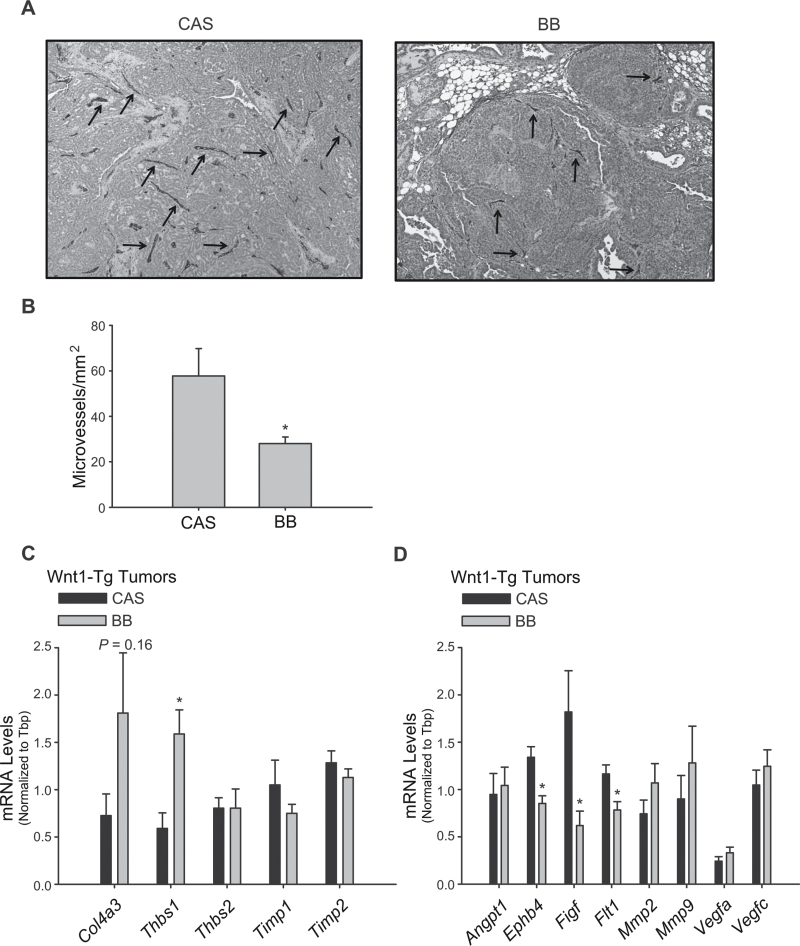
Maternal exposure to BB diet decreased microvessel density in mammary tumors of Wn1-Tg offspring
To determine whether the lower tumor volume and weight seen with maternal exposure to BB diet (Figure 2C and D) could involve regulation of growth factor supply via the vasculature, tumor microvessel density was analyzed by CD34 immunostaining (Figure 4A). CD34-reactive microvessel numbers were 2-fold lower (CAS = 57.72 ± 12.1 versus BB = 28.04 ± 2.85; P = 0.039) in tumors from Wnt1-Tg mice exposed to maternal BB diet relative to the CAS-exposed group (Figure 4B).
Fig. 4.
Maternal exposure to BB diet decreases microvessel density in mammary tumors of Wnt1-Tg offspring. (A) Representative images of CD34 staining (arrows) of microvessels in mammary tumors of Wnt1-Tg mice fed CAS or BB via maternal diet. (B) CD34-immunoreactive vessels were quantified in five random areas per tumor sample (n = 3–4 independent tumors per diet group); *P < 0.05 relative to CAS. Anti-angiogenesis (C) and proangiogenesis (D) transcript levels in mammary tumors from Wnt1-Tg offspring exposed to CAS or BB via maternal diet. mRNA levels were determined by QPCR and normalized to Tbp (n = 8–10 mice per diet group); *P < 0.05 relative to CAS, for each gene analyzed.
To evaluate possible mediators of the observed decrease in microvessel formation with maternal dietary BB exposure (Figure 4A and B), we measured the transcript levels of a panel of genes with known anti- and proangiogenic activities, in mammary tumors of Wnt1-Tg offspring. Tumors from offspring exposed to maternal BB diet relative to those exposed to control diet had higher expression of genes Col4a3 and Thbs1, which were shown previously to inhibit angiogenesis (31,32); no differences for Thbs2, Timp1 or Timp2 transcript levels were observed between the dietary groups (Figure 4C). Analysis of proangiogenic genes revealed suppression of Ephb4 (33), Figf (34) and Flt1 (34) transcript levels in tumors from offspring with maternal BB exposure (Figure 4D). Similar changes in the expression of other proangiogenic genes such as Angpt1, Mmp2, Mmp9, Vegfa and Vegfc were not observed (Figure 4D).
Early exposure to BB diet altered gene expression of DNA methylation and chromatin-modifying enzymes in mammary tumors
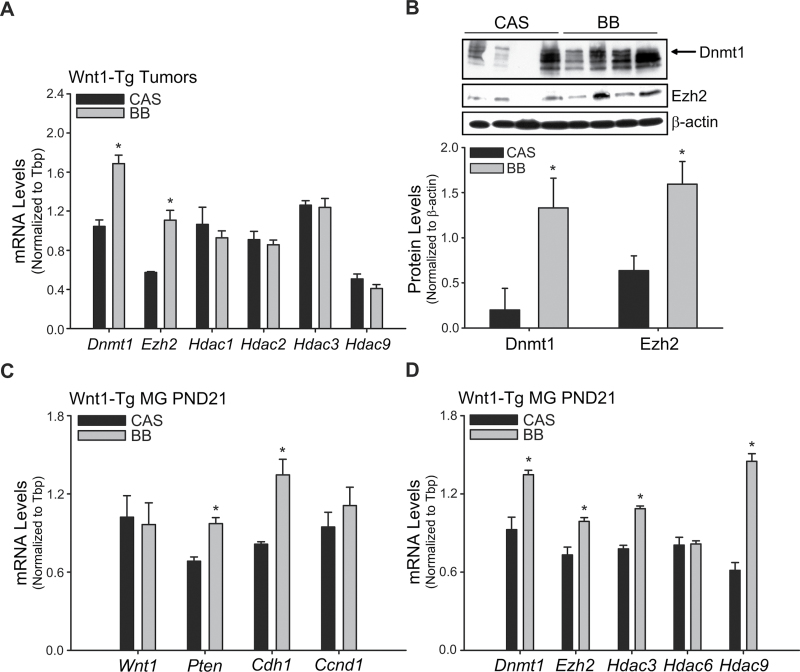
To examine potential associations between maternal BB dietary effects on offspring mammary tumor outcome and epigenetic mechanisms, the expression patterns of a select group of DNA methylation and chromatin-modifying enzyme genes were evaluated in mammary tumors by QPCR. Transcript levels of DNA methyl transferase 1 (Dnmt1) and Enhancer of zeste homolog 2 (Ezh2) were higher in tumors from BB-exposed offspring (Figure 5A); these changes in transcript abundance were confirmed at the protein level by western blots (Figure 5B). In contrast, transcript levels of enzymes involved in histone modifications namely histone deacetylases 1, 2, 3 and 9 (Hdac1, 2, 3 and 9) were not influenced by diet (Figure 5A).
Fig. 5.
Gene expression of DNA methylation and chromatin-modifying enzymes in mammary tumors and in non-tumor (preneoplastic) mammary glands. (A) Gene expression of DNA methylation (Dnmt1 and Ezh2) and histone deacetylation (Hdac1, Hdac2, Hdac3 and Hdac9) enzymes was quantified by QPCR in tumors from Wnt1-Tg mice exposed to maternal CAS or BB diets. Tbp was used as a normalizing control; *P < 0.05 relative to CAS (n = 9 per diet group). (B) Top, western blot analyses of DNMT1 (arrow) and EZH2 proteins (50 μg) in tumors from maternal CAS or BB diet groups; each lane represents an individual animal. Bottom, immunoreactive bands were quantified by densitometry and normalized to β-actin; relative values are presented as histograms (*P < 0.05 relative to CAS). (C) Gene expression of proliferative (cyclin D1, Ccnd1) and proapoptotic/prodifferentiation (Pten; E-cadherin, Cdh1) markers was evaluated in mammary glands of Wnt1-Tg mice at weaning (PND21). (D) Gene expression of DNA methylation (Dnmt1 and Ezh2) and histone deacetylation (Hdac3, Hdac6 and Hdac9) enzymes in mammary glands of weaned Wnt1-Tg mice exposed to CAS or BB via maternal diets. Tbp was used as a normalizing control; *P < 0.05 relative to CAS (n = 6 per diet group).
Preneoplastic mammary tissues of PND21 Wnt1-Tg female offspring of the two diet groups were similarly evaluated for changes in expression of tumor suppressor Pten and Cdh1, proproliferative Ccnd1 and DNA/chromatin-modifying enzyme (Dnmt1, Ezh2, Hdac3, 6 and 9) genes, by QPCR. The increase in Pten, Cdh1, Dnmt1 and Ezh2 transcript levels in mammary tumors of maternal BB-exposed offspring was also observed in corresponding non-tumor PND21 mammary tissues (Figure 5C and 5D). In contrast to mammary tumors, preneoplastic mammary tissue Ccnd1 transcript levels did not differ, whereas those for Hdac3 and Hdac9 were greater with maternal BB exposure. Diet did not alter Wnt1 gene expression in tissues (Figure 5C), as shown for mammary tumors (Figure 3A).
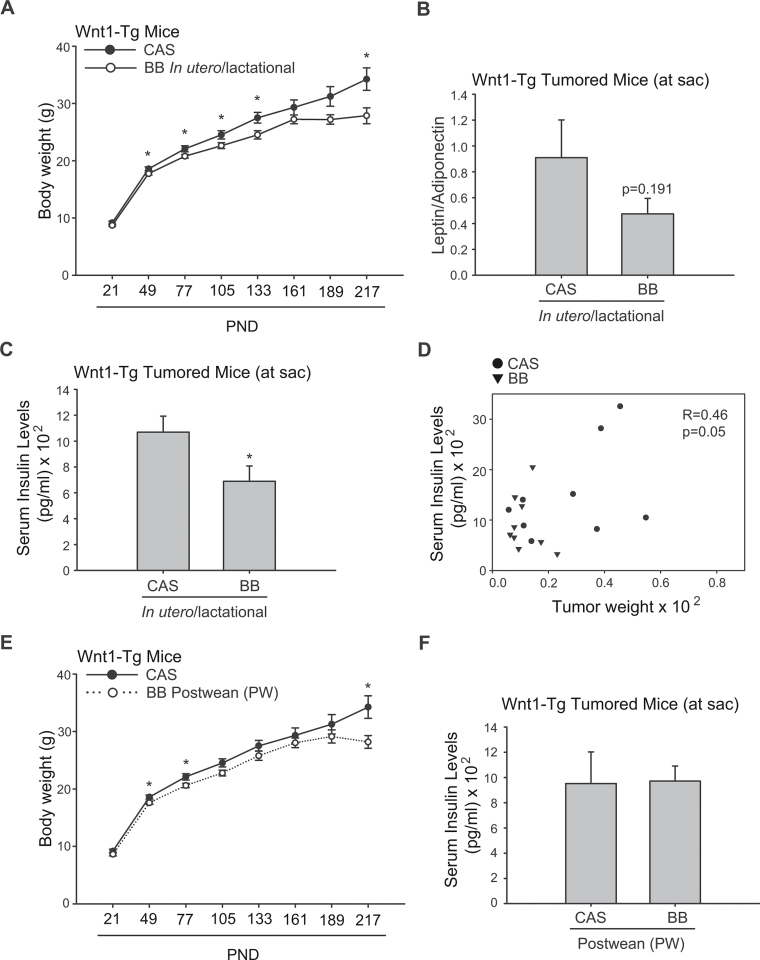
Maternal diet influenced body weight and hormone levels in tumored offspring
To determine if maternal diet affected endocrine parameters that may influence mammary tumor development long after the dietary exposure, body weights of offspring were evaluated throughout the study, and serum levels of the adipokines leptin and adiponectin and of insulin and IGF-1 were measured in tumored mice at killing. Although body weights of offspring from the two diet groups did not differ at weaning, lower body weights were found for maternal BB-exposed Wnt1-Tg females by PND50 and which persisted for lifetime, when compared with maternal CAS-exposed counterparts (Figure 6A). BB-exposed, tumor-bearing offspring showed numerically lower serum leptin/adiponectin ratio (Figure 6B), and significantly reduced serum insulin levels (Figure 6C). Serum IGF-1 levels did not differ for offspring of the two groups (CAS: 443.47 ± 23.75ng/ml; BB: 426.11 ± 52.19ng/ml). Tumor weights were positively correlated with serum insulin levels (R = 0.46; Figure 6D). Interestingly, although mice exposed to BB diets beginning at postweaning had lower body weights than those of CAS-fed mice (Figure 6E), serum insulin levels for mice in these two groups did not differ (Figure 6F).
Fig. 6.
Maternal BB exposure lowers body weight and suppresses serum insulin levels in Wnt1-Tg offspring. (A) Body weights of Wnt1-Tg mice enrolled in the tumor study were recorded monthly from weaning (PND21) until study conclusion (up to 8 months). *P < 0.05 relative to CAS. (B and C) The ratio of serum leptin to adiponectin levels and serum insulin levels, respectively, were measured for tumor-bearing Wnt1-Tg mice at killing as described in Materials and methods (n = 9 per diet group). (D) Correlation between serum insulin and tumor weight (normalized to body weight) in Wnt1-Tg tumored mice exposed to CAS (circle) or BB (triangle) via maternal diet. The Pearson correlation coefficient is 0.46; P = 0.05. (E) Body weights and (F) serum insulin levels for mice fed CAS or BB diets beginning at weaning until killing. *P < 0.05 relative to CAS.
Discussion
This study provides strong support for the significant influence of maternal diet on mammary tumor outcome in progeny. Using the Wnt1-Tg mouse model of spontaneous human breast cancer and BB powder intake at 3% of the diet, amounts which are readily achievable with half to one cup of BBs per day for an average-weight individual, we showed that maternal BB consumption during pregnancy and lactation elicited reductions in mammary tumor weight and tumor volume in adult progeny, resulting in a more favorable prognosis. The suppression of mammary tumor growth with early BB exposure, which was not recapitulated when the same BB diet was introduced during early childhood (postweaning) and continued through adulthood, suggests that the effects of early BB exposure with later adult consequence, probably occurred during an early window of mammary gland development. We further showed that the inhibitory effects of early BB exposure on mammary tumor growth are associated with: (i) elevated expression of tumor suppressors PTEN and E-cadherin and lower expression of anti-apoptotic Bcl2 and proliferative cyclin D1, genes in mammary tumors; (ii) an early increase (at weaning) of PTEN and E-cadherin gene expression in mammary tissues, which was accompanied by reduced ductal side-branching in developing (PND21) and young adult (PND50) and lower TEB numbers in young adult (PND50) mammary glands, respectively, and (iii) decreased intratumoral microvessel density and decreased and increased expression, respectively, of a subset of pro- and anti-angiogenic genes. The differences in ductal branching with early BB exposure are not likely due to differing estrous cycle stage at the time of tissue collection because these were observed even at PND21, when these mice have not begun to cycle. Moreover, we previously demonstrated that exposure to BB through maternal diet did not promote sexual maturity (22). We provided evidence to suggest that the effects of early dietary BB exposure on gene expression patterns in preneoplastic mammary tissues and mammary tumors may involve epigenetic modifications through early BB effects on EZH2 and DNMT1 expression, both of which regulate the expression of many developmental genes (36). Finally, we showed that the long-term effects of maternal BB diets are associated with altered metabolic parameters in offspring, manifested as decreased body weights, lower serum insulin levels and diminished leptin/adiponectin ratio, all of which are negatively linked to breast cancer progression (37–39).
In this study, we utilized Wnt1-Tg female mice as an in vivo model for human breast cancer because numerous targets of the Wnt signaling pathway are overexpressed or deregulated in breast tumors (40). Moreover, we have previously demonstrated that tumor suppressors PTEN and E-cadherin, which are linked by their regulation of β-catenin degradation and nuclear transport (41,42), are in vivo and in vitro targets of diets/dietary factors with breast cancer protective effects (29,30,43,44). Although maternal BB diet did not reduce Wnt1-induced tumor incidence, most likely because highly activated Wnt signaling is a powerful driver of breast cancer initiation (19) and activation of this pathway could not be inhibited or reversed by bioactive components present in BB, limited early exposure to this diet was sufficient to alter the trajectory of mammary tumor volume/weight in offspring. The latter suggests the protective effects of early exposure to BB on pathways involved in tumor progression. The lack of a similar ‘rescue’ of overactivated Wnt signaling by provision of the BB diet at postweaning and through adulthood, suggests that a critical developmental window of mammary gland development is affected by maternal nutrition. The latter possibility places the maternal (in utero/lactational) environment as a highly relevant nutritional target for breast cancer prevention (45).
Although this study did not address the in utero/lactational mechanism(s) and the precise mediators by which maternal BB exposure influences offspring mammary gene expression and hence, breast cancer risk and outcome, it is possible that maternal BB intake may reduce oxidative stress, limit the inflammatory milieu and oppose mitogenic signals within the uterine microenvironment of the developing fetus, thereby leading to metabolic changes with later consequences. In this regard, BB polyphenolic acid metabolites, which are highly bioavailable, are known to display high anti-oxidative potential (46). Further, BB intake has been recently shown to negatively affect proinflammatory gene networks involving IL-13 and IFNγ and to suppress Wnt/β-catenin signaling pathways through its induction of Wnt inhibitor SFRP4 (23). In a recent study, we demonstrated that several BB polyphenolic acids present in sera can inhibit mammosphere formation of human breast cancer cells, the latter a measure of mammary stem cell activity (47). These findings raise the interesting possibility that stem cell pathways in developing mammary glands may be influenced by early BB exposure through maternal diets. Maternal obesity and high fat/high calorie maternal diets have been shown to impose a proinflammatory state on the fetus (48), and the latter has been suggested to underlie some of the adult chronic diseases associated with metabolic syndrome in adult offspring of obese dams (12,13). Although we did not find differences in body weights of dams consuming CAS or BB diets, similar to a previous report in rats (22), the observed lower body weights, serum insulin levels and serum leptin/adiponectin ratio in adult offspring of dams fed BB diet, relative to those of dams fed CAS, are consistent with suppression of a proinflammatory environment associated with obesogenic conditions, with maternal BB intake. Interestingly, pups exposed to BB diets beginning only at postweaning, although exhibiting lower body weights than those fed CAS, did not manifest the lower insulin levels attributed to early (pre- and postnatal) BB exposure. Thus, life-long effects of maternal BB consumption in the suppression of mammary tumor progression in adult offspring may be attributed to developmental programming of insulin sensitivity as well as of anti-inflammatory response, both of which can be mediated by epigenetic mechanisms, to counter the negative effects of adipogenesis and insulin resistance.
Formation of new blood vessels (i.e. angiogenesis) is essential for breast cancer progression, and increased microvessel density in breast carcinoma has been correlated with tumor size, grade and lymph node metastasis (49). Our findings of decreased microvessel density and alterations in the expression of a subset of genes known to inhibit angiogenesis (31–35) in mammary tumors of Wnt1-Tg offspring that were exposed to maternal BB relative to control diet indicate that early BB exposure could modulate intratumoral angiogenic factor levels. The latter possibility provides a testable mechanism (to be addressed in future studies) by which maternal dietary BB intake may influence tumor outcome in women predisposed to breast cancer due to aberrant activation of Wnt signaling.
Bioactive dietary factors have been reported to be potent modifiers of the epigenome, exerting influence on the expression and/or activity of numerous chromatin-modifying enzymes (50–52). Herein, we found that exposure to BB through maternal diet increased the expression of Dnmt1, Ezh2 and Hdac3 and Hdac9 genes in non-tumor (preneoplastic) mammary tissues of weaning offspring and these effects persisted for DNMT1 and EZH2 in mammary tumors. Although these findings implicate changes in DNA methylation and histone modifications as important for the protective effects of maternal BB exposure in offspring mammary tumor outcome, the endogenous gene targets of these epigenetic changes remain unclear. An earlier study reported that dietary intake of black raspberries by patients with colon tumors led to decreased DNMT1 protein levels and reduced methylation (and hence, activation) of anti-proliferative p16 and Wnt suppressor genes (53). Paradoxically, inhibition of mammary tumor progression by maternal BB exposure was associated in this study with higher PTEN levels coincident with increased DNMT1 and EZH2 expression. In other studies, increased methylation of PTEN gene promoter by DNMT1 (54) and EZH2 (55) has been reported to result in lower PTEN expression and higher Akt activity, leading to increased breast cancer invasion and metastasis (56). Although we have no experimental data to explain our results at the present time, we suggest that dietary exposure under distinct contexts (e.g. tumor versus normal, non-tumor) leading to similar DNA methylation status might elicit different effects. Indeed, it is worth noting that an increase in DNA methylation may be beneficial under some contexts (16). In that study, in utero exposure to bisphenol A, which caused DNA hypomethylation in mouse offspring, was counteracted by supplementation of maternal diet with folic acid or the phytoestrogen genistein, leading to DNA hypermethylation. Thus, some early epigenetic marks persisting to adulthood in mammary tumors may be beneficial. Further studies to identify changes in global and promoter-specific methylation events elicited by BB in mammary tissues and tumors using more sophisticated procedures of DNA methylation profiling will address these apparent discrepancies.
In conclusion, our use of the Wnt1-Tg mouse model of human breast cancer has provided compelling support for the maternal (in utero/lactational) environment of the developing mammary gland as a modifiable determinant of breast tumor growth/progression in adult progeny. In light of the life-long effects of the limited (maternal only) dietary exposure described here, the notion of pubertal mammary development as most susceptible to ‘tumorigenic insults’ may need reconsideration. Given the many health risks that can be imposed by obesity and poor dietary choices of pregnant and nursing mothers on their progeny, further studies on nutritional epigenomics and programming and how these may underlie breast cancer outcome and influence treatment of the disease are warranted.
Supplementary material
Supplementary Tables 1–4 and Supplementary Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
United States Department of Agriculture (CRIS-6251-5100002-06S, Arkansas Children’s Nutrition Center to R.C.M.S); Department of Defense Breast Cancer Research Program Pre-doctoral Fellowship (W81XWH-10-1-0047 to O.M.R.); National Institutes of Health-National Cancer Institute (RO1 CA136493 to F.A.S.); University of Arkansas for Translational Research Institute/Arkansas Breast Cancer Research Program (1UL1RR029884).
Conflict of Interest Statement: None declared.
Supplementary Material
Glossary
Abbreviations:
- Angpt1
angiopoietin 1
- BB
blueberry
- CAS
casein
- Ccnd1
cyclin D1
- Cdh1
cadherin 1
- Col4a3
collagen type IV, alpha 3
- DNMT
DNA methyl transferase
- Ephb4
Eph receptor B4
- EZH2
enhancer of zeste homolog 2
- Figf
c-fos induced growth factor
- Flt1
FMS-like tyrosine kinase 1
- HDAC
histone deacetylase
- MG
mammary gland
- Mmp
matrix metallopeptidase
- PND
postnatal day
- PTEN
phosphatase and tensin homologue deleted on chromosome ten
- PW
postweaning
- QPCR
quantitative real-time polymerase chain reaction
- TEB
terminal end bud
- Thbs
thrombospondin
- Timp
tissue inhibitor of metalloproteinase
- Vegf
vascular endothelial growth factor
- WT
wild-type
- Wnt1-Tg
Wnt1-transgenic
References
- 1. Barker D.J., et al. (1989). The intrauterine and early postnatal origins of cardiovascular disease and chronic bronchitis. J. Epidemiol. Community Health, 43, 237–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnson R.C., et al. (2011). Early-life origins of adult disease: national longitudinal population-based study of the United States. Am. J. Public Health, 101, 2317–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stocker C.J., et al. (2005). Fetal origins of insulin resistance and obesity. Proc. Nutr. Soc., 64, 143–151 [DOI] [PubMed] [Google Scholar]
- 4. Thompson J.A., et al. (2011). In utero origins of adult insulin resistance and vascular dysfunction. Semin. Reprod. Med., 29, 211–224 [DOI] [PubMed] [Google Scholar]
- 5. Hochberg Z., et al. (2011). Child health, developmental plasticity, and epigenetic programming. Endocr. Rev., 32, 159–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeSantis C., et al. (2011). Breast cancer statistics, 2011. CA. Cancer J. Clin., 61, 409–418 [DOI] [PubMed] [Google Scholar]
- 7. Polyak K. (2007). Breast cancer: origins and evolution. J. Clin. Invest., 117, 3155–3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Troisi R., et al. (2007). Exploring the underlying hormonal mechanisms of prenatal risk factors for breast cancer: a review and commentary. Cancer Epidemiol. Biomarkers Prev., 16, 1700–1712 [DOI] [PubMed] [Google Scholar]
- 9. Palmer J.R., et al. (2002). Risk of breast cancer in women exposed to diethylstilbestrol in utero: prelimiinary results (United States). Cancer Causes Control, 13, 753–758 [DOI] [PubMed] [Google Scholar]
- 10. Sanderson M., et al. (1998). Maternal factors and breast cancer risk among young women. Paediatr. Perinat. Epidemiol., 12, 397–407 [DOI] [PubMed] [Google Scholar]
- 11. de Assis S., et al. (2006). High birth weight increases mammary tumorigenesis in rats. Int. J. Cancer, 119, 1537–1546 [DOI] [PubMed] [Google Scholar]
- 12. Hilakivi-Clarke L., et al. (1997). A maternal diet high in n - 6 polyunsaturated fats alters mammary gland development, puberty onset, and breast cancer risk among female rat offspring. Proc. Natl. Acad. Sci. U.S.A., 94, 9372–9377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walker B.E. (1990). Tumors in female offspring of control and diethylstilbestrol-exposed mice fed high-fat diets. J. Natl. Cancer Inst., 82, 50–54 [DOI] [PubMed] [Google Scholar]
- 14. Luijten M., et al. (2004). Effects of soy-derived isoflavones and a high-fat diet on spontaneous mammary tumor development in Tg.NK (MMTV/c-neu) mice. Nutr. Cancer, 50, 46–54 [DOI] [PubMed] [Google Scholar]
- 15. Fernandez-Twinn D.S., et al. (2007). Compensatory mammary growth following protein restriction during pregnancy and lactation increases early-onset mammary tumor incidence in rats. Carcinogenesis, 28, 545–552 [DOI] [PubMed] [Google Scholar]
- 16. Dolinoy D.C., et al. (2007). Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. U.S.A., 104, 13056–13061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Su Y., et al. (2007). In utero exposure to maternal diets containing soy protein isolate, but not genistein alone, protects young adult rat offspring from NMU-induced mammary tumorigenesis. Carcinogenesis, 28, 1046–1051 [DOI] [PubMed] [Google Scholar]
- 18. Doherty L.F., et al. (2010). In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm. Cancer, 1, 146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tsukamoto A.S., et al. (1988). Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell, 55, 619–625 [DOI] [PubMed] [Google Scholar]
- 20. Hayes M.J., et al. (2008). Genetic changes of Wnt pathway genes are common events in metaplastic carcinomas of the breast. Clin. Cancer Res., 14, 4038–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin S.Y., et al. (2000). Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc. Natl. Acad. Sci. U.S.A., 97, 4262–4266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu X., et al. (2009). In utero and lactational exposure to blueberry via maternal diet promotes mammary epithelial differentiation in prepubescent female rats. Nutr. Res., 29, 802–811 [DOI] [PubMed] [Google Scholar]
- 23. Adams L.S., et al. (2011). Whole blueberry powder modulates the growth and metastasis of MDA-MB-231 triple negative breast tumors in nude mice. J. Nutr., 141, 1805–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stull A.J., et al. (2010). Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr., 140, 1764–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seymour E.M., et al. (2011). Blueberry intake alters skeletal muscles and adult tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J. Med. Food, 14, 1511–1518 [DOI] [PubMed] [Google Scholar]
- 26. Davie S.A., et al. (2007). Effects of FVB/NJ and C57Bl/6J strain backgrounds on mammary tumor phenotype in inducible nitric oxide synthase deficient mice. Transgenic Res., 16, 193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kelly T., et al. (2003). High heparanase activity in multiple myeloma is associated with elevated microvessel density. Cancer Res., 63, 8749–8756 [PubMed] [Google Scholar]
- 28. Cardiff R.D., et al. (2000). The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene, 19, 968–988 [DOI] [PubMed] [Google Scholar]
- 29. Rahal O.M., et al. (2010). PTEN and p53 cross-regulation induced by soy isoflavone genistein promotes mammary epithelial cell cycle arrest and lobuloalveolar differentiation. Carcinogenesis, 31, 1491–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rahal O.M., et al. (2011). Paracrine-acting adiponectin promotes mammary epithelial differentiation and synergizes with genistein to enhance transcriptional response to estrogen receptor β signaling. Endocrinology, 152, 3409–3421 [DOI] [PubMed] [Google Scholar]
- 31. Hamano Y., et al. (2003). Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin. Cancer Cell, 3, 589–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Folkman J. (2007). Angiogenesis: an organizing principle for drug discovery? Nat. Rev. Drug Discov., 6, 273–286 [DOI] [PubMed] [Google Scholar]
- 33. Kertesz N., et al. (2006). The soluble extracellular domain of EphB4 (sEphB4) antagonizes EphB4-EphrinB2 interaction, modulates angiogenesis, and inhibits tumor growth. Blood, 107, 2330–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marconcini L., et al. (1999). c-fos-induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro . Proc. Natl. Acad. Sci. U.S.A., 96, 9671–9676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shalaby F., et al. (1995). Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature, 376, 62–66 [DOI] [PubMed] [Google Scholar]
- 36. Viré E., et al. (2006). The Polycomb group protein EZH2 directly controls DNA methylation. Nature, 439, 871–874 [DOI] [PubMed] [Google Scholar]
- 37. Vona-Davis L., et al. (2007). Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes. Rev., 8, 395–408 [DOI] [PubMed] [Google Scholar]
- 38. Gray S.G., et al. (2003). The insulin-like growth factors and insulin-signalling systems: an appealing target for breast cancer therapy? Horm. Metab. Res., 35, 857–871 [DOI] [PubMed] [Google Scholar]
- 39. Jardé T., et al. (2011). Molecular mechanisms of leptin and adiponectin in breast cancer. Eur. J. Cancer, 47, 33–43 [DOI] [PubMed] [Google Scholar]
- 40. Brennan K.R., et al. (2004). Wnt proteins in mammary development and cancer. J. Mammary Gland Biol. Neoplasia, 9, 119–131 [DOI] [PubMed] [Google Scholar]
- 41. Orsulic S., et al. (1999). E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J. Cell. Sci., 112 (Pt 8), 1237–1245 [DOI] [PubMed] [Google Scholar]
- 42. Lau M.T., et al. (2011). E-cadherin inhibits tumor cell growth by suppressing PI3K/Akt signaling via β-catenin-Egr1-mediated PTEN expression. Oncogene, 30, 2753–2766 [DOI] [PubMed] [Google Scholar]
- 43. Dave B., et al. (2005). The soy isoflavone genistein promotes apoptosis in mammary epithelial cells by inducing the tumor suppressor PTEN. Carcinogenesis, 26, 1793–1803 [DOI] [PubMed] [Google Scholar]
- 44. Su Y., et al. (2009). Soy isoflavone genistein upregulates epithelial adhesion molecule E-cadherin expression and attenuates beta-catenin signaling in mammary epithelial cells. Carcinogenesis, 30, 331–339 [DOI] [PubMed] [Google Scholar]
- 45. Simmen F.A., et al. (2011). The maternal womb: a novel target for cancer prevention in the era of the obesity pandemic? Eur. J. Cancer Prev., 20, 539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xie C., et al. (2011). Blueberries reduce pro-inflammatory cytokine TNF-alpha and IL-6 production in mouse macrophages by inhibiting NF-kappaB activation and the MAPK pathway. Mol. Nutr. Food Res., 55, 1587–1591 [DOI] [PubMed] [Google Scholar]
- 47. Montales M.T., et al. (2012). Repression of mammosphere formation of human breast cancer cells by soy isoflavone genistein and blueberry polyphenolic acids suggests diet-mediated targeting of cancer stem-like/progenitor cells. Carcinogenesis, 33, 652–660 [DOI] [PubMed] [Google Scholar]
- 48. Bouanane S., et al. (2009). Time course of changes in serum oxidant/antioxidant status in overfed obese rats and their offspring. Clin. Sci., 116, 669–680 [DOI] [PubMed] [Google Scholar]
- 49. Choi W.W., et al. (2005). Angiogenic and lymphangiogenic microvessel density in breast carcinoma: correlation with clinicopathologic parameters and VEGF-family gene expression. Mod. Pathol., 18, 143–152 [DOI] [PubMed] [Google Scholar]
- 50. Heerwagen M.J., et al. (2010). Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am. J. Physiol. Regul. Integr. Comp. Physiol., 299, R711–R722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McKay J.A., et al. (2011). Folate depletion during pregnancy and lactation reduces genomic DNA methylation in murine adult offspring. Genes Nutr., 6, 189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reuter S., et al. (2011). Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr., 6, 93–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang L.S., et al. (2011). Modulation of genetic and epigenetic biomarkers of colorectal cancer in humans by black raspberries: a phase I pilot study. Clin. Cancer Res., 17, 598–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Phuong N.T., et al. (2011). Role of PTEN promoter methylation in tamoxifen-resistant breast cancer cells. Breast Cancer Res. Treat., 130, 73–83 [DOI] [PubMed] [Google Scholar]
- 55. Zheng S., et al. (2011). DNA hypermethylation profiles associated with glioma subtypes and EZH2 and IGFBP2 mRNA expression. Neuro-Oncology, 13, 280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu D.C., et al. (2011). Overexpression of EZH2 and loss of expression of PTEN is associated with invasion, metastasis, and poor progression of gallbladder adenocarcinoma. Pathol. Res. Pract., 207, 472–478 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.