Abstract
Background
Although much evidence supports the hypothesis that cognitive function and physical function are interrelated, it is unclear whether cognitive decline with mild cognitive impairment influences trainability of physical performance in exercise intervention. The purpose of this study was to examine the association between cognitive function at baseline and change in physical performance after exercise intervention in older adults with mild cognitive impairment.
Methods
Forty-four older adults diagnosed with mild cognitive impairment based on the Peterson criteria (mean age 74.8 years) consented to and completed a 6-month twice weekly exercise intervention. The Timed Up and Go (TUG) test was used as a measure of physical performance. The Mini-Mental State Examination (MMSE), Trail Making Test Part B, Geriatric Depression Scale, baseline muscle strength of knee extension, and attendance rate of intervention, were measured as factors for predicting trainability.
Results
In the correlation analysis, the change in TUG showed modest correlations with attendance rate in the exercise program (r = −0.354, P = 0.027) and MMSE at baseline (r = −0.321, P = 0.034). A multiple regression analysis revealed that change in TUG was independently associated with attendance rate (β = −0.322, P = 0.026) and MMSE score (β = −0.295, P = 0.041), controlling for age and gender.
Conclusion
General cognitive function was associated with improvements in physical performance after exercise intervention in subjects with mild cognitive impairment. Further research is needed to examine the effects of exercise programs designed to address cognitive obstacles in older adults with mild cognitive impairment.
Keywords: exercise, mobility, rehabilitation, Timed Up and Go test
Introduction
Mild cognitive impairment is widely regarded as a transitional syndrome between normal cognitive ageing and clinical dementia.1 Deterioration in episodic learning and memory functions constitute the core characteristics of mild cognitive impairment and Alzheimer’s disease. Older adults with mild cognitive impairment demonstrate decreased physical performance, which in turn is related to the risk of Alzheimer’s disease.2 Reduced physical function leads to restricted life space mobility,3 which is associated with increased risk of Alzheimer’s disease and cognitive decline among older persons.4 Improved usual gait speed over a 12-month period predicts a substantial reduction in mortality.5 Maintaining and improving physical function may be beneficial for preventing conversion to Alzheimer’s disease in older adults with mild cognitive impairment.
A better understanding of the modifiable factors independently associated with improved physical performance is needed.6 This would ensure effective intervention based on a valid theoretical framework for increasing physical performance. Possible modifiable factors include cognitive function, mental status, and physiological function, such as muscular strength. Logsdon et al7 found that older adults with mild cognitive impairment may benefit significantly from an exercise program specifically designed to address their cognitive needs (ie, memory aids and easy to follow instructions). They suggest that cognitive impairment may prevent successful engagement in exercise. Several studies support the hypothesis that cognitive and physical function are interrelated,2,8 although it is not clear whether older adults with mild cognitive impairment have decreased trainability for improving physical performance.
The purpose of this study was to examine the association between cognitive function at baseline and trainability of physical performance using a 6-month exercise intervention in older adults with mild cognitive impairment. We used the Timed Up and Go test (TUG) as an assessment of physical performance.9 This test has frequently been used to assess lower extremity function, mobility, and risk of falls in older adults. Recent research has revealed that TUG is associated with executive function8 and periventricular leukoaraiosis10 in older adults with mild cognitive impairment. Changes in TUG before and after intervention were measured as the dependent variable. In the present study, we defined trainability as the ability to be trained and this was expressed by rate of improvement between before and after intervention.11 By means of correlation and regression analysis, we examined how cognitive function at baseline influenced change in TUG. Other factors including depression, attendance rate, and physiological function (such as muscle strength) were also investigated. Similar to the relationship between physical and cognitive function,2,8 it is also possible that improvements in physical performance are associated with cognitive function, where cognitive impairment may prevent successful engagement in exercise.
We hypothesized that reduced cognitive functioning affects trainability of physical performance by exercise intervention in older adults with mild cognitive impairment. This investigation is critical for the exploration of the modifiable factors associated with trainability in order to plan future rehabilitation programs that prevent deterioration of physical and cognitive function.
Materials and methods
Subjects
The participants were recruited from two volunteer databases (n = 1543), which included elderly participants aged 65 years and over who either attended a health check in Obu, Japan, or were selected by stratified random sampling. The strata used in our stratified random sampling were age and gender. Criteria for inclusion in this intervention study required that participants were 65 years or older, living independently in the community (ie, no impairment of activities of daily living), and Japanese-speaking, with sufficient hearing and visual acuity to participate in the examinations. In the first eligibility assessment for this study, 528 potential participants who had either a Clinical Dementia Rating of 0.5 or subjective memory complaints were screened. One hundred and thirty-five participants met the criteria for the second eligibility assessment. They also needed to meet the Peterson criteria for a diagnosis of mild cognitive impairment.1 The final sample consisted of 44 older adults (mean age 74.8 years; 20 males; mean years of education, 11.1 years). These participants were exposed to an exercise intervention and they completed a randomized controlled trial that aimed to examine the effect of multicomponent exercise on cognitive function. The design and the primary results of the study have been reported elsewhere.12
Exclusion criteria included a history of major psychiatric illness (eg, schizophrenia or bipolar disorder) and other serious neurological or musculoskeletal diagnoses. This study was approved by the ethics committee of National Center for Geriatrics and Gerontology, Japan. Appropriate written informed consent was obtained for all participants.
Procedures
The 6-month exercise program involved biweekly 90-minute sessions with a combination of aerobic exercise, muscle strength training, and postural balance retraining. In addition, the exercise program focused on promoting exercise and behavior change. Two trained physiotherapists involved in geriatric rehabilitation conducted the interventions. Each supervised session began with a 10-minute warmup period and stretching exercise, followed by 20 minutes of muscle strength exercise. The participants then practiced aerobic exercise, postural balance retraining, and a combination of both activities over a period of 60 minutes.
Before and after each session of the program, physiotherapists conducted a health check of each participant. The physiotherapists and a well trained instructor implemented risk management for adverse events during the program. In the aerobic exercise, participants performed stair stepping and endurance walking. The intensity of the aerobic exercise was prescribed at approximately 40% of maximum heart rate during weeks 1–8. The intensity was then increased to 60% of maximum heart rate from week 9. Heart rate was measured before and after exercise at every training session for risk management and estimation of the strength of training. The strength of aerobic exercise was adjusted by the height of the stair in stair stepping, walking speed, or weights put on participants’ feet in endurance walking. Eleven of the 40 classes during the 6-month intervention period included approximately 20–30 minutes of consecutive outdoor walking. Muscle strength training was mainly performed using subjects’ own weight; training equipment was not used (eg, knee extension, calf raise, squat). Postural balance exercise (such as tandem walking and side walking on balance boards) was also included in the program. There were five different widths of balance boards (4, 6, 8, 10 and 12 cm), which were narrowed progressively. Further, participants performed a combination of exercises (eg, circuit training including stair-stepping, endurance walking, and walking on balance boards). They also performed concurrent cognitive tasks during exercise (eg, walking while inventing a poem) because effects of dual tasks on brain activation have been reported.13 Between individual training sessions, participants were invited to sit and rest for about 5 minutes. Participants were required to perform daily home-based muscle strength exercises and walking. These were self-monitored using a booklet and pedometer based on the concept of promoting exercise and behavior change.
Physical performance test to assess trainability
The TUG was used to assess physical performance.9 The TUG involves rising from a chair, walking 3 meters, turning around, walking back to the chair, and sitting down. Participants were instructed to complete the task at their usual walking pace. The score for this test represents the time (in seconds) that the participant needed to complete the assessment. Lower times indicate better physical performance. The recorded TUG score was the lesser of the times measured in the two trials in order to exclude immediate change by learning effects from the measured value.14 Licensed and well-trained physical therapists assessed the physical performance tests. Interrater reliability of TUG is very high in community-dwelling older adults (intraclass coefficient of 0.98).15 Changes in TUG were calculated as trainability using the following formula:
| 11 |
Thus, a higher negative value represents greater improvement by exercise intervention.
Potential correlates
All measurements were performed before the intervention commenced. Demographic data were recorded, including age, gender, number of medications, and educational history. Depressive symptoms were measured using the 15-item Geriatric Depression Scale (GDS).16
All neuropsychological testing was conducted by well trained speech therapists. Each score was rechecked by one therapist who was blinded to all other participant data. General cognitive function was assessed with the Japanese version of 30-item Mini-Mental State Examination (MMSE),17 the most frequently used cognitive screening measure in cognitive aging research, with good test-retest reliability (intraclass coefficient = 0.827).18,19 Executive function was assessed using the Trail Making Test Part B (TMT-B).20 For this task, participants were required to navigate a series of alternating numbers and letters, and connect them in alternating sequential order. In the Japanese version of the TMT-B, letters from the Roman alphabet are exchanged for Kana characters.21 The time required to complete each task was recorded, where a higher time indicates a poorer performance. The TMT-B has demonstrated adequate test-retest reliability for use in longitudinal studies.21
The participants’ muscle strength of knee extension was measured twice using a dynamometer (MDKKS, Molten Co, Ltd, Tokyo, Japan). The recorded strength score was the higher of the strength measurements (Nm/kg) in the two trials.22
Statistical analysis
The data were analyzed using the Statistical Package for Social Sciences version 19.0 for Windows (SPSS Inc, Chicago, IL). A probability level of P < 0.05 was considered to be statistically significant. Data were expressed as mean values and standard deviations. TUG scores were compared before and after intervention using the paired Student’s t-test. The relationships between change in TUG score and potential correlates (measurements at baseline and attendance rate for exercise intervention) were investigated using Pearson’s correlation. A stepwise multivariate linear regression model was used to examine whether potential predictors were independently associated with the change in TUG. Age, gender, and other variables that were significantly correlated with change in TUG were entered as independent variables.
Results
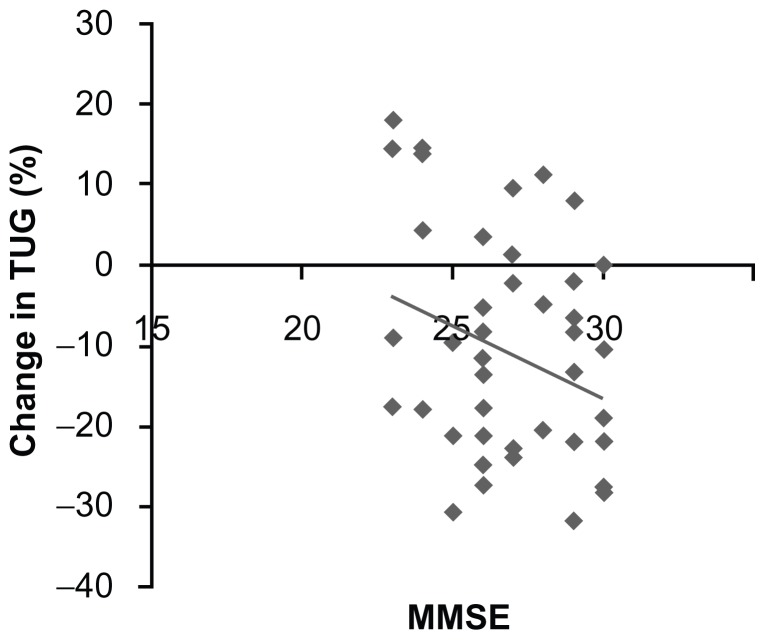
Table 1 shows the demographic and baseline clinical characteristics and attendance rate of the study participants. Mean adherence to the exercise program was 88.2%. TUG showed significant (P < 0.001) improvement between before and after intervention (8.6 ± 1.9 versus 7.6 ± 2.1 seconds, respectively). In the correlation analyses (Table 2), the change in TUG had significant negative correlations with attendance rate (r = −0.354, P = 0.027) and MMSE (r = −0.321, P = 0.034; Figure 1). The change in TUG was not correlated with age (r = 0.146, P = 0.35), TMT-B (r = 0.202, P = 0.19), GDS (r = 0.03, P = 0.85), or muscle strength of knee extension (r = −0.236, P = 0.12).
Table 1.
Demographic and clinical characteristics of study participants
| Age (years) | 74.8 ± 7.3 (65–93) |
| Education (years) | 11.1 ± 2.9 |
| Gender (males) | 20 (48) |
| Medications (n) | 2.6 ± 2.3 |
| MMSE (points) | 26.9 ± 2.3 |
| GDS (points) | 3.7 ± 2.9 |
| TMT-B (seconds) | 174.9 ± 75.3 |
| Muscle strength of knee extension (Nm/kg) | 1.19 ± 0.4 |
| TUG (seconds) | 8.6 ± 1.9 |
| Attendance rate (%) | 88.2 ± 19.2 |
Note: values presented are the mean ± standard deviation or n (%).
Abbreviations: MMSE, Mini-Mental State Examination; GDS, Geriatric Depression Scale; TMT-B, Trail Making Test Part B; TUG, Timed Up and Go test.
Table 2.
Correlation coefficients for potential correlates and change in TUG
| Potential correlates | Change ratio in TUG Correlation coefficient | |
|---|---|---|
|
|
||
| R | P value | |
| Age | 0.146 | 0.35 |
| MMSE | −0.321 | 0.034 |
| GDS | 0.03 | 0.85 |
| TMT-B | 0.202 | 0.19 |
| Muscle strength of knee extension | −0.236 | 0.12 |
| Attendance rate | −0.354 | 0.027 |
Abbreviations: MMSE, Mini-Mental State Examination; TUG, Timed Up and Go test; GDS, Geriatric Depression Scale; TMT-B, Trail Making Test Part B.
Figure 1.
Scatter graph showing the relationship between change in Timed Up and Go test and Mini-Mental State Examination scores.
Abbreviations: MMSE, Mini-Mental State Examination; TUG, Timed Up and Go test.
Table 3 shows the factors that were significantly related to change in TUG in the stepwise multiple regression. The regression model explained 24% of the change in variance of TUG. The factors retained in the final model were attendance rate (β = −0.322, P = 0.026) and MMSE (β = −0.295, P = 0.041). Age and gender did not show a significant relationship with change in TUG.
Table 3.
Factors associated with improved TUG in stepwise multiple regression
| Factors | β | P value | R2 | |
|---|---|---|---|---|
| Change ratio in TUG | Attendance rate | −0.322 | 0.026 | 0.24 |
| MMSE | −0.295 | 0.041 |
Abbreviations: TUG, Timed Up and Go test; MMSE, Mini-Mental State Examination.
Discussion
Exercise intervention was considered beneficial for older adults with mild cognitive impairment in the present study, and we showed a significant improvement in physical performance between before and after intervention. However, investigation of the association between physical performance and other variables revealed that improvement in physical performance after exercise intervention depended on general cognitive function, as assessed by the MMSE, in addition to attendance rate. Generally, it has been reported that exercise intervention in older adults improves physical performance, such as gait speed,23 dynamic balance,24 and cardiovascular fitness.25 Previous studies targeting older adults with Alzheimer’s disease or dementia have reported larger improvements in TUG (approximately 41%)26–28 than those reported in the present study (8.6%); however, we targeted relatively healthy older adults with better physical function at baseline. This is the first study to focus on baseline cognitive function and the trainability of physical performance in older adults with mild cognitive impairment.
The results of the present study indicate that general cognitive function may be important for persons with mild cognitive impairment to gain benefit from an exercise intervention. Previous studies support the relationship between cognitive and physical function.2,8 Therefore, general cognitive function may provide an important basis for improvement in physical performance, particularly in older adults with mild cognitive impairment. It is possible that persons with mild cognitive impairment may experience several cognitive obstacles, such as difficulties in learning new exercises and remembering how to perform them correctly, even if they have previously participated in the same programs with similar adherence.
Our findings suggest that it is important for therapists and health care practitioners to assess general cognitive function in order to estimate training ability at baseline and adjust their approach to exercise. Logsdon et al7 reported that older adults with mild cognitive impairment show significant benefit from an exercise program specifically designed to address their cognitive needs. The findings of the present study confirm that physical benefits vary based on the degree of general cognitive functioning. This indicates the necessity for older adults with mild cognitive impairment to engage in an exercise program specifically designed to address cognitive needs (ie, one that provides memory aids and easy to follow instructions). In addition, individually prescribed exercise is a more effective intervention strategy for the improvement of balance, gait performance, and reduction in risk of falling than group exercise in older adults.29
When an exercise program is prescribed for older adults in clinical practice, individual physical function is usually considered an important factor, by which strength and contents of exercise are regulated by therapists or practitioners. Our findings suggest that it is also important for therapists or practitioners to assess general cognitive function in order to estimate training ability at baseline and adjust their approach to exercise. Exercise programs designed to address cognitive obstacles should be developed and prescribed according to the level of cognitive functioning in older adults with mild cognitive impairment. For example, we recommend that complex exercise behaviors are broken into small steps and practiced repeatedly in class, and simple written materials are provided along with memory aids to support exercise outside of class.7 These programs, tailor-made for level of cognitive function, would be effective and efficient for improving physical function in older adults.
Improvement in some aspects of cognitive function has been related to adherence to an exercise program in older adults with mild cognitive impairment.30 This finding is in line with the present study which shows that attendance rate was associated with improvement in physical performance. Williams et al31 reported that reduced muscle strength, slow reaction time, and psychoactive drug use explained most of the variance in adherence during an exercise trial. For interventions to be effective it may be important to maintain attendance rates by addressing associated factors.
Several limitations in this study need to be mentioned, such as the small sample size and lack of a control group. Additionally, there may be other compounding factors, such as motivation and self-efficacy, which should be included in future analysis. Only 24% of the change in TUG variance could be explained in the present study. Future research should include a broader range of cognitive impairment levels, more detailed neuropsychological testing, and more extensive physical assessments, particularly those that may have more or less of a cognitive demand than TUG. These areas should be addressed to evaluate the association between various cognitive functions and trainability of physical performance in detail and to determine the cutoff points for predicting improvement in physical performance. Further research is needed to clarify whether specific exercise programs designed to address cognitive obstacles could improve physical function effectively and lead to a potentially preventive effect for conversion to Alzheimer’s disease. Our findings concerning the relationship between trainability and cognitive function are specific to older adults with mild cognitive impairment, and cannot be applied to persons with severe dementia.
In summary, despite some limitations, the present study examined the association between cognitive function at baseline and the effect of intervention on physical performance in older adults with mild cognitive impairment. Our results reveal that MMSE and attendance rate were independently associated with improvement in physical performance. A major implication of this study is that general cognitive function may be important for persons with mild cognitive impairment to gain benefit from an exercise intervention.
Acknowledgments
We would like to thank the Obu City Office for assisting with recruitment of participants, and the speech therapists of the Ukai Rehabilitation Hospital for their assistance with data collection. This work was supported by a grant from the Japanese Ministry of Health, Labour, and Welfare (programs minimizing long-term care B-3, to TS).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62(7):1160–1163. doi: 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Arch Neurol. 2006;63(12):1763–1769. doi: 10.1001/archneur.63.12.1763. [DOI] [PubMed] [Google Scholar]
- 3.Wood KM, Edwards JD, Clay OJ, Wadley VG, Roenker DL, Ball KK. Sensory and cognitive factors influencing functional ability in older adults. Gerontology. 2005;51(2):131–141. doi: 10.1159/000082199. [DOI] [PubMed] [Google Scholar]
- 4.James BD, Boyle PA, Buchman AS, Barnes LL, Bennett DA. Life space and risk of Alzheimer disease, mild cognitive impairment, and cognitive decline in old age. Am J Geriatr Psychiatry. 2011;19(11):961–969. doi: 10.1097/JGP.0b013e318211c219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55(11):1727–1734. doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu-Ambrose T, Davis JC, Nagamatsu LS, Hsu CL, Katarynych LA, Khan KM. Changes in executive functions and self-efficacy are independently associated with improved usual gait speed in older women. BMC Geriatr. 2010;10:25. doi: 10.1186/1471-2318-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logsdon RG, McCurry SM, Pike KC, Teri L. Making physical activity accessible to older adults with memory loss: a feasibility study. Gerontologist. 2009;49( Suppl 1):S94–S99. doi: 10.1093/geront/gnp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGough EL, Kelly VE, Logsdon RG, et al. Associations between physical performance and executive function in older adults with mild cognitive impairment: gait speed and the timed “Up and Go” Test. Phys Ther. 2011;91(8):1198–1207. doi: 10.2522/ptj.20100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Podsiadlo D, Richardson S. The timed “Up and Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 10.Onen F, Henry-Feugeas MC, Roy C, Baron G, Ravaud P. Mobility decline of unknown origin in mild cognitive impairment: an MRI-based clinical study of the pathogenesis. Brain Res. 2008;1222:79–86. doi: 10.1016/j.brainres.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka H. Trainability in elderly. Nihon Ronen Igakkai Zasshi. 2005;42(5):526–528. doi: 10.3143/geriatrics.42.526. Japanese. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki T, Shimada H, Makizako H, et al. Effects of multicomponent exercise on cognitive function in WMS-LM older adults with amnestic mild cognitive impairment: a randomized controlled trial. BMC Neurol. 2012;12(1):128. doi: 10.1186/1471-2377-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci. 2011;66(8):879–887. doi: 10.1093/gerona/glr068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uemura K, Shimada H, Makizako H, et al. Factors associated with life-space in older adults with amnestic mild cognitive impairment. Geriatr Gerontol Int. 2013;13(1):161–166. doi: 10.1111/j.1447-0594.2012.00878.x. [DOI] [PubMed] [Google Scholar]
- 15.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up and Go Test. Phys Ther. 2000;80(9):896–903. [PubMed] [Google Scholar]
- 16.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24(4):709–711. [PubMed] [Google Scholar]
- 17.Ideno Y, Takayama M, Hayashi K, Takagi H, Sugai Y. Evaluation of a Japanese version of the Mini-Mental State Examination in elderly persons. Geriatr Gerontol Int. 2012;12(2):310–316. doi: 10.1111/j.1447-0594.2011.00772.x. [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Uhlmann RF, Larson EB. Effect of education on the Mini-Mental State Examination as a screening test for dementia. J Am Geriatr Soc. 1991;39(9):876–880. doi: 10.1111/j.1532-5415.1991.tb04454.x. [DOI] [PubMed] [Google Scholar]
- 20.Kortte KB, Horner MD, Windham WK. The Trail Making Test, Part B: cognitive flexibility or ability to maintain set? Appl Neuropsychol. 2002;9(2):106–109. doi: 10.1207/S15324826AN0902_5. [DOI] [PubMed] [Google Scholar]
- 21.Abe M, Suzuki K, Okada K, et al. Normative data on tests for frontal lobe functions: Trail Making Test, Verbal fluency, Wisconsin Card Sorting Test (Keio version) No To Shinkei. 2004;56(7):567–574. Japanese. [PubMed] [Google Scholar]
- 22.Makizako H, Shimada H, Doi T, et al. The association between decline in physical functioning and atrophy of medial temporal areas in community-dwelling older adults with amnestic and nonamnestic mild cognitive impairment. Arch Phys Med Rehabil. 2011;92(12):1992–1999. doi: 10.1016/j.apmr.2011.07.195. [DOI] [PubMed] [Google Scholar]
- 23.Holviala JH, Sallinen JM, Kraemer WJ, Alen MJ, Hakkinen KK. Effects of strength training on muscle strength characteristics, functional capabilities, and balance in middle-aged and older women. J Strength Cond Res. 2006;20(2):336–344. doi: 10.1519/R-17885.1. [DOI] [PubMed] [Google Scholar]
- 24.Ogaya S, Ikezoe T, Soda N, Ichihashi N. Effects of balance training using wobble boards in the elderly. J Strength Cond Res. 2011;25(9):2616–2622. doi: 10.1519/JSC.0b013e31820019cf. [DOI] [PubMed] [Google Scholar]
- 25.Hallage T, Krause MP, Haile L, et al. The effects of 12 weeks of step aerobics training on functional fitness of elderly women. J Strength Cond Res. 2010;24(8):2261–2266. doi: 10.1519/JSC.0b013e3181ddacc6. [DOI] [PubMed] [Google Scholar]
- 26.Potter R, Ellard D, Rees K, Thorogood M. A systematic review of the effects of physical activity on physical functioning, quality of life and depression in older people with dementia. Int J Geriatr Psychiatry. 2011;26(10):1000–1011. doi: 10.1002/gps.2641. [DOI] [PubMed] [Google Scholar]
- 27.Netz Y, Axelrad S, Argov E. Group physical activity for demented older adults feasibility and effectiveness. Clin Rehabil. 2007;21(11):977–986. doi: 10.1177/0269215507078318. [DOI] [PubMed] [Google Scholar]
- 28.Baum EE, Jarjoura D, Polen AE, Faur D, Rutecki G. Effectiveness of a group exercise program in a long-term care facility: a randomized pilot trial. J Am Med Dir Assoc. 2003;4(2):74–80. doi: 10.1097/01.JAM.0000053513.24044.6C. [DOI] [PubMed] [Google Scholar]
- 29.Rose DJ. Reducing the risk of falls among older adults: the Fallproof Balance and Mobility Program. Curr Sports Med Rep. 2011;10(3):151–156. doi: 10.1249/JSR.0b013e31821b1984. [DOI] [PubMed] [Google Scholar]
- 30.Tak EC, van Uffelen JG, Paw MJ, van Mechelen W, Hopman-Rock M. Adherence to exercise programs and determinants of maintenance in older adults with mild cognitive impairment. J Aging Phys Act. 2012;20(1):32–46. doi: 10.1123/japa.20.1.32. [DOI] [PubMed] [Google Scholar]
- 31.Williams P, Lord SR. Predictors of adherence to a structured exercise program for older women. Psychol Aging. 1995;10(4):617–624. doi: 10.1037//0882-7974.10.4.617. [DOI] [PubMed] [Google Scholar]