Abstract
Background
The purpose of this study was to develop, characterize, and investigate a molecular inclusion complex containing rifaldazine with good solubility and antibacterial activity.
Methods
Rifaldazine, a lipophilic molecule, was encapsulated into the hydrophobic cavity of β-cyclodextrin to form a molecular inclusion complex (RAABCD) with good solubility. RAABCD was prepared in a short time using a solid-state grinding method. The inclusion ratio, binding constant, and change in Gibbs free energy were determined by a phase solubility diagram and/or ultraviolet-visible spectroscopy. Differential scanning calorimetry and Fourier transform infrared spectroscopy of RAABCD were performed. Morphological features of RAABCD were observed by photomicroscopy. The most likely optimal configuration for RAABCD was simulated by computer modeling. Broth macrodilution testing was done to investigate the antibacterial activity of RAABCD.
Results
The inclusion ratio, binding constant, and change in Gibbs free energy, determined by a phase solubility diagram and/or ultraviolet-visible spectroscopy were 1:1, 288.33/261.33 L/mol, and 32.29/31.73 kJ/mol, respectively. Differential scanning calorimetry and Fourier transformed infrared spectra of RAABCD confirmed the molecular interaction between rifaldazine and β-cyclodextrin. The morphological difference between irregular and amorphous-shaped RAABCD and columnar-shaped rifaldazine further confirmed the molecular encapsulation of rifaldazine. The most likely optimal configuration for RAABCD was confirmed by computer modeling. Broth macrodilution testing indicated that RAABCD had good antibacterial activity.
Conclusion
RAABCD had improved solubility and good activity, and might be a promising alternative for treatment of a range of bacterial infections.
Keywords: rifaldazine, cyclodextrin inclusion complex, stoichiometric relationships, differential scanning calorimetry, Fourier transform infrared spectra, computer modeling
Introduction
Rifaldazine, one of the most clinically effective antibiotics, is a semisynthetic product (Figure 1A) derived from Amycolatopsis rifamycinica. Being an inhibitor of bacterial DNA-dependent RNA polymerase, rifaldazine inhibits bacterial RNA synthesis, retards bacterial growth, and kills bacteria. Rifaldazine has been shown to be effective against Mycobacterium infections (such as tuberculosis and Hansen’s disease), other bacterial infections (such as methicillin-resistant Staphylococcus aureus, osteomyelitis, and prosthetic joint infections), and vaccinia virus.1,2 Rifaldazine is also used as a prophylactic agent for meningococcal infection.3 However, taking tuberculosis treatment as an example, being an antibiotic with poor solubility and low stability, rifaldazine must be administered at a high daily dose continuously for several months, with adverse effects such as hepatitis, breathlessness, flushing, nausea, and headache.4,5 Therefore, attempts have been made in recent decades to develop alternative delivery systems for rifaldazine, including liposomes6 and microspheres7 for injection, cyclodextrin complexes,8 chitosomes,9 and liposomes10 for lung nebulization. Disadvantages of these rifaldazine delivery systems include inconvenient administration, a complex manufacturing process, and uncertain safety.
Figure 1.
Chemical structure of (A) rifaldazine and (B) β-cyclodextrin.
A molecular inclusion complex refers to a complex compound or an unbonded association in which a guest molecule (usually a low molecular weight lipophilic drug) is contained wholly or partially within the lipophilic interior cavity of a host molecule (frequently a cyclodextrin). β-cyclodextrin (Figure 1B) is a cyclic oligosaccharide composed of seven glucopyranose units joined through α-1,4-glucosidic bonds, and is typically considered to be a truncated cone-shaped molecule with a hydrophilic external surface.11 Being a nontoxic and cost-effective pharmaceutical excipient with favorable features, β-cyclodextrin is recorded in several pharmacopoeias including the United States Pharmacopoeia, British Pharmacopoeia, and Chinese Pharmacopoeia. The safety and cost-effectiveness of β-cyclodextrin (its price is much lower than for chemically modified β-cyclodextrin) are well known. The inclusion complexation of β-cyclodextrin molecular technology has been successfully utilized to increase the solubility and/or stability of some insoluble and/or labile drugs.12 The pharmaceutical industry has already marketed a few β-cyclodextrin molecular inclusion complexes, including Cicladol® (containing piroxicam, a nonsteroidal anti-inflammatory drug) by Masterpharma (Chiesi, Italy), Ulgut® (containing benexate, an anti-ulcer drug) by Teikoku (San Jose, CA), and Surgamyl® (containing tiaprofenic acid, an analgesic) by Roussel-Maestrelli (Italy).13 Although it is currently unavailable for clinical use, β-CD molecular inclusion complexes of RAA (RAABCD) have already aroused people’s interest. It was documented in a United States patent that the RAABCD with higher solubility (twice that of free rifaldazine) could be prepared in spite of the fact that it must take a very long time (5–8 hours) to complete the preparation process.14
In addition to the above-mentioned considerations, we chose to investigate β-cyclodextrin over its derivatives for the following reasons. Although a lot of modified β-cyclodextrins were available on the market, none except hydroxypropyl-β-cyclodextrin (HCD) had been recorded in a pharmacopoeia, and all modified β-cyclodextrins (including HCD) were more expensive than the parent compound. Further, the safety of the modified β-cyclodextrins, except for HCD, was either inferior to that of β-cyclodextrin or needed further investigation of safety in oral use. HCD has been mainly used to prepare various injectable formulations so far. The reason why HCD is seldom used to prepare the oral formulation is partly due to the high price of HCD. In addition, although HCD had better aqueous solubility, it was not expected to have better solubilization effects than the parent compound, β-cyclodextrin. Also, earlier researchers15 had found that the solubilization effects of HCD on diazepam, digoxin, indomethacin, and hydroprednisone were much lower than that of β-cyclodextrin. In our preliminary study, we found that the solubility of RAAHCD and RAABCD was 2.5 and 4.4 times that of free rifaldazine, respectively (RAAHCD data not shown). Based on the above information, RAABCD were the most feasible and therefore more likely to be produced and marketed by the pharmaceutical industry and used in future clinical practice.
In this study, RAABCD with a solubility 4.4 times that of free rifaldazine was prepared in just 30 minutes using a solid-state grinding method. RAABCD was characterized by differential scanning calorimetry and Fourier transform infrared (FTIR) spectroscopy. The physical properties and antibacterial activity of RAABCD, including its complex stoichiometry (inclusion ratio, binding constant, and change in Gibbs free energy), microscopic morphology, configuration simulation, interaction energy, and minimum inhibitory concentration were investigated. Our findings indicate that RAABCD enhanced the physical properties of rifaldazine and had good antibacterial activity.
Materials and methods
Materials
Rifaldazine (purity ≥ 97.0%) was purchased from Shuainuo Medicine Supplement Co, Ltd (Shanxi, People’s Republic of China). ß-cyclodextrin (purity ≥ 98.0%) was obtained from Xinxin Pharmaceutical Excipients Co, Ltd (Taixing, People’s Republic of China). All other chemicals and solvents were of analytical grade.
Phase solubility study
An excess amount of rifaldazine was added to ß-cyclodextrin solutions of different concentrations (0-15.18 mmol/L). The resulting suspensions were sonicated for 5 minutes and shaken (100 rpm) for 5 hours in a water bath at 25°C ± 0.5°C until solubility equilibrium was established. Next, the suspensions were filtered through Millipore membrane filters (0.45 μm pore size). The filtrate was analyzed at 474 nm using an ultraviolet-visible spectrophotometer (UV-2501PC, Shimadzu, Kyoto, Japan). The binding constant (Kb) was calculated from a phase solubility diagram with the assumption of 1:1 stoichiometry using the following equation:1617
| (1) |
where Sp is the slope of the phase solubility curve ( Figure 2A) and So is the intrinsic solubility of rifaldazine.
Figure 2.
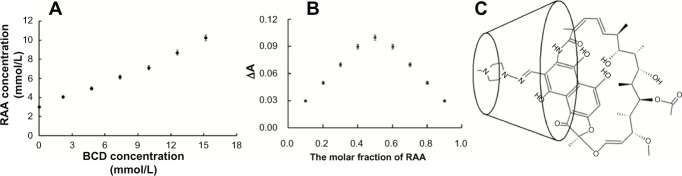
Phase solubility diagram (A) of RAABCD. Continuous variation plot (B) of RAABCD. The data represent the mean ± standard deviation (n = 6). The proposed structure model (C) of RAABCD with 1:1 stoichiometry.
Abbreviation: RAABCD, ß-cyclodextrin molecular inclusion complexes of rifaldazine.
The change in Gibbs free energy (ΔG0) on transfer of rifaldazine from aqueous solution to the cavity of ß-cyclodextrin was calculated using the following equation:18
| (2) |
where Soand Ss are the solubility of rifaldazine in the absence and presence of ß-cyclodextrin, respectively.
Determination of inclusion ratio using a continuous variation method
Various volumes of equimolar concentrations of rifaldazine and aqueous ß-cyclodextrin solution were mixed such that the total molar concentrations of rifaldazine and ß-cyclodextrin were kept constant and the molar fraction of rifaldazine (the numerator was the molar value of rifaldazine, the denominator was the sum of molar value of ß-CD and molar value of rifaldazine) varied from 0.1 to 0.9.19 Absorbance values for the mixed solutions (A1) and the corresponding concentrations of free rifaldazine solution ( A2) were measured separately at 474 nm. The difference in absorbance (ΔA = A2 - A1) was then plotted against the molar fraction. The molar fraction corresponding to the maximum difference in absorbance was considered to be the optimal inclusion ratio.
Determination of binding constant by ultraviolet-visible spectroscopy
Various quantities of rifaldazine and ß-cyclodextrin were simultaneously dissolved in distilled water to obtain a series of solutions containing the same concentration of rifaldazine (2.43 × 10−5 mol/L) and different concentrations of ß-cyclodextrin (0-1.52 × 10−2 mol/L). These solutions were then analyzed by ultraviolet-visible spectrophotometry, and the absorbance values (Ai) were recorded individually. Each experiment was done in triplicate. When the complex stoichiometry was 1:1, the apparent binding constant Kb could be calculated using the following equation:
| (3) |
If 1/ΔAi was plotted against 1/[ß—CD]i, Kb could be calculated from the linear regression intercept and slope according to the following equation:
| (4) |
where ΔA is the difference in absorbance (ΔAi = A0 - Ai) of the rifaldazine solution in the absence (A0) and presence (Ai) of ß-cyclodextrin, Δε is the difference between the molar absorption coefficient (Δε= ε0 − εRAA) of the rifaldazine solution in the absence (ε0) and presence (εi) of ß-cyclodextrin, and [ß-cyclodextrin]i and [RAA] are the concentrations of ß-cyclodextrin and RAA, respectively20
Preparation of RAABCD
RAABCD was prepared using a solid-state grinding method. Equimolar amounts of rifaldazine and ß-cyclodextrin were triturated separately by mortar and pestle for 3 minutes, and then mixed followed by grinding for 30 minutes at 25°C.
Differential scanning calorimetry
The samples (rifaldazine, ß-cyclodextrin, RAABCD, physical mixture) were analyzed using a differential scanning calorimeter (STA 449C, Netzsch, Selb, Germany). The samples were sealed in an aluminum crimp cell and heated at a rate of 10°C per minute from 30°C to 350°C under nitrogen gas.21
FTIR spectroscopy
The samples (rifaldazine, ß-cyclodextrin, RAABCD, physical mixture) were mixed with KBr powder and analyzed using an FTIR spectrometer (Nicolet 380, Thermo Electron Scientific Instruments, Madison, WI) at a resolution of 4 cm-1.22
Solubility study
An excess amount of RAABCD was added to 50 mL of distilled water and stirred for 60 minutes at 25°C until equilibrium was reached. The resulting suspension was filtered through a Millipore membrane filter (0.45 μm pore size), and the filtrate was investigated at 474 nm with an ultraviolet-visible spectrometer (UV-5130, Shimadzu).23
Morphological features
The surface morphology of the sample powders (rifaldazine, ß-cyclodextrin, RAABCD, physical mixture) was observed using a biological microscope (XSP-35-1600X, Phoenix, Shangrao, People’s Republic of China) and images were taken with a camera (C-60 Zoom, Olympus, Hong Kong, People’s Republic of China).
Computer modeling
The chemical structures of rifaldazine and ß-cyclodextrin were simulated using a CAChe 6.18 Worksystem Pro version 5.0 (Fujitsu Limited Tokyo, Japan). The minimum energies of rifaldazine and ß-cyclodextrin before and after inclusion were estimated separately, and the energy change (ie, binding energy) was then calculated to compute the most likely optimal configuration of RAABCD.
Antibacterial activity
The broth macrodilution method was used to test the antibiotic susceptibility of S. aureus ATCC25 923 and Escherichia coli ATCC25 922 according to standard procedures used in the laboratory24 Briefy, RAABCD and free rifaldazine were separately dissolved in sterile distilled water and diluted with Mueller-Hinton culture medium to obtain a series of culture media containing different rifaldazine concentrations (0.125, 0.25, 0.5, 1, 2, 4, 8, 16, 32, 64, 128, and 256 μg/mL). These culture media were then inoculated with S. aureus and E. coli for 24 hours at 37°C, respectively. Pure Mueller-Hinton culture medium inoculated with the corresponding bacterium was used as the positive reference. The antibacterial activity was observed by visual inspection of the bacterial lawn. The minimal inhibitory concentration was the lowest drug concentration at which no visible growth of bacteria was observed.
Results and discussion
Stoichiometric relationships
The inclusion ratio and binding constant, two key characteristics of molecular inclusion complexes, are usually determined by the phase solubility diagram method. However, ultraviolet-visible spectroscopy is also suitable for determination of these parameters if the encapsulated drug has an appropriate ultraviolet-visible absorption spectrum. Firstly, a phase solubility study was carried out. The phase solubility curve (Figure 2A) indicated that the concentration of rifaldazine increased with increasing ß-cyclodextrin concentration in a linear fashion (C[RAA CONCENTRATION] = 0.4638 × C[β-CD Concentration] + 2.8789, r2 = 0.9917). The phase solubility diagram was characterized as being of AL type, suggesting formation of the RAABCD complex with a 1:1 molar ratio of rifaldazine to ß-cyclodextrin. The binding constant (Kb) can reflect the strength of the binding force between rifaldazine and ß-cyclodextrin molecules. The Kb value of RAABCD calculated according to equation (1) was 288.33 ± 2.79 L/mol (mean ± standard deviation, n = 3) >200 L/mol, further suggesting that rifaldazine could be complexed with ß-cyclodextrin and become a more soluble and stable complex.20 The spontaneity and feasibility of the molecular complexation of rifaldazine and ß-cyclodextrin were further evaluated using the thermodynamic approach. The change in Gibbs free energy (AG0) on transfer of rifaldazine from aqueous solution to the cavity of β-cyclodextrin calculated according to equation (2) was -32.29 kJ/mol. The negative value of the energy change indicated energetically favorable conditions for complexation. Secondly the inclusion ratio was obtained by the classic continuous variation method used to determine complex stoichiometry experimentally. As shown in Figure 2B, the molar fraction of rifaldazine corresponding to the maximum difference of absorbance was 0.5, indicating that the inclusion ratio was 1:1. This inclusion ratio was the same as that obtained in the phase solubility study. The above results, considered together with the differential scanning calorimetry and FTIR spectroscopy results described later, indicate that the rifaldazine molecule can enter and remain in the β-cyclodextrin cavity (Figure 2C). Thirdly, the binding constant (Kb) of RAABCD was determined by ultraviolet-visible spectroscopy. The Kb value calculated by equation (3) and (4) was 261.33 ± 2.79 L/mol (n = 3), so was a little lower than that of 288.33 L/mol obtained in the phase solubility study. The corresponding ΔG0 value calculated according to equation (2) was −31.73 kJ/mol.
Preparation and characterization of RAABCD
A drug can often be molecularly encapsulated by several methods, eg, coprecipitation, sonication, and kneading. Judgment of the suitability of a given preparation method is based on several considerations, including the properties of the host and guest molecules, the type and amount of solvents, and the encapsulation effect. When RAABCD was prepared using coprecipitation or sonication, encapsulation was not successful (data not shown), which was probably attributable to the fact that rifaldazine undergoes hydrolysis and oxidation when exposed to high temperature and/or ultrasound. Further, considering the need for viable industrial production and the instability of rifaldazine in water (oxidation and hydrolysis reactions frequently occur because of the high reactivity between hydroxyl groups and amide bonds), referring to previous work11 and based on preparatory experiments, a solid-state grinding method was chosen to prepare the RAABCD. The preparation time of 30 minutes in our study is significantly shorter than the 5–8 hours documented in the US patent application.14 The water solubility of RAABCD was 13.21 ± 0.13 mmol/L (n = 5), which was 4.4 times higher than that of free rifaldazine (3.00 ± 0.16 mmol/L, n = 5), ie, the solubilization effect of RAABCD obtained here was solubility of rifaldazine was increased by two-fold compared with the method cited in the US patent application. In addition, as far as the method for improving solubility is concerned, molecular inclusion complexation technology might be equally or more effective and much simpler and easier for production scale-up than other approaches, such as emulsification and phospholipid complexation.25,26
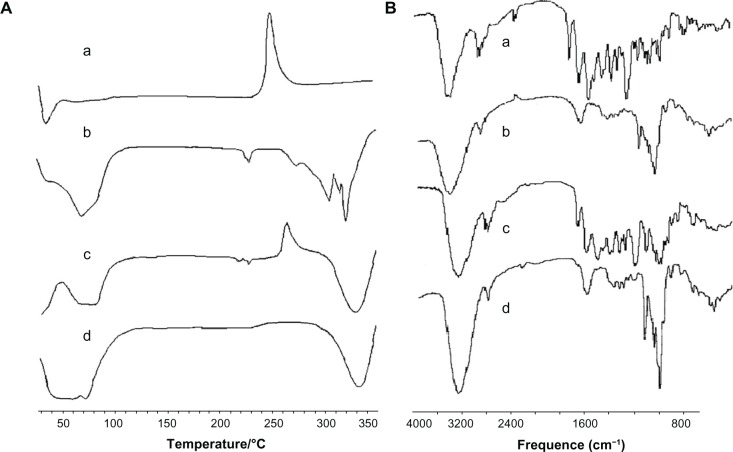
Formation of RAABCD was confirmed by differential scanning calorimetry and FTIR spectroscopy. As shown in Figure 3A, the differential scanning calorimetry thermogram for rifaldazine showed a sharp peak at 259°C, corresponding to the melting point of rifaldazine. Compared with free rifaldazine, the physical mixture of rifaldazine and β-cyclodextrin showed an intrinsic endothermal peak for rifaldazine at the same temperature with lower intensity, which might be partly due to the dilution of rifaldazine with β-cyclodextrin. However, in RAABCD, this characteristic peak for rifaldazine almost disappeared suggesting that the rifaldazine molecule might be mainly enclosed within the inner cavity of β-cyclodextrin. Similarly, the interaction between rifaldazine and β-cyclodextrin could be confirmed by the observed shifts in wave number of peaks in the FT-IR spectra. As shown in Figure 3B, the FTIR spectrum of rifaldazine showed peaks at 3476 cm-1, 2881 cm-1, 1726 cm-1, 1646 cm-1, and 1568 cm-1, corresponding to OH, N-CH3, acetyl C-O, furanone C-O, and amide C-O, respectively,14 while the peaks for β-cyclodextrin at 3434 cm-1, 2924 cm-1, 1649 cm-1, 1158 cm-1, and 1080 cm-1 were assigned to O–H stretching vibration, C–H stretching vibration, H–O–H bending vibration, C–O stretching vibration, and C–O–C stretching vibration, respectively. The FTIR spectrum of RAABCD was quite different from that of rifaldazine or the additive effects of rifaldazine and β-cyclodextrin, indicating that there might be some molecular interaction between rifaldazine and β-cyclodextrin (for example, the methylpiperazine group of rifaldazine might be entrapped in the cavity of β-cyclodextrin, accounting for the rifaldazine peaks missing in the spectrum for the complex).
Figure 3.
Differential scanning calorimetry (A) and Fourier transform infrared spectra (B) of rifaldazine (a), β-cyclodextrin (b), physical mixture (c), and RAABCD (d).
Abbreviation: RAABCD, β-cyclodextrin molecular inclusion complexes of rifaldazine.
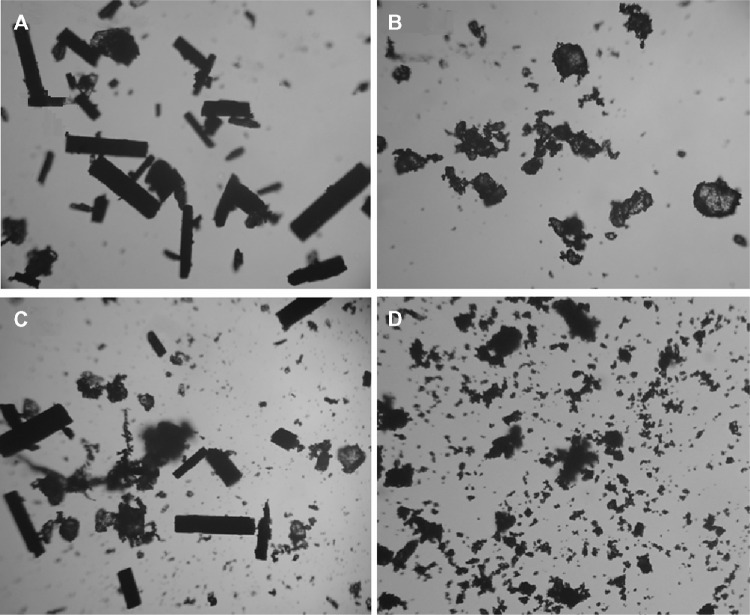
As depicted in Figure 4, the morphologies of RAABCD, rifaldazine, β-cyclodextrin, and the physical mixture were observed by optical microscopy. Free rifaldazine and β-cyclodextrin had columnar and irregular (quadrate) shapes, respectively, while the physical mixture was the simple adduct of free rifaldazine and β-cyclodextrin. In contrast with the physical mixture, RAABCD showed an irregular and amorphous shape, suggesting the formation of a molecular inclusion complex containing rifaldazine.
Figure 4.
Microscopic photographs (×400) of rifaldazine (A), β-cyclodextrin (B), physical mixture (C), and RAABCD (D).
Abbreviation: RAABCD, β-cyclodextrin molecular inclusion complexes of rifaldazine.
Computer modeling
The molecular structures of rifaldazine and β-cyclodextrin were simulated separately using the MOPAC2004 program in the computer-aided software system (CAChe 6.18 Worksystem Pro). The clathrate structure of RAABCD was constructed using a molecular docking approach. Interaction energies were computed for rifaldazine, approaching from the methylpiperazine group, to identify the most probable optimal configuration of the complex (see Figure 5). The corresponding binding energies with their electrostatic and Van der Waals contributions are shown in Table 1. The dominant driving force of the complexation process between rifaldazine and β-cyclodextrin appeared to be Van der Waals forces and hydrogen bonds. The positive binding energy value indicate the spontaneity and feasibility of the complexation of rifaldazine and β-cyclodextrin, which is in agreement with the result obtained using the above-mentioned thermodynamic approach (ie, method of calculating change in Gibbs free energy).
Figure 5.
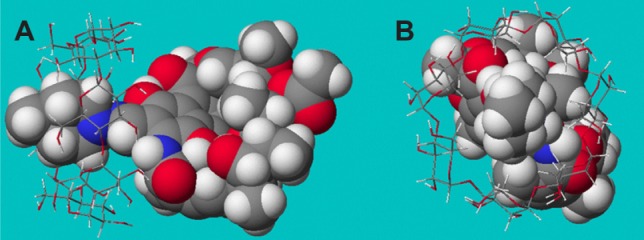
Side (A) and front (B) views of the most probable RAABCD confgurations obtained for the 1:1 complexes.
Notes: Rifaldazine is represented as a CPK model, while β-cyclodextrin is represented as a ball-and-stick model. The rifaldazine and β-cyclodextrin molecules consisted of different atoms of hydrogen (white color), carbon (gray color), oxygen (red color), and nitrogen (blue color).
Abbreviation: RAABCD, β-cyclodextrin molecular inclusion complexes of rifaldazine.
Table 1.
Molecular modeling of interaction energies corresponding to optimal configurations of RAABCD
| Parameter | Energy (kcal/mol)
|
||
|---|---|---|---|
| Before complexing (E1) | After complexing (E2) | Binding energy* (Ebinding = E1 − E2) | |
| Van der Waals | 116.230 | 105.446 | 10.784 |
| Electrostatics | 275.242 | 273.203 | 2.039 |
| Hydrogen bond | 3.735 | −3.364 | 7.099 |
| Stretch | 20.329 | 20.436 | −0.107 |
| Angle | 62.245 | 63.745 | −1.5 |
| Stretch bend | 3.179 | 3.105 | 0.074 |
| Dihedral | 48.907 | 46.481 | 2.426 |
| Improper torsion | 0.897 | 0.795 | 0.102 |
| Torsion stretch | −3.200 | −2.955 | −0.245 |
| Bend bend | 0.514 | 0.605 | −0.091 |
| Total energy | 528.0782 | 507.4965 | 20.5817 |
Note: *Binding energy was equal to the energy difference between the energy level before complexing and that after complexing.
Abbreviation: RAABCD, β-cyclodextrin molecular inclusion complexes containing rifaldazine.
Antibacterial activity
RAABCD and free rifaldazine were tested-n Gram-positive (S. aureus) and Gram-negative bacteria (E. coli) using the broth macrodilution method. Bacteria fourished in pure Mueller-Hinton culture medium (positive reference). Minimum inhibitory concentrations for RAABCD and free rifaldazine were 0.125 μg/mL and 0.125 μg/mL for S. aureus, and 32 μg/mL and 16 μg/mL for E. coli, respectively. These data suggest that RAABCD has good antibacterial activity against S. aureus and E. coli. In an earlier study, the mean minimum inhibitory concentrations of a randomly methylated RAABCD complex, RAAHCD complex, and free rifaldazine were reported to be 26.3 μg/mL, 5.63 μg/mL, and 15 μg/mL for Acinetobacter baumannii CIP7010T, respectively.8 The activity of the rifaldazine complex for bacteria clearly decreased in the order of S. aureus, E. coli, and A. baumannii. The difference between the minimum inhibitory concentrations of free rifaldazine and its molecular inclusion complex indicate a complicated relationship between the drug delivery system and type of bacteria.
Conclusion
The hydrophobic drug, rifaldazine, could be successfully molecularly encapsulated into the cavity of β-cyclodextrin to form RAABCD with better solubility (4.4 times that of free rifaldazine) in a short time frame (30 minutes) using a solidstate grinding method. The inclusion ratio, binding constant, and change in Gibbs free energy determined by a phase solubility diagram and/or ultraviolet-visible spectroscopy were 1:1, 288.33/261.33 L/mol, and -32.29/-31.73 kJ/mol, respectively. Differential scanning calorimetry and FTIR spectroscopy of RAABCD confirmed that there was a molecular interaction between rifaldazine and β-cyclodextrin. The morphological differences between irregular and amorphous-shaped RAABCD and columnar-shaped rifaldazine further confirm the molecular encapsulation of rifaldazine. The most probable optimal configuration for RAABCD with a 1:1 stoichiometry was simulated via computer modeling. Broth macrodilution tests indicate that RAABCD had good antibacterial activity. Therefore, RAABCD might be a preferred alternative for oral administration of rifaldazine when used to treat various bacterial infections. Further studies are necessary to evaluate the in vivo pharmacokinetics, biodistribution, and pharma-codynamics of RAABCD.
Acknowledgments
This research was partially supported by grants from the National Natural Science Foundation of China (30973645), Specialized Research Fund for the Doctoral Program of Higher Education (20095503120008), Chongqing Natural Science Foundation (CSTC2012JJB10027), and Chongqing Education Committee Fund (Excellent University Personnel Financial Aid Plan, KJ120321).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dooley KE, Bliven-Sizemore EE, Weiner M, et al. Safety and pharmacokinetics of escalating daily doses of the antituberculosis drug rifapen-tine in healthy volunteers. Clin Pharmacol Ther. 2012;91:881–888. doi: 10.1038/clpt.2011.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spellberg B, Lipsky BA. Systemic antibiotic therapy for chronic osteomyelitis in adults. Clin Infect Dis. 2012;54:393–407. doi: 10.1093/cid/cir842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zalmanovici Trestioreanu A, Fraser A, Gafter-Gvili A, et al. Antibiotics for preventing meningococcal infections. Cochrane Database Syst Rev. 2011;8:CD004785. doi: 10.1002/14651858.CD004785.pub4. [DOI] [PubMed] [Google Scholar]
- 4.Drugs for tuberculosis. Treat Guidel Med Lett. 2012;10:29–36. [No authors listed] [PubMed] [Google Scholar]
- 5.Steingart KR, Jotblad S, Robsky K, et al. Higher-dose rifampin for the treatment of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis. 2011;15:305–316. [PubMed] [Google Scholar]
- 6.Changsan N, Nilkaeo A, Pungrassami P, et al. Monitoring safety of liposomes containing rifampicin on respiratory cell lines and in vitro efficacy against Mycobacterium bovis in alveolar macrophages. J Drug Target. 2009;17:751–762. doi: 10.3109/10611860903079462. [DOI] [PubMed] [Google Scholar]
- 7.Quenelle DC, Staas JK, Winchester GA, et al. Efficacy of microen-capsulated rifampin in Mycobacterium tuberculosis-infected mice. Antimicrob Agents Chemother. 1999;43:1144–1151. doi: 10.1128/aac.43.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tewes F, Brillault J, Couet W, et al. Formulation of rifampicin-cyclodextrin complexes for lung nebulization. J Control Release. 2008;129:93–99. doi: 10.1016/j.jconrel.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Manca ML, Manconi M, Valenti D, et al. Liposomes coated with chitosan-xanthan gum (chitosomes) as potential carriers for pulmonary delivery of rifampicin. J Pharm Sci. 2012;101:566–575. doi: 10.1002/jps.22775. [DOI] [PubMed] [Google Scholar]
- 10.Gaur PK, Mishra S, Gupta VB, et al. Targeted drug delivery of rifam-picin to the lungs: formulation, characterization, and stability studies of preformed aerosolized liposome and in situ formed aerosolized liposome. Drug Dev Ind Pharm. 2010;36:638–646. doi: 10.3109/03639040903410300. [DOI] [PubMed] [Google Scholar]
- 11.Loftsson T, Brewster ME. Cyclodextrins as functional excipients: methods to enhance complexation efficiency. J Pharm Sci. 2012;101:3019–3032. doi: 10.1002/jps.23077. [DOI] [PubMed] [Google Scholar]
- 12.Laza-Knoerr AL, Gref R, Couvreur P. Cyclodextrins for drug delivery. J Drug Target. 2010;18:645–656. doi: 10.3109/10611861003622552. [DOI] [PubMed] [Google Scholar]
- 13.Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins: basic science and product development. J Pharm Pharmacol. 2010;62:1607–1621. doi: 10.1111/j.2042-7158.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- 14.Rao KR, Bhanumathi N, Yadav JS, et al. inventors Council of Scientific and Industrial Research, assignee. Inclusion complex of rifampicin, an anti-tubercular drug, with beta-cyclodextrin or 2-hydroxypropyl beta-cyclodextrin and a process thereof. United States patent US 7001893. Feb 21, 2006.
- 15.Ding PT. Cyclodextrin derivatives. In: He ZG, editor. Cyclodextrin Inclusion Complex Technology. Beijing, China: People’s Medical Publishing House; 2008. Chinese. [Google Scholar]
- 16.Madan J, Baruah B, Nagaraju M, et al. Molecular cycloencapsulation augments solubility and improves therapeutic index of brominated noscapine in prostate cancer cells. Mol Pharm. 2012;9:1470–1480. doi: 10.1021/mp300063v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higuchi T, Connors KA. Phase-solubility techniques. Adv Anal Chem Instrum. 1965;4:117–212. [Google Scholar]
- 18.Jiang Y, Sha X, Zhang W, et al. Complex of 9-nitro-camptothecin in hydroxypropyl-beta-cyclodextrin: in vitro and in vivo evaluation. Int J Pharm. 2010;397:116–121. doi: 10.1016/j.ijpharm.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Barooah N, Mohanty J, Pal H, et al. Supramolecular assembly of Hoechst-33258 with cucurbit[7]uril macrocycle. Phys Chem Chem Phys. 2011;13:13117–13126. doi: 10.1039/c1cp20493a. [DOI] [PubMed] [Google Scholar]
- 20.Ma XQ, Ren Y, Fu XY. Research on camptothecin-HP-β-cyclodextrin inclusion. Chinese Journal of New Drugs. 2007;16:1385–1387. Chinese. [Google Scholar]
- 21.Zhang JQ, Liu J, Li XL, et al. Preparation and characterization of solid lipid nanoparticles containing silibinin. Drug Deliv. 2007;14:381–387. doi: 10.1080/10717540701203034. [DOI] [PubMed] [Google Scholar]
- 22.Tan QY, Hu NN, Liu GD, et al. Role of a novel pyridostigmine bromide-phospholipid nanocomplex in improving oral bioavailability. Arch Pharm Res. 2012;35:499–508. doi: 10.1007/s12272-012-0313-6. [DOI] [PubMed] [Google Scholar]
- 23.Zhang JQ, Zhang ZR, Yang H, et al. Lyophilized paclitaxel magne-toliposomes as a potential drug delivery system for breast carcinoma via parenteral administration: in vitro and in vivo studies. Pharm Res. 2005;22:573–583. doi: 10.1007/s11095-005-2496-8. [DOI] [PubMed] [Google Scholar]
- 24.Klancnik A, Piskernik S, Jersek B, et al. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J Microbiol Methods. 2010;81:121–126. doi: 10.1016/j.mimet.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Kotta S, Khan AW, Pramod K, et al. Exploring oral nanoemulsions for bioavailability enhancement of poorly water-soluble drugs. Expert Opin Drug Deliv. 2012;9:585–598. doi: 10.1517/17425247.2012.668523. [DOI] [PubMed] [Google Scholar]
- 26.Tan Q, Liu S, Chen X, et al. Design and evaluation of a novel evodiamine-phospholipid complex for improved oral bioavailability. AAPS Pharm Sci Tech. 2012;13:534–547. doi: 10.1208/s12249-012-9772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]