Abstract
Human very small embryonic-like (hVSEL) cells are a resident population of multipotent stem cells in the bone marrow involved in the turnover and regeneration of tissues. The levels of VSEL cells in blood are greatly increased in response to injury, and they have been shown to repair injured tissues. Adult hVSEL cells, SSEA-4+/CD133+/CXCR4+/Lin−/CD45−, express the pluripotency markers (Oct-4 and Nanog) and may be able to differentiate into cells from all 3 germ lineages. hVSEL cells isolated from blood by apheresis following granulocyte–colony-stimulating factor mobilization were fractionated and enriched by elutriation and fluorescence activated cell sorting. Collagen sponge scaffolds containing 2,000–30,000 hVSEL cells were implanted into cranial defects generated in SCID mice. Analysis by microcomputed tomography showed that a cell population containing VSEL cells produced mineralized tissue within the cranial defects compared with controls at 3 months. Histologic studies showed significant bone formation and cellular organization within the defects compared with cellular or scaffold controls alone. Antibodies to human leukocyte antigens demonstrated that the newly generated tissues were of human origin. Moreover, human osteocalcin was identified circulating in the peripheral blood. There was evidence that some level of hVSEL cells migrated away from the defect site, using quantitative real-time polymerase chain reaction to detect for human-specific Alu sequences. This study demonstrates that hVSEL cells are able to generate human bone tissue in a mouse model of skeletal repair. These studies lay the foundation for future cell-based regenerative therapies for osseous and connective tissue disorders, including trauma and degenerative conditions, such as osteoporosis, fracture repair, and neoplastic repair.
Introduction
Bone loss due to fractures and disease is a serious medical condition that affects millions of individuals worldwide. While major efforts have been made to understand mechanisms of healing of skeletal structures and to develop therapeutics to treat overall bone loss due to the many metabolic bone diseases, information on bone remodeling is scarce in the human craniofacial skeleton. One approach to repair and regenerate bone loss is through the use of stem-cell-based therapy. Bone marrow (BM)–derived mesenchymal stem cells (MSCs) are capable of differentiating into osteoblasts and other cells of mesenchymal lineage. They can be directed to do so in vitro and when implanted in bone can also facilitate bone formation. In fact, several studies have shown that MSCs can be employed to regenerate craniofacial bone in animal studies, supporting the potential of stem-cell-based therapy for bone repair [1–5]. However, there are potential limitations to the use of autologous MSCs in bone repair in humans because most preparatory protocols require the extensive expansion of MSC populations in vitro using animal-derived or recombinant growth factors as well as modulators of transcription and cell survival.
In previous reports we described an in vivo assay to identify cells with stem-cell-like activities [6,7]. Murine marrow cells with stem-cell-like activities were found to be present in a low density fraction that was resistant to 5-fluorouracil in vivo [7]. Further characterization of these cells identified a fluorescence activated cell sorting profile that identified a very small cell type that expressed the Sca-1 antigen but did not express the pan-hematopoietic CD45 antigen or other hematopoietic lineage markers (Lin−). This Lin−Sca-1+CD45− population has previously been described as having embryonic-like features and are therefore referred to as very small embryonic-like or VSEL cells [8–11]. Freshly isolated Lin−Sca-1+CD45− cells, when used in an in vivo model, demonstrated that as few as 500 cells are able to generate bone-like tissues [12]. Importantly, when transplanted to a BM environment, the cells are able to differentiate into multiple mesenchymal lineages [6].
In the present report we evaluated the ability of human VSEL (hVSEL) cells to generate bone structures in vivo. We demonstrated that hVSEL cells were able to form cortical and trabecular osseous structures when implanted into cranial defects in immune-deficient mice. Importantly, the regenerated bone tissue is of human origin as determined by immunohistochemistry for human-specific leukocyte antigens (HLAs). These data demonstrate that hVSEL cells form bone in a preclinical model and therefore represent a novel source of adult stem cells for the regeneration of skeletal structures.
Materials and Methods
hVSEL cell isolation
hVSEL cells were collected and processed under an IRB approved protocol at the NeoStem Laboratory in Cambridge, Massachusetts. Healthy Caucasian men (age 23–27) were recruited as VSEL cell donors and screened for known diseases, use of drugs and tobacco, and obesity. Two days prior to apheresis, each donor received daily subcutaneous injections of granulocyte–colony-stimulating factor [G-CSF (Neupogen®; Amgen, Inc.)] (480 μg/day) to facilitate mobilization of VSEL cells from the BM into the peripheral blood stream. Apheresis was conducted by a certified staff technician over the course of 2 to 3 h. All the solutions, tubing, and needles were sterile, used only one time and then discarded after each donation. Subsequently, the hVSEL cells were enriched by Elutriation (CaridianBCT), followed by CD34/CD133 Microbeads (Miltenyi Biotec) positive selection, and then viable Lin−CD45−CD34+CD133+ VSEL cells were flow sorted using Moflo XDP high-speed cell sorter (Beckman Coulter). Highly purified VSEL cells from 3 donors were frozen in phosphate-buffered saline–5% human serum albumin and 5% dimethyl sulfoxide (Sigma Chemical Corp.) and shipped by overnight courier to the University of Michigan without any demographic information. Upon thawing, VSEL cells were counted using Trypan Blue exclusion (Sigma). Typically fewer than 100,000 VSEL cells were isolated and therefore to accommodate all the groups used in the study 2,000 cells were set as standard cell dose. In one case, >3,000,000 VSEL cells were isolated which afforded the opportunity to increase the cell numbers used.
Animal procedures
Five-week-old female SCID mice (CB.17. SCID; Taconic) were divided randomly into 5 groups consisting of 10–13 animals per group, except for the 500,000 cell dose in which n=5 animals were used. The animals were anesthetized by continuous isoflurane inhalation, hair was removed by shaving, and a linear scalp incision was made from the nasal bone to the occiput and full-thickness flaps were elevated. A trephine was used to create a 3-mm craniotomy defect centered in each of the parietal bones with constant irrigation with Hanks' balanced salt solution [13]. The calvarial disks were removed with care to avoid injury of the underlying dura and brain tissues. After hemostasis was established (light pressure applied with saline-soaked surgical guaze), scaffolds (Gelfoam™; Pharmacia & Upjohn) previously loaded with either vehicle or hVSEL cells were placed into the defects so that the scaffolds filled the entire defect. The incisions were closed with 4-0 chromic gut suture (Ethicon/Johnson & Johnson), and the mice were recovered from anesthesia on a heating pad. All mice were sacrificed 16 weeks after the implantation. This time frame was selected to ensure that early healing and early remodeling would have taken place. At sacrifice, intracardiac puncture and aspiration was performed under anesthesia to collect serum. All procedures were approved by the University Committee on the Use and Care of Animals, and Biologic Safety Board in compliance with State and Federal laws.
Bone marrow stromal cells
BM was isolated by flushing the femurs, tibia, and humeri of C57BL/6 mice (Jackson Laboratory) with DMEM+heat inactivated 10% fetal bovine serum (FBS) (Invitrogen). Plastic adherence at 37°C was performed in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS and penicillin/streptomycin (Life Technologies). Following overnight adherence, the nonadherent cells were removed and fresh medium was replaced. The cultures were expanded by trypsinization twice over the course of 3 weeks generating first and second passage cells (P1 or P2). Bone marrow stromal cells (BMSCs) from P1 or P2 at 80%–90% confluence were transduced with AdCMVBMP-7 ex vivo 24 h prior to transplantation at a multiplicity of infection of 500. The AdCMVBMP-7 was constructed by Cre-lox recombination as previously described and generated by Vector Core at the University of Michigan [14].
Cell transplantation
Five groups of mice were established to evaluate the ability of hVSEL cells to regenerate the cranial defects. The first group served as a negative control in which only the vehicle and Gelfoam were placed into the defect. The second group consisted of P1 or P2 murine BMSCs infected with an AdCMVBMP-7 designed to express huBMP-7 to serve as a positive control [14]. Our test groups consisted of 2,000, 10,000, 30,000, and 500,000 VSEL cells in Gelfoam isolated from 3 different human donors.18 The cell doses were derived at by estimating the frequency of these marrow human MSCs reported to be present in BM (ranging from 1/10,000 to 1/100,000 BM mononuclear cells) [15–19] and observations that ∼ 2×106 human marrow adherent cells are required to heal a 5-mm cranial defect in mice [1]. The incorporation of a 2,000 VSEL cell dose was to ensure our ability to observe a VSEL cell response assuming that only 10% of the transplanted cells were able to participate in wound repair. BMSCs transduced with AdCMVBMP-7 (2,000 cells/implant) were used as controls. Negative controls included the scaffold alone.
Microcomputed tomography
Calvariae were harvested and immediately fixed in 10% neutral buffered formalin for 48 h. The bone specimens were then scanned at 8.93 μm voxel resolution on an EVS Corp., micro-CT scanner, with a total of 667 slices per scan. GEMS MicroView® software was used to make 3-D reconstructions from the data set. Each defect was individually assessed for the region of interest and bone analysis was conducted with a fixed threshold (600) used to extract the mineralized bone phase; bone volume fraction, bone mineral density, and trabecular number were calculated.
Histologic examination
After microcomputed tomography (μCT) analysis, the bones were decalcified in 10% EDTA (pH 7.4) for 10 days and embedded in paraffin. Longitudinal sections of the calvariae were cut and stained with hematoxylin and eosin (H&E) or using Masson's trichrome staining and analyzed by light microscopy. In some cases, the slides were stained with an antibody to human HLA antigens [Anti-HLA-ABC antibody (BioLegend)] or an IgG control (Sigma-Aldrich) in conjunction with an HRPAEC staining system kit following the manufacture's protocols (R&D Systems) to identify the human cells.
Serum osteocalcin levels
Human osteocalcin levels were determined using a sandwich Mid-Tact Osteocalcin EIA (Biomedical Technologies) and using human recombinant osteocalcin as a standard (Biomedical Technologies). This sandwich EIA is highly specific for both intact human osteocalcin and the major (1–43) fragment. To normalize the osteocalcin levels in the serum, total protein was determined using the RC-DC Protein Assay Kit (BioRad Laboratories) against a bovine serum albumin standard. Human serum was purchased from Sigma as a positive control.
Real-time polymerase chain reaction evaluation for hVSEL cells
DNA isolation kits were used to prepare genomic DNA from the designated tissues [DNeasy Blood and Tissue Kit (Cat. No. 69506); Qiagen, Inc.]. All sample concentrations were standardized in each reaction to exclude false-positive results. Real-time polymerase chain reactions were performed using 15.0 μL of TaqMan PCR Master Mix (Applied Biosystems) with 100 nM of the human Alu TaqMan probes [(F: 5′-CAT GGT GAA ACC CCG TCT CTA-3′, R: 5′-GCC TCA GCC TCC CGA GTA G-3′; TaqMan probe: 5′-FAM-ATT AGC CGG GCG TGG TGG CG-TAMRA-3′); Applied Biosystems] [20,21] and 1 μg of the isolated tissue DNA in a total volume of 30 μL. The thermal conditions were 50°C for 2 min and 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The level of expression was detected as an increase in fluorescence using a sequence detection system (ABI PRISM 7700; Applied Biosystems). The DNA levels were expressed as relative copies (% control) normalized against murine β-actin (Cat. No. 4331182; Applied Biosystems), and a standard curve constructed from serial dilutions of a purified Luc/Alu cDNA fragment was cloned by classic polymerase chain reaction (PCR). Numerical data were determined against a standard curve established using mouse BM containing log-fold dilutions of hVSEL cells. Positive controls used DNA isolated from a human prostate cancer cell line (PC3). Negative controls included tissues isolated from animals not injected with hVESL cells.
Statistical analyses
Numerical data are expressed as mean±standard deviation. Statistical analysis was performed by Kruskal–Wallis one-way analysis of variance using the GraphPad Instat statistical program (GraphPad Software). Specific differences were measured by Mann–Whitney U test. The level of significance was set at P<0.05.
Results
Evaluation of bone formation by μCT
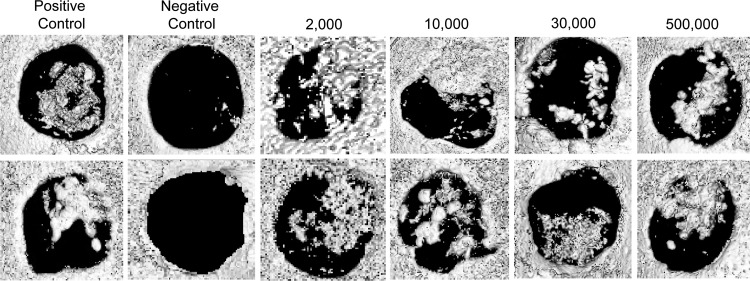
hVSEL cells were evaluated for their ability to form bone in murine calvarial defects. VSEL cells were isolated following G-CSF mobilization of normal healthy donors and placed into 3-mm-diameter calvarial defects generated in the left parietal bones of SCID mice. Transplanted cells (ranging from 2,000 to 500,000 cells) were delivered to the defects in 3×3 mm GelFoam collagen sponges. Negative cellular controls consisted of the sponge alone. Positive controls incorporated murine BMSCs engineered to overexpress human BMP7. After 3 months all the specimens were evaluated by μCT. The data demonstrate that animals implanted with carrier alone did not generate any bone compared with the mineralized tissue formed in the positive control (Fig. 1). Implantation of 2,000–500,000 VSEL cells from the 3 donors all stimulated bone formation (Fig. 1). Examination of the mineralization demonstrated incomplete closure of all of the defects. However, robust bone formation was observed in samples generated from the 2,000 and 10,000 cells/implant groups. Interestingly, bone formed in animals treated with 30,000 cells/implant did not produce significantly more osseous tissue than those treated with 2,000 cells/implant and the 500,000 cells/implant produced greater boney structures than did the 30,000 and 2,000 cells/implant groups.
FIG. 1.
Human very small embryonic-like (hVSEL) cells form bone in immune-deficient mice. Microcomputed tomography images of the calvarial specimens show several representatives of calvarial defects following VSEL cell implantation. Bone marrow stromal cells (BMSCs) from P1 or P2 at 80%–90% confluence were transduced with AdCMVBMP-7 ex vivo 24 h prior to transplantation and implanted at 2,000 cells/defect served as a positive control. The data show the lack of mineralized tissue present within the defects within the negative control (collagen carrier alone). Each of the VSEL-cell-implanted defects generated mineralized tissues.
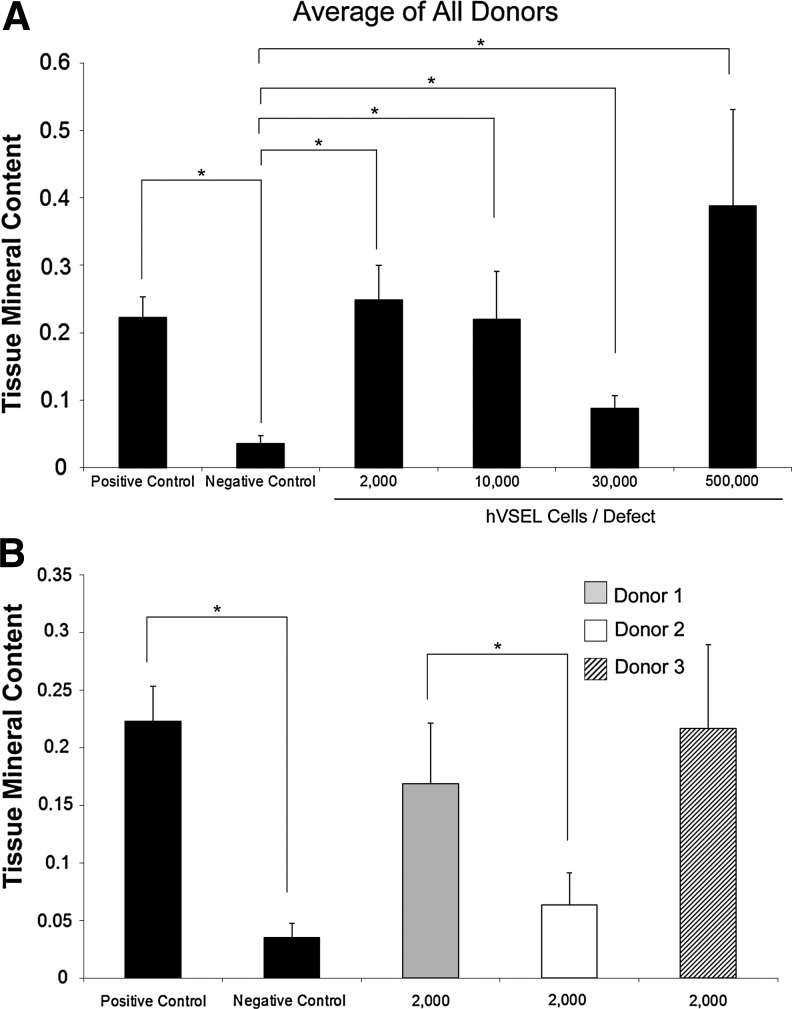
Using the GEMS MicroView software, each sample was examined for mineral content. The efficacy of cells to produce mineralized matrix was not proportional to the number of cells transplanted (Fig. 2A). For example, defects implanted with 2,000 hVSEL cells produced more mineralized tissue than those implanted with 10,000 or 30,000 cells. Interestingly, implants with 30,000 hVSEL cells produced the lowest amount of mineralized tissue. The most mineralized tissue that was formed was when 500,000 VSEL cells were implanted within the calvarial defects; however, the amount of tissue formation was not statistically different from the tissue generated by 2,000 VSEL cells.
FIG. 2.
Mineral content generated by hVSEL cells in immune-deficient mice. (A) hVSEL cells were implanted into n=10 calvarial defects over a dose range of 2,000–30,000 cells/defect and n=5 for 500,000 cells/defect. Groups consisting of 30,000 and 500,000 VSEL cells/defect were isolated from a single donor. At 3 months the tissue mineral content values within each defect were averaged for the animal groups. Positive controls included murine BMSCs expressing human BMP7; negative controls consisted of only the collagen carrier alone. (B) Averages of tissue mineral content formed within the calvarial defect by 2,000 hVSEL cells by donor (*P<0.05).
As previously described, hVSEL cell isolation was collected from 3 separate donors. Total VSEL cells isolated and generated were 3,120,000, 19,000, and 11,000 for donors 1, 2, and 3, respectively. To assess any differences in efficacy in bone formation between donors, implants from individual donors were evaluated at the same cell dose. When bone generated by 2,000 hVSEL cells/implant was compared with donors 1 and 3, results showed that these groups performed equivalently, but both generated more mineralized tissue than donor 2 (Fig. 2B). Yet donor 3 generated more hVSEL cells than the other 2 donors, suggesting that potential individual differences in hVSEL cell function are possible.
Evaluation of bone formation by histological analysis
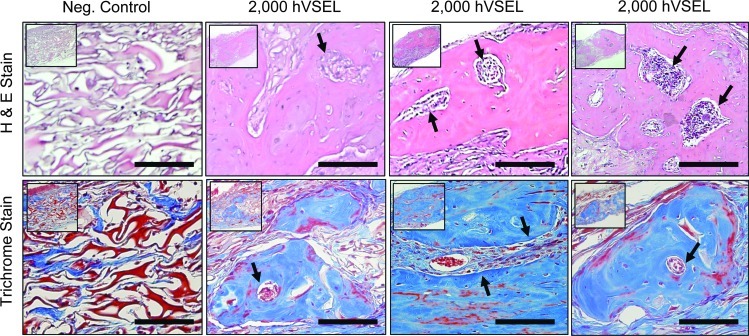
After decalcification, serial sections were generated through each defect in preparation for histologic evaluation. Implanted material demonstrated a high degree of tissue integration, with few, if any, neutrophils or macrophages observed within newly formed tissue. In the control group, the collagen carrier matrix persisted and no osteoid or mature lamellar bone could be observed by H&E or Masson's trichrome staining (Fig. 3). In experimental groups implanted with 2,000 hVSEL cells, woven bone containing marrow spaces was observed within the calvarial defect (Fig. 3). Here, the carrier matrix was largely resorbed from the defect site with few remaining particles embedded within fibrous connective tissue surrounding the lamellar bone. The bone generated was comprised predominantly of woven bone (Fig. 3).
FIG. 3.
Histologic evaluation of tissues generated by hVSEL cells within calvarial defects. Representative slides were stained with hematoxylin and eosin (top row) and Masson's trichrome (bottom row) in which collagen and bone appear blue. Positive controls included murine BMSCs expressing BMP7 (not shown); negative controls (neg. control) consisted of only the collagen carrier alone. Histology presented at 20× (insets) and 40× magnifications. Note the persistence of the collagen carrier matrix in the neg. control group as well as the absence of an inflammatory cell infiltrate. The 2,000 hVSEL cell groups demonstrate lamellar bone containing marrow spaces (arrows). Scale bar=100 μm.
Demonstration that hVSEL cells formed bone in vivo
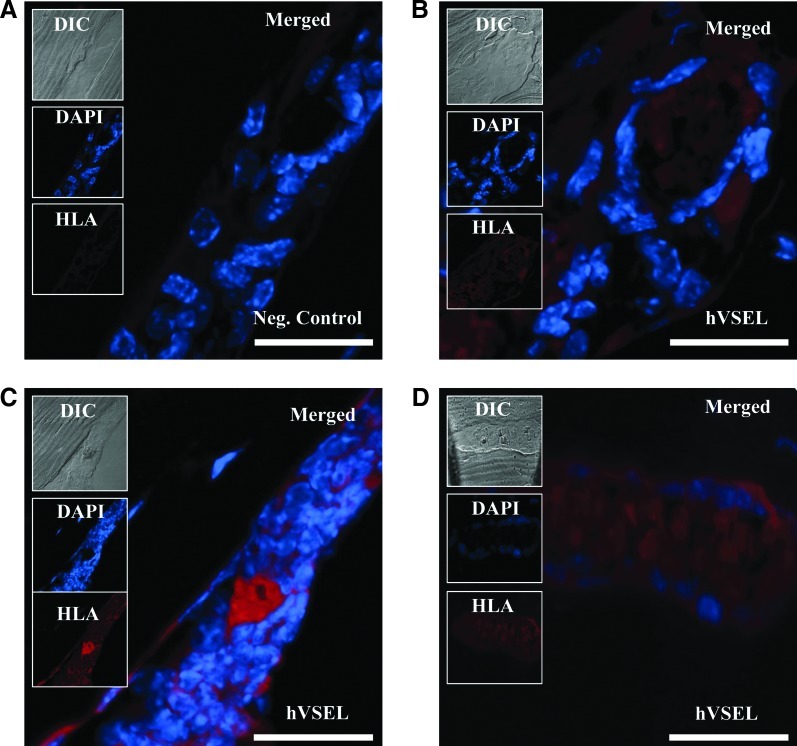
To validate that the bone formed was generated by the implanted human cells, 2 independent methods were evaluated. First, the sections were stained with a pan-HLA antibody. Mice implanted with vehicle only did not demonstrate any cross-reactivity for human HLA (Fig. 4A). Mice implanted with hVSEL cells demonstrated significant human HLA staining specifically on cells lining the bone surface and in other cells in the marrow (Fig. 4B–D). Together, these results show that injected hVSEL cells localize to mouse BM spaces adjacent to bony trabeculae and demonstrate that VSEL cells are likely to be active participants in bone formation.
FIG. 4.
Tissues generated by hVSEL cells are derived from human cells. Tissues formed by hVSEL cells within calvarial defects were stained with a fluorescent human-specific pan-human leukocyte antigen (HLA) (red) and merged with images of antinuclear stain (DAPI) and differential interference contrast (DIC) images. (A) Negative control is a longitudinal section of vessel in mice implanted with vehicle only demonstrating lack of human HLA staining. (B–D) Longitudinal section of a vessel in mice injected with hVSEL cells demonstrating cytoplasmic human HLA staining. Histologic images presented at 40×. Scale bar=100 μm.
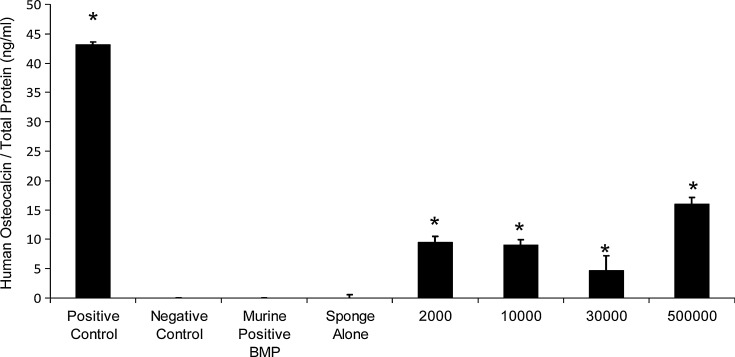
To prove that the hVSEL cells generated bone, serum from the animals collected at the time of sacrifice (3 months) was evaluated for the presence of human-specific osteocalcin. Animals that did not receive any hVSEL cells in their implants did not demonstrate any human osteocalcin in the serum (Fig. 5). Animals that were implanted with hVSEL cells did have circulating human osteocalcin present within their serum (Fig. 5). The levels of osteocalcin roughly corresponded to the amount of bone formation with animals receiving 500,000 cells/implant having the highest concentration of osteocalcin present in the serum, and animals implanted with fewer hVSEL cells demonstrating less osteocalcin in the blood (Fig. 5).
FIG. 5.
Human osteocalcin present in murine serum. Circulating levels of intact human osteocalcin present in the serum (3 months) of animals implanted with no human cells (negative control), carrier alone (sponge alone), or human serum (positive control), murine BMSCs expressing human BMP, or 2,000–500,000 hVSEL cells/defect. Osteocalcin levels are presented as the mean and standard deviation of all animals/implant group normalized against total serum protein. *P<0.05 compared with negative control.
Migration of hVSEL cells from the bone defects
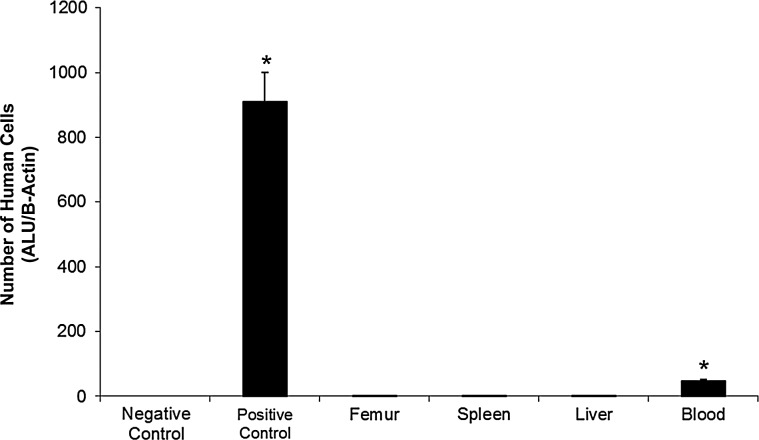
Migration of hVSEL cells from the implant site into the periphery was assessed using quantitative real-time PCR for human-specific Alu sequences. The presence of human DNA was evaluated from representative tissue samples from the spleen, femur, right lobe of the liver, and whole blood. Animals not implanted with human cells and animals implanted with murine BM served as a negative control. A human prostate cancer cell line (PC-3) mixed (1:1,000) with murine BM served as a positive control. Human-specific Alu expression was identified at low levels in the blood of the recipient animals that were implanted with hVSEL cells (Fig. 6). No evidence of human DNA was seen in the spleen, femur, or liver of the animals implanted with hVSEL cells, suggesting that the level of VSEL cells that migrated out of the defect was relatively low (Fig. 6).
FIG. 6.
Human cells are found in the blood of experimental animals. Quantitative real-time polymerase chain reaction for human-specific Alu was used to determine the presence of human cells within select murine tissues (spleen, femur, right lobe of the liver, and whole blood). Pure mouse bone marrow served as a negative control and human prostate cancer cell line (PC3) served as a positive control. Human DNA was not observed in the spleen, femur, or liver of any of the animals implanted with 2,000 hVSEL cells. Human-specific Alu was detected in the peripheral blood of the animals. *P<0.05 compared with negative control.
Discussion
Bone is a specialized and dynamic tissue that undergoes continuous remodeling throughout life [22]. Osteoblasts, derived from stem cells of mesenchymal origin, are responsible for the synthesis of the bone matrix that ultimately becomes mineralized. Their origin appears to be derived from a population of stem cells frequently described as MSCs. Numerous models have demonstrated that MSCs are capable of limited self-renewal and are able to undergo differentiation into multiple lineages, including bone, adipocytes, cartilage, and hematopoiesis-supporting cells [23]. This capacity to form new osseous tissues has many clinical applications, including treatment for patients with boney defects, poor bone mass, or deficient fracture repair. However, limitations in our ability to collect and expand MSCs along with difficulties in activating and driving MSCs in situ with current therapeutic modalities limit the local or systemic treatment of many skeletal conditions. These modalities include the use of human recombinant parathyroid hormone (PTH) treatments, calcium and vitamin D to stimulate osteoblastic bone formation, or bisphosphonate therapies to inhibit osteoclastic activities.
Recent work in regenerative medicine has demonstrated that marrow and other tissues contain a population of nonhematopoietic stem cells with many properties of pluripotent stem cells [24]. First described by Ratajczak and coworkers, these VSEL cells (<8 μm) appear to have the ability to generate all 3 germ layer cellular populations under experimental conditions [8,22–37]. They are found in BM [8,24,26,27,29], cord blood [25,38], and adult peripheral blood [30] and other adult organs [31,39,40]. Murine VSEL cells express SSEA-1+, Oct-4+, and Sca-1+ but do not express hematopoietic lineage markers (lin− and CD45−). hVSEL cells are SSEA-4+/Oct-4+/CD34+/lin+/CD45+. Morphologically, VSEL cells appear to be mostly comprised of a relatively large nucleus with scant cytoplasm, and express early embryonic transcription factors, including Oct-4, Nanog, and the chemokine receptor CXCR4. Due to their expression of CXCR4, they are highly sensitive to stromal derived factor-1 (SDF-1 or CXCL12), which is likely to be a critical feature in their ability to be mobilized and migration in response to tissue injury. VSEL cells are also highly sensitive to G-CSF, which can induce their mobilization from BM into the blood and other organs and tissues.
The results of our studies establish that hVSEL cells can be isolated from human peripheral blood and are capable of osseous regeneration in a cranial defect in immune-deficient mice. However, the ability of these cells to completely reconstitute a large skeletal defect was limited. As demonstrated by HLA staining, the bone formed was derived from the human cells and did not specifically represent cells induced from the host animal that were recruited into the osseous site. Moreover, enzyme-linked immunosorbent assay specific for human osteocalcin, a noncollagenous protein often used as a biochemical marker for bone formation, was found in the serum of the animals. Secreted specifically by the osteoblasts into the bone matrix, osteocalcin is believed to play a pivotal role in the mineralization of the bone matrix. Osteocalcin is known to circulate in the blood as the intact protein as well as the major N-terminal fragment [41]. However, only a small fraction of the total osteocalcin is known to be released into the circulation, with most being deposited within the bone matrix [42]. Thus, the level of osteocalcin in the circulation, while indicative of tissue turnover, may not be truly reflective of bone formation in this model.
A perplexing aspect of the findings is that the results did not necessarily correlate with the number of cells placed in the defect. We expected to observe greater osseous regeneration when a greater number of hVSEL cells were placed per defect. We did observe that placing 500,000 cells/implant produced the most bone, but defects grafted with 2,000 cells performed better than 10,000 and 30,000 cells. It is not clear why the tissue response did not mirror those in other regenerative studies. One possible explanation is that stem cell–to–stem cell communication is important under the experimental conditions. This concept is not unprecedented in the stem cell field where one daughter of a stem cell remains as a stem cell while the other matures and differentiates taking on a tissue-specific phenotype [43,44]. Thus, a potential decrease in regenerative capacity may have reduced the osteogenic production of 10,000 versus 30,000 cells, but may be overcome when cell concentrations are either much higher or when other exogenous factors participate in local tissue construction. Along these lines there are several instances where mature cell progeny output is facilitated by lower inoculum densities, including cells derived from hematopoietic and mesenchymal lineages [45].
Throughout our studies hVSEL cells were isolated from a number of different donors. Not unexpectedly, we observed significant differences in osseous tissue formation among donors even with equivalent cell dosing. From a therapeutic standpoint this suggests that there are likely to be inherent differences in the abilities of VSEL cells isolated from different individuals, or a spectrum of activities in the VSEL cell phenotype caused by the isolation and purification methods. This is best illustrated by the observation that equivalent numbers of frozen and thawed VSEL cells did not function as well in generating bone in our studies, as did VSEL cells that had not been frozen (unpublished observations). Yet the basis for these observations remains unclear. We did explore in this context a number of methods to improve tissue regeneration, including the delivery of exogenous PTH and erythropoietin; however, no significant differences were noted (not shown). At present, there are a multitude of operational factors that may have bearing on our results. For example, GelFoam matrix was used as a scaffold in our studies; it may not be ideal for mineralized tissue formation in conjunction with hVSEL cells. hVSELs may require a mineralized matrix for proper osseous formation to occur. In addition, we did not evaluate the ability of this material to maintain sufficient space for tissue regeneration. Thus, while the GelFoam in the formulation we used is pliable and compressible, it may have collapsed within the defect limiting the extent of the tissue that could have been generated. Alternatively, the carrier may not have been sufficient to hold hVSEL cells in the wound site such that they were able to migrate out of the defect and therefore not contribute to regeneration. This remains a distinct possibility since we were able to identify human Alu signatures in the peripheral blood of the animals.
Equally important to the results is the concept that the timing of our VSEL cell collections or animal studies may not have been optimized for calvarial regeneration. hVSEL cells may require a longer experimental period for differentiation into osteogenic cells and for the production of sufficient osseous tissue to complete the closure of the defect. There are numerous examples whereby differences in stem cell characteristics are known to contribute to short- and long-term reconstitution [43,44]. It has been proposed that VSEL cells are a quiescent population that reside within the BM [31], and after being mobilized into peripheral blood, they participate in the turnover of other tissue-specific stem cells that are located in peripheral niches, and play a role in tissue organ regeneration [24,29]. Thus, while VSEL cell numbers are normally at very low levels in blood, the ability to mobilize them into the blood is particularly attractive from the standpoint of developing a therapeutic product. This has been shown in clinical studies where VSEL cells were mobilized into peripheral blood following acute myocardial infarction [46] and stroke [47]. The mobilization appears to be a protective reaction of the body to repair heart tissue in response to injury. In fact, VSEL cells have been shown to repair cardiac tissue in an animal model of acute myocardial infarction [36]. Other studies show that VSEL cells can be used to treat retinal injuries [48] and diabetes [37]. Most importantly, procedures have been developed to isolate VSEL cells from cord blood and as in this study, hVSEL cells were mobilized into peripheral blood by G-CSF stimulation [32]. However, inherent in the mobilization process, there may be deleterious activities on the ability of VSEL cells to participate in osseous function in our model. While we did not specifically examine osteoclastic activities, G-CSF is well known to activate the formation of osteoclasts and to inhibit osteoblastic function. Thus, while exogenous G-CSF was not administered to the mice during the studies, the effects of mobilization alone on VSEL cells require closer examination.
As a prerequisite to clinical trials, it is important to determine whether the hVSEL cells were able to migrate away from the calvarial defect site. Upon end-point of the experiments, the spleen, femur, right lobe of the liver, and whole blood were removed to isolate genomic DNA. Using quantitative real-time PCR for human-specific Alu sequences, we were able to detect the presence of human-specific cell lines. We observed low levels of human-specific Alu expression in the blood of the recipient animals; however, no Alu expression was identified in the peripheral tissues examined. A possible explanation is that hVSEL cells were present in the tissues but fell below the level of detection of our assays, remaining as a reserve stem cell compartment [46,49]. Recently, it was demonstrated that culture-expanded VSEL cells have the capacity to generate cells in the hematopoietic lineages [50]. While we do not have specific data that address whether the results of the blood PCR were due to the hVSEL cells becoming hematopoietic cells, it remains a possibility, although we would have expected to see similar results in the spleens of the animals.
In conclusion, this study demonstrates that hVSEL cells can generate human bone in a mouse model of skeletal repair. These studies lay the foundations for future cell-based regenerative therapies for osseous and connective tissue disorders, including trauma and degenerative conditions, such as osteoporosis and fracture repair. While much needs to be learned pertaining to cell delivery, timing, matrices, and differences between individuals, the ability of VSEL cells to generate human bone could have numerous clinical applications, especially since alternative approaches to induce new bone formation have associated limitations. Thus, the ability to harvest and deliver these cells as a therapeutic agent has great potential to enhance bone formation in states of poor bone growth or deficient fracture repair.
Acknowledgments
This work is directly supported by the National Institute of Health (AR0568903, Rodgerson and Taichman; DK082481, Krebsbach and Taichman) and the Forestadent U.S. Scholarship (Havens).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Krebsbach PH. Mankani MH. Satomura K. Kuznetsov SA. Robey PG. Repair of craniotomy defects using bone marrow stromal cells. Transplantation. 1998;66:1272–1278. doi: 10.1097/00007890-199811270-00002. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y. Hallgrimsson B. Putnins EE. Craniofacial defect regeneration using engineered bone marrow mesenchymal stromal cells. J Biomed Mater Res A. 2011;99:74–85. doi: 10.1002/jbm.a.33155. [DOI] [PubMed] [Google Scholar]
- 3.Yang Y. Rossi FM. Putnins EE. Periodontal regeneration using engineered bone marrow mesenchymal stromal cells. Biomaterials. 2010;31:8574–8582. doi: 10.1016/j.biomaterials.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Taub PJ. Yau J. Spangler M. Mason JM. Lucas PA. Bioengineering of calvaria with adult stem cells. Plast Reconstr Surg. 2009;123:1178–1185. doi: 10.1097/PRS.0b013e31819f2949. [DOI] [PubMed] [Google Scholar]
- 5.Mizuta N. Hattori K. Suzawa Y. Iwai S. Matsumoto T. Tadokoro M. Nakano T. Akashi M. Ohgushi H. Yura Y. Mesenchymal stromal cells improve the osteogenic capabilities of mineralized agarose gels in a rat full-thickness cranial defect model. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.495. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Taichman RS. Wang Z. Shiozawa Y. Jung Y. Song J. Balduino A. Wang J. Patel LR. Havens AM, et al. Prospective identification and skeletal localization of cells capable of multilineage differentiation in vivo. Stem Cells Dev. 2010;19:1557–1570. doi: 10.1089/scd.2009.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z. Song J. Taichman RS. Krebsbach PH. Ablation of proliferating marrow with 5-fluorouracil allows partial purification of mesenchymal stem cells. Stem Cells. 2006;24:1573–1582. doi: 10.1634/stemcells.2005-0399. [DOI] [PubMed] [Google Scholar]
- 8.Kucia M. Zuba-Surma EK. Wysoczynski M. Wu W. Ratajczak J. Machalinski B. Ratajczak MZ. Adult marrow-derived very small embryonic-like stem cells and tissue engineering. Expert Opin Biol Ther. 2007;7:1499–1514. doi: 10.1517/14712598.7.10.1499. [DOI] [PubMed] [Google Scholar]
- 9.Ratajczak MZ. Kucia M. Reca R. Majka M. Janowska-Wieczorek A. Ratajczak J. Stem cell plasticity revisited: CXCR4-positive cells expressing mRNA for early muscle, liver and neural cells “hide out” in the bone marrow. Leukemia. 2004;18:29–40. doi: 10.1038/sj.leu.2403184. [DOI] [PubMed] [Google Scholar]
- 10.Ratajczak MZ. Machalinski B. Wojakowski W. Ratajczak J. Kucia M. A hypothesis for an embryonic origin of pluripotent Oct-4(+) stem cells in adult bone marrow and other tissues. Leukemia. 2007;21:860–867. doi: 10.1038/sj.leu.2404630. [DOI] [PubMed] [Google Scholar]
- 11.Ratajczak MZ. Majka M. Kucia M. Drukala J. Pietrzkowski Z. Peiper S. Janowska-Wieczorek A. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells. 2003;21:363–371. doi: 10.1634/stemcells.21-3-363. [DOI] [PubMed] [Google Scholar]
- 12.Song J. Kiel MJ. Wang Z. Wang J. Taichman RS. Morrison SJ. Krebsbach PH. An in vivo model to study and manipulate the hematopoietic stem cell niche. Blood. 2010;115:2592–2600. doi: 10.1182/blood-2009-01-200071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aybar Odstrcil A. Territoriale E. Missana L. An experimental model in calvaria to evaluate bone therapies. Acta Odontol Latinoam. 2005;18:63–67. [PubMed] [Google Scholar]
- 14.Franceschi RT. Wang D. Krebsbach PH. Rutherford RB. Gene therapy for bone formation: in vitro and in vivo osteogenic activity of an adenovirus expressing BMP7. J Cell Biochem. 2000;78:476–486. doi: 10.1002/1097-4644(20000901)78:3<476::aid-jcb12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Bianco P. Gehron Robey P. Marrow stromal stem cells. J Clin Invest. 2000;105:1663–1668. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castro-Malaspina H. Gay RE. Resnick G. Kapoor N. Meyers P. Chiarieri D. McKenzie S. Broxmeyer HE. Moore MA. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56:289–301. [PubMed] [Google Scholar]
- 17.Nussenbaum B. Rutherford RB. Krebsbach PH. Bone regeneration in cranial defects previously treated with radiation. Laryngoscope. 2005;115:1170–1177. doi: 10.1097/01.MLG.0000166513.74247.CC. [DOI] [PubMed] [Google Scholar]
- 18.Pittenger MF. Mackay AM. Beck SC. Jaiswal RK. Douglas R. Mosca JD. Moorman MA. Simonetti DW. Craig S. Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 19.Simmons PJ. Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 20.Lee RH. Hsu SC. Munoz J. Jung JS. Lee NR. Pochampally R. Prockop DJ. A subset of human rapidly self-renewing marrow stromal cells preferentially engraft in mice. Blood. 2006;107:2153–2161. doi: 10.1182/blood-2005-07-2701. [DOI] [PubMed] [Google Scholar]
- 21.Havens AM. Pedersen EA. Shiozawa Y. Ying C. Jung Y. Sun Y. Neeley C. Wang J. Mehra R, et al. An in vivo mouse model for human prostate cancer metastasis. Neoplasia. 2008;10:371–380. doi: 10.1593/neo.08154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh AJ. Demiralp B. Neiva KG. Hooten J. Nohutcu RM. Shim H. Datta NS. Taichman RS. McCauley LK. Cells of the osteoclast lineage as mediators of the anabolic actions of parathyroid hormone in bone. Endocrinology. 2005;146:4584–4596. doi: 10.1210/en.2005-0333. [DOI] [PubMed] [Google Scholar]
- 23.Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005;105:2631–2639. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 24.Kucia M. Reca R. Campbell FR. Zuba-Surma E. Majka M. Ratajczak J. Ratajczak MZ. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 25.Kucia M. Halasa M. Wysoczynski M. Baskiewicz-Masiuk M. Moldenhawer S. Zuba-Surma E. Czajka R. Wojakowski W. Machalinski B. Ratajczak MZ. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia. 2007;21:297–303. doi: 10.1038/sj.leu.2404470. [DOI] [PubMed] [Google Scholar]
- 26.Kucia M. Wu W. Ratajczak MZ. Bone marrow-derived very small embryonic-like stem cells: their developmental origin and biological significance. Dev Dyn. 2007;236:3309–3320. doi: 10.1002/dvdy.21180. [DOI] [PubMed] [Google Scholar]
- 27.Kucia M. Wysoczynski M. Ratajczak J. Ratajczak MZ. Identification of very small embryonic like (VSEL) stem cells in bone marrow. Cell Tissue Res. 2008;331:125–134. doi: 10.1007/s00441-007-0485-4. [DOI] [PubMed] [Google Scholar]
- 28.Kucia M. Zhang YP. Reca R. Wysoczynski M. Machalinski B. Majka M. Ildstad ST. Ratajczak J. Shields CB. Ratajczak MZ. Cells enriched in markers of neural tissue-committed stem cells reside in the bone marrow and are mobilized into the peripheral blood following stroke. Leukemia. 2006;20:18–28. doi: 10.1038/sj.leu.2404011. [DOI] [PubMed] [Google Scholar]
- 29.Kucia M. Zuba-Surma E. Wysoczynski M. Dobrowolska H. Reca R. Ratajczak J. Ratajczak MZ. Physiological and pathological consequences of identification of very small embryonic like (VSEL) stem cells in adult bone marrow. J Physiol Pharmacol. 2006;57(Suppl 5):5–18. [PubMed] [Google Scholar]
- 30.Kucia MJ. Wysoczynski M. Wu W. Zuba-Surma EK. Ratajczak J. Ratajczak MZ. Evidence that very small embryonic-like stem cells are mobilized into peripheral blood. Stem Cells. 2008;26:2083–2092. doi: 10.1634/stemcells.2007-0922. [DOI] [PubMed] [Google Scholar]
- 31.Ratajczak MZ. Zuba-Surma EK. Wysoczynski M. Ratajczak J. Kucia M. Very small embryonic-like stem cells: characterization, developmental origin, and biological significance. Exp Hematol. 2008;36:742–751. doi: 10.1016/j.exphem.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuba-Surma EK. Klich I. Greco N. Laughlin MJ. Ratajczak J. Ratajczak MZ. Optimization of isolation and further characterization of umbilical-cord-blood-derived very small embryonic/epiblast-like stem cells (VSELs) Eur J Haematol. 2010;84:34–46. doi: 10.1111/j.1600-0609.2009.01352.x. [DOI] [PubMed] [Google Scholar]
- 33.Zuba-Surma EK. Kucia M. Abdel-Latif A. Dawn B. Hall B. Singh R. Lillard JW., Jr. Ratajczak MZ. Morphological characterization of very small embryonic-like stem cells (VSELs) by ImageStream system analysis. J Cell Mol Med. 2008;12:292–303. doi: 10.1111/j.1582-4934.2007.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuba-Surma EK. Kucia M. Dawn B. Guo Y. Ratajczak MZ. Bolli R. Bone marrow-derived pluripotent very small embryonic-like stem cells (VSELs) are mobilized after acute myocardial infarction. J Mol Cell Cardiol. 2008;44:865–873. doi: 10.1016/j.yjmcc.2008.02.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zuba-Surma EK. Kucia M. Wu W. Klich I. Lillard JW., Jr. Ratajczak J. Ratajczak MZ. Very small embryonic-like stem cells are present in adult murine organs: imagestream-based morphological analysis and distribution studies. Cytometry A. 2008;73A:1116–1127. doi: 10.1002/cyto.a.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dawn B. Tiwari S. Kucia MJ. Zuba-Surma EK. Guo Y. Sanganalmath SK. Abdel-Latif A. Hunt G. Vincent RJ, et al. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26:1646–1655. doi: 10.1634/stemcells.2007-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y. Kucia M. Hussain LR. Wen Y. Xu H. Yan J. Ratajczak MZ. Ildstad ST. Bone marrow transplantation temporarily improves pancreatic function in streptozotocin-induced diabetes: potential involvement of very small embryonic-like cells. Transplantation. 2010;89:677–685. doi: 10.1097/TP.0b013e3181c9dc7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zuba-Surma EK. Wu W. Ratajczak J. Kucia M. Ratajczak MZ. Very small embryonic-like stem cells in adult tissues-potential implications for aging. Mech Ageing Dev. 2009;130:58–66. doi: 10.1016/j.mad.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ratajczak MZ. Zuba-Surma EK. Shin DM. Ratajczak J. Kucia M. Very small embryonic-like (VSEL) stem cells in adult organs and their potential role in rejuvenation of tissues and longevity. Exp Gerontol. 2008;43:1009–1017. doi: 10.1016/j.exger.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ratajczak MZ. Kucia M. Ratajczak J. Zuba-Surma EK. A multi-instrumental approach to identify and purify very small embryonic like stem cells (VSELs) from adult tissues. Micron. 2009;40:386–393. doi: 10.1016/j.micron.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 41.Gundberg CM. Weinstein RS. Multiple immunoreactive forms of osteocalcin in uremic serum. J Clin Invest. 1986;77:1762–1767. doi: 10.1172/JCI112499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hauschka PV. Lian JB. Cole DE. Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 43.Li L. Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137:811–819. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koller MR. Palsson MA. Manchel I. Maher RJ. Palsson BO. Tissue culture surface characteristics influence the expansion of human bone marrow cells. Biomaterials. 1998;19:1963–1972. doi: 10.1016/s0142-9612(98)00101-x. [DOI] [PubMed] [Google Scholar]
- 46.Wojakowski W. Tendera M. Kucia M. Zuba-Surma E. Paczkowska E. Ciosek J. Halasa M. Krol M. Kazmierski M, et al. Mobilization of bone marrow-derived Oct-4+ SSEA-4+ very small embryonic-like stem cells in patients with acute myocardial infarction. J Am Coll Cardiol. 2009;53:1–9. doi: 10.1016/j.jacc.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paczkowska E. Kucia M. Koziarska D. Halasa M. Safranow K. Masiuk M. Karbicka A. Nowik M. Nowacki P. Ratajczak MZ. Machalinski B. Clinical evidence that very small embryonic-like stem cells are mobilized into peripheral blood in patients after stroke. Stroke. 2009;40:1237–1244. doi: 10.1161/STROKEAHA.108.535062. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y. Gao L. Zuba-Surma EK. Peng X. Kucia M. Ratajczak MZ. Wang W. Enzmann V. Kaplan HJ. Dean DC. Identification of small Sca-1(+), Lin(-), CD45(-) multipotential cells in the neonatal murine retina. Exp Hematol. 2009;37:1096–1107. doi: 10.1016/j.exphem.2009.05.014. 1107 e1. [DOI] [PubMed] [Google Scholar]
- 49.Shin DM. Liu R. Klich I. Wu W. Ratajczak J. Kucia M. Ratajczak MZ. Molecular signature of adult bone marrow-purified very small embryonic-like stem cells supports their developmental epiblast/germ line origin. Leukemia. 2010;24:1450–1461. doi: 10.1038/leu.2010.121. [DOI] [PubMed] [Google Scholar]
- 50.Ratajczak J. Wysoczynski M. Zuba-Surma E. Wan W. Kucia M. Yoder MC. Ratajczak MZ. Adult murine bone marrow-derived very small embryonic-like stem cells differentiate into the hematopoietic lineage after coculture over OP9 stromal cells. Exp Hematol. 2011;39:225–237. doi: 10.1016/j.exphem.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]