Abstract
Pluripotent stem cells offer unprecedented potential not only for human medicine but also for veterinary medicine, particularly in relation to the horse. Induced pluripotent stem cells (iPSCs) are particularly promising, as they are functionally similar to embryonic stem cells and can be generated in vitro in a patient-specific manner. In this study, we report the generation of equine iPSCs from skin fibroblasts obtained from a foal and reprogrammed using viral vectors coding for murine Oct4, Sox2, c-Myc, and Klf4 sequences. The reprogrammed cell lines were morphologically similar to iPSCs reported from other species and could be stably maintained over more than 30 passages. Immunostaining and polymerase chain reaction analyses revealed that these cell lines expressed an array of endogenous markers associated with pluripotency, including OCT4, SOX2, NANOG, REX1, LIN28, SSEA1, SSEA4, and TRA1-60. Furthermore, under the appropriate conditions, the equine iPSCs readily formed embryoid bodies and differentiated in vitro into cells expressing markers of ectoderm, mesoderm, and endoderm, and when injected into immunodeficient mice, gave raise to tumors containing differentiated derivatives of the 3 germ layers. Finally, we also reprogrammed fibroblasts from a 2-year-old horse. The reprogrammed cells were similar to iPSCs derived from neonatal fibroblasts in terms of morphology, expression of pluripotency markers, and differentiation ability. The generation of these novel cell lines constitutes an important step toward the understanding of pluripotency in the horse, and paves the way for iPSC technology to potentially become a powerful research and clinical tool in veterinary biomedicine.
Introduction
Horses are highly valued as both companion and sporting animals; the horse industry is estimated to be worth US$300 billion worldwide. Equine health is a major concern to the horse racing industry with the cost of injuries and illnesses worldwide being about US$6.5 billion each year [1]. Musculoskeletal problems are a leading cause of poor health among race horses. For example, as many as 5% of competition horses will suffer from tendon or ligament injuries during their careers, and only 25%–50% of those will compete again [2]. Traditional therapeutic options only provide a short-term solution and are associated with a high rate of reoccurrence [3]. The therapeutic use of stem cells provides, in principle, a better alternative to achieve restoration of normal tissue function, and adult bone marrow- or adipose tissue-derived cells have been used clinically for the treatment of musculoskeletal injuries in horses during the last decade with encouraging results [4]. However, several factors severely limit the efficacy of current adult stem cell therapies, namely the very low fraction of truly multipotent precursor cells that can be obtained in vivo (<0.01%), the high heterogeneity of such cell populations, and their limited replication and differentiation potential [5,6]. In this context, the derivation of pluripotent stem cell lines from horses may provide a superior alternative, as such cells have the ability to proliferate indefinitely while maintaining an undifferentiated state, and have unrestricted differentiation potential. However, attempts to obtain bona fide embryonic stem cells (ESCs) from species other than rodents and humans have been largely unsuccessful [7]. At present, 2 different teams have reported the generation of ES-like cells from horses [8,9]. The reported cell lines displayed ESC features, but failed to form teratomas after injection into mice [9].
A major breakthrough in stem cell biology occurred in 2006 when Takahashi and Yamanaka reported the production of pluripotent stem cells in vitro by using retroviruses to force the expression of 4 transcription factors, Oct4, Sox2, Klf4, and c-Myc, into adult mouse fibroblasts [10]. Since then, induced pluripotent stem cells (iPSCs) or iPSC-like cells have been derived from different species, including human [11], rhesus monkey [12], rat [13], pig [14,15], dog [16,17], rabbit [18], marmoset [19], sheep [20–22], and more recently, horse [23] and cow [24]. Transgene-mediated reprogramming offers distinct technical advantages over other established reprogramming techniques, and the resulting cell lines are functionally comparable to ESCs [25]. Moreover, iPSCs can, in principle, be produced in a patient-specific manner, a feature that would provide these cells with considerable potential for regenerative medicine and in vitro disease modeling. However, full realization of this potential will first require addressing several limitations associated with the current iPSC technology that at present severely restrict any therapeutic prospects of available iPSC lines [26].
In this report, we describe the generation of equine pluripotent stem cell lines from nonfetal sources by reprogramming of fibroblasts obtained from a newborn foal and from a 2-year-old horse using retroviruses coding for mouse Oct4, Sox2, c-Myc, and Klf4 sequences. We show that the reprogrammed cells express several markers of pluripotent cells, including novel ones in equine, and can differentiate into derivatives of the 3 germ layers both in vitro and in vivo.
Materials and Methods
Cell culture
Fibroblast cultures were separately derived from skin samples collected from a newborn male foal and from a 2-year-old gelding at the equine hospital of the School of Veterinary Studies, University of Edinburgh. Fibroblasts were grown and expanded from skin explants in Dulbecco's modified Eagle medium (DMEM) (Sigma-Aldrich, Irvine, United Kingdom) containing 10% fetal bovine serum (FBS) gold (PAA Laboratories Ltd., Yeovil, United Kingdom), 2 mM l-glutamine (Invitrogen, Paisley, United Kingdom), 0.1 mM minimum essential medium (MEM) nonessential amino acids (Invitrogen), and 1% penicillin–streptomycin (Invitrogen) at 37°C in 5% CO2. Once cells reached 90% confluence, they were passaged using TrypLE™ (Invitrogen). For reprogramming experiments, fibroblasts at passage <7 were used.
Putative iPSCs were grown on mitotically inactivated SNL feeder cells (CBA-316; Cell Biolabs, San Diego, CA) on 6-well plates coated with 0.1% gelatin (Sigma-Aldrich) using a medium containing either the DMEM (Invitrogen) with 20% fetal calf serum or a knockout DMEM (Invitrogen) supplemented with 20% knockout serum replacement (Invitrogen). Medium preparations also contained 2 mM l-glutamine, 0.1 mM β-mercaptoethanol (Invitrogen), 0.1 mM MEM nonessential amino acids and 1% penicillin–streptomycin, and were supplemented with 8 ng/mL human basic fibroblast growth factor (bFGF) (Peprotech, London, United Kingdom) and/or 1,000 U/mL human leukemia inhibitory factor (LIF) (Millipore, Watford, United Kingdom). Cells were kept at 37°C in 5% CO2. The medium was changed every other day, and the cells were passaged every 3 days using Accutase™ (Sigma-Aldrich).
SNL feeder cells [27] were maintained in the DMEM containing 10% FBS gold, 2 mM l-glutamine, 0.1 mM MEM nonessential amino acids, and 1% penicillin–streptomycin. These cells were mitotically inactivated by incubation with mitomycin C (10 μg/mL; Sigma-Aldrich) for 2 h, followed by dissociation with TrypLE and incubation for at least 1 day in iPSC medium described above before seeding of putative iPSCs.
Viral constructs and cell reprogramming
Mouse cDNA sequences for Oct4, Sox2, Klf4, and c-Myc that had been cloned into a Moloney Murine Leukemia Virus backbone (pMXs) [28] were obtained from Addgene (Cambridge, MA). Viral particles were produced by individually transfecting each of these constructs with the retroviral packaging vector, pCL-10A1 (Imgenex; Cambridge Bioscience, Cambridge, United Kingdom) using FuGENE (Roche, West Sussex, United Kingdom) in human embryonic kidney (HEK) cells (American Type Culture Collection, Manassas, VA). In addition, the AcGFP1 sequence was excised from a pAcGFP1-C1 vector (Clontech, Mountain View, CA) and amplified before being inserted in the retroviral packaging vector pCLXSN (Imgenex).
Two days after transfection of HEK cells, supernatants containing concentrates of each of the viral particles encoding for the reprogramming factors were collected, mixed, filtered, and added to equine fibroblasts that had been seeded 1 day earlier on gelatin-coated 6-well plates (1.3×105cells/well). To assess transduction efficiency, some fibroblasts were transduced with the pCLXSN-GFP vector only. In all cases, the transduction procedure was repeated 1 day later, as described [11]. Cells were passaged onto a feeder layer in 10-cm dishes (5×104 cells/dish) the following day and transferred to iPSC medium 2 days later. Beginning 2 weeks after transduction, appearing colonies were mechanically passaged to 96-well plates and individually expanded by further passaging. Three different reprogramming experiments were performed, and colonies with similar characteristics were obtained in all cases.
Polymerase chain reaction analyses
Endogenous expression of pluripotency genes in reprogrammed cells was determined by reverse transcription-polymerase chain reaction (RT-PCR) using primers specifically recognizing equine sequences (Table 1). Genomic integration and expression of the viral transgenes were assessed by PCR on gDNA and cDNA, respectively, using primers specific for each of the mouse transcription factors and a primer complementary to a common flanking sequence in the viral backbone (Table 1). Genomic DNA and total RNA were extracted with a DNeasy Blood and Tissue Kit (Qiagen, Crawley, United Kingdom) and an RNeasy mini kit (Qiagen), respectively. RNA was reverse-transcribed using Superscript III (Invitrogen) as per the manufacturer's instructions, and PCR was performed with BIOTAQ™ DNA polymerase (Bioline, London, United Kingdom) using an annealing temperature of 60°C–65°C and 40 cycles. PCR products were resolved in 3% agarose gels stained with Sybr Safe (Invitrogen) and visualized with the Kodak Gel Logic 200 imaging system.
Table 1.
List of Primers Used for Polymerase Chain Reaction Analyses
| Primer | Sequence | Product (bp) |
|---|---|---|
| OCT4 (For) | ATTGAGACCCGAGTGAGAGG | 74 |
| OCT4 (Rev) | CTGATCTGCTGCAGTGTGG | |
| SOX2 (For)a | CACCCACAGCAAATGACAGC | 252 |
| SOX2 (Rev)a | TTTCTGCAAAGCTCCTACCG | |
| NANOG (For)b | TCCTCAATGACAGATTTCAGAGA | 323 |
| NANOG (Rev)b | GAGCACCAGGTCTGACTGTT | |
| DNMT3b (For) | CTTCTGCGTGGAGTGTCTGG | 169 |
| DNMT3b (Rev) | GGTGTCGCTGGTAAAGAAGG | |
| LIN28 (For) | CATGGGCTCTGTGTCAAACC | 167 |
| LIN28 (Rev) | CGGTCATGGACAGGAAGC | |
| REX1 (For) | GACGGGAAAGGCCTGGATAGAAG | 297 |
| REX1 (Rev) | GGCGGTAAGAAGCTGTTGAGAAAGG | |
| GAPDH (For) | CATCATCCCTGCTTCTACTGG | 117 |
| GAPDH (Rev) | TCCACGACTGACACGTTAGG | |
| Viral Common (For) | GGATCCCAGTGTGGTGGTACG | |
| Viral Oct4 (Rev) | CTGTAGGGAGGGCTTCGGGCACTT | 850 |
| Viral Sox2 (Rev) | TCACATGTGCGACAGGGGCAG | 1,000 |
| Viral Klf4 (Rev) | TTAGGCTGTTCTTTTCCGGGGCCACGA | 1,200 |
| Viral c-Myc (Rev) | TTATGCACCAGAGTTTCGAAGCTGTTC | 1,400 |
Southern blotting
Ten micrograms of genomic DNA were digested overnight with BamHI and then electrophoresed in a 0.8% agarose/Tris-Acetate-EDTA gel and transferred to a Hybond-N membrane (GE Healthcare, Little Chalfont, United Kingdom). This was then hybridized overnight at 65°C with probes isolated from pMXs-Oct4 and pMXs-Klf4 constructs and labeled using a High Prime DNA labeling kit (Roche) and 32P dCTP (Perkin Elmer, Cambridge, United Kingdom). After washing, membranes were exposed to a phosphor screen for 3 days and visualized using a Typhoon phosphorimage (GE Healthcare). Before rehybridization, membranes were stripped using boiling 0.1% sodium dodecyl sulfate.
Karyotyping
Cells were synchronized by incubation with thymidine (10 mg/mL; Sigma-Aldrich) for 15 h. After washings with the DMEM containing 10% FBS, cells were incubated for a further 5 h, followed by a second synchronization with thymidine. After washing, cells were incubated with colcemid (10 μg/mL; Invitrogen) for 2.5 h, and the supernatant was aspirated, and cells were washed with phosphate-buffered saline (PBS) before being harvested using TrypLE. After centrifugation at 1,200 rpm for 8 min, the cell pellet was incubated with 0.56% KCl for 8 min at room temperature, and an ice-cold fixative mixture (acetic acid and methanol 1:3) was added. Centrifugation followed by addition of ice-cold fixative was repeated 2 more times. Finally, 10–20 μL of suspension was smeared onto a slide, allowed to dry overnight at 37°C, stained with a Giemsa solution for 15 min, and washed in PBS before mounting a coverslip.
Alkaline phosphatase staining
Alkaline phosphatase activity was determined in reprogrammed cells using a commercial kit (86R; Sigma-Aldrich) following the manufacturer's instructions.
Immunocytochemistry
Cells were fixed in 4% paraformaldehyde for 15 min at room temperature and then permeabilized in a 0.5% Triton–PBS solution, followed by blocking for 50 min in PBS containing 2% bovine serum albumin, 0.05% Tween20, and 0.05% Triton. Primary antibody was then incubated overnight at 4°C. The next day, after 3 washes in PBS, 1-h incubation with secondary antibody (Alexa Fluor 568 or 488; Invitrogen) was performed at room temperature. Cells were then washed in PBS, and a mounting solution containing 4′,6-diamidino-2-phenylindole (Sigma-Aldrich) was added before sealing with a coverslip. Specific antibodies and dilutions used are indicated in Table 2. The slides were observed on an Axiovert 25 inverted microscope and a Nikon EC1 confocal microscope using the 20×, 40×, and 63× lenses. The images obtained were processed with ImageJ software version 1.42q (http://rsb.info.nih.gov/ij).
Table 2.
List of Antibodies Used for Immunocytochemistry
| Marker | Supplier | Product no. | Dilution |
|---|---|---|---|
| Oct4 | Santa Cruz | Sc-5279 | 1/300 |
| Sox2 | Santa Cruz | Sc-17320 | 1/200 |
| SSEA1 | DSHB | MC480 | 1/100 |
| SSEA4 | Abcam | ab16287 | 1/300 |
| Nanog | Abcam | ab80892 | 1/200 |
| Lin28 | Abcam | ab63740 | 1/200 |
| TRA1-60 | Cell Signalling | 9656 | 1/200 |
| Rex 1 | Millipore | MAB4316 | 1/200 |
| TUJ-1 | R&D Systems | MAB1195 | 1/100 |
| AFP | R&D Systems | MAB1368 | 1/200 |
| Troponin I | Abcam | ab19615 | 1/100 |
| Vimentin | Abcam | ab8978 | 1/300 |
| Pan-cytokeratin | Sigma | C5992 | 1/200 |
| Nestin | Millipore | 07-449 | 1/100 |
| GATA4 | Abcam | ab5694 | 1/300 |
| ASMA | Abcam | ab56944 | 1/100 |
Induction of cell differentiation in vitro
Putative equine iPSCs were harvested, passaged in a bacterial culture dish, and allowed to grow in suspension for 7 days in the DMEM containing 10% FBS. The resulting embryoid bodies (EBs) were then transferred onto gelatin-coated 6-well plates in the same medium and allowed to differentiate for 3 weeks, after which they were fixed before immunostaining.
Induction of cell differentiation in vivo
Putative equine iPSCs were harvested with TrypLE, and 5×106 cells resuspended in 100 μL of the DMEM medium containing 10 mM HEPES, 10% FBS, and 200 nM l-glutamine. Fifteen microliters of the resulting cell slurry was surgically grafted under the kidney capsule of NOD/SCID mice. Animals were euthanized between 5 and 8 weeks after injection, and tumors recovered and processed for histological analysis. All animal procedures were carried out under the United Kingdom Home Office Animals (Scientific Procedures) Act 1986, after approval by the Ethics Review Committee, University of Edinburgh (Project License no. 60/3715).
Results
Reprogramming of equine fibroblasts
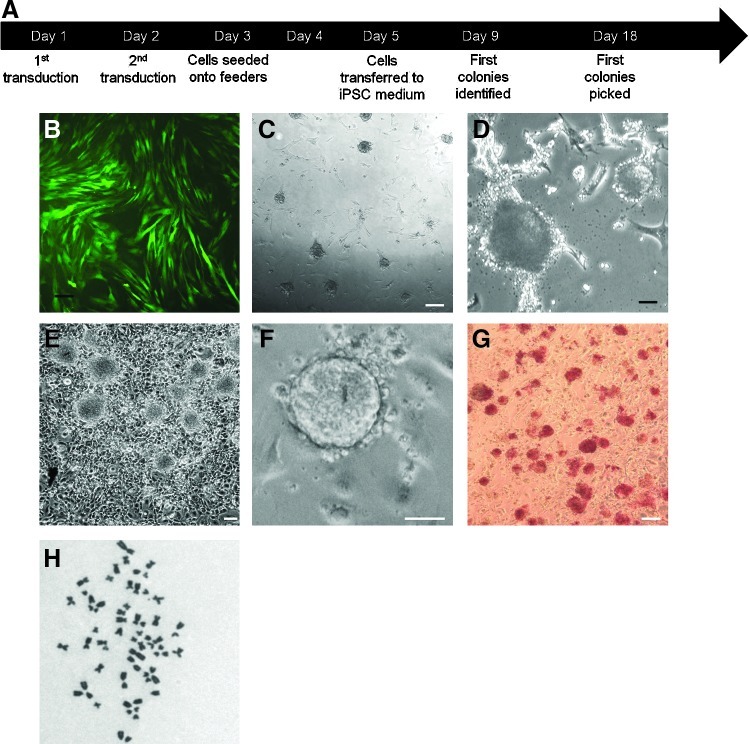
We initially reprogrammed fibroblasts derived from a newborn foal. Chronological events during cell reprogramming are schematically shown in Fig. 1A. Over 75% of fibroblasts transduced with control virus showed clear expression of green fluorescence protein (Fig. 1B), indicating that the viral vectors used were able to induce efficient transgene expression in these cells. Nine days after transduction with the 4 transcription factor-coding viruses, the first iPSC-like colonies distinctly appeared in the culture dishes (Fig. 1C). These consisted of tight, dome-shaped cell aggregates with well-defined borders, somewhat resembling naïve-type pluripotent stem cell colonies generated from rodents [10,13] and also reported from sheep [22] (Fig. 1D). This was followed by new colonies appearing periodically over the next 4 weeks. Beginning on day 18 after transduction, colonies were individually selected on the basis of their ESC-like morphology, picked, dissociated, and transferred onto feeder layers in 96-well plates. New colonies continued to be collected until day 40, and were in all cases expanded by sequential passaging onto fresh feeder layers. When expanded in a medium supplemented with FBS and LIF, putative equine iPSC colonies acquired a relatively loose, granulated appearance after a few passages, with abundant detaching cells visible at the edges, suggestive of differentiation. In contrast, after being transferred onto a serum-free medium supplemented with both bFGF and LIF, colonies maintained their iPS-like appearance, characterized by tight colonies with defined edges, a high nucleus-to-cytoplasm ratio, and prominent nucleoli (Fig. 1E, F).
FIG. 1.
Reprogramming of equine fibroblasts. (A) Chronological outline of equine fibroblast reprogramming. (B) Photomicrograph of parental fibroblasts after transduction with pCLXSN-GFP. (C) Early cell colonies appearing the second week after transduction of parental fibroblasts. (D) Close-up of colonies in (C). (E) Typical morphology of an established iPSC line (line H, passage 14). (F) Close-up of a colony from the same cell line. (G) Equine iPSCs (passage 6) stained for alkaline phosphatase. (H) Metaphasic spread of equine iPSCs (line H) stained with Giemsa showing a normal chromosomal complement of 64. White scale bar=100 μm; black scale bar=20 μm. iPSCs, induced pluripotent stem cells. Color images available online at www.liebertpub.com/scd
A total of 103 colonies were initially picked, and 27 of those were expanded. Twelve colonies could be maintained for up to at least 6 passages and all showed alkaline phosphatase staining (Fig. 1G). Results from characterization of 4 of these cell lines (H, U, E, B) are shown below. These 4 lines have now been robustly expanded (over 30 passages in the case of lines H and U), and they display a typical equine karyotype (Fig. 1H).
Expression of pluripotency markers by reprogrammed cells
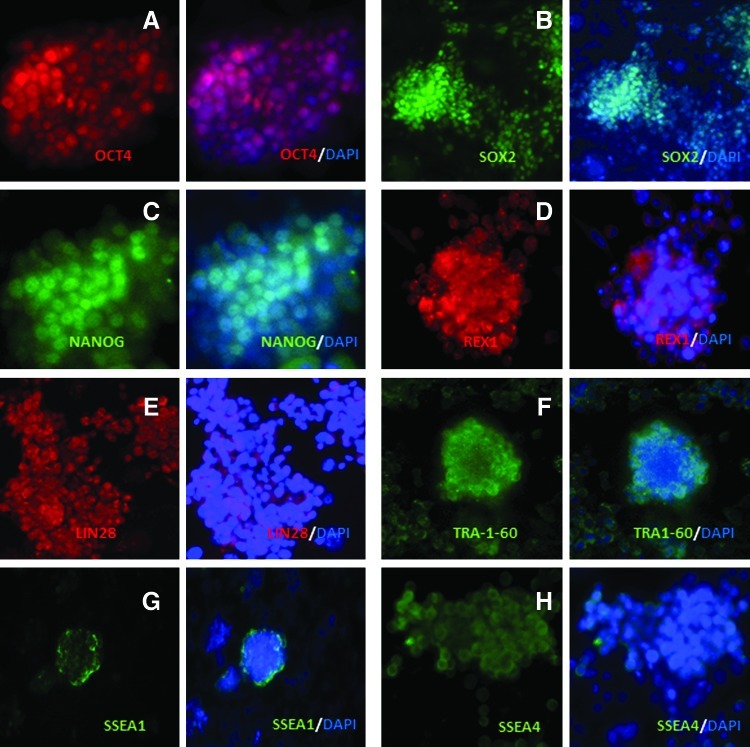
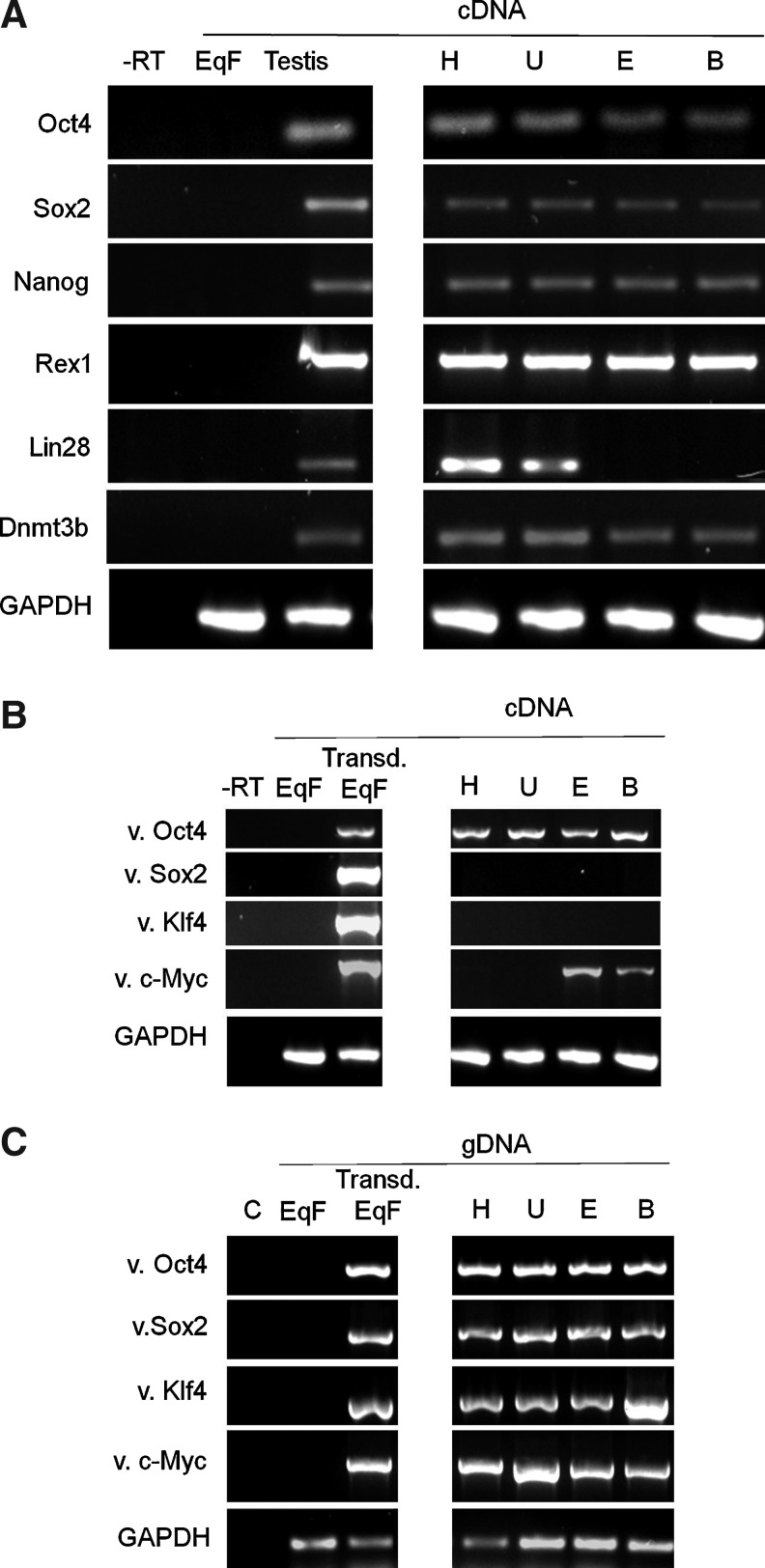
Immunofluorescence and RT-PCR analyses of the 4 putative iPSC lines generated from foal fibroblasts revealed expression of the endogenous pluripotency markers, OCT4, SOX2, NANOG, and REX1, with 2 of the lines also showing detectable expression of LIN28 (Figs. 2 and 3A). Additionally, all lines showed transcriptional activation of DNMT3B (Fig. 3A), although this could not be confirmed at the protein level, as a suitable antibody was not available. Immunofluorescence analyses also revealed the presence of the pluripotency-associated cell surface antigens, TRA1-60, SSEA1, and SSEA4, in the cell lines (Fig. 2). The parental equine fibroblasts did not show immunostaining for any of these markers (Supplementary Fig. S1A–H; Supplementary Data are available online at www.liebertpub.com/scd), whereas mouse ESCs stained positively for all the markers analyzed, except TRA-1-60 and SSEA-4 (Supplementary Fig. S2, left and middle panels), consistent with previous reports [29]. PCR was also used to determine the expression of the virus-encoded factors in the established cell lines (Fig. 3B). As expected, the 4 transgenes were expressed in equine fibroblasts 3 days after transduction with viruses. In addition, all 4 putative iPSC lines showed persistent expression of Oct4, whereas 2 of the lines also displayed detectable levels of c-Myc. Despite variable expression of the transgenes in the reprogrammed cell lines, further analyses revealed effective genomic integration of the 4 viral sequences in all lines (Fig. 3C). Southern blot analyses showed the same viral integration pattern in 3 of the 4 lines examined (H, U, and B), indicating that these lines had originally derived clonally from the same integration event, whereas line E originated from a different event (data not shown). Nonetheless, taken together, the results of expression analyses indicate effective transcriptional activation of the endogenous pluripotency machinery in the reprogrammed equine cells.
FIG. 2.
Pluripotency marker immunostaining of reprogrammed cell lines. Representative images showing immunostaining of equine iPSCs (line H) for (A) OCT4, (B) SOX2 (C) NANOG, (D) REX1, (E) LIN28, (F) TRA1-60, (G) SSEA1, and (H) SSEA4. Secondary antibody was conjugated to Alexa Fluor 568 (red) or Alexa Fluor 488 (green), and DAPI was used for nuclear counterstaining (blue). The second and fourth columns of the panel display the merged images for Alexa Fluor and DAPI. Scale bar=50 μm. DAPI, 4′,6-diamidino-2-phenylindole. Color images available online at www.liebertpub.com/scd
FIG. 3.
PCR analysis of reprogrammed cell lines. (A) Messenger RNA levels of equine-specific pluripotency gene sequences in equine testis (positive control) and in reprogrammed cell lines (H, U, E, and B). In all cases, nontransduced equine fibroblasts (EqF) were used as negative controls, and a no-RT control (-RT) was also included. GAPDH was used as loading control. (B) Messenger RNA levels of viral transgenes determined by RT-PCR in equine fibroblasts 3 days after transduction with retroviruses (Transd EqF) and in reprogrammed cell lines. (C) Genomic DNA levels of viral transgenes determined by PCR in equine fibroblasts 3 days after transduction and in reprogrammed cell lines. A no-template control (C) was included. RT-PCR, RT-polymerase chain reaction.
In vitro differentiation potential of reprogrammed cells
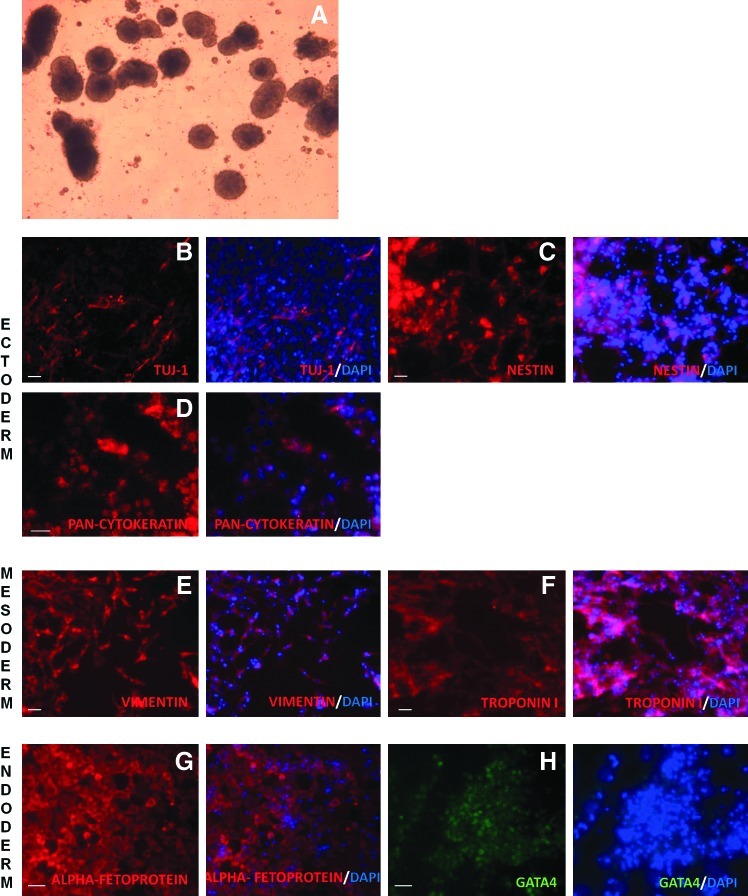
The capacity of putative iPSCs generated from foal fibroblasts to undergo differentiation in vitro was investigated by placing the cells on a nonadherent Petri dish with FBS in the absence of growth factors. Under such conditions, these cells were able to readily form EBs (Fig. 4A), which were then seeded on gelatin-coated plates to allow differentiation. Outgrowths of heterogeneous populations of differentiating cells began to appear soon after, and 28 days after plating, the differentiated cells were immunostained. Different populations of cells were identified that expressed markers of ectoderm (Fig. 4B–D), mesoderm (Fig. 4E, F), or endoderm (Fig. 4G, H). Adult equine tissues were also stained as positive controls for the different differentiation markers analyzed (Supplementary Fig. S3).
FIG. 4.
In vitro differentiation potential of reprogrammed cell lines. (A) Embryoid bodies forming 5 days after equine iPSCs (line H, passage 12) were placed in suspension culture. (B–H) Immunostaining of differentiated cells for markers of ectoderm (B–D), mesoderm (E, F), or endoderm (G, H). Secondary antibody was conjugated to Alexa Fluor 568 (red) or Alexa Fluor 488 (green), and DAPI was used for nuclear counterstaining (blue). The second and fourth columns of the panel display the merged images for Alexa Fluor and DAPI. Scale bar=20 μm. Color images available online at www.liebertpub.com/scd
Ability of equine iPSCs to undergo multilineage differentiation in vivo
To determine whether putative equine iPSCs displayed in vivo pluripotency, line H (passage 17) was injected into the kidney capsule of 3 SCID mice. All injected mice developed tumors, which appeared as large globular solid masses about 2 cm in diameter at 5–8 weeks after injection (Fig. 5A). These tumors showed discrete areas of necrosis, which may have resulted from their relatively high growth rate. Further, all tumors showed clear evidence of differentiation into derivatives of the 3 germ layers (Fig. 5B–D), including keratinized epithelium (ectoderm), bone (mesoderm), and gut-like epithelium (endoderm).
FIG. 5.
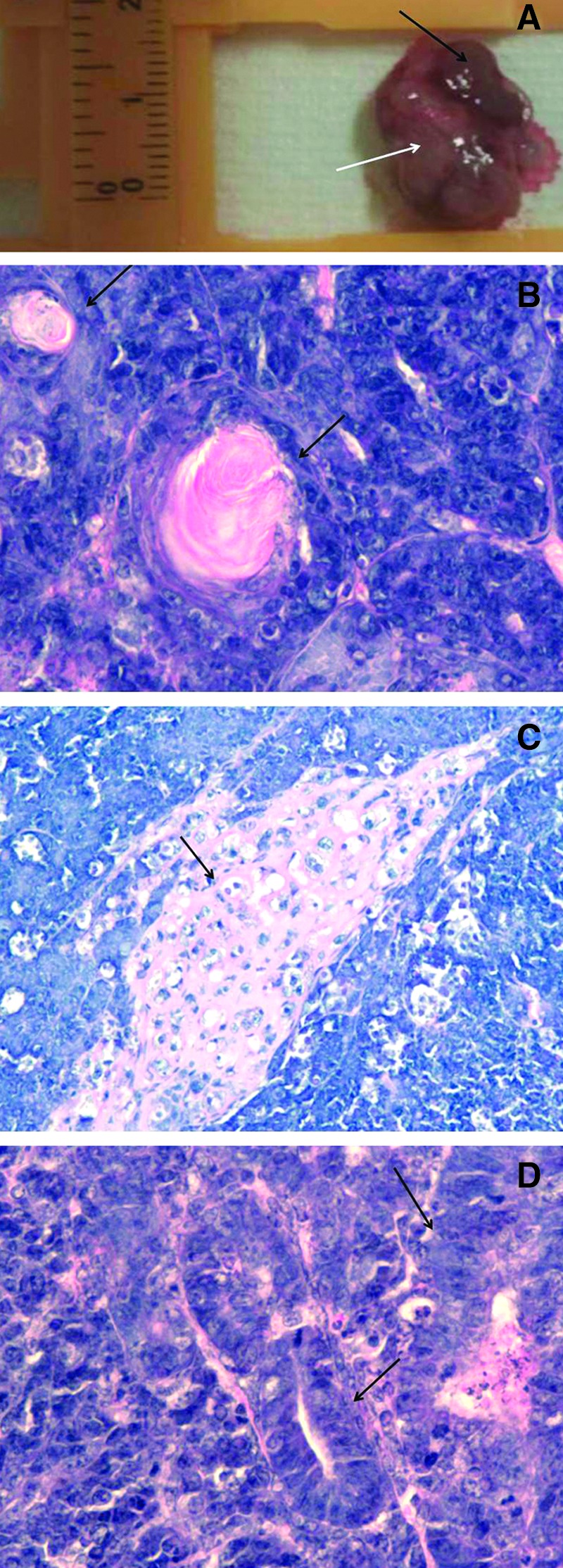
In vivo differentiation potential of reprogrammed cell lines. (A) Tumor resulting from injection of equine iPSCs into SCID mice is pictured. The tumor can be seen (white arrow) growing adjacent to the kidney (identified by a black arrow). Tumors obtained contained differentiated derivatives of the 3 germ layers (stained with hematoxylin and eosin), including keratinized epithelium (B), bone (C), and gut-like epithelium (D) structures (identified in each photo by black arrows). Color images available online at www.liebertpub.com/scd
Reprogramming of fibroblasts from an adult horse
Finally, since the eventual application of iPSC technology in equine regenerative medicine would most often involve derivation of iPSCs from young performance horses rather than neonates, we aimed to determine whether fibroblasts obtained from a 2-year-old gelding could also be reprogrammed to pluripotency. Reprogrammed colonies were obtained that were morphologically similar to iPSCs derived from foal fibroblasts. Six different colonies were picked and gave rise to different putative clonal populations. One of the resulting cell lines (Fig. 6A) was further analyzed for the expression of selected pluripotency markers, namely ALP, OCT4, SOX2, LIN28, and TRA-1-60, and for its ability to differentiate into derivatives of the 3 germ layers. The putative adult derived iPSCs stained positively for the above pluripotency markers (Fig. 6B–F), consistent with results with iPSCs derived from foal fibroblasts. Further, they readily formed EBs in vitro (Fig. 6G) that were able to give rise to cells expressing markers of ectoderm (TUJ-1), mesoderm (alpha-smooth muscle actin), and endoderm (alpha-fetoprotein) (Fig. 6H–J), demonstrating their capacity to differentiate into multiple cell lineages.
FIG. 6.
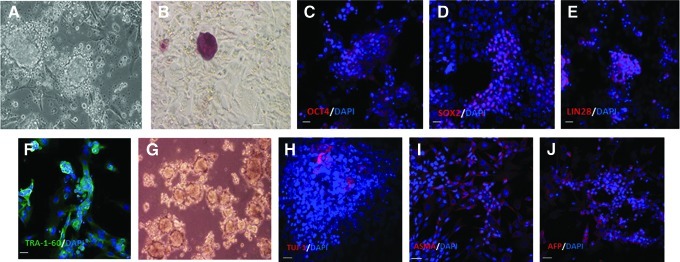
Characteristics of reprogrammed cell lines from adult equine fibroblasts. (A) Colonies derived from reprogramming of fibroblasts from a 2-year-old gelding and which stained positively for (B) alkaline phosphatase, (C) OCT4, (D) SOX2, (E) LIN28, and (F) TRA-1-60. (G) These cells readily generated embryoid bodies in vitro, which produced cells expressing markers of (H) ectoderm, (I) mesoderm, and (J) endoderm. Secondary antibody was conjugated to Alexa Fluor 568 (red) or Alexa Fluor 488 (green), and DAPI was used for nuclear counterstaining (blue). Merged images for Alexa Fluor and DAPI are shown. Scale bar=20 μm. Color images available online at www.liebertpub.com/scd
Discussion
In this report, we describe the establishment of stable equine iPSC lines using the 4-transcription factor technique, initially described by Takahashi and Yamanaka [10]. Unlike previous reports, we used nonfetal cell sources to generate equine pluripotent cell lines. Embryonic stem-like cells have been derived from horse blastocysts [8,9], whereas in a recent study, equine iPSCs were obtained from retrotransposon-mediated reprogramming of fibroblasts taken from 55-day-old conceptuses, which correspond to a very early stage during the 11-month long period of prenatal development in the horse [23]. In other species, iPSCs were also generated, at least initially, using fetal fibroblasts, as they represent a relatively undifferentiated cell type compared to later developmental stages, and therefore they are, in principle, more readily reprogrammable [10,14,15,21]. Consistent with this, it has been shown that, relative to adult mesenchymal cells, equine fetal fibroblasts express high levels of key reprogramming factors [30] that very likely facilitate the reprogramming of these cells. In contrast, in the present study, we could not detect expression of any of the pluripotency factors analyzed in parental fibroblasts, either by immunocytochemistry or by PCR, consistent with their postfetal origin. The present results represent a step forward toward the generation and biomedical application of reprogrammed cells from clinical equine patients.
The equine cell lines generated in this study showed numerous features associated with pluripotency. These include transcriptional reactivation of endogenous OCT4 and expression of 3 different cell surface antigens, all of which have been reported in the inner cell mass of equine blastocysts [31] as well as in equine ESC-like cells [8,9,32] and iPSCs [23]. Equine iPSCs in the present report also expressed NANOG, in agreement with the earlier iPSC report by Nagy et al. [23], as well as other ESC-associated factors, namely SOX2, REX1, LIN28, and DNMT3B, which are transcriptionally activated during reprogramming of human, mouse, and pig cells [10,15,33], but have not been previously reported in equine pluripotent cells. The reactivation of endogenous pluripotency markers is well known to be associated with late stages of reprogramming [34]. Furthermore, Chan et al. [35] demonstrated that 3 of the markers found in our equine iPSCs, namely TRA-1-60, REX1, and DNMT3B, were bona fide indicators of fully reprogrammed human iPSCs, a conclusion that is consistent with the ability of the equine iPSCs reported in the present study to readily produce differentiated teratomas upon injection into SCID mice. In a previous study [23], equine cells reportedly resembling primed-type PSCs were generated using an inducible transposon-based expression system and culture conditions that included LIF and bFGF as well as a combination of different signaling pathway inhibitors that are reportedly necessary to generate naïve-type PSCs [36]. In the present study, stable equine pluripotent cell lines could be generated and maintained without the need to use signaling inhibitors. Compared with the cells reported by Nagy et al. [23], in general, our cell lines did not display obvious morphological features of typical primed-type PSCs, but they predominantly grew as relatively tight, dome-like shaped cell aggregates, which is more typical of naïve-type PSCs. These apparent differences in the cells obtained between the 2 studies may have been derived from differences in the conditions used to generate and maintain the reprogrammed cells and/or from differences in the criteria used to select ESC-like colonies for expansion. Similar discrepancies in the morphology of iPSCs from the same species have been reported for sheep and pig [21,22,37,38], and they could be attributed to the use of human ESC versus mouse ESC culture conditions in some studies [38], but not in others [21,22]. Clearly, greater understanding of the molecular pathways involved in pluripotency in these species, as well as of the different pluripotent states that may be potentially generated in vitro [38], is required to reconcile these discrepancies.
The MMLV vector used for reprogramming in this study was similar to the one used to generate the first mouse and human iPSC lines [10,11]. Because, in principle, such vectors become transcriptionally repressed during the late stages of reprogramming [10,11], in some studies, stable iPSC lines could not be generated using MMLV vectors, but the use of lentiviral vectors, which may not undergo the same levels of transcriptional repression, was required [13]. Further, many successful attempts to derive iPSC lines from domestic species, including horse, have involved the use of lentiviral or other expression vectors under transcriptional control by an inducible promoter, which ensures sustained transgene expression required to achieve and maintain the pluripotent state [14,15,20,23]. The equine iPSCs reported in the present study showed partial silencing of transgenes characterized by sustained expression of Oct4 in all the lines examined and expression of c-Myc in some of the lines. This pattern is consistent with that reported in sheep iPSCs produced using the same vectors [22]. In addition, studies using a similar retroviral expression system in other species often reported silencing of one or several of the transgenes in the iPSC lines generated, except for Oct4, whose expression was usually maintained in all or most of the lines [13,33,39,40]. These findings suggest that clonal populations that fail to silence viral Oct4 may be distinctly selected for during reprogramming. In that context, the ectopic Oct4 may critically contribute, together with the reactivated endogenous pluripotency genes, to the attainment and maintenance of the induced pluripotent state. So far, all iPSC lines reported from domestic species have shown to be dependent on continuous transgene expression for long-term propagation, as demonstrated with the use of inducible reprogramming vectors [14,20,21,41,42]. Similarly, equine iPSCs described in an earlier study quickly underwent differentiation after the expression of reprogramming genes from a transposon-based vector was turned off [23]. Significant risks associated with insertional mutagenesis and incomplete transgene silencing severely restrict the clinical potential of iPSCs generated with current virus-based technology, a limitation that will only be overcome once robust iPSC lines can be efficiently generated using integration-free or nongenetic approaches [34]. An interesting observation in the present study was that 3 of the foal-derived iPSC lines characterized (H, U, and B) appeared to be clonally derived from the same integration event, yet these 3 lines were not phenotypically identical as showed by differences in the expression of pluripotency genes (LIN28) and viral c-Myc (Fig. 3); since the 3 lines were expanded using the same culture conditions, the observed differences likely reflect the stochastic nature of iPSCs derivation.
In the study by Nagy et al. [23], a lack of suitable antibodies prevented the authors from assessing the in vitro pluripotency of the reported equine iPSCs. In the present study, we used a panel of antibodies for various differentiation markers, which we validated in adult equine tissues (Supplementary Fig. S3), to demonstrate the capacity of reprogrammed equine cells to undergo differentiation into derivatives of the 3 germ layers in vitro. The pluripotency of putative equine iPSCs in vivo was demonstrated by injecting these cells into the kidney capsule of SCID mice, a route that is technically more demanding than subcutaneous or intramuscular injection, but that facilitates discrimination between the resulting tumor and host tissues. In previous studies, equine ESC-like cells failed to produce tumors when injected into the testes of SCID mice [9] whereas subcutaneous injection of putative equine iPSCs resulted in growth of tumors after 4 months and only after injected mice had been temporarily fed with doxycycline to maintain expression of the reprogramming transgenes, a strategy that was reportedly necessary to avoid premature cell differentiation in vivo [23]. Equine iPSCs in the present study were able to spontaneously maintain their pluripotency in vivo and generated tumors that grew over a period, 5–8 weeks, which was intermediate between periods normally reported for teratomas derived from mouse and human iPSCs [10,11]. Outgrowths resulting from the injected cell line in our study were confirmed to be teratomas, as they contained differentiated derivatives of the 3 germ layers, constituting to this date the most stringent proof of pluripotency for equine iPSCs.
Finally, we also showed that adult equine fibroblasts can be reprogrammed to generate cell lines that are morphologically similar to iPSCs produced from neonatal fibroblasts, express similar pluripotency factors, and readily differentiate into derivatives of the 3 germ layers in vitro. The age of the donor animal in this experiment, 2 years, corresponds to the career peak of most racing horses, providing support to the prospect of using iPSCs for equine regenerative medicine in the future.
The potential of pluripotent stem cells in veterinary medicine, and particularly in equine health, is similar to that in human medicine. Although significant technical advances still need to be made to eliminate constraints associated with genetic, epigenetic, and immunogenic aspects of iPSCs that at the moment severely restrict their clinical potential [43], huge progress has already been made in the application of iPSC technology for in vitro modeling of diseases and therapeutics [26]. In that regard, there are a number of equine diseases that would be amenable to experimental modeling using iPSCs. Further, horses could be used as preclinical models for human stem cell-based therapies [1]. Our results provide an important step toward that goal by demonstrating that nonfetal equine somatic cells can be reprogrammed to cells that are pluripotent both in vitro and in vivo. These established cell lines should facilitate the realization of the veterinary potential of the iPSC technology.
Supplementary Material
Acknowledgments
We thank Drs. Simon Lilico, Chiara Sartori, and Alexandra DiDomenico for assisting with viral preparations and Sothern blotting, and for providing reagents for gene expression analyses. We are also grateful to Drs. Joe Mee, Rosa Rabanal, and Christopher Palgrave, and Prof. Dolors Fondevila for assistance and advice during in vivo work and tissue analyses, to Matt Hanks, Stephanie Schauer, and Ben Wentink for providing equine tissues, and to Drs. Tom Burdon and Alison Thomson for thoughtful comments throughout the project and during preparation of this manuscript. This work was supported by grants from the Horserace Betting Levy Board (Prj 744), the Royal College of Veterinary Surgeons Trust (grant no. 707), and the Biotechnology and Biological Sciences Research Council (BBSRC ISPG) to F.X.D.
Author Disclosure Statement
The authors have declared that no competing interests exist.
References
- 1.Tecirlioglu RT. Trounson AO. Embryonic stem cells in companion animals (horses, dogs and cats): present status and future prospects. Reprod Fertil Dev. 2007;19:740–747. doi: 10.1071/rd07039. [DOI] [PubMed] [Google Scholar]
- 2.Perkins NR. Reid SW. Morris RS. Risk factors for injury to the superficial digital flexor tendon and suspensory apparatus in Thoroughbred racehorses in New Zealand. N Z Vet J. 2005;53:184–192. doi: 10.1080/00480169.2005.36503. [DOI] [PubMed] [Google Scholar]
- 3.Fortier LA. Potter HG. Rickey EJ. Schnabel LV. Foo LF. Chong LR. Stokol T. Cheetham J. Nixon AJ. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92:1927–1937. doi: 10.2106/JBJS.I.01284. [DOI] [PubMed] [Google Scholar]
- 4.Ribitsch I. Burk J. Delling U. Geissler C. Gittel C. Julke H. Brehm W. Basic science and clinical application of stem cells in veterinary medicine. Adv Biochem Eng Biotechnol. 2010;123:219–263. doi: 10.1007/10_2010_66. [DOI] [PubMed] [Google Scholar]
- 5.Vidal MA. Kilroy GE. Johnson JR. Lopez MJ. Moore RM. Gimble JM. Cell growth characteristics and differentiation frequency of adherent equine bone marrow-derived mesenchymal stromal cells: adipogenic and osteogenic capacity. Vet Surg. 2006;35:601–610. doi: 10.1111/j.1532-950X.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- 6.Martins-Taylor K. Xu RH. Determinants of pluripotency: from avian, rodents, to primates. J Cell Biochem. 2010;109:16–25. doi: 10.1002/jcb.22402. [DOI] [PubMed] [Google Scholar]
- 7.Talbot NC. Blomberg Le A. The pursuit of ES cell lines of domesticated ungulates. Stem Cell Rev. 2008;4:235–254. doi: 10.1007/s12015-008-9026-0. [DOI] [PubMed] [Google Scholar]
- 8.Saito S. Ugai H. Sawai K. Yamamoto Y. Minamihashi A. Kurosaka K. Kobayashi Y. Murata T. Obata Y. Yokoyama K. Isolation of embryonic stem-like cells from equine blastocysts and their differentiation in vitro. FEBS Lett. 2002;531:389–396. doi: 10.1016/s0014-5793(02)03550-0. [DOI] [PubMed] [Google Scholar]
- 9.Li X. Zhou SG. Imreh MP. Ahrlund-Richter L. Allen WR. Horse embryonic stem cell lines from the proliferation of inner cell mass cells. Stem Cells Dev. 2006;15:523–531. doi: 10.1089/scd.2006.15.523. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Liu H. Zhu F. Yong J. Zhang P. Hou P. Li H. Jiang W. Cai J. Liu M, et al. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell. 2008;3:587–590. doi: 10.1016/j.stem.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Liao J. Cui C. Chen S. Ren J. Chen J. Gao Y. Li H. Jia N. Cheng L. Xiao H. Xiao L. Generation of induced pluripotent stem cell lines from adult rat cells. Cell Stem Cell. 2009;4:11–15. doi: 10.1016/j.stem.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Ezashi T. Telugu BP. Alexenko AP. Sachdev S. Sinha S. Roberts RM. Derivation of induced pluripotent stem cells from pig somatic cells. Proc Natl Acad Sci U S A. 2009;106:10993–10998. doi: 10.1073/pnas.0905284106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esteban MA. Xu J. Yang J. Peng M. Qin D. Li W. Jiang Z. Chen J. Deng K, et al. Generation of induced pluripotent stem cell lines from Tibetan miniature pig. J Biol Chem. 2009;284:17634–17640. doi: 10.1074/jbc.M109.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaags AK. Rosic-Kablar S. Gartley CJ. Zheng YZ. Chesney A. Villagomez DA. Kruth SA. Hough MR. Derivation and characterization of canine embryonic stem cell lines with in vitro and in vivo differentiation potential. Stem Cells. 2009;27:329–340. doi: 10.1634/stemcells.2008-0433. [DOI] [PubMed] [Google Scholar]
- 17.Luo J. Suhr ST. Chang EA. Wang K. Ross PJ. Nelson LL. Venta PJ. Knott JG. Cibelli JB. Generation of leukemia inhibitory factor and basic fibroblast growth factor-dependent induced pluripotent stem cells from canine adult somatic cells. Stem Cells Dev. 2011;20:1669–1678. doi: 10.1089/scd.2011.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Honda A. Hirose M. Hatori M. Matoba S. Miyoshi H. Inoue K. Ogura A. Generation of induced pluripotent stem cells in rabbits: potential experimental models for human regenerative medicine. J Biol Chem. 2010;285:31362–31369. doi: 10.1074/jbc.M110.150540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y. Zhang Y. Mishra A. Tardif SD. Hornsby PJ. Generation of induced pluripotent stem cells from newborn marmoset skin fibroblasts. Stem Cell Res. 2010;4:180–188. doi: 10.1016/j.scr.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao L. He L. Chen J. Wu Z. Liao J. Rao L. Ren J. Li H. Zhu H, et al. Reprogramming of ovine adult fibroblasts to pluripotency via drug-inducible expression of defined factors. Cell Res. 2011;21:600–608. doi: 10.1038/cr.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y. Cang M. Lee AS. Zhang K. Liu D. Reprogramming of sheep fibroblasts into pluripotency under a drug-inducible expression of mouse-derived defined factors. PLoS One. 2011;6:e15947. doi: 10.1371/journal.pone.0015947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sartori C. Didomenico AI. Thomson AJ. Milne E. Lillico SG. Burdon TG. Whitelaw CB. Ovine-induced pluripotent stem cells can contribute to chimeric lambs. Cell Reprogram. 2012;14:8–19. doi: 10.1089/cell.2011.0050. [DOI] [PubMed] [Google Scholar]
- 23.Nagy K. Sung HK. Zhang P. Laflamme S. Vincent P. Agha-Mohammadi S. Woltjen K. Monetti C. Michael IP. Smith LC. Nagy A. Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell Rev. 2011;7:693–702. doi: 10.1007/s12015-011-9239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumer H. Liu J. Malaver Ortega LF. Lim ML. Khodadadi K. Verma PJ. NANOG is a key factor for induction of pluripotency in bovine adult fibroblasts. J Anim Sci. 2011;89:2708–2716. doi: 10.2527/jas.2010-3666. [DOI] [PubMed] [Google Scholar]
- 25.Hochedlinger K. Jaenisch R. Nuclear reprogramming and pluripotency. Nature. 2006;441:1061–1067. doi: 10.1038/nature04955. [DOI] [PubMed] [Google Scholar]
- 26.Saha K. Jaenisch R. Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell. 2009;5:584–595. doi: 10.1016/j.stem.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan C. Hicks A. Guan X. Chen H. Bishop CE. SNL fibroblast feeder layers support derivation and maintenance of human induced pluripotent stem cells. J Genet Genomics. 2010;37:241–248. doi: 10.1016/S1673-8527(09)60042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitamura T. Koshino Y. Shibata F. Oki T. Nakajima H. Nosaka T. Kumagai H. Retrovirus-mediated gene transfer and expression cloning: powerful tools in functional genomics. Exp Hematol. 2003;31:1007–1014. [PubMed] [Google Scholar]
- 29.De Miguel MP. Fuentes-Julian S. Alcaina Y. Pluripotent stem cells: origin, maintenance and induction. Stem Cell Rev. 2010;6:633–649. doi: 10.1007/s12015-010-9170-1. [DOI] [PubMed] [Google Scholar]
- 30.Hackett CH. Greve L. Novakofski KD. Fortier LA. Comparison of gene-specific DNA methylation patterns in equine induced pluripotent stem cell lines with cells derived from equine adult and fetal tissues. Stem Cells Dev. 2011;21:1803–1811. doi: 10.1089/scd.2011.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guest DJ. Allen WR. Expression of cell-surface antigens and embryonic stem cell pluripotency genes in equine blastocysts. Stem Cells Dev. 2007;16:789–796. doi: 10.1089/scd.2007.0032. [DOI] [PubMed] [Google Scholar]
- 32.Saito S. Sawai K. Minamihashi A. Ugai H. Murata T. Yokoyama KK. Derivation, maintenance, and induction of the differentiation in vitro of equine embryonic stem cells. Methods Mol Biol. 2006;329:59–79. doi: 10.1385/1-59745-037-5:59. [DOI] [PubMed] [Google Scholar]
- 33.Lowry WE. Richter L. Yachechko R. Pyle AD. Tchieu J. Sridharan R. Clark AT. Plath K. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci U S A. 2008;105:2883–2888. doi: 10.1073/pnas.0711983105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amabile G. Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends Mol Med. 2009;15:59–68. doi: 10.1016/j.molmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Chan EM. Ratanasirintrawoot S. Park IH. Manos PD. Loh YH. Huo H. Miller JD. Hartung O. Rho J, et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol. 2009;27:1033–1037. doi: 10.1038/nbt.1580. [DOI] [PubMed] [Google Scholar]
- 36.Buehr M. Meek S. Blair K. Yang J. Ure J. Silva J. McLay R. Hall J. Ying QL. Smith A. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 37.Telugu BP. Ezashi T. Roberts RM. The promise of stem cell research in pigs and other ungulate species. Stem Cell Rev. 2010;6:31–41. doi: 10.1007/s12015-009-9101-1. [DOI] [PubMed] [Google Scholar]
- 38.Telugu BP. Ezashi T. Roberts RM. Porcine induced pluripotent stem cells analogous to naive and primed embryonic stem cells of the mouse. Int J Dev Biol. 2010;54:1703–1711. doi: 10.1387/ijdb.103200bt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W. Wei W. Zhu S. Zhu J. Shi Y. Lin T. Hao E. Hayek A. Deng H. Ding S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4:16–19. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 40.Aasen T. Raya A. Barrero MJ. Garreta E. Consiglio A. Gonzalez F. Vassena R. Bilic J. Pekarik V, et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 41.Li W. Ding S. Generation of novel rat and human pluripotent stem cells by reprogramming and chemical approaches. Methods Mol Biol. 2010;636:293–300. doi: 10.1007/978-1-60761-691-7_18. [DOI] [PubMed] [Google Scholar]
- 42.Wu Z. Chen J. Ren J. Bao L. Liao J. Cui C. Rao L. Li H. Gu Y, et al. Generation of pig induced pluripotent stem cells with a drug-inducible system. J Mol Cell Biol. 2009;1:46–54. doi: 10.1093/jmcb/mjp003. [DOI] [PubMed] [Google Scholar]
- 43.Barrilleaux B. Knoepfler PS. Inducing iPSCs to escape the dish. Cell Stem Cell. 2011;9:103–111. doi: 10.1016/j.stem.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.