Abstract
Free light chains are proteins produced by B lymphocytes during the process of antibody synthesis. Their production, as a reflection of B cell activation, can give insight into the activity of the adaptive immune system. In recent years, an automated immunoassay that provides quantitative measurement of free light chains in the serum has been developed. This assay has not only revolutionised the investigation of monoclonal light chain overproduction in plasma cell diseases, but has also allowed for the quantification of polyclonal free light chains in serum. The discovery of high levels of polyclonal free light chains in a number of inflammatory and auto-immune conditions has led to the examination of their value as a biomarker of disease activity. Research into their bio-activity has also highlighted their potential role in the pathogenesis of inflammatory disease, making them an attractive target for novel therapies.
Introduction
Dr H. Bence Jones first described free light chains when he linked the presence of a urinary protein to the diagnosis of "mollities ossium" in 1847 [1]. Immunoglobulin free light chains are a by-product of antibody synthesis by terminally differentiated B lymphocytes, a key element of the adaptive immune system. Antibodies are immunoglobulins with a tetrameric structure composed of two identical heavy chains and two identical light chains linked by disulphide bonds (Figure 1). There are two light chain isotypes: Kappa (κ) and Lambda (λ). Heavy chain and light chain proteins are assembled in the endoplasmic reticulum during immunoglobulin synthesis. During this process there is an excess of light chain production in the region of 500 mg per day [2,3]. Excess free light chains are secreted into the circulation, where rapid renal clearance results in a short half-life of 2-6 hours. In recent years, our advancing knowledge of their diverse immunological functions has sparked new interest in their potential pathogenic role in chronic inflammatory and autoimmune diseases. In this article we describe the recent advances in our ability to measure free light chains and explore their utility as a novel biomarker and potential therapeutic target.
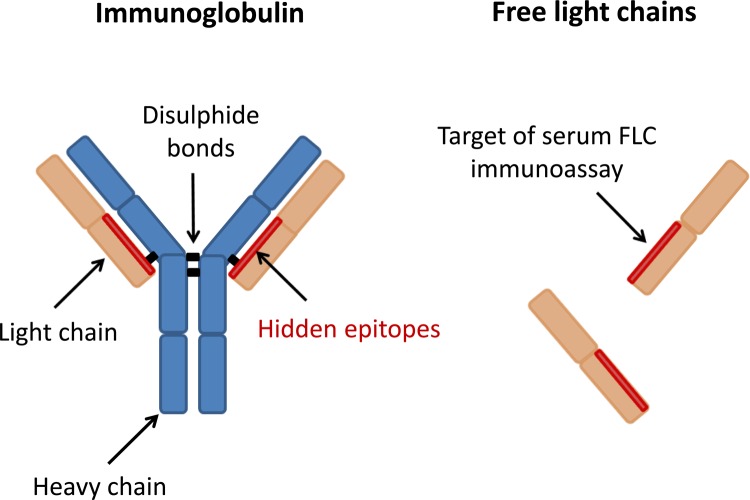
Figure 1. Intact immunoglobulin and free light chain structure.
Each immunoglobulin is composed of two heavy chains and two light chains linked by disulphide bonds. The variability of the amino acid sequence of the "variable region" is responsible for the antigen binding specificity of the antibody. There are two types of light chain termed kappa (κ) and lambda (λ). The serum immunoassay targets "hidden epitopes" found on the interface between the light and heavy chains in the intact immunoglobulin molecule.
Measurement of free light chains
Commercial methods for identifying free light chains utilising serum and urine protein electrophoresis and immunofixation electrophoresis have been problematic due to their lack of sensitivity and cumbersome methodology [4]. The advent of a highly sensitive nephelometric immunoassay that uses antibodies that bind to epitopes of free light chains that are “hidden” in intact immunoglobulin molecules has had a significant impact on research in this field [5] . Using this assay, reference and diagnostic ranges for serum free light chains and the κ/λ ratio were determined by analysing the sera of healthy donors and patients with monoclonal gammopathies [6]. Some analytical performance limitations have been identified, such as variation in free light chain concentration from the same sample assayed using different batches of polyclonal free light chain antiserum, and non-linear dilution of some monoclonal free light chains [7,8]. If there are large quantities of free light chain present in the serum, the phenomenon of “antigen excess”, where non-precipitating immune complexes can form and result in falsely low free light chain concentrations, is also well recognised [9-11]. Awareness of these issues and close links between biologists and clinicians involved has been highlighted as crucial for the optimal interpretation of results.
Free light chains and disease
Concentrations of serum free light chains are dependent on the balance between production and renal clearance [12]. There is extensive knowledge of monoclonal free light chain overproduction in haematological disorders due to clonal plasma cell proliferation, which is beyond the scope of this article. Polyclonal free light chain overproduction can also occur when there is an excess production of multiple immunoglobulins, usually as a result of chronic immune stimulation. In the context of polyclonal hypergammaglobulinamia or renal impairment the κ/λ ratio should remain unchanged [12].
Polyclonal free light chains: a biomarker for disease activity?
Increased free light chain concentrations have been described in a variety of inflammatory and autoimmune diseases including systemic lupus erythematosus (SLE) [13,14], rheumatoid arthritis, Sjögren’s syndrome [15], atopic dermatitis [16], asthma [17], rhinitis [18,19], food allergy [20], idiopathic pulmonary fibrosis, hypersensitivity pneumonitis [21], chronic obstructive pulmonary disease (COPD) [22], inflammatory bowel disease [23] and multiple sclerosis [24-26]. Evidence of the relationship of free light chain levels to disease activity in these conditions is emerging.
Gottenberg et al. were the first to demonstrate a relationship between free light chain concentrations and disease activity in patients with rheumatoid arthritis as measured by the Disease Activity Score 28 (DAS28) [15]. In this small study of 50 patients, they also demonstrated correlations between free light chains and other markers of B cell activation, such as total gammaglobulin, IgG and rheumatoid factor. Interestingly, total gammaglobulin and IgG levels did not correlate with the DAS28 in the same way as free light chains, which the authors felt may be accounted for by the short half-life of free light chains in comparison, making them a better marker of current disease activity. A larger prospective study of 710 patients with early arthritis also found elevated polyclonal free light chains in patients with early rheumatoid arthritis to correlate with DAS28 [27]. Correlation with disease activity has also been studied in patients with SLE. Hopper et al. demonstrated that clinical relapses can be associated with an antedescent elevation in urinary free light chains four to eight weeks prior to the onset of symptoms [14]. A larger study of 75 patients found serum free light chain levels (in addition to complement C3) correlated strongly with the SLE disease activity index (SLEDAI) and modified SLEDAI [13].
The use of free light chains as a biomarker of B cell activation has prompted evaluation of their potential role for monitoring the response to treatments, such as Rituximab (a monoclonal antibody that causes B cell depletion). In patients with rheumatoid arthritis treated with Rituximab, a significant reduction in serum free light chain concentrations has been seen only in patients that respond clinically. However, baseline levels have not been found to be a predictor of response [28]. A small study of 11 patients with SLE similarly found a significant reduction in free light chain concentrations following Rituximab therapy, which correlated with C3 consumption [29]. The growing enthusiasm for B cell-targeted therapies in inflammatory disease promotes the need for larger prospective studies to establish the importance of free light chain monitoring in these patients.
Serum free light chains have also been found to be raised in type 2 diabetes prior to the development of renal impairment, suggesting their possible role for predicting early diabetic nephropathy [30]. The fact that polyclonal free light chains are higher in patients with chronic kidney disease may seem unsurprising given their renal clearance but their pathogenic role in worsening tubular injury is also the subject of ongoing research [31].
Polyclonal free light chains and mortality
Dispenzieri et al. [32] have reported that a polyclonal increase in free light chains (measured by the combined sum of κ and λ concentrations) is a predictor of mortality in the general population. The study of 15,859 patients over the age of 50 without plasma cell dyscrasias found the excess risk of death was independent of age, sex and renal impairment [32]. Another study followed up 527 patients sent for routine haematological investigations (which had excluded a monoclonal gammopathy) and found the relative risk of death increased proportionally with combined free light chain concentrations. A combined concentration of >65 mg/l was found to be a risk factor for death with the highest prognostic value within the first 100 days. Forty one percent of patient deaths within this period were attributable to cardiovascular causes, suggesting that polyclonal free light chains might serve as a “cardiovascular risk factor” [33]. Given the established links between chronic inflammation and cardiovascular disease this certainly warrants further investigation.
Pathogenic role of free light chains: could they be a therapeutic target?
As evidence of the biological activity of free light chains is emerging, it has challenged the concept that their excess production is inconsequential (Figure 2) [34]. Hutchinson et al. have recently demonstrated that free light chains can bind to a broad range of cell membranes but have a high binding affinity with monocytes [35]. Given the important role that monocytes play in antigen presentation, this has prompted them to question whether one of the roles of free light chains is to assist antigen uptake by cells and hence promote the associated immune response. The ability of free light chains to bind to antigen independently is controversial [36,37], although a recent study by Thio et al. used a variety of techniques to show that free light chains could bind to antigen with significant affinity [38]. To date no specific free light chain receptor has been identified.
Figure 2. Free light chain production, biological interactions and metabolism.
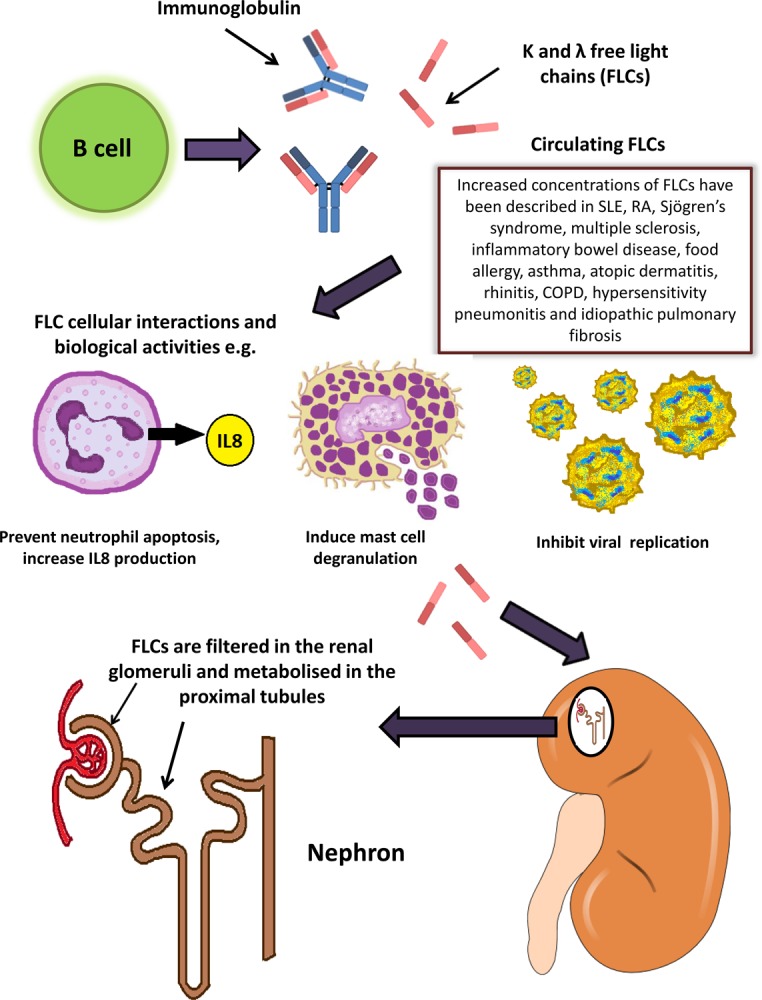
Free light chains are produced by B cells, released into the circulation and have been shown to exert a variety of biological functions, including inhibition of neutrophil apoptosis and viral replication, and induction of mast cell degranulation. Free light chains are filtered in the renal glomeruli and metabolised in the proximal tubule of the kidney.
Free light chains and hypersensitivity reactions
In 2002, Redegeld et al. demonstrated that free light chains can elicit immediate hypersensitivity responses in sensitised mice and described how in vitro cross-linking of free light chains and mast cell surface proteins can induce degranulation [39]. F991 is a 9-mer peptide that acts as an immunoglobulin free light chain antagonist. It was derived using the amino acid sequence responsible for binding immunoglobulin light chains to Tamm-Horsfall glycoprotein, which is secreted from the ascending loop of Henle [40]. The potential role of free light chains in the pathogenesis of non-atopic asthma was subsequently raised by using a murine model to show that the use of F991 can abate the development of air flow obstruction, airway hyperresponsiveness and inflammation [17]. Similarly, the use of F991 in a murine model for inflammatory colitis has been shown to inhibit mast cell activation and prevent the development of diarrhoea [23].
Free light chain interaction with neutrophils
Free light chains have been shown to inhibit the apoptosis of neutrophils, which are key effector cells in the inflammatory process [41]. The increased concentration of free light chains in patients with end-stage renal disease has therefore been implicated as a cause for the pro-inflammatory state of these patients [42]. More recently, Braber et al. have described a link between free light chains and neutrophils in the pathogenesis of COPD. Increased serum free light chain concentrations were also found in three different murine models with emphysema. Antagonising the free light chain using F991 led to a reduction in neutrophil influx in the murine lungs [22]. The researchers also demonstrated that the binding of free light chains to human neutrophils results in in vitro production of interleukin-8, which can cause neutrophil activation, a key pathological feature in lung disease [22].
Protective role of free light chains in disease
It is important to point out that free light chains have also been shown to have a potentially protective role. Matsumori et al. [43] demonstrated increased free light chain expression in mice infected with encephalomyocarditis virus. However, the application of F991 worsened the degree of myocarditis, whereas supplementation with free light chains led to reduced necrosis and improved outcome. Free light chains were shown to inhibit encephalomyocarditis viral replication in vitro [43].
Conclusion
Measuring polyclonal free light chains as a marker of B cell activation can give new insight into the activity of the adaptive immune system in a variety of inflammatory conditions [44]. However, further large prospective trials are needed to establish their role as a biomarker of disease activity and predictor of mortality in different patient populations. The increasing use of B-cell-targeted therapies in the treatment of autoimmune diseases means the potential utility of free light chains in risk-stratifying patients for treatment initiation, as well as monitoring response to therapy, is an exciting prospect for future research.
Free light chains have been shown to exert a variety of biological activities, and as our knowledge of their immunological function increases, the growing evidence suggesting their involvement in pathogenesis makes them an attractive target for novel therapies. Preclinical models using free light chain antagonists seem promising, but we do not yet know if this will translate clinically, and given the evidence of their protective effect to certain infections, their safety will need to be monitored closely. There are still many important questions that remain unanswered, such as whether, and where, free light chain receptors exist and their role, as well as antigen specificity of the light chains. Further investigation is needed to increase our understanding of the biological role of free light chains in inflammatory disease.
Abbreviations
- SLE
systemic lupus erythematosus
- COPD
chronic obstructive pulmonary disease
- DAS28
disease activity score 28
- SLEDAI
SLE disease activity index
Disclosures
The authors declare that they have no disclosures.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/m/5/4/
References
- 1.Jones HB. Papers on chemical pathology, lecture III. The Lancet. 1847;11:88–92. doi: 10.1016/S0140-6736(02)86528-X. [DOI] [Google Scholar]
- 2.Solomon A. Light chains of human immunoglobulins. Meth. Enzymol. 1985;116:101–21. doi: 10.1016/S0076-6879(85)16008-8. [DOI] [PubMed] [Google Scholar]
- 3.Waldmann TA, Strober W, Mogielnicki RP. The renal handling of low molecular weight proteins. II. Disorders of serum protein catabolism in patients with tubular proteinuria, the nephrotic syndrome, or uremia. J. Clin. Invest. 1972;51:2162–74. doi: 10.1172/JCI107023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levinson SS, Keren DF. Free light chains of immunoglobulins: clinical laboratory analysis. Clin. Chem. 1994;40:1869–78. [PubMed] [Google Scholar]
- 5.Bradwell AR, Carr-Smith HD, Mead GP, Tang LX, Showell PJ, Drayson MT, Drew R. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin. Chem. 2001;47:673–80. [PubMed] [Google Scholar]; http://f1000.com/prime/717971155
- 6.Katzmann JA, Clark RJ, Abraham RS, Bryant S, Lymp JF, Bradwell AR, Kyle RA. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin. Chem. 2002;48:1437–44. [PubMed] [Google Scholar]
- 7.Tate JR, Mollee P, Dimeski G, Carter AC, Gill D. Analytical performance of serum free light-chain assay during monitoring of patients with monoclonal light-chain diseases. Clin. Chim. Acta. 2007;376:30–6. doi: 10.1016/j.cca.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Briand P, Decaux O, Caillon H, Grosbois B, Le Treut A, Guenet L. Analytical performance of the serum free light chain assay. Clin. Chem. Lab. Med. 2010;48:73–9. doi: 10.1515/cclm.2010.012. [DOI] [PubMed] [Google Scholar]
- 9.Bosmann M, Kössler J, Stolz H, Walter U, Knop S, Steigerwald U. Detection of serum free light chains: the problem with antigen excess. Clin. Chem. Lab. Med. 2010;48:1419–22. doi: 10.1515/cclm.2010.283. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971157
- 10.Murata K, Clark RJ, Lockington KS, Tostrud LJ, Greipp PR, Katzmann JA. Sharply increased serum free light-chain concentrations after treatment for multiple myeloma. Clin. Chem. 2010;56:16–8. doi: 10.1373/clinchem.2009.133041. [DOI] [PubMed] [Google Scholar]
- 11.Vercammen M, Meirlaen P, Broodtaerts L, Vande Broek I, Bossuyt X. Effect of sample dilution on serum free light chain concentration by immunonephelometric assay. Clin. Chim. Acta. 2011;412:1798–804. doi: 10.1016/j.cca.2011.06.021. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971158
- 12.Bradwell AR. Serum free light chain measurements move to center stage. Clin. Chem. 2005;51:805–7. doi: 10.1373/clinchem.2005.048017. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal R, Sequeira W, Kokebie R, Mikolaitis RA, Fogg L, Finnegan A, Plaas A, Block JA, Jolly M. Serum free light chains as biomarkers for systemic lupus erythematosus disease activity. Arthritis Care Res (Hoboken) 2011;63:891–8. doi: 10.1002/acr.20446. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971159
- 14.Hopper JE, Sequeira W, Martellotto J, Papagiannes E, Perna L, Skosey JL. Clinical relapse in systemic lupus erythematosus: correlation with antecedent elevation of urinary free light-chain immunoglobulin. J. Clin. Immunol. 1989;9:338–50. doi: 10.1007/BF00918666. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971160
- 15.Gottenberg J, Aucouturier F, Goetz J, Sordet C, Jahn I, Busson M, Cayuela J, Sibilia J, Mariette X. Serum immunoglobulin free light chain assessment in rheumatoid arthritis and primary Sjogren's syndrome. Ann. Rheum. Dis. 2007;66:23–7. doi: 10.1136/ard.2006.052159. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717971161
- 16.Kayserova J, Capkova S, Skalicka A, Vernerova E, Polouckova A, Malinova V, Bartunkova J, Sediva A. Serum immunoglobulin free light chains in severe forms of atopic dermatitis. Scand. J. Immunol. 2010;71:312–6. doi: 10.1111/j.1365-3083.2010.02376.x. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971162
- 17.Kraneveld AD, Kool M, van Houwelingen AH, Roholl P, Solomon A, Postma DS, Nijkamp FP, Redegeld FA. Elicitation of allergic asthma by immunoglobulin free light chains. Proc. Natl. Acad. Sci. U.S.A. 2005;102:1578–83. doi: 10.1073/pnas.0406808102. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717971163
- 18.Groot Kormelink T, Calus L, Ruyck N de, Holtappels G, Bachert C, Redegeld FA, Gevaert P. Local free light chain expression is increased in chronic rhinosinusitis with nasal polyps. Allergy. 2012;67:1165–72. doi: 10.1111/j.1398-9995.2012.02866.x. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971164
- 19.Powe DG, Groot Kormelink T, Sisson M, Blokhuis BJ, Kramer MF, Jones NS, Redegeld FA. Evidence for the involvement of free light chain immunoglobulins in allergic and nonallergic rhinitis. J. Allergy Clin. Immunol. 2010;125:139–45. doi: 10.1016/j.jaci.2009.07.025. e1-3. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971165
- 20.Schouten B, van Esch BCAM, van Thuijl AOJ, Blokhuis BRJ, Groot Kormelink T, Hofman GA, Moro GE, Boehm G, Arslanoglu S, Sprikkelman AB, Willemsen LEM, Knippels LMJ, Redegeld FA, Garssen J. Contribution of IgE and immunoglobulin free light chain in the allergic reaction to cow's milk proteins. J. Allergy Clin. Immunol. 2010;125:1308–14. doi: 10.1016/j.jaci.2010.02.039. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971166
- 21.Groot Kormelink T, Pardo A, Knipping K, Buendía-Roldán I, García-de-Alba C, Blokhuis BR, Selman M, Redegeld FA. Immunoglobulin free light chains are increased in hypersensitivity pneumonitis and idiopathic pulmonary fibrosis. PLoS ONE. 2011;6:e25392. doi: 10.1371/journal.pone.0025392. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717971167
- 22.Braber S, Thio M, Blokhuis BR, Henricks PAJ, Koelink PJ, Groot Kormelink T, Bezemer GFG, Kerstjens HAM, Postma DS, Garssen J, Kraneveld AD, Redegeld FA, Folkerts G. An association between neutrophils and immunoglobulin free light chains in the pathogenesis of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012;185:817–24. doi: 10.1164/rccm.201104-0761OC. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971168
- 23.Rijnierse A, Redegeld FA, Blokhuis BR, van der Heijden MW, Te Velde AA, Pronk I, Hommes DW, Nijkamp FP, Koster AS, Kraneveld AD. Ig-free light chains play a crucial role in murine mast cell-dependent colitis and are associated with human inflammatory bowel diseases. J. Immunol. 2010;185:653–9. doi: 10.4049/jimmunol.0901129. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971169
- 24.Fagnart OC, Sindic CJ, Laterre C. Free kappa and lambda light chain levels in the cerebrospinal fluid of patients with multiple sclerosis and other neurological diseases. J. Neuroimmunol. 1988;19:119–32. doi: 10.1016/0165-5728(88)90041-0. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971170
- 25.Kaplan B, Aizenbud BM, Golderman S, Yaskariev R, Sela B. Free light chain monomers in the diagnosis of multiple sclerosis. J. Neuroimmunol. 2010;229:263–71. doi: 10.1016/j.jneuroim.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Presslauer S, Milosavljevic D, Brücke T, Bayer P, Hübl W, Hübl W. Elevated levels of kappa free light chains in CSF support the diagnosis of multiple sclerosis. J. Neurol. 2008;255:1508–14. doi: 10.1007/s00415-008-0954-z. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971171
- 27.Gottenberg J, Miceli-Richard C, Ducot B, Goupille P, Combe B, Mariette X. Markers of B-lymphocyte activation are elevated in patients with early rheumatoid arthritis and correlated with disease activity in the ESPOIR cohort. Arthritis Res. Ther. 2009;11:R114. doi: 10.1186/ar2773. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1165520
- 28.Kormelink TG, Tekstra J, Thurlings RM, Boumans MHJ, Vos K, Tak PP, Bijlsma JWJ, Lafeber FPJG, Redegeld FA, van Roon JAG. Decrease in immunoglobulin free light chains in patients with rheumatoid arthritis upon rituximab (anti-CD20) treatment correlates with decrease in disease activity. Ann. Rheum. Dis. 2010;69:2137–44. doi: 10.1136/ard.2009.126441. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971172
- 29.Chiche L, Cournac JM, Mancini J, Bardin N, Thomas G, Jean R, Schleinitz N, Kaplanski G, Durand JM, Boucraut J, Harlé JR. Normalization of serum-free light chains in patients with systemic lupus erythematosus upon rituximab treatment and correlation with biological disease activity. Clin. Rheumatol. 2011;30:685–9. doi: 10.1007/s10067-010-1674-1. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971173
- 30.Hutchison CA, Cockwell P, Harding S, Mead GP, Bradwell AR, Barnett AH. Quantitative assessment of serum and urinary polyclonal free light chains in patients with type II diabetes: an early marker of diabetic kidney disease? Expert Opin. Ther. Targets. 2008;12:667–76. doi: 10.1517/14728222.12.6.667. [DOI] [PubMed] [Google Scholar]
- 31.Hutchison CA, Harding S, Hewins P, Mead GP, Townsend J, Bradwell AR, Cockwell P. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1684–90. doi: 10.2215/CJN.02290508. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717971174
- 32.Dispenzieri A, Katzmann JA, Kyle RA, Larson DR, Therneau TM, Colby CL, Clark RJ, Mead GP, Kumar S, Melton LJ, Rajkumar SV. Use of nonclonal serum immunoglobulin free light chains to predict overall survival in the general population. Mayo Clin. Proc. 2012;87:517–23. doi: 10.1016/j.mayocp.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717971175
- 33.Anandram S, Assi LK, Lovatt T, Parkes J, Taylor J, Macwhannell A, Jacob A, Handa S, Harding S, Basu S. Elevated, combined serum free light chain levels and increased mortality: a 5-year follow-up, UK study. J. Clin. Pathol. 2012;65:1036–42. doi: 10.1136/jclinpath-2012-200910. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971176
- 34.Nakano T, Matsui M, Inoue I, Awata T, Katayama S, Murakoshi T. Free immunoglobulin light chain: its biology and implications in diseases. Clin. Chim. Acta. 2011;412:843–9. doi: 10.1016/j.cca.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Hutchinson AT, Jones DR, Raison RL. The ability to interact with cell membranes suggests possible biological roles for free light chain. Immunol. Lett. 2012;142:75–7. doi: 10.1016/j.imlet.2011.10.013. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971178
- 36.Nakano T, Takahashi H, Miyazaki S, Kawai S, Shinozaki R, Komoda T, Nagata A. Antigen-specific free immunoglobulin light-chain antibodies: could it be a new diagnostic marker for patients with allergy? Clin. Biochem. 2006;39:955–9. doi: 10.1016/j.clinbiochem.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Sun M, Li L, Gao QS, Paul S. Antigen recognition by an antibody light chain. J. Biol. Chem. 1994;269:734–8. [PubMed] [Google Scholar]
- 38.Thio M, Groot Kormelink T, Fischer MJ, Blokhuis BR, Nijkamp FP, Redegeld FA. Antigen binding characteristics of immunoglobulin free light chains: crosslinking by antigen is essential to induce allergic inflammation. PLoS ONE. 2012;7:e40986. doi: 10.1371/journal.pone.0040986. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717971179
- 39.Redegeld FA, van der Heijden MW, Kool M, Heijdra BM, Garssen J, Kraneveld AD, van Loveren H, Roholl P, Saito T, Verbeek JS, Claassens J, Koster AS, Nijkamp FP. Immunoglobulin-free light chains elicit immediate hypersensitivity-like responses. Nat. Med. 2002;8:694–701. doi: 10.1038/nm722. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971180
- 40.Huang ZQ, Sanders PW. Localization of a single binding site for immunoglobulin light chains on human Tamm-Horsfall glycoprotein. J. Clin. Invest. 1997;99:732–6. doi: 10.1172/JCI119218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen G, Rudnicki M, Hörl WH. Uremic toxins modulate the spontaneous apoptotic cell death and essential functions of neutrophils. Kidney Int. Suppl. 2001;78:S48–52. doi: 10.1046/j.1523-1755.59.s78.20.x. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971181
- 42.Cohen G, Rudnicki M, Deicher R, Hörl WH. Immunoglobulin light chains modulate polymorphonuclear leucocyte apoptosis. Eur. J. Clin. Invest. 2003;33:669–76. doi: 10.1046/j.1365-2362.2003.01191.x. [DOI] [PubMed] [Google Scholar]
- 43.Matsumori A, Shimada M, Jie X, Higuchi H, Groot Kormelink T, Redegeld FA. Effects of free immunoglobulin light chains on viral myocarditis. Circ. Res. 2010;106:1533–40. doi: 10.1161/CIRCRESAHA.110.218438. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717971182
- 44.Hutchison CA, Landgren O. Polyclonal immunoglobulin free light chains as a potential biomarker of immune stimulation and inflammation. Clin. Chem. 2011;57:1387–9. doi: 10.1373/clinchem.2011.169433. [DOI] [PMC free article] [PubMed] [Google Scholar]