Abstract
This study was carried out to better understand the epidemiology of hantaviruses in a province of Turkey (Giresun) where human hantavirus disease has recently been detected. In this cross-sectional study, a total of 626 blood samples from healthy people aged 15 and 84 years old were collected both in urban and rural areas in 2009. The sera were tested by enzyme-linked immunosorbent assay (ELISA), immunoblotting assay, and the focus reduction neutralization test (FRNT). We screened the samples by an ELISA and found that 65/626 samples reacted positively for the presence of hantavirus-reactive immunoglobulin G (IgG). Twenty of the 65 ELISA-positive samples could be confirmed by an immunobloting assay, and the overall seroprevalence was thereby calculated to 3.2% (20/626). The seroprevalence of the people living in wood areas or adobe houses 9/17 (52.9%) was significantly higher than among people living in concrete houses 10/47 (21.3%) (p=0.014). Finally, 3 of the 20 immunoblot-positive sera were confirmed as specific for the Puumala hantavirus serotype by FRNT, 1 serum was confirmed as Dobrava virus-specific, whereas 1 serum was found to be equally reactive to Dobrava and Saaremaa viruses. We will now focus on further investigations of the ecology and epidemiology of hantaviruses in humans and their carrier animals in Turkey, studies that have already been started and will be further intensified.
Key Words: Hantavirus, Serology, Turkey
Introduction
Hantaviruses (family Bunyaviridae, genus Hantavirus) are enveloped, single-stranded RNA viruses, carried primarily by rodents or insectivores of specific host species. The major route for humans to become infected is through contact with saliva, urine, or feces from infected rodents. This primarily happens through inhalation of aerosols stemming from rodent excreta that are contaminated with the virus. Hantaviruses causes two different types of disease in humans: Hemorrhagic fever with renal syndrome (HFRS) and hantavirus cardiopulmonary syndrome (HCPS). There are at present 7 hantaviruses known to be associated with HFRS, among them Hantaan virus (HTNV) in Asia and Dobrava virus (DOBV) in Europe, that cause the most severe form of HFRS with a mortality rate from 3% to 12% (Vapalahti et al. 2003, Bi et al. 2008). Saaremaa virus (SAAV) was recently recognized by the International Committee on Taxonomy of Viruses (ICTV) as a unique hantavirus species distinguished from DOBV and, along with Puumala virus (PUUV), reported to cause a milder form of HFRS in Europe (Sironen et al. 2005, Bi et al. 2008).
During the 1993 epidemic in the United States of America, Sin Nombre virus (SNV) was first identified as the causative agent of HCPS (Nichol et al. 1993). To date, at least 15 unique hantaviruses have been associated with HCPS, causing high mortality in the New World. While approximately 150.000 cases of HFRS are estimated to occur annually on a worldwide basis, in total only around 2000 HCPS cases have been reported to date (Vapalahti et al. 2003, Bi et al. 2008).
In Turkey, the epidemiology of hantavirus disease is poorly known. To our knowledge, only a few reports concerning hantaviruses in our country have been published before 2009 (Kavukçu et al. 1997, Laakkonen et al. 2006). The first report of a serologic survey for rodent-borne viruses in their natural hosts dates from year 2006. These preliminary results indicated that antibodies reactive to PUUV were present in Microtus voles by an immunofluorescence assay (IFA) (Laakkonen et al. 2006). In 1997, a sero-survey in humans, also based on IFA, suggested hantavirus immunoglobulin G (IgG) reactivity in 7.3% of the cases with nephropathy and in 2.6% of the cases without nephropathy or without any infectious disease. Kavukçu et al. concluded that hantaviruses circulate in the Aegean Region in Anatolia, but may only cause an abortive infection (Kavukçu et al. 1997).
Early in 2009, the human HFRS cases in Turkey were notified from 2 neighboring provinces (Zonguldak and Bartin) in the western Black Sea region of the country. The cases were interpreted as caused by PUUV (Ertek et al. 2009). From this date, sera taken from hantavirus-suspected cases have been sent to the Refik Saydam National Public Health Agency (RSNPHA) from different parts of the country, especially from the Black Sea regions. In August, 2009, a number of HFRS cases were detected in the Giresun province located in the eastern Black Sea region of the country (Kaya et al. 2010). In this study, we investigated the presence of hantavirus infections and identified possible risk factors of its occurrence in the Giresun province.
Materials and Methods
Study design
A cross-sectional study was conducted in 2009 in the Giresun province of Turkey, located at 40°55’ north latitude and 38°30’ east longitude. Reported seroprevalences of hantaviruses in the neighboring countries are approximately 2% (Bi et al. 2008). The sample size was estimated using Epi Info 2000 StatCalc software with a known prevalence of 2% and 0.75% worst acceptable prevalence within a 95% confidence level from a total population of 327,000 individuals aged between 15 and 84 years. The estimated sample size was 722 people. Assuming an average of 3 people living in 1 household, 215 households were sampled randomly from the address-based population counting system of the Turkish Statistical Institute (TurkStat).
All participants were over 15 years of age and healthy. Written informed consent was obtained from all the subjects who expressed willingness to participate in the study. Trained health staff interviewed every participant by using a standardized questionnaire. The questionnaire included demographic and epidemiological variables on residential area, housing condition, cutting or handling of wood, contact with rodents and/or rodent droppings, etc.
Serology
Serum samples were collected, transported at +4°C and stored at −25°C until tested. Laboratory tests were performed in the RSNPHA, Virology and Reference Laboratory, Novel and Dangerous Pathogens Unit in Ankara, Turkey, and the Swedish Institute for Communicable Disease Control/Karolinska Institutet in Stockholm, Sweden.
Anti-Hantavirus IgG enzyme-linked immunosorbent assay
The Hantavirus enzyme-linked immunosorbent assay (ELISA) test (Focus Diagnostics, DxSelectTM, USA) detects antibodies to the most clinically relevant pathogenic strains of hantaviruses, i.e, Seoul virus (SEOV), HTNV, PUUV, DOBV, SAAV, and SNV. The kit uses a cocktail of baculovirus-derived recombinant nucleoprotein (rNP) of various hantavirus strains. The test was performed according to the protocol provided by the manufacturer. Results were reported as index values (IV) relative to the cutoff calibrator. The IV was calculated by dividing specimen optical density (OD) values by the mean of the cutoff calibrator OD values. Samples that exhibited an IV of >1.1, were considered IgG positive to 1 or more hantavirus species.
Immunoblotting assay for Hantavirus
An immunoblot assay was used for confirmation of the ELISA IgG-positive samples. The “Hanta Profile 1 EUROLINE” tests were performed according to the protocol of the manufacturer (Euroimmun Medizinische Labordiagnostika AG, Germany). The strip provides a quantitative assay for detection of human antibodies of the IgG class to three different hantavirus serotypes—PUUV, DOBV, and HTNV. The processed strips were visually evaluated from (0) to (+++). Medium (+) to strongly (++/+++) colored bands were considered to indicate positivity.
Focus reduction neutralization test
For analyses by the focus reduction neutralization test (FRNT), PUUV strain Sotkamo (Brummer-Korvenkontio et al. 1982), SAAV strain Saaremaa (Nemirov et al. 1999), and DOBV strain Slovenia (Avsic-Zupanc et al. 1992) were used. The test was performed as described earlier (Lundkvist et al. 1997). Briefly, sera were serially diluted and mixed with an equal volume of diluted virus, containing 30–70 focus forming units/100 μL. The mixture was incubated at 37°C for 1 h and subsequently inoculated into 6-well tissue culture plates containing confluent Vero E6 cell monolayers. The wells were overlaid with a mixture of agarose and tissue culture medium and incubated for 9 days (DOBV, SAAV) or 13 days (PUUV). The agarose was removed from the wells, and the cells were fixed in methanol. Rabbit anti-hantavirus sera, followed by peroxidase-labeled goat antibodies to rabbit IgG (BioRad Laboratories, Hercules, CA), were added to indicate virus-infected cells. 3,3′,5,5′-tetramethylbenzidine substrate (Sigma) was used as substrate, and foci were enumerated. An 80% reduction of the number of foci, as compared to the virus control, was used as the criterion for virus neutralization titers.
Statistical analysis
The analysis of the results was carried out using SPSS for Windows (version 17.0). Correlates between seropositivity and variables were calculated with chi-squared and Fisher exact tests. All p values were two-tailed and the statistical significance was set at p<0.05.
Results
In this study, 626 samples were screened by an ELISA, and 65 were found positive for the presence of hantaviruses-reactive IgG; seroprevalence was 10.4%. Twenty of these 65 samples were also found to be positive by immunoblotting. According to the results of the immunblot, the hantavirus IgG seroprevalence was calculated to 3.2% (20/626) in Giresun province.
The characteristics of the subjects, the results of the ELISA, and the immunoblotting are summarized in Table 1. The seropositivity of the people living in wood or adobe houses 9/17(52.9%) was significantly higher than that among the people living in concrete houses 10/47 (21.3%) (p=0.014) by immunoblotting and 17/106 (16%) and 47/506 (9.3%) (p=0.039) by ELISA, respectively. According to the immunoblotting results, although the prevalence of hantavirus-reactive IgG was higher among males 9/24 (37.5%), in the age group 45 years and older 10/24 (41.7%), among people with a history of contact with rodents 17/47 (36.2%), and among people cutting or handling wood 16/49 (32.7%) than others, these differences were not significant (p=0.368, p=0.178, p=0.148, and p=1.000, respectively). An individual who had a habit of drinking water from lakes or streams was 1.42 times more likely to have been infected, but the difference was not significant statistically (p=0.545). Although the seropositivity was higher among the people working in fields and/or pastures 18/56 (32.1%) compared to people not working 2/7 (28.6 %), the differences were not statistically significant (p=1.000).
Table 1.
Relationship Between Independent Variables and Antibody to Hantaviruses
| |
ELISAa |
Immunoblotting assay |
|
|||
|---|---|---|---|---|---|---|
| Variable | No. positive/ no. tested (% antibody positive) | Odds ratio | p value | No. positive/ no. tested (% antibody positive) | Odds ratio | p value |
| Sex | ||||||
| Male | 24/256 (9.4) | 1.21 | 0.491 | 9/24 (37.5) | 0.61 | 0.368 |
| Female | 41/370 (11.1) | 11/41 (26.8) | ||||
| Age | ||||||
| 15–44 | 40/326 (12.3) | 0.63 | 0.085 | 10/40 (25.0) | 2.14 | 0.178 |
| ≥45 | 24/297 (8.1) | 10/24 (41.7) | ||||
| Living in wood or adobe house | ||||||
| Yes | 17/106 (16.0) | 1.87 | 0.039 | 9/17 (52.9) | 4.16 | 0.014 |
| No | 47/506 (9.3) | 10/47 (21.3) | ||||
| Exposure to rodentsb | ||||||
| Yes | 47/462 (10.2) | 0.92 | 0.772 | 17/47 (36.2) | 2.83 | 0.148c |
| No | 18/164 (11.0) | 3/18 (16.7) | ||||
| Cleaning lofts, cellars, sheds | ||||||
| Yes | 45/405 (11.1) | 1.26 | 0.419 | 13/45 (28.9) | 0.75 | 0.622 |
| No | 20/221 (9.0) | 7/20 (35.0) | ||||
| Cleaning barns, haylofts | ||||||
| Yes | 26/211 (12.3) | 1.36 | 0.257 | 5/26 (19.2) | 0.38 | 0.169c |
| No | 39/415 (9.4) | 15/39 (38.5) | ||||
| Cutting or handling wood | ||||||
| Yes | 49/424 (11.6) | 1.63 | 0.118 | 16/49 (32.7) | 1.21 | 1.000c |
| No | 14/189 (7.4) | 4/14 (28.6) | ||||
Enzyme-linked immunosorbent assay (ELISA).
Contact with rodents and/or rodent droppings in pastures, lofts, cellars, sheds, barns, or haylofts.
Determined by using two-tailed Fisher exact test.
The 20 sera that were found to be positive by immunoblotting were tested further by FRNT. One sample (serum no. 20) was confirmed as DOBV-specific by FRNT. Another sample (serum no.19) was confirmed as DOBV/SAAV-specific by FRNT. Serum numbers 19 and 20 were further tested against SEOV and HTNV viruses by FRNT and shown to be negative or only low reactive (maximum titer 1:40), thus confirming the DOBV, or DOBV/SAAV, specificity. Three (sera no. 16, 17, and 18) of the 20 immunoblot-positive samples could be further confirmed as PUUV-specific by FRNT. The results of the immunoblotting, ELISA, and FRNT are summarized in Table 2. In Table 2, the sample numbers, the serotype, and their titers of the FRNT-positive samples are in bold.
Table 2.
Some Characteristics and Reactivity of Immunoblotting, ELISA, and FRNT Assay in 20 Immunoblotting-Positive Sera
| |
|
|
|
|
|
Hantavirus IgG ELISA (Focus Diagnostic) |
Hantavirus IgG Immunoblotting assay (Euroimmun) |
Immunoblotting assay |
FRNTa |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sera nos. | Age/gender | Contact with rodents at home | Contact with rodents in outbuildings | Working in pastures | Cutting or Handling wood | Test value | Serotype PUUV, DOBV, HTNV | Probable serotype | PUUV | DOBV | SAAV |
| 1 | 41/m | + | + | + | 5,15 | −, ++, − | DOBV | <40 | <40 | <40 | |
| 2 | 54/m | − | + | + | + | 1,53 | −, +,+ | DOBV/HTNV | <40 | <40 | <40 |
| 3 | 54/f | − | + | − | − | 1,76 | −, +,+ | DOBV/HTNV | <40 | <40 | <40 |
| 4 | 81/f | − | + | + | + | 1,97 | −, ++, − | DOBV | <40 | <40 | <40 |
| 5 | 70/m | + | + | + | + | 2,32 | −, ++,+ | DOBV | <40 | <40 | <40 |
| 6 | 17/f | − | + | − | + | 1,45 | −, +, − | DOBV | <40 | <40 | <40 |
| 7 | 39/f | + | + | − | + | 3,57 | −, +, − | DOBV | <40 | <40 | <40 |
| 8 | 20/f | − | − | − | − | 1,18 | +,+++,+++ | DOBV/HTNV | <40 | <40 | <40 |
| 9 | 20/f | − | − | − | − | 1,16 | +,+, − | DOBV /PUUV | <40 | <40 | <40 |
| 10 | 71/f | + | + | + | + | 3,21 | +,++,+ | DOBV | <40 | <40 | <40 |
| 11 | 75/m | − | − | + | + | 1,26 | +, −, ++ | HTNV | <40 | <40 | <40 |
| 12 | 41/m | − | + | + | + | 1,41 | ++,+,+ | PUUV | <40 | <40 | <40 |
| 13 | 36/f | − | + | + | − | 2,04 | +,+++,+++ | DOBV/HTNV | <40 | <40 | <40 |
| 14 | 54/m | − | + | + | + | 1,62 | −, ++,+ | DOBV | <40 | <40 | <40 |
| 15 | 44/f | − | + | + | + | 1,11 | ++,++, − | PUUV/DOBV | <40 | <40 | <40 |
| 16 | 48/f | − | + | + | + | 1,59 | ++, −, − | PUUV | >40 | <40 | <40 |
| 17 | 18/m | + | + | + | + | 2,34 | +,+, − | PUUV/DOBV | >40 | <40 | <40 |
| 18 | 37/m | − | + | + | + | 1,42 | +,+, − | PUUV/DOBV | >40 | <40 | <40 |
| 19 | 75/m | + | + | + | + | 8,51 | ++,+++,+++ | DOBV/HTNV | <40 | 160 | 160 |
| 20 | 80/f | + | + | + | + | 9,73 | +,+++,+++ | DOBV/HTNV | 40 | 640 | 160 |
For the positive samples, the sample numbers, the serotype, and their titers are in boldface.
Cutoff value=1:40. Sera 16–18 are confirmed as specific for the Puumala virus, serum no. 19 is equally reactive to Dobrava and Saaremaa viruses, serum no. 20 is Dobrava virus specific.
ELISA, Enzyme-linked immunosorbent assay; FRNT, focus reduction neutralization test; IgG, immunoglobulin G; PUUV, Puumala virus; DOBV, Dobrava virus; HTNV, Hantaan virus; m, male; f, female; −, negative; +, positive.
The 3 individuals who were confirmed as seropositive for PUUV-specific antibodies were all working in pastures, cutting or handling wood, and reported previous contact with rodents at least during the outbuilding activity. Both individuals who were found positive for DOBV-specific antibodies were older than 75 years and reported several risk factors for hantavirus infections, such as contact with rodents at home and in outbuildings, working in pastures, and cutting or handling wood.
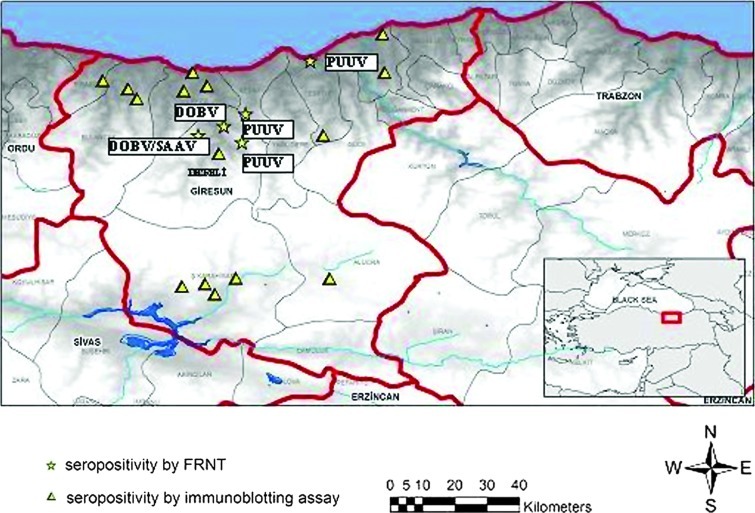
Four confirmed subjects (1 DOBV, 1 DOBV/SAAV, and 2 PUUV cases) were living in the Dereli village (Fig. 1). In parallel, seroprevalance was higher in Dereli by ELISA 8/36 (22.2%) and by immunoblotting 5/8 (62.5%) than the other villages in Giresun province. A stream passes through the boundaries of Dereli. Approximately 40% of the village land is landscaping and forest and 30% is grassland and pasture. The village has mixed forest with dense vegetation, optimal for bank voles. Only 3% of the total land is agricultural land; the most common product is hazelnuts. There are cattle and sheep farming in the region.
FIG. 1.
Map of Giresun, results of the seropositivity by using immunoblotting assay and focus reduction neutralization test (FRNT). (Color image available online at www.liebertpub.com/vbz).
Discussion
Hantavirus disease or HFRS is an endemic zoonosis that affects tens to thousands of individuals in Europe and Asia. PUUV and DOBV have caused the vast majority of human cases in Europe, with the virus transmitted to humans mainly through persistently infected bank voles (Myodes glareolus, previously known as Clethrionomys glareolus) and the yellow-necked field mouse (Apodemus flavicollis), respectively (Vapalahti et al. 2003, Bi et al. 2008, Heyman et al. 2008, 2009). In western and northern Europe, only PUUV infections have been reported, with the largest number observed in Finland. The DOBV/PUUV infection ratio varied from 3.6% in Southern Germany to more than 50% in Slovenia and up to 100% in Greece from the west and north to eastern Europe. Although HFRS caused by PUUV is usually seen as a milder form of HFRS (nephropathia epidemica=NE), DOBV is associated with severe HFRS with high rates of mortality (Heyman et al. 2009). Recently, another European hantavirus, genetically closely related to DOBV, was found in the striped field mice (Apodemus agrarius) (Nemirov et al. 1999) and named SAAV (or DOBV-A.a.). The virus has been found to circulate in Estonia, Russia, Denmark, Slovenia, and Slovakia (Heyman et al. 2009, Jonsson et al. 2010, Kaya et al. 2010) and to be associated with human disease in outbreaks in Russia and cases in Germany and Slovakia, with mild, NE-like HFRS (Sibold et al. 1999, Klempa et al. 2003, 2008, Heyman et al. 2009, Jonsson et al. 2010). SAAV was recently recognized by the ICTV as a unique hantavirus species.
Of the 626 participants involved in the survey, 20 sera had hantavirus-specific antibodies detected by immunoblotting. Three of 20 immunoblot-positive sera were confirmed as PUUV-specific by FRNT, and 2 samples were found as specific for DOBV/SAAV (serum no. 19) or for DOBV (serum no. 20) by FRNT. Sample 19 was strongly positive by ELISA (TV: 8.51) and immunoblotting (PUUV, DOBV, HTNV: ++, +++, +++) and the other (sample 20) was also found strongly positive by ELISA (TV: 9.73) and immunoblotting (+, +++, +++). The other probable DOBV serotype positivities detected by immunoblotting could not be confirmed by FRNT. One possible reason for the low numbers of samples confirmed by FRNT (total 5) as compared to 20 immunoblot-positive samples might be that there exist (unknown) hantavirus strains/viruses that are distinguished from the different strains included in the FRNT. Therefore, further studies are needed, and RSNPHA is at present carrying out analyses of further potential unknown hantaviruses/strains circulating in Turkey.
We calculated the hantavirus seroprevalance 0.8% by using FRNT. Asymptomatic or mild infections with nonspecific symptoms may have caused the real number of hantavirus infections to be largely underestimated in Turkey until 2009. In Europe, the seroprevalence for hantaviruses is reported to be up to 24.0% in some areas, predominantly by PUUV (Bi et al. 2008). In our previous study, carried out among risk groups for hantavirus infections in Bartin located in the western part of the Black Sea region of Turkey, we found 5.2% PUUV seropositivity by immunoblotting (testing by RSNPHA, Virology and Reference Laboratory, Novel and Dangerous Pathogens Unit) and 4.2% PUUV seropositivity by FRNT (testing by the Swedish Institute for Communicable Disease Control, Stockholm, Sweden) (our unpublished data).
According to earlier studies, the average age for developing NE is 35–42 years; the male-to-female ratio of clinical cases varies from 5/1 to 2/1 (Vapalahti et al. 2003). In the present study, although the positivity by immunoblotting increased in males and in the age group 45 years and older, there was no statistically significant difference. The variables that included cleaning lofts, cellars, sheds, and barns/haylofts were not associated with an increased hantavirus seropositivity. However, it has earlier been reported that the incidence of hantavirus infection is higher among individuals with low socioeconomic status because of poor housing conditions and is also related to gardening and cleaning closed areas (Schmaljohn et al. 1997, Crowcroft et al. 1999, Abu Sin et al. 2007, Zhang et al. 2009).
Occupations including forestry work, farming, and hunting that favor human–rodent contact are associated with a higher risk of hantavirus infection (Schmaljohn et al. 1997, Crowcroft et al. 1999, Vapalahti et al. 2003, Abu Sin et al. 2007, Schultze et al. 2007, Gledovic et al. 2008). However, there was no increased seropositivity among persons cutting or handling wood in the present study, either among people who were living close to or into forest areas and spending more time in the forest, which is a major risk factor according to earlier studies (Crowcroft et al. 1999, Abu Sin et al. 2007). However, the immunoblotting seropositivity of people living in wood or adobe houses 9/17 (52.9%) was significantly higher than the people living in concrete houses 10/47 (21.3%) (p=0.014). Interestingly, we found that individuals who had a habit of drinking water from lakes or streams were 1.42 times (p=0.545) more likely to have been infected, indicating that this may be a risk factor for hantavirus infection (Gledovic et al. 2008, Mesić et al. 2008).
According to the results of the FRNT, 3 sera were confirmed to be PUUV specific, whereas one of them was confirmed as DOBV-specific, and one of them reacted equally to DOBV/SAAV. It is known that the main reservoir for PUUV is M. glareolus and for DOBV A. flavicollis. M. glareolus is distributed mainly in the Black sea area in northern Turkey (including Giresun), and A. flavicollis is present all over Turkey, except for inner Anatolia, southeastern Anatolia, and the southern part of eastern Anatolia (including Giresun). We suggest that the sample found as specific for DOBV/SAAV was likely DOBV because A. agrarius, known to be the reservoir for SAAV, is distributed only in the European part of Turkey (Kryštufek et al. 2005, Yiğit et al. 2006, Kryštufek et al. 2009), far away from Giresun.
The survey was carried out in the Giresun province located in the eastern part of the Black Sea region where the climate is characterized by mild–humid and cold–humid, with an average of 1500–2500 millimeters annual precipitation (Atalay and Efe 2010). The region has mountains extending parallel to the Black Sea coast. The altitude of the mountains is as high as 3331 meters in some areas. The altitude, exposure, and the extended direction of the mountain belt plays an important role in the distribution of precipitation and temperature, and consequently on the vegetation. As a result, the vegetation type is mainly constituted of broadleaf deciduous humid forests appearing along the coastal belt of the mountain and is related to the both solar radiation and the precipitation, while the upper parts of the mountains are the main areas of Picea abies forests (Atalay 2006, Atalay and Efe 2010).
We concluded that the deforestation, the changing of agricultural practice during the last decade, the switch from hazelnuts to kiwi since 2000, and the climate change can differentially impact the rodent population densities and, consequently, the risk of disease transmission to humans in Giresun (Goodin 2006, Klempa 2009, Jonsson et al. 2010). For example, the agricultural switch might have forced the rodents, normally preferring hazelnuts, to human dwellings, putting them into closer contact with the human population. On the other hand, maize cultivation had been decreased due to corn-feed livestock having been decreased by 30–45% in the area, and, consequently, rodents began to break into homes to find food (unpublished results).
The meteorological data of the past 30 years suggest that the average summer (June, July, and August) and autumn (September, October, and November) temperatures are 22.1°C and 16.2°C, respectively. Mean annual precipitation was 106.2 mm and annual humidity was 73.7%. The average of total precipitation in 2007 (119.0 mm) was higher than many years in the past, with flooding in July. We found, when analyzing the data from the Turkish State Meteorological Service, that the high autumn temperatures followed high summer temperatures in 2000, 2002, 2004, 2006, and 2008. The high rodent density has been related to a mast year, with high seed production of oak and beech, that is induced by high summer temperatures 2 years and high autumn temperatures 1 year prior to hantavirus epidemics in central and western Europe (Vapalahti et al. 2003, Heyman et al. 2007, Schilling et al. 2007, Klempa 2009, Jonsson et al. 2010, Heyman et al. 2011). According to the information provided by agriculture and forestry experts, the rodent density in Giresun began to rise in 2008, and the increase continued during 2009. The first human case in the province was reported in 2009 (Kaya et al. 2010). Therefore, an increase in human contact with these animals and consequently the frequency of hantavirus infections is possibly related to changes in the habitat of those rodents in the last years in the region. It is not clear, however, whether the virus had been present previously in these areas at very low levels or in small ecological niches, thereby presenting only a negligible risk for humans, or whether it has been newly introduced.
Conclusions
Further studies regarding the emergence and expansion of hantavirus infection in the country are needed both in human and rodent populations. However, it is important that the detection of antibodies to hantaviruses among the asymptomatic subjects in this study should increase awareness of this disease in Turkey. It is obvious that hantavirus infections are not recognized by the medical community in many countries, including large areas of South America and Europe, even though hantaviruses are globally important “emerging and re-emerging pathogens,” with a seroprevalences of several percent in the population of those areas (Vaheri et al. 2008).
We now need to focus on analytical studies to clarify the risk factors and identify sero/genotyping of hantaviruses in humans and their natural hosts in different geographical regions of Turkey. In the current study, we evaluated data from 626 people who provided serum samples that resulted a 87% response rate (626 out of 722 people targeted). In population surveys, a response rate over 85% is desirable. We have not considered design effect for sampling from the households rather than individulas due to time and resource constraints. This clustering may have an effect on results presented in Table 1. However, we think this effect is modest given the small number of people living in each household. On the other hand geographic clustering and design effects should be considered in the future population studies.
Acknowledgments
This study was supported with a grant by the Refik Saydam National Public Health Agency in Ankara and Turkey, the Swedish Research Council (Project Number 121 77), and the European Union grant FP7-261504EDENext. The paper is catalogued by the EDENext Steering Committee as EDENext 00xx (http://www.edenext.eu).
We thank the Giresun Provincial Health Directorate staff for their kind technical support to the study: in alphabetical order, Ayşe Ceren Altıntaş, Emrullah Baş, Rukiye Pelin Başar, İlker Bay, Gülüzar Çağla, Ayşe Çekiç, Özgül Çınarcık, Ayşegül Çulfaz, Çiğdem Demir, Veysel Demir, Özge Demirci, Serdar Demiröz, Nezahat Diner, Hikmet Doma, Neşe Doma, Elif Epsileli, Şebnem Erdoğan, Aysel Gügercin, İrem Güleç, Zennuriye Gürel, Kamil Emre Gürgün, Deniz Kamer, Aynur Karaca, Güler Karameşe, Ali Volkan Kaya, Gönül Kekül, Neslihan Kemal, Celal Köse, Nafiz Ömer Namazcı, Emine Özdemir, Banu Özge, Ayla Patan, Saime Pekdemir, Zehra Sayın, Seval Sıvalı, Cem Sundu, Onur Şenel, Muammer Şenol, Aysun Tan, Engin Temur, Canan Terzi, Uğur Topal, Ruşen Topallı, Abdullah Turan, Ayla Turan, Mustafa Turan, Selda Türker, Deniz Usta, Esen Usta, Mevhibe Ülker, Arife Sarı Yılmaz, Arife Yılmaz, Murat Yılmaz, Emel Yüce, and Canan Zehir Terzi. We thank Ahmet Aydemir and Şukran Kara for technical support from the Refik Saydam National Public Health Agency.
Author Disclosure Statement
No competing financial interests exist.
References
- Abu Sin M. Stark K. van Treeck U. Dieckmann H, et al. Risk factors for hantavirus infection in Germany,2005. Emerg Infect Dis. 2007;13:1364–1346. doi: 10.3201/eid1309.070552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atalay I. The effects of mountainous areas on biodiversity: A case study from the Northern Anatolian mountains and the Taurus mountains. Grazer Schriften der Geographie und Raumforschung Bant 41/2006. Graz, Austria: Institute for Geography and Regional Science. 2006:17–26. Karl Franzens University. [Google Scholar]
- Atalay I. Efe R. Structural and distributional evaluation of forest ecosystems in Turkey. J Environ Biol. 2010;31:61–70. [PubMed] [Google Scholar]
- Avsic-Zupanc T. Xiao SY. Stojanovic R. Gligic A, et al. Characterization of Dobrava virus: A Hantavirus from Slovenia, Yugoslavia. J Med Virol. 1992;38:132–137. doi: 10.1002/jmv.1890380211. [DOI] [PubMed] [Google Scholar]
- Bi Z. Formenty PB. Roth CE. Hantavirus infection: A review and global update. J Infect Dev Ctries. 2008;2:3–23. doi: 10.3855/jidc.317. [DOI] [PubMed] [Google Scholar]
- Brummer-Korvenkontio M. Henttonen H. Vaheri A. Hemorrhagic fever with renal syndrome in Finland: Ecology and virology of nephropathia epidemica. Scand J Infect Dis Suppl. 1982;36:88–91. [PubMed] [Google Scholar]
- Crowcroft NS. Infuso A. Ilef D. Le Guenno B, et al. Risk factors for human hantavirus infection: Franco-Belgian collaborative case-control study during 1995–6 epidemic. Br Med J. 1999;318:1737–1738. doi: 10.1136/bmj.318.7200.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertek M. Buzgan T. Refik Saydam National Public Health Agency; Ministry of Health Ankara Turkey. An outbreak caused by hantavirus in the Black Sea region of Turkey, January–May 2009. Euro Surveill. 2009;14:1–2. doi: 10.2807/ese.14.20.19214-en. [DOI] [PubMed] [Google Scholar]
- Gledovic ZB. Jeknic AS. Grgurevic AD. Rakocevic BB, et al. Hemorrhagic fever with renal syndrome in Montenegro. Jpn J Infect Dis. 2008;61:386–387. [PubMed] [Google Scholar]
- Goodin DG. Koch DE. Owen RD. Chu YK, et al. Land cover associated with hantavirus presence in Paraguay. Global Ecol Biogeogr. 2006;15:519–527. [Google Scholar]
- Heyman P. Vaheri A. ENVID members. Situation of hantavirus infections and haemorrhagic fever with renal syndrome in European countries as of December 2006. Euro Surveill. 2008;13:1–7. [PubMed] [Google Scholar]
- Heyman P. Cochez C. Ducoffre G. Mailles A, et al. Haemorrhagic fever with renal syndrome: An analysis of the outbreaks in Belgium, France, Germany, the Netherlands and Luxembourg in 2005. Euro Surveill. 2007;12:E15–E16. doi: 10.2807/esm.12.05.00712-en. [DOI] [PubMed] [Google Scholar]
- Heyman P. Vaheri A. Lundkvist A. Avsic-Zupanc T. Hantavirus infections in Europe: From virus carriers to a major public-health problem. Expert Rev Anti Infect Ther. 2009;7:205–217. doi: 10.1586/14787210.7.2.205. [DOI] [PubMed] [Google Scholar]
- Heyman P. Cochez C. Korukluoğlu G. Gözalan A, et al. Bridging continents; Hantaviruses of Europe and Asia Minor. Turk Hij Den Biyol Derg. 2011;68:41–48. [Google Scholar]
- Jonsson CB. Figueiredo LT. Vapahaldi O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin Microbiol Rev. 2010;23:412–441. doi: 10.1128/CMR.00062-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavukçu S. Türkmen M. Salman Ş. Soylu A, et al. What is the risk of nephropathy associated with hantavirus in Aegean region? Office Journal of the Turkish Nephrology, Association. 1997:3–4. 131–135. [Google Scholar]
- Kaya S. Yılmaz G. Erensoy S. Yağcı Çağlayık D, et al. Hantavirus infection: Two case reports from a province in the Eastern Blacksea Region, Turkey. Mikrobiyol Bul. 2010;3:479–487. [PubMed] [Google Scholar]
- Klempa B. Hantaviruses and climate change. Clin Microbiol Infect. 2009;15:518–523. doi: 10.1111/j.1469-0691.2009.02848.x. [DOI] [PubMed] [Google Scholar]
- Klempa B. Meisel H. Räth S. Bartel J, et al. Occurrence of renal and pulmonary syndrome in a region of northeast Germany where Tula hantavirus circulates. J Clin Microbiol. 2003;41:4894–4897. doi: 10.1128/JCM.41.10.4894-4897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempa B. Tkachenko EA. Dzagurova TK. Yunicheva YV, et al. Hemorrhagic fever with renal syndrome caused by 2 lineages of Dobrava hantavirus, Russia. Emerg Infect Dis. 2008;14:617–625. doi: 10.3201/eid1404.071310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryštufek B. Vohralík V. Knjiznica Annales Majora; Koper, Slovenia: 2005. Mammals of Turkey and Cyprus: Rodentia I: Sciuridae, Dipodidae, Gliridae, Arvicolinae; p. 292. [Google Scholar]
- Kryštufek B. Vohralik V. University of Primorska; Koper, Slovenia: 2009. Mammals of Turkey and Cyprus, Rodentia II: Cricetinae, Muridae, Spalacidae, Calomyscidae, Capromyidae, Hystricidae, Castoridae; pp. 1–372. [Google Scholar]
- Laakkonen J. Kallio-Kokko H. Oktem MA. Blasdell K, et al. Serological survey for viral pathogens in Turkish rodents. J Wildl Dis. 2006;42:672–676. doi: 10.7589/0090-3558-42.3.672. [DOI] [PubMed] [Google Scholar]
- Lundkvist A. Hukic M. Hörling J. Gilljam M, et al. Puumala and Dobrava viruses cause hemorrhagic fever with renal syndrome in Bosnia-Herzegovina: Evidence of highly cross-neutralizing antibody responses in early patient sera. J Med Virol. 1997;53:51–59. [PubMed] [Google Scholar]
- Mesić S. Almedin H. Investigation of modes of hantavirus infection transmission from rodents to humans. Med Arh. 2008;6:229–230. [PubMed] [Google Scholar]
- Nemirov K. Vapalahti O. Lundkvist A. Vasilenko V, et al. Isolation and characterization of Dobrava hantavirus carried by the striped field mouse (Apodemus agrarius) in Estonia. J Gen Virol. 1999;80(Pt 2):371–379. doi: 10.1099/0022-1317-80-2-371. [DOI] [PubMed] [Google Scholar]
- Nichol ST. Spiropoulou CF. Morzunov S. Rollin PE, et al. Genetic identification of hantavirus associated with an outbreak of acute respiratory illness. Science. 1993;262:914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- Schilling S. Emmerich P. Klempa B. Auste B, et al. Hantavirus disease outbreak in Germany: Limitations of routine serological diagnostics and clustering of virus sequences of human and rodent origin. J Clin Microbiol. 2007;45:3008–3014. doi: 10.1128/JCM.02573-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohn C. Hjelle B. Hantaviruses: A global disease problem. Emerg Infect Dis. 1997;3:95–104. doi: 10.3201/eid0302.970202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze D. Fierz W. Matter HC. Bankoul S, et al. Cross-sectional survey on hantavirus seroprevalence in Canton St. Gallen, Switzerland. Swiss Med Wkly. 2007;137:21–26. doi: 10.4414/smw.2007.11594. [DOI] [PubMed] [Google Scholar]
- Sibold C. Meisel H. Lundkvist A. Schulz A, et al. Short report: Simultaneous occurence of Dobrava, Puumala, and Tula Hantaviruses in Slovakia. Am J Trop Med Hyg. 1999;61:409–411. doi: 10.4269/ajtmh.1999.61.409. [DOI] [PubMed] [Google Scholar]
- Sironen T. Vaheri A. Plyusnin A. Phylogenetic evidence for the distinction of Saaremaa and Dobrava hantaviruses. Virol J. 2005;2:90. doi: 10.1186/1743-422X-2-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A. Vapalahti O. Plyusnin A. How to diagnose hantavirus infections and detect them in rodents and insectivores. Rev Med Virol. 2008;18:277–288. doi: 10.1002/rmv.581. [DOI] [PubMed] [Google Scholar]
- Vapalahti O. Mustonen J. Lundkvist A. Henttonen H, et al. Hantavirus infections in Europe. Lancet Infect Dis. 2003;3:653–661. doi: 10.1016/s1473-3099(03)00774-6. [DOI] [PubMed] [Google Scholar]
- Yiğit N. Çolak E. Sözen M. Karataş A. Rodents of Türkiye: Türkiye Kemiricileri. In: Demirsoy A, editor. 1th. Ankara: Meteksan Yayınevi; 2006. [Google Scholar]
- Zhang YZ. Zhang FX. Wang JB. Zhao ZW, et al. Hantaviruses in rodents and humans, Inner Mongolia Autonomous Region, China. Emerg Infect Dis. 2009;15:885–891. doi: 10.3201/eid1506.081126. [DOI] [PMC free article] [PubMed] [Google Scholar]